Abstract
Background
Salmonella enterica subspecies enterica serovar Oranienburg (SO) is a foodborne pathogen but rarely causes systemic infections such as bacteremia. Between July and September 2018, bacteremia cases caused by SO were identified in 12 persons without any underlying medical conditions in the southern Kyushu area of Japan.
Methods
Randomly amplified polymorphic DNA (RAPD) analysis was performed to investigate the genetic similarity of the 12 bacteremia-related strains and other Japanese isolates. Furthermore, a series of whole-genome sequence (WGS)–based phylogenetic analyses was performed with a global SO strain set (n = 1648).
Results
The resolution power of RAPD was insufficient to investigate the genetic similarity between the bacteremia-related strains and other strains. WGS-based phylogenetic analyses revealed that the bacteremia-related strains formed a tight cluster along with 2 strains isolated from asymptomatic carriers in 2018 in the same area, with a maximum within-cluster single-nucleotide polymorphism (SNP) distance of 11. While several strains isolated in the United States and the United Kingdom were found to be closely related to the bacteremia-related strains, 2 strains isolated in 2016 in the southern Kyushu area were most closely related, with SNP distances of 4–11 and 5–10, and had the same plasmids as the bacteremia-related strains.
Conclusions
The 12 bacteremia cases identified were caused by a single SO clone. As none of the bacteremia patients had any underlying diseases, this clone may be prone to cause bacteremia. Although further analyses are required to understand its virulence, particular attention should be given to this clone and its close relatives in the surveillance of nontyphoidal salmonellae.
Keywords: bacteremia cluster, Salmonella enterica subsp enterica serovar Oranienburg
Nontyphoidal salmonellae (NTS) are one of the leading causes of acute bacterial gastroenteritis worldwide [1]. Although NTS are usually associated with gastroenteritis in healthy people, individuals who are very young (<5 years old), elderly (≥70 years old), or immunocompromised are at risk for higher mortality as a consequence of invasive diseases [2]. Among NTS, Salmonella Enteritidis is the most prevalent serovar causing human infection in the United States (US) and the European Union, followed by various other serovars, such as Typhimurium and Infantis [3, 4]. In Japan, NTS account for 12.6% of the cases of bacterial food poisoning reported in food poisoning statistics (2000–2021) by the Ministry of Health, Labour and Welfare of Japan. Among these NTS, S Enteritidis dominated until 2015, but a wide variety of serovars have been isolated since 2016 [5] (see Supplementary Table 1 for the summary data).
Salmonella Oranienburg (SO) is one of the NTS that have been recognized as foodborne pathogens. Although this serovar is relatively uncommon in NTS-associated food poisoning, it is sometimes associated with outbreaks. In Japan, 2 outbreaks have been reported thus far [6, 7], 1 of which was a nationwide outbreak caused by contaminated processed squid products in 1999. In this outbreak, while most patients showed gastrointestinal symptoms, some patients developed bacteremia; however, there is no information on whether the patients with bacteremia had underlying diseases [6].
Recently, we identified 12 SO strains isolated from a cluster of bacteremia cases occurring in persons without any underlying medical conditions according to medical records in the southern Kyushu area in Japan in 2018. Although we suspected the possibility of an outbreak of SO bacteremia, the genetic relationship of these strains was unknown in routine laboratory examinations. To clarify it, we performed randomly amplified polymorphic DNA (RAPD) analysis and whole-genome sequencing (WGS)–based phylogenetic analyses of these strains and show that the cluster was caused by a single SO clone.
METHODS
Bacterial Strains
The 12 SO strains were isolated from 12 bacteremia patients during routine laboratory investigations in 6 hospitals in Kagoshima, Miyazaki, and Okinawa prefectures (cases 1–12, Table 1). In addition to these bacteremia-related strains, we used an additional 25 SO strains and a serotype O8 strain isolated in Japan in this study (Supplementary Table 2). A total of 1622 S. Oranienburg strains that were registered in the EnteroBase website version 1.1.2 (https://enterobase.warwick.ac.uk [8]), which included strain 0250 as a reference, were also used for WGS-based phylogenetic analysis (listed in Supplementary Table 3). Although the EnteroBase included 1763 strains registered as SO (accessed on 10 May 2021), 110 were excluded for lack of genome sequence information, and 31 were excluded due to low completeness (<99%) or high contamination (>1%), as estimated by CheckM version 1.0.11 [9].
Table 1.
