Abstract
We report a case of a person with human immunodeficiency virus with disseminated Mycobacterium avium infection, in whom antiretroviral therapy combined with all drugs of anti–M avium activity failed to clear the pathogen. After PD-1 inhibitor treatment, T-cell exhaustion was reversed and M avium–specific T-cell response was boosted, together with M avium clearance.
Keywords: HIV, immunotherapy, mycobacteria, Mycobacterium avium, PD-1 blockade
Immune checkpoint inhibitors (ICIs), including those that enhance T-cell function by blockade of coinhibitory receptor programmed cell death 1 (PD-1) and one of its ligands, programmed cell death ligand 1 (PD-L1), are now widely used for multiple malignant tumors. The PD-1/PD-L1 pathway also plays a major role in T-cell exhaustion during chronic infections, for example, human immunodeficiency virus (HIV) infection [1]. Therefore, there is an increasing interest in targeting the PD-1/PD-L1 pathway for the treatment of chronic infectious diseases [2].
Mycobacterium avium complex (MAC), mainly comprising M avium and Mycobacterium intracellulare, is the most common etiology of nontuberculous mycobacteria (NTM) disease in people with HIV (PWH) [3]. In the modern antiretroviral therapy (ART) era, the 5-year mortality of PWH with disseminated NTM disease is as high as 26.7% despite aggressive treatment [4].
Given the markedly increased risk of NTM caused by loss of CD4+ T cells, enhancing T-cell function by PD-1/PD-L1 blockage may be a promising therapy to combat intracellular mycobacterial infections. However, the role of PD-1 in mycobacterial infection is still controversial. Several cases of tuberculosis and M avium lung disease have been reported in patients with multiple cancers who were treated with PD-1 inhibitors, suggesting that boosting type 1 T-helper cell function with PD-1 blockade may increase the risk or severity of tuberculosis in humans [5, 6]. In contrast, a recent study suggested that nivolumab improved Mycobacterium abscessus lung disease in a patient with advanced lung cancer [7]. Taken together, this evidence suggests that enhanced pathogen-specific T-cell function through PD-1 blockade may either control infection or drive lethal immunopathology, depending on the particular microbe present and the immune status of the host.
We report a case of a patient with HIV with disseminated and refractory M avium infection, who was treated successfully with a combination of PD-1 inhibitor, anti-mycobacterial therapy, and ART.
A 25-year-old Chinese man was referred to Shanghai Public Health Clinical Center on 17 July 2020, due to fever and fatigue for 2 months. He was recently diagnosed with HIV infection and disseminated M avium infection, which could explain his symptoms. Upon admission, his CD4 T-cell count was 10 cells/mm3 with CD4/CD8 T-cell ratio of 0.08. The HIV viral load was 8.71 × 104 copies/mL. Mycobacterium avium was identified by blood culture with a time to positivity (TTP) of 14 days. Computed tomography of the abdomen showed enlarged liver and spleen, as well as multiple swollen lymph nodes at the retroperitoneal and mesenteric root (Supplementary Figure 1A). The patient then received azithromycin, ethambutol, and rifabutin together with moxifloxacin. At the same time, ART with emtricitabine/tenofovir and dolutegravir was initiated. However, the patient's symptoms continued, with the highest temperature of 40°C. Then, linezolid and amikacin were also added to the regimen after 4 weeks of anti-mycobacterial therapy. Taking immune reconstitution inflammatory syndrome (IRIS) into consideration, dexamethasone 5 mg daily intravenously was given. However, blood culture for M avium continued to be positive with a TTP of 13 days after 6 weeks of anti-mycobacterial therapy. In addition to persistent fever, the patient also suffered from pancytopenia. Despite elevated serum ferritin level (4678 ng/mL), levels of triglycerides and fibrinogen were normal and hemophagocytosis was also not identified on bone marrow aspirate cytology. Therefore, his symptoms were attributed to the chronic M avium infection and side effects of linezolid, but not IRIS or hemophagocytic lymphohistiocytosis (HLH). Drug susceptibility testing reported that the M avium isolated was susceptible to clarithromycin and intermediate to moxifloxacin but resistant to amikacin and linezolid. Thus, dexamethasone was tapered while the anti-mycobacterial regimen was switched to azithromycin, ethambutol, rifabutin, moxifloxacin, bedaquiline, and clofazimine. After that, vomiting occurred, which was attributed to clofazimine, while the patient continued to suffer from a high fever. Therefore, clofazimine was discontinued, and meropenem and amikacin were added. Despite continuous anti-mycobacterial therapy for 24 weeks, blood culture for M avium was still positive, with TTP ranging from 13 days to 28 days, and radiological imaging was unchanged (Supplementary Figure 1B and Figure 1A). The CD4 T-cell count increased slightly to 33 cells/mm3 and viral load was under the limit of detection (Figure 1B).
