Abstract
Attenuated Salmonella strains are of interest as new vaccine candidates and as vectors of cloned genes of other organisms. Attenuated strains expressing specific cytokines were constructed as a means of manipulating the immune response in various disease settings. In the present study, interleukin-2 (IL-2)-expressing (GIDIL2) or tumor necrosis factor alpha (TNF-α)-expressing (GIDTNF) strains were compared with the parent strain (BRD509) for the effect of cytokines on anti-Salmonella immunity. Expression of IL-2 resulted in a rapid clearance of the organism soon after vaccination. The reduction in GIDIL2 CFU was 50- to 300-fold higher than that of BRD509 and correlated with a markedly decreased splenomegaly. Furthermore, no evidence for any significant activation, including upregulation of surface markers and production of nitric oxide (NO), was observed in spleens of GIDIL2-injected mice. In contrast, the host response to GIDTNF was marked by an early, strong, splenic cellular influx, but surprisingly, the degree of induced splenomegaly and NO secretion was only 50% of that observed in BRD509-treated mice. Despite this, bacterial colonization of the spleen in GIDTNF-immunized animals was either slightly decreased from or equivalent to that of the BRD509-treated group, suggesting the induction of additional antimicrobial mechanisms by TNF-α. In vivo protection studies demonstrated that, at limiting doses, GIDIL2 was inferior to GIDTNF and BRD509 in its capacity to protect against virulent challenge. At high doses, however, all three strains exhibited equal protective efficacy. These results demonstrate that the immune response against intracellular bacteria can be manipulated by pathogen-expressed cytokines and open the way for further fine tuning of immune responses not only to Salmonella strains themselves but also to the heterologous gene(s) carried by them.
Salmonellae comprise a family of enteric pathogens characterized as facultative intracellular bacteria capable of inducing a range of systemic diseases, including typhoid fever, gastroenteritis, and bacteremia in human and animal hosts. Attenuated strains of Salmonella have been developed by genetic targeting of genes involved in essential metabolic pathways. The most widely used approach has been the introduction of defined deletions into genes encoding enzymes involved in the biosynthetic pathway of aromatic amino acids (16). The resultant mutants are thus rendered avirulent by virtue of their inability to synthesize several critically needed aromatic compounds not found in mammalian hosts. Such mutants have become of great interest not only for their potential as the next generation of new and improved vaccines against Salmonella infections but also as mucosal vaccine delivery systems of a wide range of heterologous antigens of other pathogens (reviewed in reference 8). Salmonella enterica strains of both the Typhimurium and Typhi serovars carrying single or double mutations in aro genes were shown to be effective in providing long-term protection in the mouse model of typhoid fever as well as in inducing good cell-mediated and humoral immunity in human volunteers (21, 42, 43). Additionally, several aroA/aroD double mutants of serovar Typhimurium have been engineered to express defined cytokines, such as interleukin-1β (IL-1β), IL-2, IL-4, IL-6, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), and were shown to be efficient vectors for the delivery of these cytokines in certain in vivo experimental conditions (7, 9, 12, 46). Thus, targeted delivery of cytokines can potentially be useful as a therapeutic approach in the treatment of autoimmune conditions, infectious diseases, and cancer.
Several studies have demonstrated the importance of the innate immune response in limiting bacterial multiplication in the early phase of Salmonella infection, ascribing roles for macrophages, NK cells, and γδ T cells in this process (reviewed in reference 18). Salmonella pathogenicity is strongly correlated with the ability to resist the antimicrobial defense mechanisms of and survive within the tissue macrophages of the host (13). In addition to serving as host cells to Salmonella organisms, macrophages also initiate the innate immune response by producing IL-12, a known growth factor for NK cells (44). The importance of NK cells has been shown to be largely due to their ability to rapidly secrete IFN-γ, a cytokine with potent macrophage activation properties, following infection (35, 37). The early release of IFN-γ and TNF-α results in the activation of tissue macrophages, enabling them to control bacterial growth and multiplication (28, 29). Tissue macrophages utilize two main antimicrobial mechanisms: production of reactive nitrogen intermediates and reactive oxygen intermediates, catalyzed by inducible nitric oxide synthase (NOS2) and phagocyte oxidase enzymes, respectively (6, 38). Both pathways contribute to defense against Salmonella infection and have largely overlapping functions in maintaining host resistance to endogenous microbial flora (38). In addition to the direct cytotoxic function of reactive nitrogen intermediates against microbial pathogens, recent evidence demonstrated an important regulatory role for nitric oxide (NO) in the innate immune response. Within a few hours of pathogen entry, NOS2 is activated transiently, an event shown to be critical for the activation and maturation of NK cells (10). This regulatory role of NOS2 appears to relate to its participation in the IL-12 signaling pathway in NK cells, enabling these cells to secrete large amounts of IFN-γ (11). Thus, the secretion of IFN-γ by NK cells during the innate phase of the immune response, which is critical for empowering host macrophages with the ability to control microbial infection via the production of NO, is itself dependent on an early and transient activation of NOS2. This pattern of interaction illustrates a hitherto unappreciated degree of sophistication governing innate immune responses.
We undertook a comprehensive analysis of the regulation of immunity to facultative intracellular bacteria by studying the host response to Salmonella following immunization with Salmonella strains expressing defined inflammatory as well as regulatory cytokines. In this report, the outcome of administering attenuated serovar Typhimurium strains expressing IL-2 or TNF-α was compared with that for the non-cytokine-expressing parent strain by using a number of parameters. The results demonstrate that the immune response can be readily and rapidly influenced by bacterially expressed cytokines. Furthermore, the differential activation of immune response parameters is shown to have a profound effect on the overall level of protection afforded by these vaccine strains of Salmonella.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Harlan Olac (Bicester, United Kingdom) and bred in the animal facility of the Faculty of Medicine and Health Sciences, UAE University. Female mice aged 8 to 12 weeks of age were used.
Bacterial strains and growth conditions.