Clinical Features and Medical Records of Bacteremia Patients
| Case | Sex | Age, y | Hospital | Place | Symptomsa | Onset of Symptoms | Date of Hospitalization | Date of Blood Culture | Days From Onset of Symptoms to Date of Blood Culture | Antibiotic Treatment | Food Exposure History (Within 1 wk) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 35 | A | Kagoshima | F, HA | 14 Jul | 21 Jul | 21 Jul | 7 | CTRX | … |
| 2 | M | 50 | A | Kagoshima | F, HA, DI | 18 Jul | OC | 30 Jul | 12 | CTRX | Raw egg |
| 3 | F | 15 | A | Kagoshima | F, DI | 21 Jul | 31 Jul | 31 Jul | 10 | CTRX | Raw chicken meat |
| 4 | M | 21 | A | Kagoshima | F, HA | 27 Jul | 6 Aug | 30 Jul | 3 | CTRX | Raw egg |
| 5 | F | 29 | A | Kagoshima | F, AP | 30 Jul | OC | 9 Aug | 10 | LVFX | Raw chicken meat |
| 6 | F | 18 | B | Kagoshima | F, DI, AR | 1 Aug | 6 Aug | 6 Aug | 5 | CEZ | Bowl of rice with chicken and eggs |
| 7 | M | 37 | A | Kagoshima | F, CH | 9 Aug | OC | 20 Aug | 11 | CTRX | Raw chicken meat |
| 8 | M | 52 | C | Miyazakib | F, DI | 9 Aug | 14 Aug | 14 Aug | 5 | CTRX | Chicken meat |
| 9 | M | 32 | D | Okinawac | F, D, DI | 13 Aug | 23 Aug | 23 Aug | 10 | CPFX → ABPC | … |
| 10 | M | 2 | E | Kagoshima | F, EM, AP, DI | 24 Aug | 3 Sep | 4 Sep | 11 | CTRX | Raw chicken meat and raw egg |
| 11 | M | 24 | A | Kagoshima | F, HA | 22 Sep | OC | 27 Sep | 5 | LVFX | … |
| 12 | F | 2 | F | Kagoshima | F, DI | 28 Sep | 5 Oct | 5 Oct | 7 | CTRX | … |
Abbreviations: …, no information; ABPC, Ampicillin; CPFX, Ciprofloxacin; CTRX, Ceftriaxone; F, female; LVFX, Levofloxacin; M, male; OC, outpatient care.
AP, abdominal pain; AR, arthralgia; CH, chills; DI, diarrhea; EM, emesis; F, fever; HA, headache.
The patient did not have any history of visiting Kagoshima Prefecture.
The patient developed symptoms during a business trip to Kagoshima Prefecture.
RAPD Polymerase Chain Reaction
Template genomic DNA was prepared by an alkaline boiling method from a 1-mL culture grown overnight at 37°C in lysogeny broth. RAPD polymerase chain reaction (PCR) was performed as previously described by Hashemi et al [10] using 3 primers, namely, OPP-16 (5′-CCAAGCTGCC-3′) [11], OPS-11 (5′-AGTCGGGTGG-3′), and P1254 (5′-CCGCAGCCAA-3′) [12], in conjunction with KAPATaq EXtra (NIPPON Genetics Co, Ltd). Each reaction mixture (25 μL) contained 5 µL of 5× KAPATaq EXtra buffer, 2.5 mM MgCl2, 300 mM dNTPs, 1 μL of template DNA, 0.4 μM primer, and 0.6 U of KAPATaq DNA polymerase. The PCR amplification steps employed were as follows: 25 cycles of initial denaturation at 94°C for 5 minutes, followed by 40 cycles of denaturation at 94°C for 1 minute, annealing at 35°C for 1 minute, and extension at 72°C for 2 minutes and a final extension step at 72°C for 10 minutes. After PCR amplification, 10 µL of each PCR product was analyzed by agarose gel electrophoresis using 1.2% agarose S (Nippon Gene).
Whole-Genome Sequencing
Genomic DNA was purified from 2 mL overnight cultures using a DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer's instructions. Sequencing libraries were prepared using the Nextera XT DNA Sample Prep Kit (Illumina) to obtain paired-end sequences (300 bp × 2) on the Illumina MiSeq platform. Draft genome sequences were obtained by assembling Illumina reads using Platanus version 1.1.4 with default parameters [13]. In addition, 1 bacteremia-related strain (strain 11548) was further sequenced by Nanopore MinION (Oxford Nanopore Technologies) to obtain the finished genome sequence. To this end, high-molecular-weight genomic DNA was prepared from a 2-mL overnight culture using the Genomic-tip 20/G and Genomic DNA buffer set (Qiagen) according to the manufacturer's instructions. A long-read sequencing library was prepared using a Rapid Barcoding Kit (Oxford Nanopore Technologies) and sequenced using an R9.4.1 flow cell. The bases were called using Guppy GPU version 3.4.5 (Oxford Nanopore Technologies), and hybrid assembly was performed by Unicycler version 0.4.8 [14] with Illumina reads.
The draft and complete genome sequences obtained in this study have been deposited in the GenBank/European Molecular Biology Laboratory/DNA Data Bank of Japan database under Bioproject number PRJDB11207 (see Supplementary Tables 2 and 4 for the accession numbers and the sequencing statistics of each strain, respectively).
