Summary
Here, we provide a protocol for an intrasplenic injection model to establish pancreatic tumors in the mouse liver. We describe the steps to inject tumor cells into mouse spleen and to perform a splenectomy, followed by animal recovery and end point analysis of tumors in the liver. This model allows rapid and reproducible tumor growth in a clinically relevant metastatic site, providing a platform to evaluate the efficacy of anti-cancer drugs. This technique can be expanded to other cancer cell lines.
For complete details on the use and execution of this protocol, please refer to Poh et al. (2022).1
Subject areas: Cell Biology, Cell culture, Cancer, Immunology, Model Organisms
Graphical abstract
Highlights
-
•
A clinically relevant model to study pancreatic cancer metastasis in the mouse liver
-
•
Steps for tumor cell injection, splenectomy, animal recovery, and end point analysis
-
•
A platform to evaluate anti-cancer drugs and expandable to other cancer cell lines
-
•
Can be used to study tumor seeding, tumor cell-niche interactions, and cancer dormancy
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here, we provide a protocol for an intrasplenic injection model to establish pancreatic tumors in the mouse liver. We describe the steps to inject tumor cells into mouse spleen and to perform a splenectomy, followed by animal recovery and end point analysis of tumors in the liver. This model allows rapid and reproducible tumor growth in a clinically relevant metastatic site, providing a platform to evaluate the efficacy of anti-cancer drugs. This technique can be expanded to other cancer cell lines.
Before you begin
In this step, users will prepare the surgical area, tools, and reagents required for intrasplenic tumor cell injection.
This protocol uses syngeneic pancreatic ductal adenocarcinoma (PDAC) cells derived from KRasG12D;Trp53R172H;Pdx-1Cre compound mutant (KPC) mice.2 However, we have also used this protocol for other cancer cell lines, including the MC38 colon cancer cell line.
This protocol uses C57BL/6 mice of either sex that are 10-weeks of age. Users are recommended to use animals that are at least 8 weeks of age of either sex due to larger spleen size (easier to inject) and better post-operative outcomes. No sex differences were observed.
Institutional permissions
All animal studies were approved and conducted in accordance with the Animal Ethics Committee at La Trobe University and the Austin Health precinct, Melbourne, Australia. Users are reminded to seek appropriate permissions from their relevant institutions to conduct animal studies.
Preparation of surgical instruments and items
Timing: 30 min
In this step, users will autoclave surgical tools and prepare gauze for surgery.
-
1.Prepare surgical tools by autoclaving at 121°C for 15 min prior to commencing animal surgeries. These include:
-
a.2× forceps.
-
b.1× scissors.
-
c.1× hemostat.
-
a.
Note: Step 1 relates to surgical tools that require autoclaving. The full list of tools and equipment required for this protocol is listed in step 7 and in the key resources table.
-
2.
Cut surgical gauze into 1 × 1″ squares in a laminar flow hood.
Preparation and administration of pain relief
Timing: 30 min
In this step, users will prepare and administer pain relief to animals.
-
3.30 min prior to surgery, administer carprofen analgesic (5 mg/kg) via subcutaneous injection for pain management.
-
a.Carprofen is provided as a 50 mg/mL solution and must be diluted with sterile saline before injection to prevent skin irritation and ulceration.
-
a.
-
4.
Prepare 0.12% bupivacaine (diluted in sterile saline) for local anesthesia. Administer 50 μL topically (2 mg/kg) following surgery.
-
5.
Weigh the mouse as a reference point for recovery.
Note: Weight loss of 5%–10% can be expected a day after surgery, but is expected to recover within 2 days. Loss of body weight is also used as a clinical sign for disease progression throughout the experiment (Refer to step 23).
Note: Users are required to confirm the anesthesia requirements of their Animal Ethics Committee.
Preparation of surgical and recovery area
Timing: 30 min
In this step, users will prepare the surgical and recovery area.
-
6.
Prepare an aseptic surgical area by wiping down all surfaces with 80% ethanol and F10sc veterinary disinfectant.
Note: Surfaces include heat pads, light source, anesthesia chamber / tubing / nose cone.
Note: If users are performing this procedure in a biosafety cabinet / surgical hood, thorough disinfection of surfaces and walls is required.
-
7.Set up the aseptic surgical area. Tools and items required include:
-
a.Surgical area:
-
i.Electrical heat pads with sterile drape.
-
ii.Light source.
-
iii.Anesthesia chamber placed on top of an electrical heat pad.
-
iv.Anesthesia tubing / nosecone.
-
i.
