Abstract
Brucella spp. are facultative intracellular parasites of various mammals, including humans, typically infecting lymphoid as well as reproductive organs. We have investigated how B. suis and B. melitensis enter human monocytes and in which compartment they survive. Peripheral blood monocytes readily internalized nonopsonized brucellae and killed most of them within 12 to 18 h. The presence of Brucella-specific antibodies (but not complement) increased the uptake of bacteria without increasing their intracellular survival, whereas adherence of the monocytes or incubation in Ca2+- and Mg2+-free medium reduced the uptake. Engulfment of all Brucella organisms (regardless of bacterial viability or virulence) initially resulted in phagosomes with tightly apposed walls (TP). Most TP were fully fusiogenic and matured to spacious phagolysosomes containing degraded bacteria, whereas some TP (more in monocyte-derived macrophages, HeLa cells, and CHO cells than in monocytes) remained tightly apposed to intact bacteria. Immediate treatment of infected host cells with the lysosomotropic base ammonium chloride caused a swelling of all phagosomes and a rise in the intraphagosomal pH, abolishing the intracellular survival of Brucella. These results indicate that (i) human monocytes readily internalize Brucella in a conventional way using various phagocytosis-promoting receptors, (ii) the maturation of some Brucella phagosomes is passively arrested between the steps of acidification and phagosome-lysosome fusion, (iii) brucellae are killed in maturing but not in arrested phagosomes, and (iv) survival of internalized Brucella depends on an acidic intraphagosomal pH and/or close contact with the phagosomal wall.
Bacteria of the genus Brucella are facultative intracellular parasites in various wildlife and domestic mammals, causing a debilitating zoonotic infection in humans. Traditionally, three major Brucella species are distinguished on the basis of their virulence for humans and their predilection for certain animal hosts: B. abortus for cattle, B. melitensis for caprines, and B. suis for hogs, with B. melitensis and B. suis accounting for the majority of clinical cases in humans (13, 42). In its mammalian hosts, the pathogen typically persists in tissues with a prominent reticuloendothelial component as well as in the reproductive organs of both genders (18). The mechanisms underlying this tissue tropism and the way in which Brucella organisms are able to enter and survive within such different host cells as resident macrophages and epithelial cells are not yet clear.
With regard to the uptake of brucellae by their host cells, studies on the phagocytosis-promoting host cell receptors and bacterial ligands are rare and gave contradictory results (reviewed in references 29 and 42). However, opsonized and nonopsonized brucellae were internalized at different rates, replicated at different rates, and induced differences in the killing mechanisms and the reactive cytokine pattern of the host cell (5, 9, 23, 24, 30). These findings suggest that phagocytosis-promoting receptors which will not intrinsically activate the host cells, thus avoiding the onset of antimicrobial activities, may lead to a “silent” uptake of Brucella without resulting intracellular killing.
The majority of studies addressing the intracellular survival of internalized Brucella focused on a pathogen-induced reduced killing capacity of the macrophages, such as blockade of the oxidative burst or inhibition of cytokine release (reviewed in reference 29). However, Brucella may also establish specific survival-permitting compartments. Data indicated that in murine macrophages (3, 19, 31) Brucella inhibits phagosome-lysosome fusion, and in nonprofessional phagocytes (1, 2, 15–17, 46) these pathogens replicate within cisternae of the rough endoplasmic reticulum (ER)
Due to the tremendous diversity of the experimental conditions used, it is difficult to assess which of the aforementioned results may reflect specific experimental conditions and which may describe general attributes of Brucella infection. With regard to the pathogen, studies have been performed primarily with B. abortus and less with B. melitensis and B. suis, the latter being more pathogenic for humans (13, 42). With regard to the host, cells derived from domestic ruminants or mice were mainly used, with the former showing a brucellosis pathophysiology different from that of humans and the latter even being able to control a Brucella infection (20). The presence and absence of opsonins as well as the use of virulent and attenuated strains or primary host cells and cell lines furthermore complicate the picture.
The present study using the human system thus systematically addressed the question of whether or not virulent Brucella spp. are internalized via conventional or special uptake mechanisms and are located in conventional or special intracellular compartments. The uptake of B. suis and B. melitensis by freshly isolated or subcultivated human monocytes under opsonic and nonopsonic conditions was investigated in detail by means of electron and fluorescence microscopy and by counting of internalized as well as surviving brucellae. The uptake of B. suis by epithelioid HeLa and CHO cells was included for comparison. The novel results obtained reveal that brucellae enter their host cells via conventional phagocytosis but locate in conventional and special compartments in parallel, with only the latter one allowing for intraphagosomal survival.