Phylogenetic Analysis
For the construction of a core gene–based phylogenetic tree, the genome assemblies of a total of 1660 genomes (37 Japanese SO genomes determined in this study, 1622 SO genomes from EnteroBase, and one O8 genome) were annotated by Prokka version 1.13 [15], and core genes were identified using Roary version 3.11.2 [16] with a 90% amino acid sequence identity cutoff. Core gene single-nucleotide polymorphisms (SNPs) were extracted using the core gene alignment tool in Roary, and a maximum-likelihood (ML) phylogenetic tree was constructed using IQ-TREE with a model inferred by ModelTest (https://github.com/ddarriba/modeltest/) and 5000 bootstrap replicates [17]. The tree was displayed as a midpoint-rooted tree and annotated using iTOL version 4 (https://itol.embl.de) [18].
For the phylogenetic analysis focusing more on 12 bacteremia-related strains, these strains and 26 closely related strains identified by core gene–based analysis were analyzed as follows. Draft sequences (contigs ≥1 kb in length were used) were aligned to the finished genome sequence of strain 11548 using NUCmer version 3.1 [19] with the parameter “—mum” to identify ≥500 bp regions shared by all tested strains. After removing recombinogenic SNPs using Gubbins with default parameters [20], a neighbor-joining tree was constructed based on the identified core genome SNPs using the p-distance method in MEGA 7 [21].
Illumina Read Mapping Analysis
Illumina read mapping to the finished genome of strain 11548 was performed with the BWA-MEM algorithm implemented in BWA-0.7.17 [22], and the results were viewed with Integrative Genomics Viewer software (IGV; https://software.broadinstitute.org/software/igv/).
Ethical Approval
This study was approved by the Medical Ethical Review Board Committee of Kagoshima University (permission number 190195 EKI) and carried out according to the ethical guidelines for Medical and Health Research Involving Human Subjects.
RESULTS
Cases and Study Design
From July to August 2018, the information that 5 bacteremia cases caused by serogroup O7 NTS (cases 1–5 in Table 1) were identified in hospital A in Kagoshima Prefecture, Japan. With increased attention to NTS O7 bacteremia in hospitals in Kagoshima and neighboring prefectures in mid-August, an additional 7 bacteremia cases caused by NTS O7 (cases 6–12), which occurred from July to September 2018, were reported to KICNet from 4 hospitals (A, B, E, F) in Kagoshima Prefecture, 1 hospital (C) in Miyazaki Prefecture, and 1 hospital (D) in Okinawa Prefecture. In case 9, the patient developed symptoms during a business trip to Kagoshima Prefecture and visited hospital D after returning to Okinawa Prefecture. Salmonella species were isolated from blood cultures of 12 bacteremia patients by routine bacteriological examination in each hospital; all isolates belonged to the serotype “O7:m, t:-” and were identified as SO. Although we collected detailed information on food exposure histories, residences, and professions through interviews with the patients, no epidemiological link was identified (Table 1) [23]. The geographic locations of the 6 hospitals in the Kyushu area in Japan are shown in Supplementary Figure 1.
The mean age of the 12 patients was 27 years (range from 2 to 52 years); 8 (67%) were males, and none had any underlying diseases. The mean duration from the onset of symptoms to diagnosis by blood culture was 8.5 days (range from 3 to 12 days). The symptoms observed were fever (38°C–39°C, 100%), diarrhea (58%), and abdominal pain (17%). Four patients (33%) had no gastrointestinal symptoms. Regarding food exposure histories within 1 week before the onset of symptoms, 6 patients consumed raw chicken eggs and/or meat, but the sources or restaurants that provided the food did not overlap.
To determine the genetic relationship of the 12 SO isolates, we first performed a RAPD analysis. Then, we performed a WGS-based phylogenetic analysis of these strains along with a global set of SO strains to determine the phylogenetic position of the SO isolates in the global population structure of SO and a more focused WGS-based phylogenetic analysis with closely related SO strains to determine the precise genetic relationship of the 12 SO isolates and closely related SO strains.
RAPD Analysis
In the RAPD analysis of the 12 SO isolates, we included an additional 25 SO strains isolated from multiple sources and geographic regions in Japan to understand the genomic diversity of SO circulating in Japan. A serotype O8 strain was also included as an outgroup. In this analysis, while the 12 bacteremia isolates exhibited identical or similar amplification patterns, several strains with no apparent epidemiological links also exhibited amplification patterns that were identical or very similar to that of bacteremia isolates (Supplementary Figure 2), indicating that the resolution power of the RAPD method was insufficient to investigate the genetic similarities and differences between these SO strains.