-
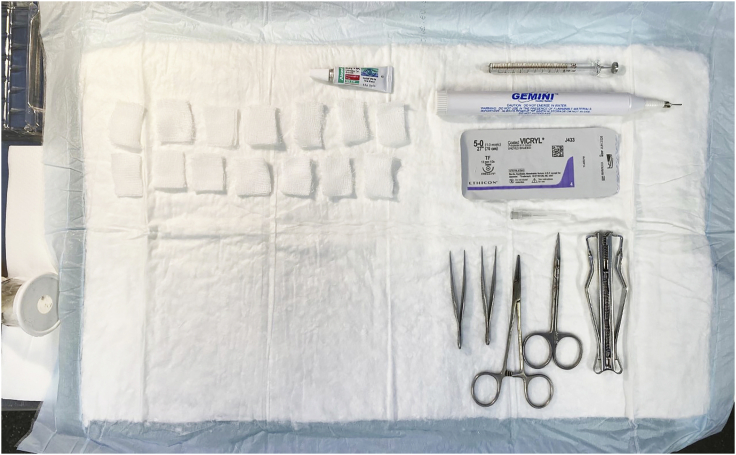
b.Surgical tools (refer to Figure 1 for example):
-
i.2× forceps.
-
ii.1× scissors.
-
iii.1× hemostat.
-
iv.27G × 1/2″ hypodermic needles.
-
v.100 μL Hamilton syringe.
-
vi.Sterile gauze (pre-cut into 1 × 1″ squares in a laminar flow hood).
-
vii.Plastic reagent tray (containing sterile PBS).
-
viii.Cauterizer.
-
ix.Absorbable sutures.
-
x.Wound stapler, staple remover, and staples.
-
xi.Timer.
-
i.
-
c.Other:
-
i.0.12% bupivacaine.
-
ii.1 mL syringes (for topical administration of bupivacaine).
-
iii.Polyvisc eye ointment (or artificial tears equivalent).
-
iv.27G insulin syringes pre-filled with 300 μL of sterile saline (one syringe per mouse).
-
v.1 mL pipette and tips (for resuspending cancer cells).
-
i.
-
d.On the bench adjacent to the surgical area, prepare a recovery area with clean cages containing sterile bedding.
-
i.Place clean cages on top of electrical heat pads, and ensure sufficient heat pads are available as mice will be kept on heat pads for 24 h.
-
i.
-
a.
CRITICAL: Heat pads are required during surgery and recovery to avoid hypothermia and death.
Figure 1.
Example set-up of surgical area
Preparation of tumor cells
Timing: 30 min
In this step, users will culture and prepare KRasG12D;Trp53R172H;Pdx-1Cre (KPC) pancreatic cancer cells for injection.2
Note: Users are encouraged to optimize this section according to the growth patterns of their selected cancer cell lines. The instructions provided below specifically relate to the KPC pancreatic cancer cell line.
-
8.Thaw KPC pancreatic cancer cells into T-175 tissue culture flasks five days prior to injection so that cells will be 80% confluent a day before surgery.
-
a.KPC cells are maintained in complete media (DMEM/F-12 supplemented with 10% FCS) at 37°C with 10% CO2.
-
a.
-
9.
One day before surgery, wash KPC cells once with 1× PBS and trypsinize confluent cells using TrypLE™ Express Enzyme.
-
10.
Passage cells 1:5 into a new T-175 tissue culture flask in complete media.
-
11.
On the day of injections, wash KPC cells 1× with PBS and trypsinize as described above.
-
12.
Resuspend trypsinized KPC cells in 50 mL of complete media.
-
13.
Centrifuge sample at 300 × g for 5 min at 4°C.
-
14.
Remove the media and resuspend the cell pellet in 5 mL of 1× PBS.
-
15.
Count KPC cells using trypan blue exclusion for viability assessment. In this protocol, an automated cell counter was used.
-
16.
Resuspend KPC cells for injection in 1× PBS at 1 106 live cells per 50 μL.
-
17.
Maintain KPC cells on ice for the duration of surgeries.
CRITICAL: It is advisable to prepare twice the number of cancer cells necessary to account for spillage and dead volume in the syringe and needle.
CRITICAL: Users are recommended to use fresh vials of KPC aliquots per experiment, and avoid passaging cells more than 3 times.