The aroA/aroD mutant strain of S. enterica serovar Typhimurium, designated BRD509, has been described previously (41). The derivation of cytokine-expressing strains GIDIL2 and GIDTNF, producers of murine IL-2 and TNF-α, respectively, was fully described elsewhere (46). Cytokine expression is under the control of the anaerobic growth-induced nirB promoter, and cytokine expression plasmids were maintained in Salmonella by selection on 100 μg of ampicillin/ml. The fully virulent Salmonella strain, SL1344, is the parental wild-type strain from which BRD509 was originally derived (16, 41). The 50% lethal dose (LD50) for BALB/c mice of BRD509, GIDIL2, or GIDTNF, given intraperitoneally (i.p.), is >2 × 106 CFU; in contrast, the LD50 of SL1344 is <5 CFU (our unpublished data). Aliquots of frozen bacteria were plated on Trypticase soy agar (Oxoid, Basingstoke, United Kingdom) plates with (GIDIL2 and GIDTNF) or without (BRD509 and SL1344) ampicillin. Five to ten CFU was cultured overnight in Trypticase soy broth and then diluted 1:10 in fresh medium and grown for a further 2 to 3 h at 37°C. Appropriate dilutions were made in pyrogen-free phosphorus-buffered saline (PBS) (Sigma Chemical Co., St. Louis, Mo.), and 0.5-ml aliquots were given i.p. per mouse. Bacterial doses were confirmed by CFU plate counts.
Determination of in vitro bacterial growth rate.
Bacterial CFU were inoculated into 20 ml of Trypticase soy broth. The density of bacterial suspension was adjusted to an optical density at 600 nm (OD600) of 0.03 and grown to log phase. Samples were incubated at 37°C with gentle shaking, and OD600 readings were taken every hour for a total of 5 h.
Enumeration of bacteria in spleen homogenates.
Groups of mice (five mice per group) were sacrificed by cervical dislocation at different time points after infection. Spleens were removed aseptically, individually weighed, and homogenized in 5 ml of cold sterile water in an Ultra-terrax T25 tissue homogenizer (Janke & Kunkle, Staufenim Breisgau, Germany). A 100-μl aliquot of the homogenate or an appropriate dilution was plated on Trypticase soy agar in the presence or absence of ampicillin, and the number of viable CFU was determined after an overnight incubation.
Flow cytometry analysis.
Spleen cell suspensions were prepared from animals infected i.p. 7 days previously with a dose of 0.5 × 106 to 1.0 × 106 BRD509, GIDIL2, or GIDTNF bacteria per mouse. Following hypotonic shock to eliminate red blood cells, cells were washed and resuspended in staining buffer (PBS–1% fetal calf serum–0.1% NaN3) to a concentration of 107/ml. Aliquots of 100 μl (106 cells) were dispensed into wells of a round-bottomed 96-well plate and incubated with specific anti-CD16/CD32 monoclonal antibody (MAb) for 15 min on ice to block all FcγR sites. Cells were then doubly stained with a combination of directly conjugated MAbs to the T-cell receptor Cβ chain, B220, and CD11b (αm subunit of Mac-1) (purchased from PharMingen, San Diego, Calif.). Anti-Sca-1 (Ly-6A/E) MAb (30) was affinity purified on a protein G-Sepharose column and biotinylated in our laboratory using a standard method. All antibodies were pretitrated in preliminary experiments and used at saturating concentrations. Cell staining was done for 30 min on ice, and washed cells were analyzed on a FACSort (Beckton Dickinson, Mountain View, Calif.). Data collected on 25,000 cells were analyzed using CELLQUEST software.
Cell preparation.
Erythrocyte-depleted single-cell suspensions of spleen cells were prepared as previously described (1, 2) and suspended in RPMI medium supplemented with 5% fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 1× essential amino acids (catalog no. 21135), 1× nonessential amino acids (catalog no. 11140), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 50 μg of gentamicin/ml, and 2 × 10−5 M 2-mercaptoethanol (2-ME) (Gibco BRL, Paisley, United Kingdom). Cells were cultured without further stimulation at a concentration of 107 cells/ml in 24-well plates and were incubated for 48 h at 37°C with 5% CO2. Culture supernatants were then collected, spun free of any cells, and kept at −20°C until assayed for nitrite content.
Nitric oxide determination.
Accumulation of NO2− was used to determine production of NO according to the Griess method, as detailed elsewhere (3). Briefly, 100 μl of cell-free culture supernatant was mixed with an equal volume of Griess reagent and incubated at room temperature for 10 to 15 min. The absorbance at 562 nm was measured in an automated microplate reader. Nitrite concentration was quantitated using NaNO2 as the standard and expressed as the micromolar concentration of NO2− per 107 spleen cells.
Cytokine ELISAs.
Overnight cultures of the BRD509, GIDIL2, and GIDTNF bacteria were diluted 1:5 and grown for a further 2 h at 37°C in the presence (GIDIL2 and GIDTNF) or absence (BRD509) of ampicillin. Cytokine expression was also tested after induction of the nirB promoter by growing bacterial colonies in the presence of ampicillin and 4 mg of glucose/ml in a closed screw-cap tube, as described (46). At the end of the incubation period, bacteria were spun down at 6,000 × g 8,000 rpm for 10 min at 4°C. Bacterial pellets were sonicated in 0.5 ml of lysis buffer containing 1% Triton X-100 supplemented with NaF, phenylmethylsulfonyl fluoride, aprotinin, and leupeptin, as described previously (4). Bacterial lysates were cleared by a 15-min high-speed centrifugation at 13,400 × g at 4°C and analyzed for cytokine content by specific enzyme-linked immunosorbent assays (ELISAs). IL-2 and TNF-α content was quantitated by ELISA using specific kits from Endogen (Cambridge, Mass.) following the manufacturer's instructions.
Vaccination and challenge experiments.
For these experiments, groups of mice (five per group) were immunized i.p. with strains GIDIL2 and GIDTNF with doses ranging from 6 × 103 to 6 × 105 bacteria per mouse. Four weeks later, mice were challenged i.p. with the virulent SL1344 strain (LD50 < 5), and deaths were scored for 60 days after challenge.
Statistical analysis.
Statistical significance was analyzed using Student's t test. Differences between experimental groups were considered significant when P was <0.05.
RESULTS
Cytokine expression by Salmonella strains.
The BRD509 strain of serovar Typhimurium is attenuated as a result of deletions in the aroA and aroD genes. It is very efficient in affording protection against virulent Salmonella challenge, with an immunizing dose of 105 bacteria per mouse i.p., resulting in 100% protection against up to 10,000 LD50s of virulent Salmonella (unpublished data). Two recombinant strains of BRD509, GIDIL2 and GIDTNF, were derived that expressed IL-2 and TNF, respectively, under the control of the nirB promoter (46). Given that both innate immunity and adaptive immunity are important for controlling infections by intracellular bacteria, we wished to study the influence of bacterially expressed cytokines on the anti-Salmonella immunity in the susceptible BALB/c mouse strain.