Core Gene–Based Phylogenetic Analysis Using a Global SO Strain Set
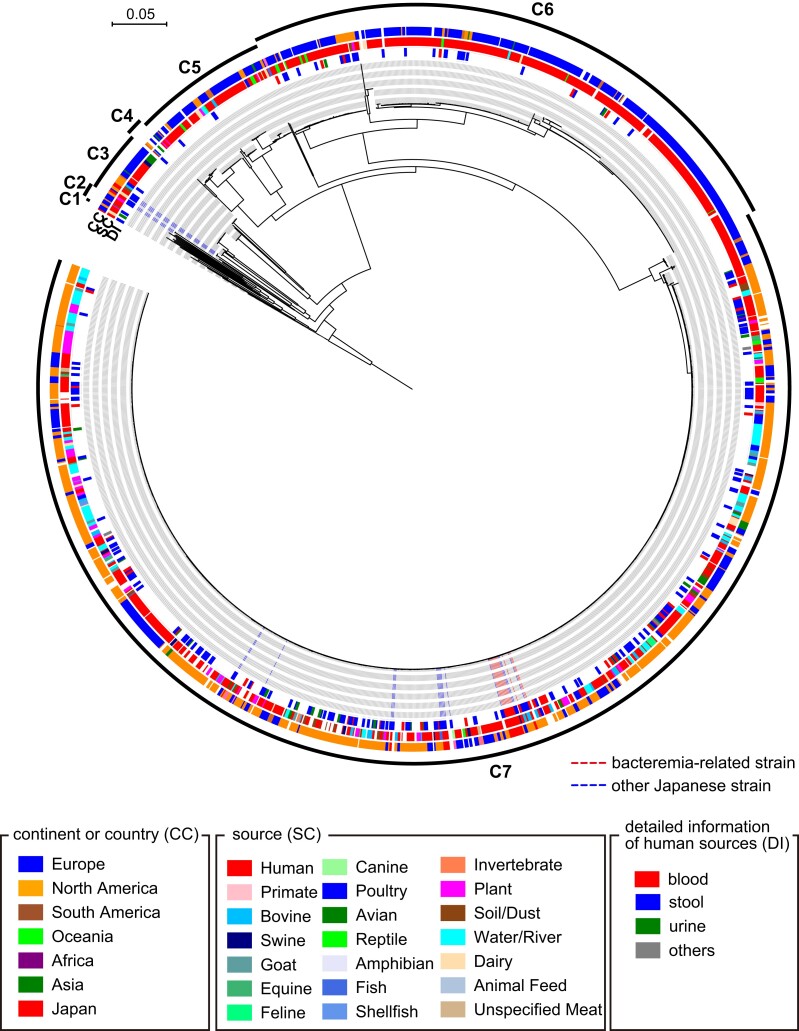
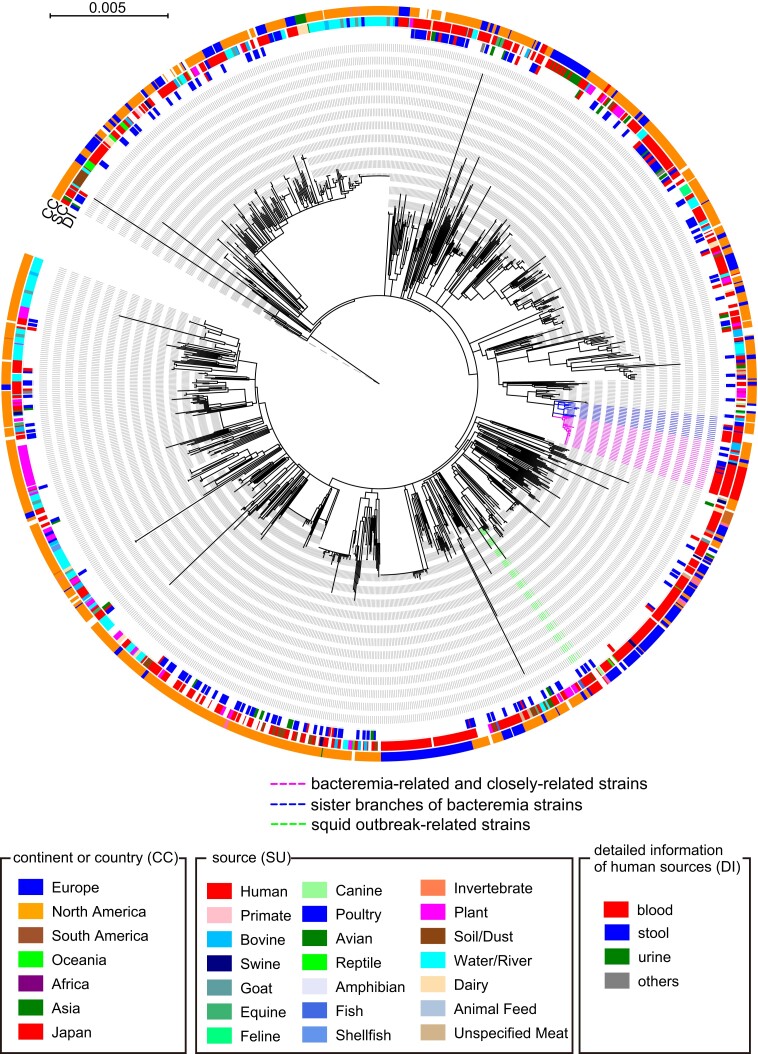
To determine the phylogenetic positions of the 12 bacteremia isolates and 25 SO strains analyzed by RAPD in the global SO population, we performed a core gene–based analysis of the 37 Japanese SO strains and 1622 SO strains (including the SO reference strain 0250), whose genome sequences were obtained from EnteroBase, with the serotype O8 strain as an outgroup. By employing a 90% amino acid sequence identity cutoff, 2660 core genes were identified, and 106 958 SNPs were detected in the sequence alignment of these core genes. In the midpoint-rooted ML tree constructed based on these SNPs (Figure 1 and Supplementary Figure 3), SO strains were divided into 7 clades (C1–C7), and 30 of the 37 Japanese strains, including 12 bacteremia-related strains, belonged to the largest clade, C7. We therefore performed a second-step phylogenetic analysis targeting the C7 clade (1050 strains) to increase the resolution power. In this analysis, 3158 core genes and 11 412 core gene SNPs were identified. In the ML tree based on 11 412 SNPs identified in the 3158 core genes (Figure 2 and Supplementary Figure 4), the 12 bacteremia-related strains formed a cluster with 4 other Japanese strains (strains 19467, 24350, 53675, and 53687) and 9 strains isolated in the US and the United Kingdom (UK) (Figure 2). The processed squid product–related outbreak strains described in the introduction belonged to clade C7 but occupied a branch completely different from that of the bacteremia-related strains (Figure 2).
Figure 1.
Core gene–based phylogenetic analysis of the global Salmonella Oranienburg strain set, which includes 37 Japanese strains sequenced in this study and 1622 strains whose genome sequences were obtained from the EnteroBase website. Single-nucleotide polymorphisms (SNPs) were identified using the finished genome sequence of strain 0250 as a reference. Based on the 106 958 SNPs identified in 2660 core genes, a maximum-likelihood tree was constructed using IQ-TREE with a model inferred by ModelTest and 5000 bootstrap replicates. Bars represented the number of substitutions per site. Salmonella enterica serotype O8 strain 11556 was used as an outlier. Abbreviations: CC, continent or country; DI, detailed information of human sources; SC, sources.