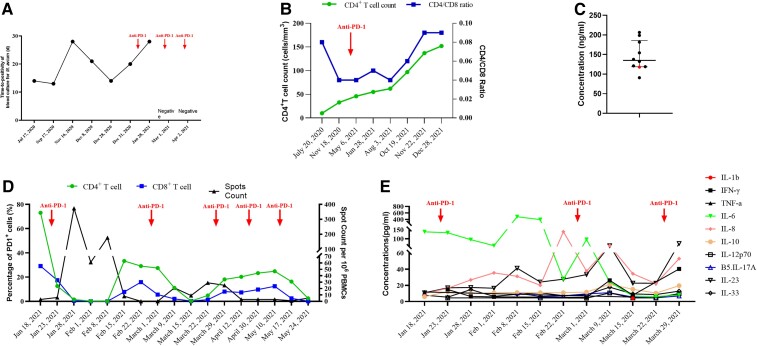
Figure 1.
Trajectories of the patient’s laboratory tests results over time. A, Time to positivity of blood culture for Mycobacterium avium. B, CD4+ T-cell count and CD4/CD8 T-cell ratio. C. Concentrations of anti-IFN-γ autoantibody in the patient and healthy controls. D. Proportions of PD-1–expressing CD4+ and CD8+ T cells in peripheral blood mononuclear cells, as well as spot count quantified by enzyme-linked immunosorbent spot assay after protein from M avium lysate stimulation. E, Concentrations of different cytokines in plasma. Abbreviations: IFN, interferon; IL, interleukin; PD-1, programmed cell death protein 1; TNF, tumor necrosis factor.
We then quantified the anti–interferon-γ autoantibodies in his plasma and found that the level was comparable to that in healthy controls (Figure 1C). We believed that the patient may not be unable to clear M avium with anti-mycobacterial therapy alone under such advanced immunosuppression. We then hypothesized that due to HIV and chronic M avium infection, the M avium–specific T cells could be exhausted and PD-1 inhibitors may be effective to restore their function. We determined the PD-1 expression on his peripheral T cells (Supplementary Figure 2). As high as 73.1% of the CD4 T cells and 29.1% of the CD8 T cells from his peripheral blood mononuclear cells (PBMCs) expressed PD-1, which further supported our hypothesis. Therefore, based on the availability, the patient received intravenous sintilimab, a PD-1 inhibitor, for 5 cycles starting from 22 January 2021. A dose of 200 mg/day was chosen as it was recommended in cancer treatment and it also has a good safety profile in our experience in treating PWH with cancers.
On the second day after sintilimab administration, the proportion of PD-1–expressing CD4 T cells decreased to 12.6%, while the proportion of CD8 T cells also decreased to 17.4% (Figure 1D). Meanwhile, the M avium–specific spot number, counted as previously described, increased from 3 to 374 per million PBMCs (Figure 1D) [8]. Plasma levels of interleukin (IL)–6 tended to decrease, while levels of IL-8 and IL-23 tended to increase (Figure 1E). The TTP of blood culture for M avium increased to 28 days after the first cycle of PD-1 inhibitor therapy and blood culture for M avium was negative after the second cycle (Figure 1A). However, due to the long incubation time (7 weeks) before a negative result was reported, the patient continued to receive another 3 cycles of sintilimab. The frequency of fever, as well as the maximum temperature, decreased gradually after PD-1 inhibitor therapy. The patient's temperature was finally normalized at 51 days since the first PD-1 inhibitor therapy (around 2 weeks after the second PD-1 inhibitor cycle) and fatigue also disappeared. The radiological image showed that the swollen lymph nodes at the retroperitoneal and mesenteric root had shrunken compared to pre–PD-1 inhibitor treatment (Supplementary Figure 1C and 1D). During the PD-1 inhibitor therapy, no sintilimab-related adverse event was observed.
The patients continued ART and anti-mycobacterial therapy thereafter. He was healthy and had returned to work at the latest visit on December 2021. His CD4 T-cell count had increased to 152 cells/mm3 with CD4/CD8 T-cell ratio of 0.09, while HIV viral load continued to be undetectable (Figure 1B).
In the current case, all the available drugs of anti–M avium activity had been tried but failed to clear the pathogen. However, after adding a PD-1 inhibitor, the M avium–specific T-cell response was boosted. Consistently, the patient's blood culture for M avium became negative and the patient's symptoms were relieved. Remarkably, in several patients with progressive multifocal leukoencephalopathy, a central nervous system disease caused by JC virus, PD-1 blockade reduces JC viral load while increasing CD4 and CD8 T-cell activity against the JC virus, together with clinical improvement or stabilization [9]. Herein, PD-1/PD-L1 inhibitors may be used in treating refractory infectious disease (eg, drug resistance) and in those cases where drugs are not available against the pathogen.
PD-1 blockade in patients with a high burden of mycobacteria and immunodeficiency may lead to severe inflammatory reactions or IRIS. Indeed, tuberculosis reactivation and development of MAC lung disease in patients with cancer receiving immune checkpoint inhibitors have been reported. Severe inflammatory reactions were not observed in our case. It is possible that after 24 weeks of anti-mycobacterial therapy, the IRIS risk was decreased due to lowering the mycobacterial burden to some degree even though TTP did not change, and this allowed PD-1 inhibitors to be safely used to boost anti-mycobacterial T-cell responses. Interestingly, PD-1 inhibitors have been used successfully to treat Epstein-Barr virus (EBV)–induced HLH by restoring a defective anti-EBV response [10]. In the current case, PD-1 inhibitor also boosted M avium–specific immune response. Therefore, it is possible that PD-1 inhibitors could be useful even in the setting of IRIS.