The extent of cytokine expression by the recombinant strains was first determined using specific ELISAs. The two recombinant strains (GIDIL2 and GIDTNF) were found to express their respective cytokines specifically, while the parental BRD509 strain is negative for both IL-2 and TNF-α. Moreover, under uninduced growth conditions, the level of expression was calculated to be 4,000 and 1,250 pg/1010 organisms for the GIDIL2 and GIDTNF strains, respectively. Expression of the cytokines increased by ∼1,000-fold when the bacteria were grown under anaerobic conditions, as described in Materials and Methods, to induce the nirB promoter. The values for the mean plus the standard error of the mean for GIDIL2 and GIDTNF under inducing growth conditions were 4,315 + 835 and 922 + 149 ng/1010 organisms, respectively. These levels are presumably a better reflection of the situation in vivo, as these bacteria are intracellular pathogens and reside mainly inside macrophages.
In vitro growth rate of recombinant Salmonella strains.
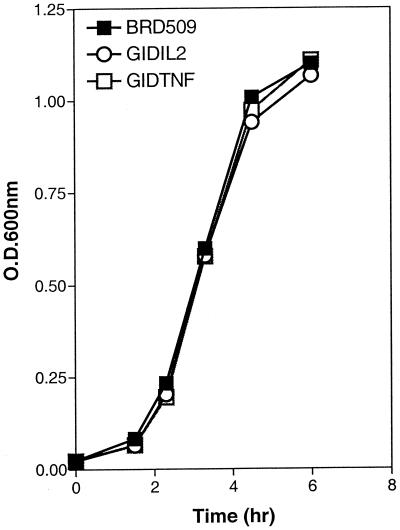
The growth rate of BRD509 was compared with those of the cytokine-expressing derivatives. Bacterial colonies were inoculated into fresh medium, and the suspension density was adjusted to be equal among all three strains. The in vitro rate of growth was followed over a period of 5 h, and the results are shown in Fig. 1. As can be clearly seen, all three strains exhibit nearly identical growth rates in vitro.
FIG. 1.
Cytokine-expressing Salmonella strains show growth rates identical to that of the parental strain in vitro. Five to 10 CFU from each strain was used to inoculate a 20-ml broth. The cell density of all three cultures was adjusted to an OD600 of 0.03 and incubated over a 6-h period to log phase. OD readings were taken at different intervals and plotted against time of culture. The growth curve of BRD509 was compared with those of the GIDIL2 and GIDTNF strains. Each data point represents the mean of two readings. Standard deviation was <10%. The results are representative of four independent experiments.
Phenotypic analysis of immunized spleen cell populations.
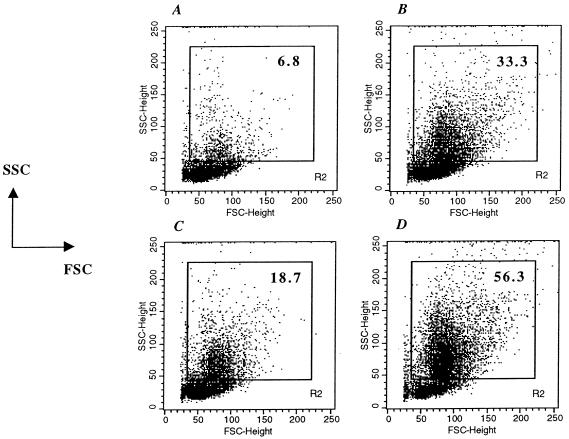
Four groups of mice were immunized with either BRD509, GIDIL2, GIDTNF, or PBS as a control. Spleens were harvested 7 days later and analyzed by flow cytometry to ascertain any alteration in the cellular composition of the spleen and to determine the extent and makeup of the inflammatory cell infiltrate. As shown in Fig. 2, the extent of the splenic cellular influx, delineated on the basis of the forward scatter/side scatter (FSC/SSC) profile by the indicated R2 gate, differs among the four groups of animals. In PBS-treated mice (Fig. 2A), the majority of splenocytes (93.2% of total) are observed outside this gate, reflecting the standard phenotype of resting spleen lymphocytes. The percentage of cells exhibiting a relatively elevated FSC/SSC profile is 6.8%, which reflects most of the macrophage and polymorphonuclear leukocyte populations (2). In vaccinated mice, the percentage of cells within the R2 gate rises significantly to 33.3, 18.7, and 56.3% in BRD509-, GIDIL2-, and GIDTNF-injected mice, respectively (Fig. 2B to D). This rise is mostly attributed to the influx of inflammatory cells (mainly macrophages and neutrophils) into the spleen and/or the activation of resident splenic lymphocytes (see below) (2, 26). It is noteworthy that the percentage seen in GIDIL2-immunized mice is reduced (18.7%), while that of GIDTNF-injected mice is substantially increased (56.3%), compared to the percentage for animals immunized with BRD509 (compare Fig. 2C and D with 2B).
FIG. 2.
Extent of splenic cellular influx in immunized animals. Spleens were analyzed at day 7 postimmunization with 1 × 106 to 2 × 106 organisms of BRD509 (B), GIDIL2 (C), or GIDTNF (D). As a control, splenocytes of PBS-treated mice are also shown (A). The results are shown as dot plots depicting FSC level versus SSC level. The percentage of cells with elevated SSC (representing the inflammatory influx) is shown in each panel. Data from a total of 25,000 cells per group were collected and analyzed. The results are representative of four independent experiments.