Figure 2.
Core gene–based phylogenetic analysis of the 1050 Salmonella Oranienburg strains classified as clade 7 in Figure 1. Single-nucleotide polymorphisms (SNPs) were identified using the finished genome sequence of strain 0250 as a reference. Based on the 11 412 SNPs identified in 3158 core genes, a midpoint-rooted maximum-likelihood tree was constructed using IQ-TREE with a model inferred by ModelTest and 2000 bootstrap replicates. Bars represent the number of substitutions per site. The total 38 colored strains containing the 12 bacteremia-related strains, 13 strains that were clustered with the 12 bacteremia-related strains, and the 13 strains in sister branches are analyzed in Figure 3. Abbreviations: CC, continent or country; DI, detailed information of human sources; SC, sources.
Core Genome–Based Phylogenetic Analysis of Bacteremia-Related Strains and Closely Related Strains
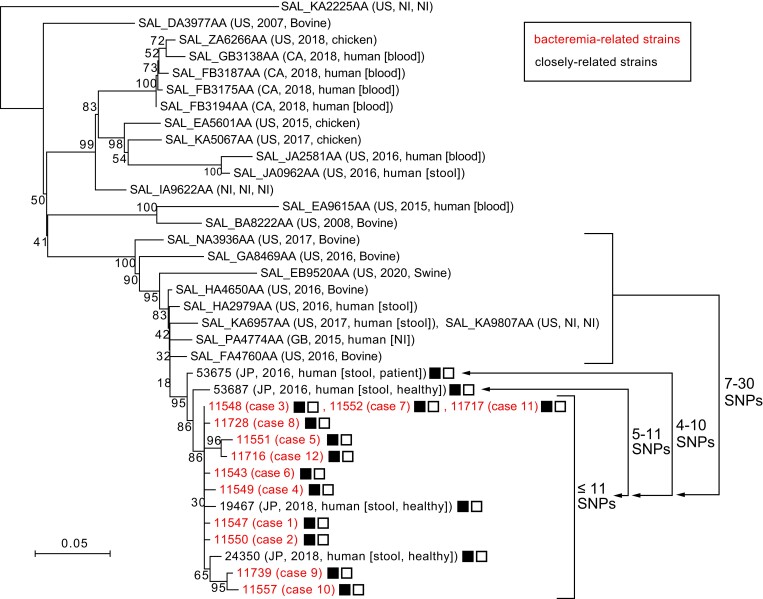
To better understand the genetic relationships between the 12 bacteremia-related strains and the closely related strains identified by the phylogenetic analysis of clade C7 (13 strains in the abovementioned cluster and the 13 strains in sister clusters; see Figure 2), we determined the complete genome sequence of strain 11548, one of the bacteremia-related strains. Using the finished chromosome sequence of this strain (the chromosome length was 4 686 981 bp, and 2 small plasmids designated pSO11548-1 [3428 bp] and pSO11548-2 [2495 bp] were present) as a reference, we identified 276 recombination-free SNPs in the core genome shared by the 38 strains (4 686 981 bp in total length). In the neighbor-joining tree constructed based on the 276 SNPs (Figure 3), the 12 bacteremia isolates, along with 2 strains isolated during stool examination of food handlers in 2018 in Kagoshima Prefecture (strains 19467 and 24350), formed a subcluster with a maximum SNP distance of 11. Three strains isolated in hospital A (from cases 3, 7, and 11) shared an identical core genome sequence. The 2 food handlers had no symptoms and no epidemiological link to any bacteremia cases. In addition, Illumina read mapping analysis revealed that all 14 strains carried the same types of plasmids as strain 11548.