MATERIALS AND METHODS
Reagents.
Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) and vitamin D3 (VD3) were kindly provided by Jacques Dornand, thapsigargin was kindly provided by Michel Vignes, and neuraminidase (Behring catalog no. ORKD 04) was kindly provided by Virginie Lafont (all at the University of Montpellier, Montpellier, France). Dextran 500 (Nycograde 500) was obtained from Pharmacia Biotech Europe, Saclay, France; Ficoll-Hypaque (1.077 g/liter) was from Eurobio, Les Ulis, France; and tryptic soy broth (TSB) was from Difco, Detroit, Mich. All cell culture reagents were purchased from Gibco BRL, Cergy-Pontoise, France, or Sigma-Aldrich, St. Quentin Fallavier, France; the latter company also supplied the chemical compounds, except for the N-hydroxysuccinimidyl esters of 5- and 6-carboxyfluorescein (CF), 5- and 6-carboxytetramethylrhodamine (Rho), and LysoTracker Red DND-99, which were purchased from Molecular Probes, Eugene, Oreg.
Host cells.
Peripheral blood monocytes (PBM) were isolated from buffy coats of healthy donors obtained from the Etablissement Transfusion Sanguine Languedoc-Roussillon, Montpellier, France, using established methods resulting in low activation (12). Briefly, red blood cells (RBC) were sedimented with Nycograde 500; mononuclear cells were isolated and platelets were removed by buoyant-density-based centrifugation using MSL and heat-inactivated fetal calf serum (hiFCS), respectively, as separation media; CD2+ lymphocytes were rosetted with neuraminidase-desialinated RBC (BAG, Lich, Germany) on ice; and the remaining RBC were osmotically lysed by exposing them to distilled water. The remaining cells were resuspended in RPMI 1640 cell culture medium supplemented with 10% hiFCS (RPMI+). Trypan blue-excluding cells were counted and split into aliquots. One buffy coat usually would give a yield of 60 × 106 to 100 × 106 viable monocytes among about 20% of other cells, mainly B lymphocytes (28).
Some monocytes were maintained for up to 7 days in RPMI+ with or without addition of 500 U of GM-CSF per ml or 100 nM VD3 to further promote differentiation into monocyte-derived macrophages (MDM). Murine macrophage-like J774.A1 cells (ATCC TIB 67) and human cervix HeLa cells (ATCC CCL-2) were grown in RPMI+, and Chinese hamster ovary (CHO) cells were grown in α-MEM supplemented with 10% hiFCS (α-MEM+). CHO cells expressing the human complement receptor type 3 (CHO/Mac1+ transfectants) (45) were maintained in α-MEM without ribo- and deoxyribonucleotides but with 0.1 mM l-glutamine, 18 mM thymidine, and 10% FCS dialyzed against a cutoff of 10,000 molecular weight. Both types of CHO cells were a kind gift from David Mosser, Philadelphia, Pa., to Marina Cinco, Trieste, Italy, and were used as part of a joint study. Culture took place in T75 polystyrene Falcon flasks in a humidified incubator at 37°C and 5% CO2 in the presence of 100 U of penicillin G per ml and 100 mg of streptomycin per ml.
Growth conditions, opsonization, and killing of bacteria.
The Brucella strains B. melitensis 16 M (ATCC 23456), B. suis 1330 (ATCC 23444), B. suis p/sog (a green fluorescent protein [GFP]-expressing mutant of strain 1330) (33), and B. suis D1 (a GFP-expressing virB9 mutant of strain 1330) (32) were maintained at 4°C on plates containing agar-solidified TSB and appropriate antibiotics. The evening before an experiment, brucellae were transferred to liquid TSB and grown with agitation in an incubator at 37°C overnight to stationary phase. Immediately prior to the experiments, the optical density of the bacterial solutions was adjusted using a spectrophotometer at 600 nm. For studying the effect of opsonins, washed bacterial aliquots were resuspended in RPMI supplemented or not with 10% hiFCS, fresh FCS, or hiFCS containing 5 μl of heat-inactivated human B. suis-specific antiserum per ml (all at room temperature for 30 min). Brucellae were killed by exposing aliquots either to a temperature of 60°C or to 4% freshly prepared paraformaldehyde for 1 h; successful killing was confirmed by the absence of bacterial growth on plates.
Inoculation of host cells.