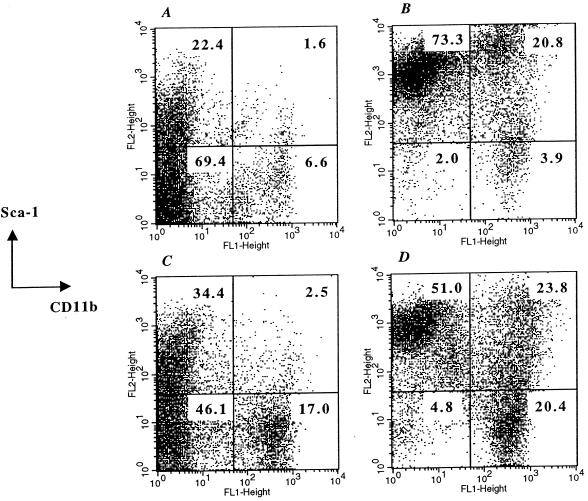
The cellular composition of the various spleen populations was also analyzed. Figure 3 shows the profile of the total, ungated splenocyte population after staining with MAbs to CD11b and Sca-1 (Ly-6A/E). CD11b is widely expressed on myeloid lineage cells, including macrophages and neutrophils, as well as on NK cells (15, 24). Sca-1 is expressed on all hematopoietic stem cells and is also an activation marker of diverse types of lymphoid and myeloid lineage cells (23, 31, 33, 40). In PBS-treated mice, 6.6% of splenocytes were CD11b+/Sca-1− (Fig. 3A), representing resting cells of myeloid origin. In addition, 22.4% of cells were CD11b−/Sca-1lo, indicating cells of lymphoid origin with low levels of Sca-1 expression. In BRD509-injected mice, the level of Sca-1 expression increased dramatically on both lymphoid and myeloid cells, resulting in 73.3% CD11b−/Sca-1hi and 20.8% CD11b+/Sca-1+ cells, respectively (Fig. 3B). Moreover, despite being clearly positive for Sca-1, CD11b+ cells exhibited a bimodal pattern of Sca-1 expression (Fig. 3B). Gating of the respective populations of CD11b+/Sca-1+ cells indicated that the great majority (∼78%) of these cells expressed very high Sca-1 levels, while ∼22% showed relatively lower levels (analysis not shown). In sharp contrast, GIDIL2-treated spleens exhibited an overall pattern of expression that was similar to that of control spleens (compare Fig. 3A and C). The two main changes observed in GIDIL2-injected mice were the increased percentage of CD11b−/Sca-1+ (34.4% compared to 22.4% in control mice) and CD11b+/Sca-1− cell populations (17.0% compared to 6.6% in control mice). Importantly, despite the evidence of a small increase in the number of CD11b+ cells in GIDIL2-injected spleens, there was no upregulation of Sca-1 expression on these cells. Moreover, splenocytes of GIDIL2-treated animals were noticeably devoid of any cells exhibiting high levels of Sca-1 expression (Fig. 3C). GIDTNF-injected mice showed a different pattern of Sca-1/CD11b expression from that of any of the other three experimental groups (compare Fig. 3D with Fig. 3A to C). The percentage of splenic CD11b+ cells increased to >44%, nearly twice the number observed in BRD509-treated animals and sevenfold that of control mice (Fig. 3D). Of the CD11b+ cells, nearly half were negative for Sca-1 (CD11b+/Sca-1−, 20.4%) while the other half (CD11b+/Sca-1+, 23.8%) expressed Sca-1 at levels varying from intermediate to high. The remaining splenocytes of lymphoid origin (CD11b−) expressed Sca-1 at high levels, very similar to what was observed in BRD509-injected animals (compare Fig. 3B and D). Furthermore, a similar analysis of the cell population within the R2 gate indicated that CD11b+ cells were the predominant cell type, accounting for ∼54.4, 58.7, 74.3, and 76.3% of cells in PBS-, BRD509-, GIDIL2-, and GIDTNF-treated mice, respectively (data not shown). Taken together, it can be concluded that (i) the different recombinant bacterial strains induce distinct cellular phenotypic changes in spleens of vaccinated animals, (ii) expression of IL-2 (GIDIL2 strain) results in a marked attenuation of the inflammatory influx as compared to the non-cytokine-expressing strain (BRD509), and (iii) immunization with GIDTNF induces several changes in the cellular composition and phenotypes which are quite distinct from those in other groups.
FIG. 3.
Phenotypic analysis of spleen cells. Single-cell suspensions were prepared from spleens of animals immunized 7 days previously with either PBS (A), BRD509 (B), GIDIL2 (C), or GIDTNF (D). The results are shown as dot plots depicting staining with Sca-1 versus CD11b. The cell percentages within each quadrant are given in the figure. Data from a total of 25,000 cells per group were collected and analyzed. The results are representative of four independent experiments.
Bacterial colonization and splenomegaly.
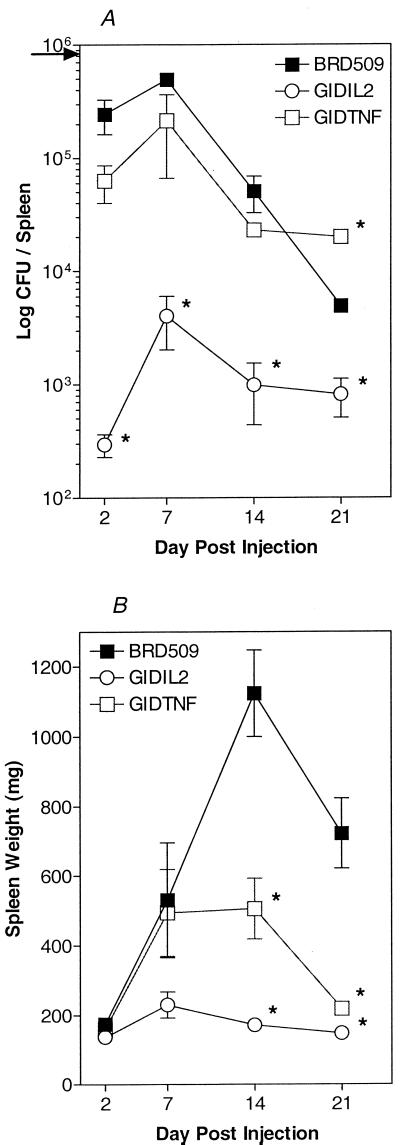
Inherently susceptible BALB/c mice were immunized with a dose of 0.8 × 106 organisms of the BRD509, GIDIL2, or GIDTNF strain. Enumeration of viable bacteria was done on homogenates of spleens obtained at 2, 7, 14, and 21 days after immunization. As illustrated in Fig. 4A, maximal numbers of CFU in the spleen were observed at day 7 postimmunization for all three bacterial strains. At day 21 postimmunization, BRD509 CFU were <1% of the injected dose and those of GIDTNF were reduced by >40-fold. The bacterial growth curves of BRD509 and GIDTNF strains were very similar. However, GIDTNF bacteria appeared to persist at higher levels in the spleen at day 21 postimmunization, as evidenced by the highly reproducible 5- to 10-fold-higher number of CFU seen colonizing the spleens of GIDTNF-treated mice. Moreover, the most striking feature, which was also consistently observed, is the rapid clearance of the GIDIL2 transductant in vivo. Even at the height of the observed bacterial CFU (day 7 postinjection), the number of GIDIL2 bacteria never exceeded 0.5% of the injected dose. These findings are consistent with the reduced inflammatory influx observed in GIDIL2-injected mice by flow cytometry (Fig. 2). Since all bacterial strains were shown to grow equally well in vitro (Fig. 1), these results point to an increased capacity of susceptible BALB/c mice to specifically limit the growth of the GIDIL2 bacterial transductants.