Figure 3.
Core genome–based phylogenetic analysis of the 12 bacteremia-related strains and the 26 closely related strains. Based on the 276 single-nucleotide polymorphisms (SNPs) identified in the core genome (4 686 981 bp in total length), a neighbor-joining tree was constructed based on the identified core genome SNPs using the p-distance method in MEGA7. Strain names (Japanese strains) or strain IDs in the EnteroBase database are indicated along with other strain information (country, isolation year, and source). The maximum SNP distance within the subcluster that included bacteremia-related strains was 11. The ranges of SNP distances from the subcluster to the 2 most closely related strains are also indicated. The presence of the plasmids pSO11548-1 (3428 bp in size) and pSO11548-2 (2495 bp in size) is indicated by solid and outlined squares, respectively. Abbreviation: NI, no information.
Among the other strains analyzed, 2 strains isolated in 2016 in Miyazaki Prefecture (strains 53687 and 53675) were most closely related to the subcluster, showing SNP distances of 5–11 and 4–10, respectively, from the strains in the subcluster. These 2 strains were isolated from a patient with gastroenteritis and a healthy carrier, respectively. Interestingly, the 2 strains also carried the 2 plasmids identified in the 14 strains (12 isolates from bacteremia patients and 2 from food handlers). However, the US and UK strains did not carry these plasmids, although they were also closely related to bacteremia-related strains, with SNP distances ranging from 7 to 30 (Figure 3). These results indicate that the 12 bacteremia cases identified in this study were caused by a single clone that was recently derived from a common ancestor shared with the Miyazaki 2016 strains.
DISCUSSION
We identified a cluster of bacteremia cases (12 in total) caused by SO in the southern Kyushu area, Japan, during a 2.5-month period in 2018. The RAPD method we first employed to examine 12 SO strains isolated from bacteremia cases was insufficient in resolution power, but core gene SNP–based phylogenetic analyses with a global SO strain set revealed that the strains are very closely related. In the core genome SNP–based analysis that was performed to maximize resolution, the 12 bacteremia-related strains formed a subcluster along with 2 strains isolated from asymptomatic carriers in 2018 in the same area (Kagoshima Prefecture) with a maximum within-subcluster SNP distance of 11. This result indicates that the 12 bacteremia cases were caused by a single SO clone. The strains in this subcluster were very closely related to 2 strains isolated in 2016 in Miyazaki Prefecture, which is located in the southern Kyushu area (SNP distances were 5–11 or 4–10). All 14 strains carried 2 common plasmids, but the strains in the sister branch did not. These results indicated that the bacteremia-related clone was derived from a common ancestor shared with these Miyazaki strains. The infection source(s) and route(s) of bacteremia cases were not identified from epidemiological investigations. As the 3 strains with an identical core genome sequence were isolated from 3 patients admitted to the same hospital (hospital A), these 3 patients might have been infected by this clone from the same source or by sources with an identical or similar route of bacterial contamination.
Several SO-associated outbreaks of gastroenteritis have been reported worldwide [6, 7, 24–28]. NTS-related gastroenteritis cases are occasionally accompanied by bacteremia, but its frequency is generally not very high in persons without any underlying diseases [2], although Tamber et al reported that SO was one of the 19 NTS serovars identified as having higher rates of bacteremia in all NTS serovars in Canada (5.5% in the cases of SO) [29]. In the nationwide SO outbreak in 1999 in Japan [5], which was caused by contaminated processed squid products, several cases of bacteremia and bacteremia-related symptoms, such as retroperitoneal abscess and purulent spondylitis, were reported individually from different local laboratories [6, 30]. Among these, Chiba Prefectural Institute of Public Health reported that 8 of 76 (10.5%) SO-positive individuals (61 symptomatic and 15 asymptomatic) showed bacteremia or bacteremia-related symptoms [30]. In the other reports, the rates of bacteremia were 80% (4 of 5 patients) [6]. However, no aggregated information on the rate of bacteremia in this outbreak is available. For the other large SO-associated outbreak of gastroenteritis that occurred in Japan [7], the rate of bacteremia was also unknown. We were also unable to estimate the rate of bacteremia in the present case, because we have no data on how many (if any) patients with only gastroenteritis caused by this clone were present during the same time period (at least no records of SO-related gastroenteritis clusters were reported from the Kagoshima and Miyazaki prefectural public health institutes). However, a remarkable feature of the bacteremia cluster was that all 12 bacteremia patients had no recognizable underlying diseases and the mean age was 27 years (range from 2 to 52 years). In addition, 85.7% (12/16) of the SO-positive individuals developed bacteremia. These findings suggest a possibility that the SO clone may be prone to cause bacteremia. Although the potential virulence of this clone needs to be analyzed in future studies, particular attention should be given to the SO clone and its close relative in NTS surveillance worldwide.