To infect nonadherent monocytes, aliquots of freshly isolated PBM were challenged with Brucella in tubes, washed once in a large volume of medium to remove extracellular bacteria, seeded, and chased. To infect adherent monocytes, aliquots of freshly isolated PBM were seeded first, rinsed after 30 min to remove nonadherent cells, challenged with Brucella, rinsed thoroughly to remove extracellular bacteria, and chased. For studying the effect of prolonged culture of PBM and resulting differentiation into MDM, aliquots of adherent monocytes were infected after various periods of culture. The cell lines were grown to semiconfluency before being challenged with Brucella. RPMI+ containing 60 μg of gentamicin per ml was used as the culture medium during the chasing period in order to kill remaining extracellular bacteria.
Uptake of Brucella and intracellular survival.
For the quantitative evaluation of infection, aliquots of monocytes (2 × 105 each) were challenged with GFP-expressing B. suis in a total of 0.2 ml of medium at a bacterium/host cell ratio (multiplicity of infection) (MOI) of 500, with a pulse time of 20 min and a chase time of 30 min. The monocytes were seeded in Lab-Tek eight-well chamber slides (Nunc Inc., Naperville, Ill.). At the end of the chasing period, the cells were thoroughly rinsed and fixed with 2% paraformaldehyde. Slides with coverslips were viewed with a Leica DM IRB epifluorescence microscope. Both the percentage of infected monocytes for at least 100 monocytes (relative infection index) and the mean number of GFP-expressing bacteria for at least 50 infected monocytes (phagocytosis index) were evaluated in duplicate chambers, screening cells from different sites of the chambers. For statistical analysis, two-way analysis of variance was performed.
For the determination of intracellular bacterial survival, aliquots of monocytes (5 × 105 each) were challenged with Brucella in a total of 0.5 ml of medium at an MOI of 500 with a pulse time of 20 min and a chase time of 30 min. The monocytes were seeded in Falcon Primaria 24-well tissue culture plates. At the end of chasing periods of 1 to 36 h, the wells were carefully rinsed, the monocytes were osmotically lysed with 0.5 ml of Triton X-100 per well in distilled water, TSB agar plates were inoculated in duplicate with 100 μl (each) of supernatant in serial dilutions, and the CFU from duplicate wells were evaluated after 3 to 5 days of growth.
Phagosomal pH.
The phagosomal pH was measured by fluorescence microscopy as described in full detail before (37). Briefly, B. suis which had been labeled with CF and Rho and opsonized with Brucella-specific human antiserum was used to infect J774 cells in Lab-Tek chamber coverslides with an MOI of 100, a pulse time of 45 min, and a chase time of 90 min. An in situ calibration curve of the CF/Rho emission ratio versus pH was obtained by exposing the infected J774 cells to nigericin-containing buffers of defined pH. Alternatively, J774 cells which had been preinfected with opsonized GFP-expressing Brucella were incubated with 0.1 or 1 μM LysoTracker Red. Fluorescence was measured with a Cool View camera (Photonic Science, Robertsbridge, United Kingdom) and an image processor linked to a Leica DM IRB epifluorescence microscope. For each reading, usually four paired images were acquired at probe-specific wavelengths and analyzed by the VISIOLAB 1000 (Biochem, Les Ulis, France) imaging system.
Ultrastructural analysis of infected cells.
Aliquots of nonadherent PBM (106 each) were challenged with Brucella in a total of 1 ml of medium at an MOI of 20 to 500 with a pulse time of 2 to 30 min and a chase time of up to 36 h. The infected PBM were either kept in 15-ml Falcon tubes (for chasing periods of less than 2 h) or seeded in T25 Falcon flasks (for periods exceeding 2 h). Aliquots of adherent PBM, MDM, and the semiconfluent epithelioid cells (about 4 × 106 cells/flask with 5 × 106 bacteria/cm2) were challenged in T25 Falcon flasks in a total of 5 ml of medium; some of the flasks containing epithelioid cells were gently centrifuged (400 × g for 10 min). The chase was stopped by adding an excess amount of cold Ito's fixative (22) to the tubes or flasks, and fixation was continued overnight at 4°C. Adherent cells were then gently scraped off the flasks and transferred to tubes.