FIG. 4.
Cytokine expression alters the rate of bacterial colonization and splenomegaly in Salmonella-immunized mice. The levels of bacterial load (A) and splenomegaly (B) were compared over a period of 3 weeks in mice injected with ∼106 organisms of the BRD509, GIDIL2, or GIDTNF strain. Each data point represents the mean ± standard error of three to five mice per group. The arrow in panel A indicates the bacterial dose used for injection. The results are representative of five independent experiments. Asterisks denote significant differences (P ≤ 0.05) between the indicated groups and the corresponding BRD509 group.
Next we investigated the ability of the different bacterial strains to induce splenomegaly. As can be seen in Fig. 4B, the degree of splenomegaly induced by BRD509 was maximal at day 14 postimmunization, reaching a level that was more than seven times the normal size, and began to decrease thereafter. The GIDTNF-induced splenomegaly followed a kinetic pattern identical to that of BRD509 up to 7 days postimmunization. However, unlike the case with BRD509, no further increase in splenomegaly was observed at later time points, with the maximal level reached never exceeding four times the normal spleen size. Strikingly, the degree of splenomegaly observed at day 21 posttreatment in BRD509-injected animals was approximately threefold that of GIDTNF-injected counterparts, even though the number of bacterial CFU was significantly higher in the latter mice (Fig. 4A). In contrast, the GIDIL2 strain induced only a small degree (less than two times the normal size) of splenomegaly throughout the experiment, consistent with the flow cytometry analysis data.
Production of nitric oxide.
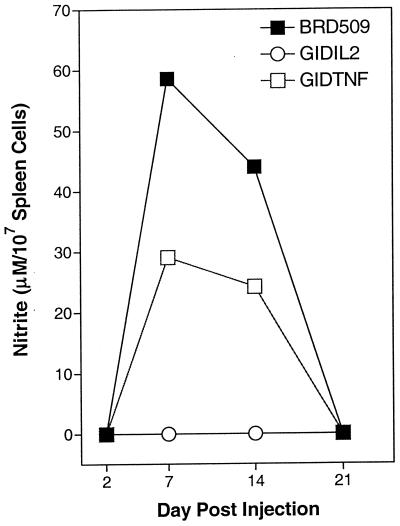
Single-spleen-cell suspensions were prepared at different time points following the administration of BRD509, GIDTNF, GIDIL2, or PBS as control. Cells were cultured for 48 h without any further stimulation, and cell-free culture supernatants were assayed for content of NO by the Griess assay. The results are shown in Fig. 5. At day 2 postimmunization, no nitrite was detected in any experimental group. At day 7, maximal nitrite production was observed in BRD509-injected mice, reaching a level of ∼60 μM per 107 cells. At day 14 the level was slightly reduced to ∼44 μM, and by day 21 nitrite was undetectable. Nitric oxide production followed a similar time course in GIDTNF-immunized mice, with the exception that the amount produced was only ∼50% of that observed in BRD509-treated animals. Strikingly, production of NO could not be detected in GIDIL2-treated mice at any of the time points examined. Similar results were obtained in spleens of PBS-treated control mice (data not shown).
FIG. 5.
Production of NO by splenocytes of immunized animals. Spleen cells were obtained 7 days after immunization with 106 organisms of the BRD509, GIDIL2, or GIDTNF strain and were cultured without further stimulation for 48 h. Collected cell-free supernatants were assayed for nitrite content as a measure of production of NO. Each data point represents the mean of three mice per group. The standard deviation was <15%. The results are representative of three independent experiments.
In vivo challenge with virulent Salmonella.
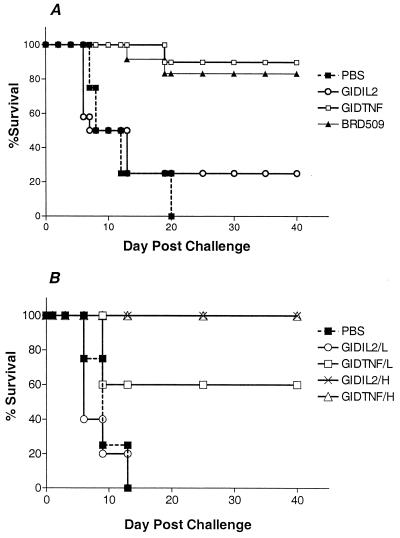
Experiments were carried out to ascertain potential functional consequence of the rapid clearance of GIDIL2 bacteria. Mice were immunized with a single i.p. injection of ∼7.0 × 103 BRD509, GIDIL2, or GIDTNF organisms per mouse. Four weeks later, all mice were challenged with the highly virulent SL1344 strain and scored for survival for up to 60 days after the challenge. The results of a representative experiment are summarized in Fig. 6A. All control (PBS-injected) mice succumbed to an overwhelming Salmonella infection, with death occurring between days 6 and 20. A very similar survival curve was observed in the GIDIL2-vaccinated group, with 80% of animals dying over the same period of time. In sharp contrast, 90% of the mice vaccinated with BRD509 organisms and 83% of those injected with GIDTNF organisms survived the lethal challenge (Fig. 6A). To test the influence of bacterial dose on protection, the experiment was repeated using a high (6 × 105 per mouse) or low (6 × 103 per mouse) dose of GIDTNF or GIDIL2 bacteria for vaccination (Fig. 6B). All mice injected with saline or with a low dose of GIDIL2 strain succumbed to their infection between days 6 and 13 after challenge (Fig. 6B). By contrast, 60% of the mice immunized with a low dose of the GIDTNF strain survived the lethal challenge (two of five mice died on day 9 following challenge). When the vaccination dose was increased by 2 logs, 100% survival was observed in mice receiving either GIDTNF or GIDIL2. It can be concluded that expression of IL-2 by Salmonella induces its rapid clearance in a mouse strain inherently susceptible to the infection. Furthermore, this clearance can reduce the protective efficacy of the bacterial strain when given in limiting doses.
FIG. 6.