Supplementary Material
Contributor Information
Tadasuke Ooka, Department of Microbiology, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
Yasuhiro Gotoh, Department of Bacteriology, Faculty of Medical Sciences, Kyushu University, Fukuoka, Japan.
Shigeki Hatanaka, Department of Emergency and General Internal Medicine, Imamura General Hospital, Kagoshima, Japan.
Miyuki Yoshimori, Department of Infection Control and Prevention, Imamura General Hospital, Kagoshima, Japan.
Kazutaka Nishitarumizu, Department of Emergency and General Internal Medicine, Imamura General Hospital, Kagoshima, Japan.
Kanami Kojo, Department of Pediatrics, Kagoshima Children's Hospital, Kagoshima, Japan.
Hiroki Kosakamoto, Department of Internal Medicine, Kokubu Seikyo Hospital, Kagoshima, Japan.
Koji Sameshima, Department of Pediatrics, Kagoshima City Hospital, Kagoshima, Japan.
Yoichi Kuroki, Department of Internal Medicine, Koga General Hospital, Miyazaki, Japan.
Naomi Chibana, Department of General Internal Medicine, Naha City Hospital, Okinawa, Japan.
Yuriko Doi, Kagoshima City Public Health Center, Kagoshima, Japan.
Shuji Yoshino, Clinical Microbiology Section, Miyazaki Prefectural Institute for Public Health and Environment, Miyazaki, Japan.
Tetsuya Harada, Division of Microbiology, Osaka Institute of Public Health, Osaka, Japan.
Kazuko Seto, Division of Microbiology, Osaka Institute of Public Health, Osaka, Japan.
Tetsuya Ikeda, Department of Infectious Diseases, Hokkaido Institute of Public Health, Hokkaido, Japan.
Hiroaki Miyanohara, Department of Clinical Laboratory, Kagoshima Prefectural Comprehensive Health Center, Kagoshima, Japan.
Koichiro Nakayama, Microorganism Section, Kagoshima Prefectural Institute for Environmental Research and Public Health, Kagoshima, Japan.
Mutsuyo Gokuden, Microorganism Section, Kagoshima Prefectural Institute for Environmental Research and Public Health, Kagoshima, Japan.
Naoko Imuta, Department of Microbiology, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
Hideki Kawamura, Department of Infection Control and Prevention, Kagoshima University Hospital, Kagoshima, Japan.
Yoshitoshi Ogura, Department of Bacteriology, Faculty of Medical Sciences, Kyushu University, Fukuoka, Japan; Division of Microbiology, Department of Infectious Medicine, Kurume University, Fukuoka, Japan.
Tetsuya Hayashi, Department of Bacteriology, Faculty of Medical Sciences, Kyushu University, Fukuoka, Japan.
Junichiro Nishi, Department of Microbiology, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. T. O., T. Hay., and J. N. were involved in the conception of the study. T. O., Y. G., and Y. O. were involved in the analysis. S. H., M. Y., K. N., K. K., H. Ko., K. S., Y. K., N. C., Y. D., S. Y., T. Har., K. S., T. I., H. M., K. N., M. G., N. I., and H. Kaw. were involved in the initial detection of cases and provided the resources. All authors were involved in the interpretation of the data. T. O., T. Hay., and J. N. were involved in the preparation and revision of the original draft manuscript. All authors made substantive intellectual contributions to the development of this manuscript and approved the final version.
Acknowledgments. We thank Y. Iwamoto, H. Uchikado, S. Oku, Y. Matsuura, Y. Uechi, J. Yamashita, N. Tanaka, H. Hokajo, K. Wada, M. Shinohara, H. Uda, S. Miyahara, T. Sugimoto, and M. Otsubo for providing materials and epidemiological information; we also thank F. Funakura for technical assistance.
Financial support. This work was supported by the Association for Research on Lactic Acid Bacteria.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Majowicz SE, Musto J, Scallan E, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 2010; 50:882–9. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 . Non-Typhoidal Salmonella invasive disease collaborators. The global burden of non-typhoidal Salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2019; 19:1312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . National enteric disease surveillance: Salmonella annual report. 2016. Available at: https://www.cdc.gov/nationalsurveillance/pdfs/2016-Salmonella-report-508.pdf. Accessed 20 September 2022.