The samples were further processed according to established protocols (22). Briefly, they were postfixed with ferricyanide-reduced osmium tetroxide, embedded in agarose, en bloc stained with an alcoholic mixture of phosphotungstic acid and uranyl acetate, physically dehydrated with a graded series of alcoholic solutions followed by pure acetone, and embedded in Epon 812 resin. Ultrathin sections were on-grid stained with a mixture of uranyl acetate and lead citrate and viewed with a Zeiss type 906 transmission electron microscope. The Brucella-containing phagosomes of at least 100 infected monocytes from different areas of two nonconsecutive sections were classified according to the intraphagosomal space as tight, loose, or other, with the last group comprising all equivocal phagosomes and macropinosomes.
For the cytochemical demonstration of reactive oxygen intermediates, the method of Briggs et al. was used (7). Briefly, 1 mg of 3,3′-diaminobenzidine (DAB) was dissolved in RPMI+. After the pH of the medium was readjusted to 7.4 with 0.2 N NaOH, PBM were challenged with antibody-opsonized or nonopsonized bacteria in RPMI+–DAB for 15 min, chased in RPMI+ for another 120 min, and fixed and further processed for electron microscopy as described above, except that the on-grid staining was done at half strength or was totally omitted. Since monomeric DAB will be oxidatively linked to highly osmiophilic polymers in presence of endogenously produced reactive oxygen intermediates, thus revealing sites of oxidative burst (26), the different types of Brucella-bearing phagosomes were qualitatively screened for the presence of electron-dense precipitates.
For the demonstration of phagosome-endosome fusion, a combination of the methods described by Rabinowitz et al. (38) and Strasser et al. (44) was used. To load early endosomal compartments, PBM were pulsed either with bovine serum albumin (BSA)-conjugated colloidal 10-nm-diameter gold particles (BSA-gold) at an optical density at 520 nm of 10, with 0.5 mg of cationized ferritin per ml, or with 0.1% ruthenium red for 10 min and chased for another 10 min before incubation with Brucella for another 20 min. To load late endosomal compartments, PBM were pulsed with BSA-gold overnight, chased for 6 h, pulsed with Brucella for 20 min, and chased again for another 6 h. The different types of Brucella-bearing phagosomes were qualitatively screened for the presence of electron-dense markers.
RESULTS
Ultrastructural analysis of the uptake of Brucella by human monocytes and epithelioid cell lines reveals conventional uptake but special phagosomes.
We decided first to evaluate the uptake of Brucella by PBM using electron microscopy. In view of the contradictory results described in the literature, various experimental settings in terms of bacterial strains, opsonization, MOI, and periods of pulse and chase were screened, and uptake of Brucella by MDM and the epithelioid HeLa and CHO cells was studied for comparison.
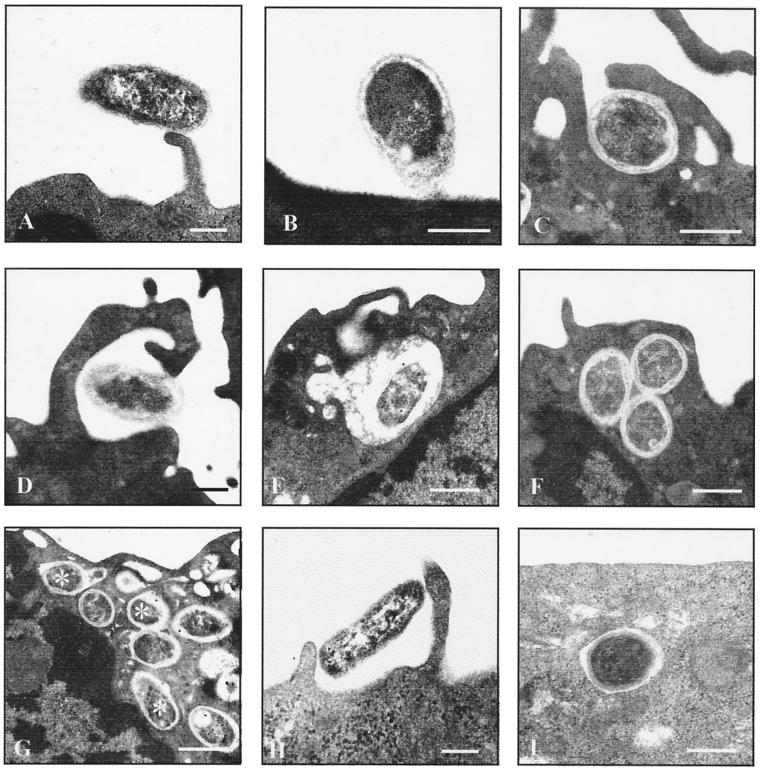
Internalization started immediately with the attachment of Brucella to either protrusions of the cell surface or the plain plasma membrane of the PBM (Fig. 1A and B). The emerging phagocytic cups had either continuous or focal contacts with the enclosed bacteria (Fig. 1C and D). A chasing period of 15 min was found to be sufficient for almost complete internalization of the bound bacteria; only very few monocytes (<1% of the total population) then had bacteria still attached to their cell surface. Engulfment resulted in individual phagosomes with either tightly (tight-fitting phagosomes [TP]) or loosely (spacious phagosomes [SP]) apposed walls, with part of them fusing with other intracellular vesicles (Fig. 1E and G).