CRITICAL: A cancer cell viability of ≥90% is required for intrasplenic injections. If viability is poor, cells will clump and block the splenic vessels, resulting in premature death during surgery.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Cell lines | ||
| Mouse KPC pancreatic cancer cells (fresh aliquot of cells with a maximum of 3 passages were used per experiment). | A/Prof Marina Pajic, The Garvan Institute of Medical Research, Australia. | PMID: 28381539 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6 mice of either sex at 10 weeks of age. No sex differences were observed. | In-house (Prof Matthias Ernst), The Olivia Newton-John Cancer Research Institute | N/A |
| Other | ||
| DMEM/F-12 media | Gibco | Cat#11320033 |
| Dulbecco’s phosphate-buffered saline (PBS) | Gibco | Cat#14190144 |
| TrypLE™ Express | Gibco | Cat#12604-021 |
| 175 cm2 Rectangular Straight Neck Cell Culture Flask with Vented Cap | Falcon | Cat#353112 |
| Countess® Automated Cell Counter | Thermo Fisher | Cat#AMQAF2000 |
| Cell Counting Slides | Bio-Rad | Cat#1450011 |
| Trypan Blue Stain (0.4%) for use with the Countess™ Automated Cell Counter | ThermoFisher | Cat#T10282 |
| Isoflurane | Baxter | N/A |
| Carprofen | Zoetis | N/A |
| Bupivacaine | AstraZeneca | N/A |
| Polyvisc eye ointment | Alcon | N/A |
| 0.9% Saline solution | Pfizer | N/A |
| Electrical heat pads | Pet stores | N/A |
| Sterile drape | Abena | Cat#4115 |
| Anesthesia chamber/tubing/nose cone | In-house (Austin Health Bioresources Facility) | N/A |
| Forceps | Braun | Cat#BD952R |
| Scissors | Braun | Cat#BC106R |
| Hemostat | Able Scientific | Cat#AS13008-12 |
| 27G × 1/2″ hypodermic needles | Terumo | Cat#NN+2713R |
| 100 μL Hamilton syringe | Sigma-Aldrich | Cat#20702 |
| 1 mL insulin syringe | Terumo | Cat#SS∗05M2713KA |
| Gauze | Multigate | Cat#11-559B |
| Reusable cautery kit (includes cauterizer and 10 mm cautery tips) | Able Scientific | Cat#ASCCK-1700 |
| 5-0 coated Vicryl sutures (absorbable) | Ethicon | Cat#J433H |
| Wound stapler and remover (BD Autoclip™ Wound Closing System) | Fisher Scientific | Cat#22-275998 |
| Wound staples | Fisher Scientific | Cat#01-804-5 |
Step-by-step method details
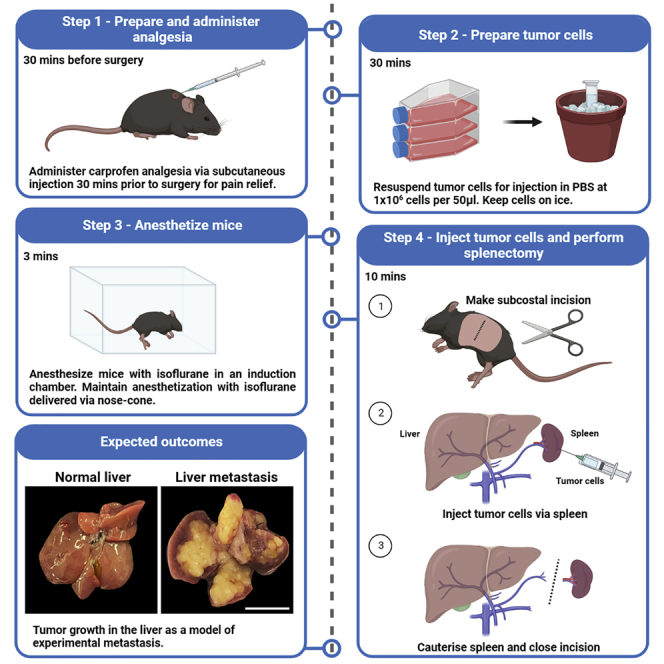
Anesthetize mice
Timing: 3 min
In this step, users will anesthetize animals via isoflurane inhalation.
-
1.Place the mouse in an anesthesia chamber with 2% isoflurane delivered with 2% oxygen.
-
a.Maintain body temperature by placing the anesthesia chamber on top of an electrical heat pad.
-
a.
-
2.
Monitor the mouse closely and remove once consciousness is lost.
Note: Sufficient anesthetization can be judged by the loss of withdrawal response to toe pinch.
-
3.
Administer a small amount of eye ointment to avoid corneal drying and trauma.
-
4.
Transfer the mouse to the aseptic surgical area, and maintain anesthetization with 2% isoflurane with 2% oxygen delivered via nose-cone.
CRITICAL: Body temperature should be maintained throughout the surgical procedure using an electrical heat pad.
Note: Users are required to monitor the breathing rate of animals and adjust the isoflurane flow-rate accordingly throughout surgery. The amount of isoflurane administered may need to be optimized for different mouse strains.
Intrasplenic injection of tumor cells
Timing: 10 min
In this step, users will inject cancer cells into the spleen and perform a splenectomy.
-
5.
Position the mouse in a lateral position on its right side.
-
6.