Effect of cytokine expression on protective efficacy of Salmonella strains. (A) Mice were vaccinated with ∼7.0 × 103 organisms of the BRD509, GIDIL2, or GIDTNF strain. Four weeks later, all mice were challenged with 6.4 × 103 organisms of the highly virulent SL1344 strain (LD50 < 5). The control group received PBS for immunization and were challenged like the other groups. The number of mice used in each group was 12 (BRD509 and GIDIL2), 10 (GIDTNF), or 4 (PBS). (B) Mice were immunized with either 6 × 103 (low dose; L) or 6 × 105 (high dose; H) organisms of the GIDIL2 or GIDTNF strain and were challenged 4 weeks later with 5 × 103 SL1344 organisms. There were five mice per group (except for the PBS group which had four mice). Survival was monitored for a total of 60 days. Results are scored as percent survival and are representative of a total of five independent experiments.
DISCUSSION
Attenuated Salmonella strains are becoming increasingly attractive as vehicles to deliver heterologous proteins of diverse microbial origin for the purpose of generating protective immunity against pathogens. In addition, several attenuated strains have been engineered to express specific cytokines that can be targeted in vivo to enhance immunity or to manipulate immune responses in a variety of disease conditions. It has been previously shown that bacterially expressed cytokines continue to be expressed for up to 2 weeks following oral administration, demonstrating the stability of the cytokine-encoding plasmids in vivo (46). However, the effect of cytokine expression on the immunogenicity of the engineered bacterial strains has not been previously addressed. This report is the first description of the influence of bacterially expressed cytokines on anti-Salmonella immunity. The data presented in this report demonstrate that cytokines expressed by the bacterial pathogen can have a profound effect on the nature of the ensuing immune response. Specifically, expression of IL-2 led to the rapid clearance of the pathogen by the host. In contrast, expression of TNF-α was observed to bring about divergent effects. Thus, despite the induction of a stronger inflammatory response initially (compared to the BRD509 parental strain), expression of TNF-α results in decreased splenomegaly and nitric oxide secretion. Moreover, while the in vivo bacterial load of GIDTNF was similar to that of BRD509 over the first 2 weeks, the former strain appeared to persist for longer time periods over the long run (Fig. 4A; data not shown). The exact mechanism(s) responsible for cytokine-induced alterations in immune responsiveness is the subject of investigation at the present time.
The marked decrease in the in vivo survival rate of GIDIL2 organisms is unlikely to be due to intrinsic, strain-specific growth defects. This is most aptly shown by the almost identical in vitro growth kinetics of GIDIL2, GIDTNF, and BRD509 bacterial strains. Instead, we believe that the significantly smaller bacterial load of GIDIL2 is due to a rapid clearance of the organism by the immune system. As early as 2 days postimmunization, the splenic bacterial load of the GIDIL2 strain was 2.5 to 3 logs lower than for GIDTNF or the parental BRD509 strain. This strongly indicates that a substantial degree of GIDIL2 bacterial clearance has taken place in the first 48 h in the peritoneal cavity, the site of immunization in our experiments. This conclusion is further supported by preliminary findings indicating the rapid induction, in the peritoneal cavity, of several host immune response mechanisms by GIDIL2 within a few hours of immunization (B. K. al-Ramadi et al., data not shown). Preliminary evidence demonstrates that GIDIL2 can very rapidly induce peritoneal cavity cells to upregulate inducible nitric oxide synthase gene transcription as well as to secrete NO and IFN-γ (data not shown). It is noteworthy that at no time point could production of NO be demonstrated in cultures of spleen cells of GIDIL2-treated mice, excluding any role for activated splenocytes in GIDIL2 clearance. Thus, Salmonella-expressed IL-2 leads to an early upregulation of host defense mechanisms in the peritoneal cavity that, in turn, results in rapid clearance of the invading organisms.
It is intriguing to note that the differences in host response to BRD509 and to GIDIL2 in susceptible BALB/c mice appear to be very similar to the various responses against virulent Salmonella observed in susceptible versus resistant mouse strains. Following infection with virulent Salmonella, early bacterial replication is regulated by the nramp1 gene, which encodes the natural-resistance-associated macrophage protein (Nramp1) expressed on macrophages (45). This gene appears to have pleiotropic effects on the function of macrophages, including antigen-processing capacity and production of proinflammatory cytokines (22). This has been directly linked to the capacity of macrophages to control the replication of a number of intracellular pathogens (5, 25). In Salmonella-resistant (nramp1 wild-type) mouse strains, bacterial proliferation in the early phase of infection (1 to 7 days postinfection) is kept under control by the action of the Nramp1 protein. In contrast, rapid proliferation of bacteria is observed in nramp1 mutant (Salmonella-susceptible) mouse strains (17, 34). In our study, attenuated Salmonella strain BRD509 continues to proliferate in susceptible BALB/c mice, reaching a maximum at 7 days postimmunization. However, expression of IL-2 by the bacteria induces a very rapid and efficient host defense response that effectively eliminates the vast majority of invading organisms. Although the identity of the target cell(s) is not yet known, these findings suggest that the expression of IL-2 by the pathogen may partly compensate for the defective function of Nramp1 protein in BALB/c phagocytes. Alternatively, expression of IL-2 may lead to a rapid activation of another cell type(s), such as NK and/or γδ T cells, which can contribute to antibacterial immune response by effectively limiting the growth of the organism in vivo (18).
The IL-2-induced increase in the rate of Salmonella clearance appears to be analogous to previous findings using recombinant strains of vaccinia virus. Infection of nude, immunodeficient mice with vaccinia virus normally results in a lethal infection. However, expression of IL-2 by the vaccinia virus induces a rapid host immune response that allows for the successful vaccination of mice, even in immunodeficient recipients (14, 36). Virus-encoded IL-2 was subsequently shown to induce elevated levels of NK cell activity and could be partly responsible for the attenuation of viral virulence in immunodeficient recipients (19). Despite the differences in the nature of the pathogens and their in vivo habitat, the rapid clearance of IL-2-expressing Salmonella may well be induced through a mechanism similar to that observed in the vaccinia virus system.