FIG. 1.
Ultrastructural observations on the phagocytosis of Brucella by human monocytes and epithelioid cells. Freshly isolated monocytes (A to G) or epithelioid cells (H and I) were challenged with nonopsonized B. suis 1330 for 15 min and chased for up to 24 h. Bars, 0.2 μm. Brucellae attached to various parts of the plasma membrane, such as cell surface projections (A) or the plain cell surface (B). The emerging phagocytic cups which formed within the first minutes had either continuous (C) or focal (D) contact with the bacteria. The resulting phagosomes either were spacious (E) or had tightly apposed walls (F). Both types of phagosomes showed fusion with small intracellular vesicles, with the fusion events for the latter type being more numerous after longer chasing periods (G) (asterisks indicate phagosome-lysosome fusion; chase was for 2 h). Uptake of Brucella by HeLa or CHO cells also took place via the usual phagocytic mechanisms (H) (HeLa cells) and resulted in membrane-bound compartments which predominantly had tightly apposed walls (I) (CHO cells).
To ascertain which type of phagosomes would permit the survival of the parasites, PBM were infected with Brucella and chased for up to 36 h. It was found that after increasing chasing periods, Brucella organisms were degraded inside the SP but remained structurally intact inside the TP. With increasing chasing periods the relative proportions of the two types of phagosomes changed in favor of the SP (from 83% TP, 11% SP, and 6% others after 15 min of chase to 47% TP, 49% SP, and 4% others after 8 h of chase), suggesting that only a minor proportion of the phagosomes had a continuous arrest in their maturation.
All of these features were observed under both opsonic and nonopsonic conditions and with GFP-expressing and wild-type B. suis as well as wild-type B. melitensis (data not shown), thus ruling out morphological effects of certain phagocytosis-promoting receptors, transformation of B. suis with the vector carrying the GFP gene, or species-specific differences. Also, no qualitative differences in the uptake by adherent or nonadherent PBM were seen, although nonadherent PBM usually had internalized higher numbers of bacteria than adherent ones. An MOI of at least 100 was necessary in order to study a sufficient number of bacterium-host cell interactions; at a lower MOI the phagocytic events were too rare to allow for proper analysis. Differences in the MOI did not cause qualitative differences in the uptake of Brucella except that, in addition to the attachment-mediated uptake described above, bacteria were more often trapped in macropinosomes with increasing MOI.
Both the engulfment and intracellular localization of Brucella were similar with the epithelioid cells (Fig. 1H and I) and MDM (Fig. 2A to C) compared to the PBM. No ultrastructural features of unusual phagosome trafficking were observed during the incubation periods used (8 h with epithelial cells and 36 h with MDM), especially no indication of an association between Brucella-bearing phagosomes and the rough ER or autophagosomes. The experiment was repeated using a different batch of HeLa cells obtained from a collaborating laboratory in order to exclude batch-specific peculiarities; however, the second batch yielded the same results. Quantitatively, (i) the number of phagocytosing epithelioid cells was much lower than the number of phagocytosing PBM (about 3 to 5% of HeLa cells and 10 to 15% of CHO cells, compared to 64 to 83% of the PBM); (ii) many bacteria remained adherent to the cell surfaces of the epithelioid cells even after several hours of chase, and in our experience centrifugation during challenge tended to increase the number of adherent but not internalized brucellae; and (iii) the majority of the phagosomes maintained a very narrow intraphagosomal space. In MDM, too, the bacteria were located almost exclusively in TP, regardless of the protocol used for differentiation (Fig. 2A to C), suggesting an effect of long-term culture itself rather than of certain differentiation-promoting factors.