Shave fur along the torso of the mouse (Figure 2), and clean the skin with 80% ethanol.
Note: Users may wish to perform this step 1–2 days in advance to save time if numerous surgeries are planned.
-
7.
Wipe the site of fur removal with gauze soaked in 2% Chlorhexidine, then with 80% ethanol.
-
8.
Dry the skin with a clean piece of gauze.
-
9.
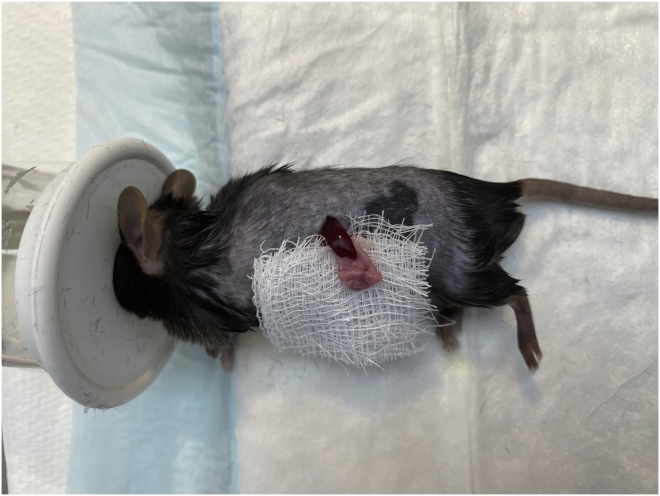
Palpate the region just below the ribs and make a single subcostal incision (approx. 1.5 cm) to access the peritoneal cavity (Figure 2).
-
10.
Gently expose the spleen and associated splenic ligaments using forceps, and place a dry square of gauze underneath the organ to hold it in place (Figure 3).
-
11.
Resuspend KPC pancreatic cancer cells by gently pipetting up and down.
-
12.
Draw up 50 μL of cancer cells in a Hamilton syringe, and attach a 27G needle tip. Ensure that air bubbles are absent.
CRITICAL: It is imperative that cancer cells are properly resuspended, as clumping of cells can block the splenic vessels and result in premature death during surgery.
-
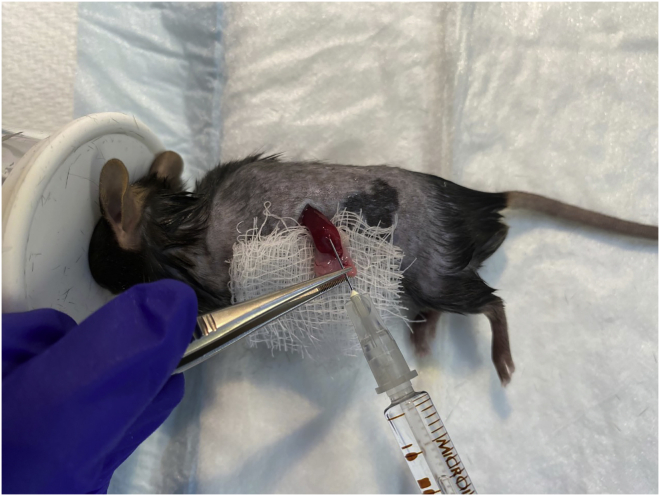
13.
Gently insert the tip of the needle (3 mm) into the spleen, and slowly inject cancer cells over a period of 40 s. Splenic distension/paling should be visible (Figure 4).
Note: Avoid moving the needle during injections as this may result in back flow.
-
14.
Place a piece of wet gauze over the injection site, and slowly retract the needle while holding the wet gauze in place (Figure 5).
CRITICAL: Wet gauze is required to soak up any cancer cells during removal of the needle.
-
15.
Compress the injection site for 1 min using either firm finger pressure or instruments (e.g., cauterizer, 1 mL pipette) as weights (Figure 6).
Note: This step is critical to allow cancer cells to enter the portal system and prevent back flow.
-
16.Discard the wet gauze, and remove the spleen by cautery (splenectomy) (Figure 7). To do this:
-
a.Hold the spleen with forceps in your left hand and the cautery in your right hand.
-
b.Cauterize the splenic ligaments to remove the spleen.
-
a.
Optional: Ask an assistant to gently retract the splenic ligaments while you hold the spleen to gain better access.
CRITICAL: If bleeding is observed following splenectomy, place a few pieces of gauze at the site and add pressure for 3 min. Once bleeding has stopped, remove the gauze.
-
17.
Once the spleen has been removed and no bleeding is observed, gently return the connective tissue (i.e., splenic ligaments) into the peritoneal cavity.