Immunization using the TNF-α-expressing Salmonella strain resulted in several unique alterations in the immune response, quite distinct from those observed in GIDIL2-immunized animals. Despite being a prototypical proinflammatory cytokine, expression of bacterially borne TNF-α in GIDTNF mice induced significantly decreased splenomegaly and secretion of NO compared to those associated with BRD509 (responses in the former were approximately 50% of the latter). Marked differences in production of NO by splenocytes of GIDTNF- versus BRD509-immunized mice were apparent at both days 7 and 14 postimmunization. However, despite the decreased level of secretion of NO in GIDTNF-immunized mice, bacterial replication was kept under control. In fact, the CFU counts at days 2, 7, and 14 postimmunization for the GIDTNF group were either equivalent to or lower than those for the BRD509 group. We conclude that expression of TNF-α leads to efficient induction of additional antibacterial defense mechanisms, such as production of reactive oxygen intermediates (32), which act in conjunction with NO and limit bacterial multiplication despite decreased inflammatory influx and NO production.
Further evidence for the differential influence of cytokine expression on immune response in vivo is seen from the flow cytometry data. Of particular importance in this regard are the results obtained by staining with antibodies to CD11b and Sca-1 markers. CD11b is expressed on macrophages, granulocytes, NK cells, and dendritic cells. Sca-1 (Ly-6A/E) is constitutively expressed on hematopoietic stem cells and is also upregulated in peripheral lymphocytes and monocytes upon activation in BALB/c mice (27, 33). One of the most studied aspects of the Ly-6 family of proteins, and particularly Ly-6A/E, is their exquisite sensitivity to induction by IFN-α/β and IFN-γ (20, 39). Our findings confirm that Sca-1 expression is dramatically upregulated on B and T lymphocytes at day 7 postimmunization. This upregulation was mostly observed in mice immunized with BRD509 or GIDTNF but not with GIDIL2 (Fig. 3). Moreover, expression of Sca-1 was also upregulated on a CD11b+, nonlymphoid population of spleen cells. This was clearly seen in BRD509-immunized animals where the majority (>84%) of CD11b+ cells were also Sca-1 positive. Surprisingly, despite the increased ratio of CD11b+ cells in GIDTNF-treated mice (44.2% compared with 24.7% in BRD509-immunized mice), a significant percentage (∼46%) of those cells failed to upregulate Sca-1 expression. Although the exact reasons for these differences are unknown, two possible scenarios can be offered to explain the findings. First, BRD509 and GIDTNF bacterial strains may induce not only quantitative but also qualitative differences in the cell types in the course of the immune response. Consequently, bacterially expressed TNF-α may lead to the accumulation of a distinct population of CD11b+ cells in the spleen that lack Sca-1 expression. This hypothesis is supported by the significant differences in the extent of splenomegaly as well as production of NO observed over a period of 3 to 4 weeks following immunization. Alternatively, differences in cellular phenotypes reflect the different kinetics of the immune response in the two experimental groups. Thus, it is conceivable that spleen cells modulate Sca-1 expression in GIDTNF-treated mice more rapidly than in BRD509-injected animals and that by day 7 postimmunization (the time point at which the flow cytometry analysis was carried out), Sca-1 has already been downregulated. Experiments are currently under way to answer these questions by probing with a wider spectrum of antibodies to cell-specific markers and examining cells at different time points following immunization.
Whatever the explanation for the variant responses observed in this study, it is clearly evident that vector-encoded cytokines can alter the immune response in a profound and specific manner. Perhaps this is best illustrated by the results of the protection studies. The rapid clearance of GIDIL2 organisms by the enhanced immune response was shown to indeed reduce the protective efficacy of the strain. As would be expected, this occurred when the immunizing dose was limiting. At high doses, both GIDIL2 and GIDTNF strains afforded a good level of protection that was comparable to what was seen in BRD509-injected mice. Thus, it is feasible to utilize recombinant GIDIL2 bacteria at an appropriate concentration to induce a good level of protection while, at the same time, minimizing the side effects associated with the administration of large doses of gram-negative bacteria, including bacteremia and sepsis (43). It must be emphasized, however, that all of the studies carried out for this report used the i.p. route for immunization and challenge. It would be important to carry out similar studies using the oral route, since this is the natural route of acquiring Salmonella in humans. Based on previous work, which demonstrated the efficacy of orally administered Salmonella-expressed cytokines in enhancing the immune response against Leishmania infection (46), we hypothesize that cytokine-specific modulation of immunity seen in the present report would still be effective. These studies are currently being undertaken. Finally, given the increasingly wide usage of Salmonella strains as vectors of virulence genes of other pathogens, incorporation of appropriate cytokines in the vectors may provide a means by which the desirable immune responses can be differentially induced.
ACKNOWLEDGMENTS
We thank Toby K. Eisenstein, Temple University School of Medicine, for critical review of the manuscript. We are grateful to Bruce Stocker, Stanford University School of Medicine, for providing the serovar Typhimurium SL1344 strain.
This work was funded by a grant from the Research Committee of the Faculty of Medicine and Health Sciences, UAE University, United Arab Emirates. Additional support was provided by a grant from the UAE Branch of the Terry Fox Fund for Cancer Research (Canada) to B. K. al-Ramadi.