The present case was a patient with advanced immunodeficiency due to HIV infection. In our case, the CD4 T-cell count remained very low after 24 weeks of ART, despite virological success. It is well established that chronic HIV infection is associated with exhausted T-cell function, which was also observed in our patient [11]. After PD-1 inhibitor administration, the proportion of T cells expressing PD-1 decreased while the function of M avium–specific T cells was enhanced, with symptoms relieved thereafter. Interestingly, PD-L1 expression on tumor cells is a valuable predictor of the efficacy of anti-PD-1/PD-L1 therapy in patients with advanced non-small cell lung cancer [12, 13]. Those patients with higher PD-L1 expression on tumor cells have more clinical benefits in PD-1 blockade [14]. Along this line, patients with high PD-1/PD-L1 expression may benefit more from PD-1 blockade, and PD-1 level on T cells may serve as a surrogate marker to guide patient selection in treating infectious diseases.
The T-cell response after PD-1 inhibitor administration waned gradually in our patient. A significant decrease in the proportion of PD-1 expression in T cells and enhanced M avium–specific T-cell response after the first dose of sintilimab was observed, while the magnitude of the changes was decreased after the second cycle. However, the following 2 infusions did not show any improvement in T-cell exhaustion and boost of M avium–specific T-cell response. This could be explained by the clearance of M avium in the blood after the second administration of sintilimab. Therefore, the duration of PD-1 blockade should be guided by microbiological results in treating infectious diseases.
There are several limitations to our study. First, during PD-1 inhibitor treatment, the patient continued ART and anti-mycobacterial therapy. In addition, the first negative blood culture was performed after 3 cycles of anti-PD-1 infusion, so the clinical improvement and clearance of the pathogen could not solely be attributed to PD-1 blockade. Second, the use of M avium lysate as a stimulation antigen may have limited our ability to detect M avium–specific CD8 T cells, as whole protein antigens may preferentially stimulate CD4 T cells. There might also be some cross-activation between M avium and other Mycobacterium species. Last, clinical trials with controls are needed to validate our results to support the hypothesis that boosting T-cell responses through PD-1 blockade can treat infectious diseases.
Supplementary Material
Contributor Information
Li Liu, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Zichen Song, Department of Scientific Research Center, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Jingna Xun, Department of Scientific Research Center, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Danping Liu, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Jianhao Wei, Department of Laboratory Medicine, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Zhenyan Wang, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Yang Tang, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Jianjun Sun, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Jun Chen, Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the patient and his family for participating in this study. We also thank Jiadang Gu for her coordination.
Patient consent. This study was approved by the Ethics Committee of Shanghai Public Health Clinical Center. Written informed consent was obtained from the patient for the therapy and the case report.
Financial support. This work was supported by the Science and Technology Commission of Shanghai Municipality (grant number 21Y11901200); the Shanghai Human Resources and Social Security Bureau (grant number 2020089); and the Shanghai Hospital Development Center (grant numbers SHDC2020CR4005 and SHDC2020CR6025 ).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J Immunol 2012; 188:2957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018; 18:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015; 36:13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu J, Gu L, Shao Y, et al. Long-term case-fatality rate of nontuberculous mycobacterial disease in people living with HIV. Infect Dis Poverty 2022; 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu K, Wang D, Yao C, et al. Increased tuberculosis incidence due to immunotherapy based on PD-1 and PD-L1 blockade: a systematic review and meta-analysis. Front Immunol 2022; 13:727220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barber DL, Sakai S, Kudchadkar RR, et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med 2019; 11:eaat2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishii S, Tamiya A, Taniguchi Y, et al. Improvement of Mycobacterium abscessus pulmonary disease after nivolumab administration in a patient with advanced non-small cell lung cancer. Intern Med 2018; 57:3625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Della Bella C, Venturini E, Devente S, et al. Role of Mycobacterium avium lysate INF-gamma, IL-17, and IL-2 ELISPOT assays in diagnosing nontuberculous mycobacteria lymphadenitis in children. Eur J Clin Microbiol Infect Dis 2019; 38:1113–22. [DOI] [PubMed] [Google Scholar]
- 9. Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med 2019; 380:1597–605. [DOI] [PubMed] [Google Scholar]
- 10. Liu P, Pan X, Chen C, et al. Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults. Blood 2020; 135:826–33. [DOI] [PubMed] [Google Scholar]
- 11. Fenwick C, Joo V, Jacquier P, et al. T-cell exhaustion in HIV infection. Immunol Rev 2019; 292:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375:1823–33. [DOI] [PubMed] [Google Scholar]
- 13. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393:1819–30. [DOI] [PubMed] [Google Scholar]
- 14. Xu Y, Wan B, Chen X, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res 2019; 8:413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.