-
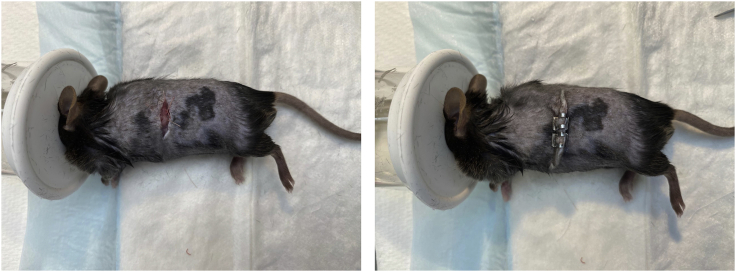
18.
Suture the peritoneum, then staple the skin layer with wound clips (Figure 8).
-
19.
Topically administer 50 μL of 0.12% bupivacaine along the incision site using a 1 mL syringe.
-
20.
Administer 300 μL of sterile saline via intraperitoneal injection to reduce dehydration from blood loss.
Note: If excessive bleeding is observed, users may consider injecting more saline to improve post-surgery recovery.
-
21.
Place the mouse in a clean cage on top of an electrical heat pad and monitor until the animal has recovered from surgery (i.e., consciousness is regained).
CRITICAL: Ensure that surgical tools are disinfected between mice by thoroughly wiping with 80% ethanol and F10sc veterinary disinfectant.
Figure 2.
Subcostal incision
Figure 3.
Expose spleen and place on gauze
Figure 4.
Intrasplenic injection of cancer cells
Splenic distension/paling should be visible.
Figure 5.
Apply wet gauze while gently removing syringe to prevent back flow
Figure 6.
Apply firm pressure for 1 min
Figure 7.
Perform splenectomy by cauterizing the spleen
Figure 8.
Close the incision with sutures and wound clips
Animal recovery, monitoring, and endpoint analysis
Timing: 15 min
In this step, users will monitor animals following surgery and up until the experimental endpoint.
-
22.After the surgical procedure, return mice to clean cages containing sterile bedding.
-
a.Cages should be placed on top of a heat pad for 24 h.
-
a.
Optional: Provide sunflower seeds, peanut butter, and Hydrogel to aid with recovery.
Optional: Administer an additional dose of carprofen every 24 h, for up to 72 h.
-
23.Perform daily welfare checks and detailed clinical monitoring (including weighing of mice) at least twice a week. Be alert for clinical signs that indicate progression of metastatic disease, which include but are not limited to:
-
a.Weight loss.
-
i.Weight loss is a key indicator of disease progression and correlates with an increased tumor burden.
-
ii.Perform daily clinical monitoring if ≥10% weight loss is observed.Note: Weight loss of 5%–10% can be expected a day after surgery. However, this should stabilize and recover within 2 days.Note: In our experiments, untreated C57BL/6 wild-type mice reproducibly demonstrate ≥15% weight loss within 3 weeks of tumor injection (experimental endpoint), at which point overt liver metastases are observed (Figure 9A).
-
i.
-
b.Jaundice.
-
i.Users may wish to keep the surgical area and/or abdominal region of mice shaved to improve detection of jaundice.
-
i.
-
c.Reduced activity / unresponsive to surroundings.
-
d.Abdominal edema / swelling.
-
i.It may be possible to detect the presence of liver metastases by gently palpating the abdomen of mice. KPC tumors are highly fibrotic and can be felt as hard nodules.
-
i.
-
e.Pale eyes, ears, and feet.
-
f.Hunched posture.Note: Users are encouraged to develop a monitoring plan for scoring clinical signs with guidance from their Animal Ethics Committee to ensure humane intervention and/or experimental endpoints are met according to Institutional guidelines and parameters.Note: In addition to clinical signs, users can also monitor tumor growth via live imaging if their cancer cells express a reporter (e.g., luciferase, mCherry, GFP). Alternatively, PET/MRI imaging may also be used.Optional: Users may wish to incorporate drug treatments to this protocol. We commence treatment on the 5th day following intrasplenic tumor cell injection to allow ample time for cancer cells to establish in the liver (refer to Expected Outcomes below). However, users are advised to optimize the timing of their treatment schedules depending on their selected cancer cell line and number of cells injected.
-
a.
-
24.At the experimental endpoint, euthanize mice by CO2 inhalation (or alternative methods approved by the investigator’s Animal Ethics Committee).
-
a.Carefully remove the liver and rinse 3× in 1× PBS.
-
b.Weigh the liver as a surrogate measure of tumor burden.
-
c.Process the liver as appropriate for downstream analysis (e.g., formalin fix, snap frozen, digestion for flow-cytometry).
-
a.
Note: For this protocol, mice reached clinical endpoint by 3 weeks following cancer cell injection.
Note: Endpoints will vary based on the study design, including the type of cancer cell line and number of cancer cells injected. Users are advised to optimize the protocol to suit their experimental needs.