REFERENCES
- 1.al-Ramadi B K, Al-Dhaheri M H, Fernandez-Cabezudo M J. Novel superantigenic properties are associated with the nosocomial pathogen Acinetobacter baumannii. Emirates Med J. 2000;18:35–40. [Google Scholar]
- 2.al-Ramadi B K, Brodkin M A, Mosser D M, Eisenstein T K. Immunosuppression induced by attenuated Salmonella: evidence for mediation by macrophage precursors. J Immunol. 1991;146:2737–2746. [PubMed] [Google Scholar]
- 3.al-Ramadi B K, Meissler J J, Jr, Huang D, Eisenstein T K. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur J Immunol. 1992;22:2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]
- 4.al-Ramadi B K, Nakamura T, Leitenberg D, Bothwell A L M. Deficient expression of p56lck in Th2 cells leads to partial TCR signaling and a dysregulation in lymphokine mRNA levels. J Immunol. 1996;157:4751–4761. [PubMed] [Google Scholar]
- 5.Blackwell J M, Searle S. Genetic regulation of macrophage activation: understanding the function of Nramp1 (=Ity/Lsh/Bcg) Immunol Lett. 1999;65:73–80. doi: 10.1016/s0165-2478(98)00127-8. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 7.Carrier J M, Chatfield S N, Dougan G, Nowicka U T, O'Callaghan D, Beesley J E, Milano S, Cillari E, Liew F Y. Expression of human IL-1β in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992;148:1176–1181. [PubMed] [Google Scholar]
- 8.Chatfield S N, Dougan G. Expressing bacterial antigens. 2nd ed. New York, N.Y: Marcel Dekker; 1997. Attenuated Salmonella as a live vector for expression of foreign antigens, part i; pp. 331–341. [Google Scholar]
- 9.Denich K, Börlin P, O'Hanley P D, Howard M, Heath A W. Expression of the murine interleukin-4 gene in an attenuated aroA strain of Salmonella typhimurium: persistence and immune response in BALB/c mice and susceptibility to macrophage killing. Infect Immun. 1993;61:4818–4827. doi: 10.1128/iai.61.11.4818-4827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Rollinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 11.Diefenbach A, Schindler H, Rollinghoff M, Yokoyama W M, Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science. 1999;282:951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan S J, Ramsay A J, Strugnell R A. Studies of immunity and bacterial invasiveness in mice given a recombinant Salmonella vector encoding murine interleukin-6. Infect Immun. 1996;64:2730–2736. doi: 10.1128/iai.64.7.2730-2736.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flexner C, Hugin A, Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987;330:259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- 15.Gahmberg C G, Valmu L, Fagerholm S, Kotovuori P, Ihanus E, Tian L, Pessa-Morikawa T. Leukocyte integrins and inflammation. Cell Mol Life Sci. 1998;54:549–555. doi: 10.1007/s000180050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are nonvirulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 17.Hormaeche C E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979;37:311–318. [PMC free article] [PubMed] [Google Scholar]
- 18.Jones B D, Falkow S. Salmonellosis: host responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 19.Karupiah G, Coupar B E H, Andrew M E, Boyle D B, Phillips S M, Mullbacher A, Blanden R V, Ramshaw I A. Elevated natural killer cell responses in mice infected with recombinant vaccinia virus encoding murine IL-2. J Immunol. 1990;144:290–298. [PubMed] [Google Scholar]
- 20.Khodadoust M M, Khan K D, Bothwell A L M. Complex regulation of Ly-6E gene transcription in T cells by IFNs. J Immunol. 1999;163:811–819. [PubMed] [Google Scholar]
- 21.Killar L M, Eisenstein T K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCr1BR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985;47:605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang T, Prina E, Sibthrope D, Blackwell J M. Nramp1 transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on antigen processing and presentation. Infect Immun. 1997;65:380–386. doi: 10.1128/iai.65.2.380-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeClair K P, Bridgett M M, Dumont F J, Palfree R G E, Hammerling U, Bothwell A L M. Kinetic analysis of Ly-6 gene induction in a T lymphoma by interferons and interleukin-1 and demonstration of Ly-6 inducibility in diverse cell types. Eur J Immunol. 1989;19:1233–1239. doi: 10.1002/eji.1830190713. [DOI] [PubMed] [Google Scholar]
- 24.Leenen P J M, de Bruijn M F T R, Voerman J S A, Campbell P A, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 25.Lissner C R, Swanson R N, O'Brien A D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- 26.MacFarlane A S, Schwacha M G, Eisenstein T K. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect Immun. 1999;67:891–898. doi: 10.1128/iai.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malek T R, Danis K M, Codias E K. Tumor necrosis factor synergistically acts with IFN-γ to regulate Ly-6A/E expression in T lymphocytes, thymocytes, and bone marrow cells. J Immunol. 1989;142:1929–1936. [PubMed] [Google Scholar]
- 28.Mastroeni P, Harrison J A, Robinson J H, Clare S, Khan S, Maskell D J, Dougan G, Hormaeche C E. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega G, Korty P E, Shevach E M, Malek T R. Role of Ly-6 in lymphocyte activation. I. Characterization of a monoclonal antibody to a nonpolymorphic Ly-6 specificity. J Immunol. 1986;137:3240–3246. [PubMed] [Google Scholar]
- 31.Patterson J M M, Johnson M H, Zimonjic D B, Graubert T A. Characterization of Ly-6M, a novel member of the Ly-6 family of hematopoietic proteins. Blood. 2000;95:3125–3132. [PubMed] [Google Scholar]
- 32.Paulnock D M. Macrophage activation by T cells. Curr Opin Immunol. 1992;4:344–349. doi: 10.1016/0952-7915(92)90087-u. [DOI] [PubMed] [Google Scholar]
- 33.Pflugh D L, Maher S E, Bothwell A L M. Ly-6I, a new member of the murine Ly-6 superfamily with a distinct pattern of expression. J Immunol. 2000;165:313–321. doi: 10.4049/jimmunol.165.1.313. [DOI] [PubMed] [Google Scholar]
- 34.Plant J, Glynn A A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976;133:72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- 35.Ramarathinam L, Niesel D W, Klimpel G R. Salmonella typhimurium induces IFN-γ production in murine splenocytes: role of natural killer cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 36.Ramshaw I A, Andrew M E, Phillips S M, Boyle D B, Coupar B E H. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987;329:545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- 37.Schafer R, Eisenstein T K. Natural killer cells mediate protection induced by a Salmonella aroA mutant. Infect Immun. 1992;60:791–797. doi: 10.1128/iai.60.3.791-797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiloh U M, MacMicking J D, Nicholson S, Brause J E, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 39.Snapper C M, Yamaguchi H, Urban J F, Jr, Finkelman F D. Induction of Ly-6A/E expression by murine lymphocytes after in vivo immunization is strictly dependent upon the action of IFN-α/β and/or IFN-γ. Int Immunol. 1991;3:845–852. doi: 10.1093/intimm/3.9.845. [DOI] [PubMed] [Google Scholar]
- 40.Spangrude G J, Brooks D M. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- 41.Strugnell R, Dougan G, Chatfield S, Charles I, Fairweather N, Tite J, Li J L, Beesley J, Roberts M. Characterization of a Salmonella typhimurium aro vaccine strain expressing the P.69 antigen of Bordetella pertussis. Infect Immun. 1992;60:3994–4002. doi: 10.1128/iai.60.10.3994-4002.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sztein B M, Wasserman S S, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 43.Tacket C O, Sztein M B, Wasserman S S, Losonsky G, Kotloff K L, Wyant T L, Nataro J P, Edelman R, Perry J, Bedford P, Brown D, Chatfield S, Dougan G, Levine M M. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect Immun. 2000;68:1196–1201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 45.Vidal S, Tremblay K L, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of Nramp1 gene. J Exp Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu D, McSorley S J, Tetley L, Chatfield S, Dougan G, Chan W L, Satoskar A, David J R, Liew F Y. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-α, and IFN-γ administered orally via attenuated Salmonella typhimurium. J Immunol. 1998;160:1285–1289. [PubMed] [Google Scholar]