- 4. European Food Safety Authority . The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J 2018; 16:e05500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institute of Infectious Diseases . By year, Top 15 most common Salmonella serovars in Summary of infectious agents surveillance report (IASR). 2022. Available at: https://www.niid.go.jp/niid/en/iasr-table/2345-iasrtbe.html. Accessed 20 September 2022.
- 6. Miyakawa S, Takahashi K, Hattori M, et al. Outbreak of Salmonella Oranienburg infection in Japan. J Environ Biol 2006; 27:157–8. [PubMed] [Google Scholar]
- 7. Nakajima H, Ohata R, Kariya H. A rare outbreak of food poisoning caused by Salmonella enterica serovar. Oranienburg—a case report and features of isolates. Kansenshogaku Zasshi 2007; 81:242–8. [DOI] [PubMed] [Google Scholar]
- 8. Zhou Z, Alikhan NF, Mohamed K, et al. The user’s guide to comparative genomics with EnteroBase. Three case studies: micro-clades within Salmonella enterica serovar Agama, ancient and modern populations of Yersinia pestis, and core genomic diversity of all Escherichia. Genome Res 2020; 30:138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parks DH, Imelfort M, Skennerton CT, et al. Checkm: assessing the quality of microbial genomes recovered from isolates, single cells and metagenomes. Genome Res 2015; 25:1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hashemi A, Baghbani-Arani F. The effective differentiation of Salmonella isolates using four PCR-based typing methods. J Appl Microbiol 2015; 118:1530–40. [DOI] [PubMed] [Google Scholar]
- 11. Albufera U, Bhugaloo-Vial P, Issack M, et al. Molecular characterization of Salmonella isolates by REP-PCR and RAPD analysis. Infect Genet Evol 2009; 9:322–7. [DOI] [PubMed] [Google Scholar]
- 12. Tikoo A, Tripathi AK, Verma SC, et al. Application of PCR fingerprinting techniques for identification and discrimination of Salmonella isolates. Curr Sci 2001; 80:1049–52. [Google Scholar]
- 13. Kajitani R, Toshimoto K, Noguchi H, et al. Efficient de novo assembly of highly heterozygous genomes from whole-genome shotgun short reads. Genome Res 2014; 24:1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wick RR, Judd LM, Gorrie CL, et al. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 2017; 13:e1005595XXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30:2068–9. [DOI] [PubMed] [Google Scholar]
- 16. Page AJ, Cummins CA, Hunt M, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015; 31:3691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen LT, Schmidt HA, von Haeseler A, et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 2015; 32:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 2016; 44:W242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurtz S, Phillippy A, Delcher AL, et al. Versatile and open software for comparing large genomes. Genome Biol 2004; 5:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Croucher NJ, Page AJ, Connor TR, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 2015; 43:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv [Preprint]. Posted online 26 May2013. doi: 10.48550/arXiv.1303.3997 [DOI] [Google Scholar]
- 23. Infectious Agents Surveillance Report (IASR). Cluster of Salmonella enterica serovar Oranienburg bacteremia mainly in Kagoshima City, July–September 2018. 2019; 40:91–2. [Google Scholar]
- 24. Gustavsen S, Breen O. Investigation of an outbreak of Salmonella Oranienburg infections in Norway, caused by contaminated black pepper. Am J Epidemiol 1984; 119:806–12. [DOI] [PubMed] [Google Scholar]
- 25. Allerberger F, Kreidl P, Dierich MP, et al. Salmonella enterica serovar Oranienburg infections associated with consumption of locally produced Tyrolean cheese. Euro Surveill 2000; 5:123–6. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention . Salmonella Oranienburg infections associated with fruit salad served in health-care facilities—northeastern United States and Canada, 2006. MMWR Morb Mortal Wkly Rep 2007; 56:1025–8. [PubMed] [Google Scholar]
- 27. Werber D, Dreesman J, Feil F, et al. International outbreak of Salmonella Oranienburg due to German chocolate. BMC Infect Dis 2005; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vázquez-Garcidueñas MS, Romero-Pérez NL, Figueroa-Aguilar GA, et al. Investigation of a food-borne Salmonella Oranienburg outbreak in a Mexican prison. J Infect Dev Ctries 2104; 8:143–53. [DOI] [PubMed] [Google Scholar]
- 29. Tamber S, Dougherty B, Nguy K. Salmonella enterica serovars associated with bacteremia in Canada, 2006–2019. Can Commun Dis Rep 2021; 47:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoda K, Koiwai K. An outobreak of Salmonella Oranienburg and Salmonella chester food poisoning due to squid snucks in 1999. Bull Public Health Lab Chiba Prefecture [in Japanese] 1999; 23:20–2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.