Note: Depending on the study design, overt liver metastases may or may not be apparent at necropsy. Instead, micro-metastases may be observed via histological analysis.
CRITICAL: The intrasplenic injection model results in tumor growth exclusively in the liver. If tumors are observed in other organs and/or the incision site, this indicates that cancer cell leakage occurred during injection. Do not use these mice for analysis.
Figure 9.
Stromal and immune cell profiling of intrasplenic KPC liver metastases
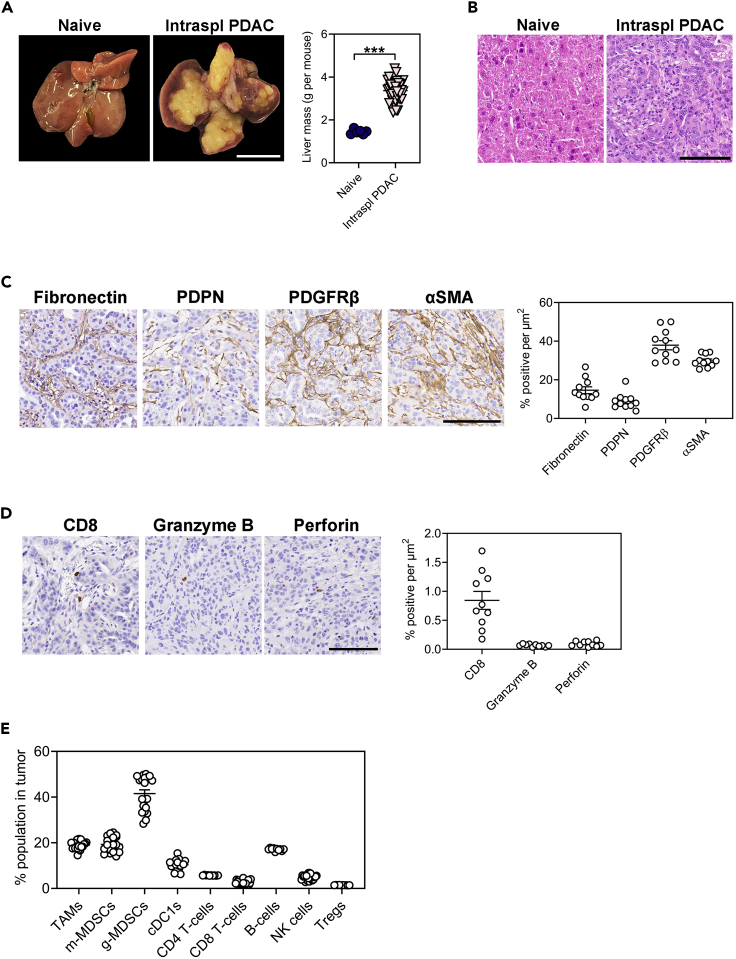
(A) Representative whole mounts and corresponding liver weights of C57BL/6 WT hosts following intrasplenic injection of KPC pancreatic ductal adenocarcinoma (PDAC) cells. Mice were collected 3 weeks following intrasplenic tumor cell injection. Livers of naïve mice are shown for comparison. Scale bar: 1 cm. Each symbol represents an individual mouse.
(B) Representative H&E-stained liver sections of mice described in (A). Scale bar: 100 μm.
(C) Representative immunohistochemical staining and quantification of fibronectin and cancer-associated fibroblast markers (i.e., PDPN, PDGFRβ, αSMA) in KPC liver metastases of WT hosts described in (A). Scale bar: 100 μm. Each symbol represents an individual mouse.
(D) Representative immunohistochemical staining and quantification of CD8+ T-cells and associated immune cell activation markers (i.e., Granzyme B, Perforin) in KPC liver metastases of WT hosts described in (A). Scale bar: 100 μm. Each symbol represents an individual mouse.
(E) Flow cytometry quantification of tumor-associated macrophages (CD11b+F4/80High of CD45+), monocytic myeloid-derived suppressor cells (Ly6c+Ly6g- of CD45+CD11b+), granulocytic myeloid-derived suppressor cells (Ly6cintLy6g+ of CD45+CD11b+), conventional type 1 dendritic cells (CD103+MHCII+ of CD45+CD11c+F4/80-), CD4 T-cells (CD4+TCRβ+ of CD45), CD8 T-cells (CD8+TCRβ+ of CD45), B-cells (CD19+ of CD45), natural killer cells (NK1.1+ of CD45) and Tregs (Foxp3+ of CD45+) in KPC liver metastases of WT hosts described in (A). Each symbol represents an individual mouse. Data represent mean ± SEM; ∗∗∗p < 0.001, with statistical significance determined by an unpaired Student’s t-test for comparison between two means. Data were re-analyzed from Poh et al.1
Expected outcomes
The intrasplenic injection model results in tumor formation exclusively in the liver, and is used as a model of experimental metastasis. In this protocol, 10-week-old C57BL/6 wild-type (WT) mice were injected with 1 106 KPC pancreatic cancer cells and reached clinical endpoint at 3 weeks following intrasplenic tumor cell injection (Figure 9A).
KPC tumors established in the liver show poorly differentiated morphology (Figure 9B) with a dense desmoplastic stroma, as indicated by extensive staining for extracellular matrix proteins (e.g., fibronectin) and cancer-associated fibroblasts (e.g., PDPN, PDGFβ, αSMA) (Figure 9C). Additionally, CD8 T-cells and cytotoxic effector molecules (e.g., Granzyme B, Perforin) are largely absent (Figure 9D).
Flow-cytometry was also performed to assess the relative abundance of immune cell populations in KPC liver metastases, including tumor-associated macrophages, myeloid-derived suppressor cells, dendritic cells, T-cells, B-cells, NK cells, and Tregs (Figure 9E).
Users may wish to incorporate drug treatments to this protocol. In the examples shown in Figures 10A and 10B, 10-week-old C57BL/6 WT mice were treated with anti-PD1 immunotherapy (200 μg, once every 3 days) or gemcitabine chemotherapy (120 mg/kg, weekly) on the 5th day following intrasplenic tumor cell injection for 2 weeks. Consistent with previous studies utilizing autochthonous and orthotopic KPC tumor models,3,4 KPC tumors established in the liver are also refractory to immune checkpoint blockade and moderately responsive to gemcitabine chemotherapy.1
Figure 10.
KPC liver metastases are refractory to anti-PD1 immune checkpoint blockade and moderately sensitive to gemcitabine chemotherapy
(A) Representative whole mounts and corresponding liver weights of WT hosts treated once every 3 days with anti-PD1 or a matched IgG isotype control. Treatment commenced on the 5th day after intrasplenic KPC pancreatic cancer cell injection and continued for 2 weeks. Scale bar: 1 cm. Each symbol represents an individual mouse.
(B) Representative whole mounts and corresponding liver weights of WT hosts treated weekly with Gemcitabine or PBS vehicle. Treatment commenced on the 5th day after intrasplenic KPC pancreatic cancer cell injection and continued for 2 weeks. Scale bar: 1 cm. Each symbol represents an individual mouse. Data represent mean ± SEM; ∗∗∗p < 0.001, with statistical significance determined by an unpaired Student’s t-test for comparison between two means. Data were re-analyzed from Poh et al.1
Quantification and statistical analysis
Data represent mean ± SEM; ∗∗∗p < 0.001, with statistical significance determined by an unpaired Student’s t-test for comparison between two means.
Limitations
One major limitation of the intrasplenic tumor model is that it bypasses the initial steps of metastasis, including dissemination of cancer cells from the primary tumor and the intravasation and extravasation from blood vessels. While orthotopic and spontaneous metastasis models recapitulate the full metastatic cascade, their limitations include the unpredictable speed of metastasis, the low frequency of metastasis, and rapid growth of the primary tumor resulting in premature death. By contrast, the intrasplenic injection technique allows investigation into later steps of the metastatic cascade, including establishment of tumors at a clinically-relevant metastatic site. It also results in robust tumor growth in the liver, and allows users to control the timing of their experiments by adjusting the number of injected cells. In turn, this leads to more reproducible results and fewer animals required per experiment.
Another limitation of this model is that a splenectomy is performed to prevent the overgrowth of splenic tumors. Splenic tumors will result in the premature death of hosts before overt liver metastases are visible. However, splenectomy may impact the function of the immune system. For example, splenectomy has been shown to decrease the mobilization of wound-healing monocytes to injured tissues5 and impair the growth of non-small cell lung cancer by reducing the presence of circulating and intratumoral myeloid-derived suppressor cells.6 Thus, splenectomy may inadvertently influence tumor progression by impacting the activity of the immune system.
Troubleshooting
Problem 1
Tumors are observed in other organs and/or the incision site (Related to step 23).
Potential solution
If performed correctly, this model will result in tumor growth exclusively in the liver. If tumors are observed at other organs/sites, cancer cell leakage may have occurred during injection. These mice should be excluded from analysis.
To reduce the chance of cancer cell leakage, insert the needle into the spleen at an appropriate depth (3 mm), inject slowly, and ensure adequate pressure is placed on the injection site. Splenic distension/paling should be visible, and will confirm that cancer cells have been injected properly.
Problem 2
Overt liver metastases are not apparent at necropsy (Related to step 23).
Potential solution
This may indicate that the procedure was not performed properly. However, the absence or delay in tumor formation may also be attributed to other factors, including the cancer cell line selected, number of cancer cells injected, or treatment with therapies that suppress tumor growth.
Possible solutions include:
-
•
Improve injection technique (e.g., do not move the needle during injections, ensure splenic distension/paling is visible).
-
•
Titrate the number of cancer cells injected until all mice develop liver metastasis. Importantly, within the same experiment, untreated mice should develop liver metastasis and reach clinical endpoint reproducibly within a similar time frame. The presence of micro-metastases can also be confirmed via histological analysis.
-
•
Ensure that cancer cells are ≥90% viable before injecting.
Problem 3
Cancer cells are clumping during surgery, which can result in blockage of splenic vessels and pre-mature death (Related to step 13).
Potential solution
-
•
Ensure that cancer cells are ≥90% viable before injecting. Users are required to optimize this protocol according to the growth patterns of their selected cell lines. Potential solutions include using cell stocks that have been sub-cultured fewer times, regular testing for mycoplasma, and ensuring cells are not over-confluent when harvesting.
-
•
Maintain cancer cells on ice throughout surgery.
-
•
Thoroughly resuspend cancer cells by gently pipetting up and down before injections.
Problem 4
Death during intrasplenic injection of tumor cells (Related to step 1).
Potential solution
-
•
Ensure that the mouse’s body temperature is maintained using heat pads during surgery and recovery to avoid hypothermia and death.
-
•
Monitor the breathing rate of animals and adjust the isoflurane flow-rate accordingly. The amount of isoflurane administered may need to be altered for different mouse strains to avoid overdosing.
-
•
It is imperative that cancer cells are properly resuspended since cell clumps can block the splenic vessels and cause premature death.
-
•
Confirm that bleeding has stopped after splenectomy as excess blood loss during or after surgery can contribute to premature death.
Problem 5
Excessive bleeding following removal of spleen (Related to step 16).
Potential solution
Ensure sufficient gauze and pressure is held on the splenectomy site. If blood loss is still observed after 3 min, replace gauze and hold pressure for an additional 3 min. Users may consider injecting mice with more saline to improve post-surgery recovery.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr Ashleigh Poh (ashleigh.poh@onjcri.org.au).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
M.E. received funding from Ludwig Cancer Research and is an NHMRC Investigator grant recipient (1173814). A.R.P. received support from 2018 Priority-driven Collaborative Cancer Research Scheme Grant (1157894) co-funded by Cancer Australia, Cure Cancer, and Pancare Foundation; a Tour de Cure Early Career Seed Grant (RSP-060-18/19); and an Avner Collaboration Grant from PanKind, The Australian Pancreatic Cancer Foundation in collaboration with Tour de Cure, and Woolworths Limited through Woolies on Wheels and Walks. A.R.P. is an NHMRC Peter Doherty Early Career Fellow (1166447).
Author contributions
Investigation, M.O’B., A.R.P., M.E.; writing, M.O’B., A.R.P., M.E.; review & editing, M.O’B., A.R.P., M.E.; supervision: A.R.P and M.E.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate new data or analyze code. Re-analyzed datasets and figures were previously published in Poh et al.1
References
- 1.Poh A.R., O'Brien M., Chisanga D., He H., Baloyan D., Traichel J., Dijkstra C., Chopin M., Nutt S., Whitehead L., et al. Inhibition of HCK in myeloid cells restricts pancreatic tumor growth and metastasis. Cell Rep. 2022;41:111479. doi: 10.1016/j.celrep.2022.111479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A., Ross S., Conrads T.P., Veenstra T.D., Hitt B.A., et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Winograd R., Byrne K.T., Evans R.A., Odorizzi P.M., Meyer A.R.L., Bajor D.L., Clendenin C., Stanger B.Z., Furth E.E., Wherry E.J., Vonderheide R.H. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 2015;3:399–411. doi: 10.1158/2326-6066.Cir-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y., Li J., Wang H., Chiu Y., Kingsley C.V., Fry D., Delaney S.N., Wei S.C., Zhang J., Maitra A., Yee C. Combination of PD-1 inhibitor and OX40 agonist induces tumor rejection and immune memory in mouse models of pancreatic cancer. Gastroenterology. 2020;159:306–319.e12. doi: 10.1053/j.gastro.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.L., Kohler R.H., Chudnovskiy A., Waterman P., et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy L., Mishalian I., Bayuch R., Zolotarov L., Michaeli J., Fridlender Z.G. Splenectomy inhibits non-small cell lung cancer growth by modulating anti-tumor adaptive and innate immune response. OncoImmunology. 2015;4:e998469. doi: 10.1080/2162402x.2014.998469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate new data or analyze code. Re-analyzed datasets and figures were previously published in Poh et al.1