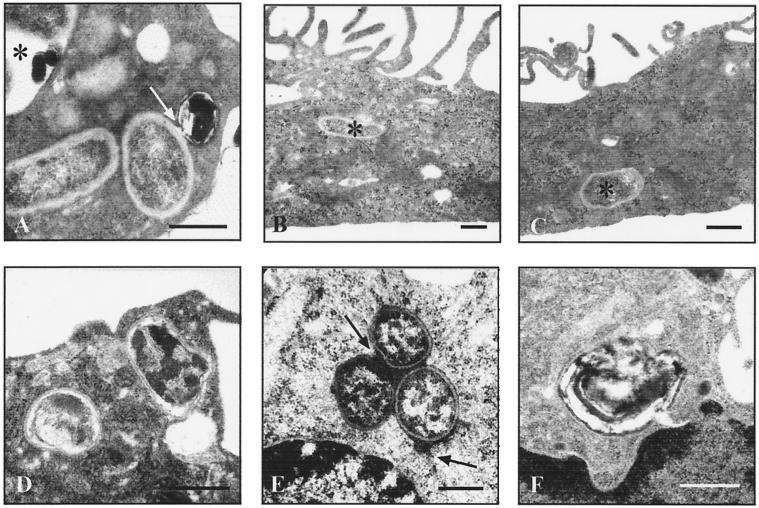
FIG. 2.
Ultrastructural observations on Brucella-bearing phagosomes in human monocytes. Monocyte-derived macrophages (A to C) or freshly isolated monocytes (D to F) were challenged with B. suis 1330 for 15 min and chased for up to 8 h. Bars, 0.2 μm. In MDM differentiated for 7 days in the presence of FCS without additions (A) or with either 100 nM VD3 (B) or 500 U of GM-CSF per ml (C), brucellae were located almost exclusively in TP (asterisks in panels B and C). Especially after longer chasing periods, Brucella-bearing phagosomes occasionally contained electron-dense membrane remnants, as indicated for an SP (asterisk in panel A) and for a vesicle fusing with a TP (arrow in panel A). Formation of TP did not depend on bacterial viability or virulence, as shown with two TP enclosing heat-killed brucellae (D). Preloading endosomal compartments of the host cells with electron-dense markers revealed that TP were able to fuse with early but not late endosomes (E), with three TP grouped around a centrally placed early endosome (upper arrow) and one TP fusing with an additional early endosome (lower arrow). Osmiophilic precipitations of polymerized DAB, indicative of oxidative burst, were occasionally present in some SP but not in TP (F), even when using antibody-opsonized brucellae.
Heat- or formaldehyde-killed virulent Brucella, as well as the nonvirulent virB9 mutant, induced TP with similar morphology (Fig. 2D) and at a similar relative frequency as the live virulent parental strain (34% TP, 57% SP, and 9% others for formaldehyde-killed Brucella and 37% TP, 53% SP, and 10% others for heat-killed Brucella after 8 h of chase). This suggests that neither bacterial viability nor virulence was required for inducing TP. With regard to the fusiogenicity of the Brucella-bearing phagosomes, both TP and SP obviously fused with intracellular vesicles (Fig. 1E and G and 2A). When prelabeled vesicles were used, freshly formed TP were found to fuse only with early and not with late endosomes (Fig. 2E). On the other hand, SP fused not only with both types of endosomes but, especially after longer incubation periods (≥18 h), also with vesicles possibly representing intracellular storage compartments or autophagosomes, as concluded from the colocalization of osmiophilic membrane whorls and degraded brucellae in SP (Fig. 2A). When monocytes were challenged with Brucella in the presence of DAB, oxidatively polymerized DAB (indicative of an oxidative burst) was seen only in some SP and never in TP (Fig. 2F). Even when the bacteria had been antibody opsonized in order to trigger intrinsic NADPH oxidase assembly, the number of DAB polymers did not increase, which calls into question the significance of the oxidative burst for the killing of internalized Brucella.
Quantification of the uptake and intracellular survival of Brucella in human monocytes and MDM reveals striking differences in opsonization, adherence, and differentiation.
In the next step we correlated the electron microscopic findings with the light microscopic quantification of the uptake and survival of Brucella. First, the effect of opsonization and monocyte differentiation was investigated by comparing the uptake of Brucella (i) in opsonic versus nonopsonic incubation media, (ii) by nonadherent versus adherent PBM, and (iii) by PBM versus MDM at different stages of differentiation. Based on our ultrastructural results, an MOI of 500, pulse time of 20 min, and chase time of 30 min were chosen to make sure that the uptake was saturated and that the bacteria were internalized. Control experiments using increasing MOIs confirmed that the uptake of Brucella reached a plateau at an MOI of 300 to 400 (data not shown).
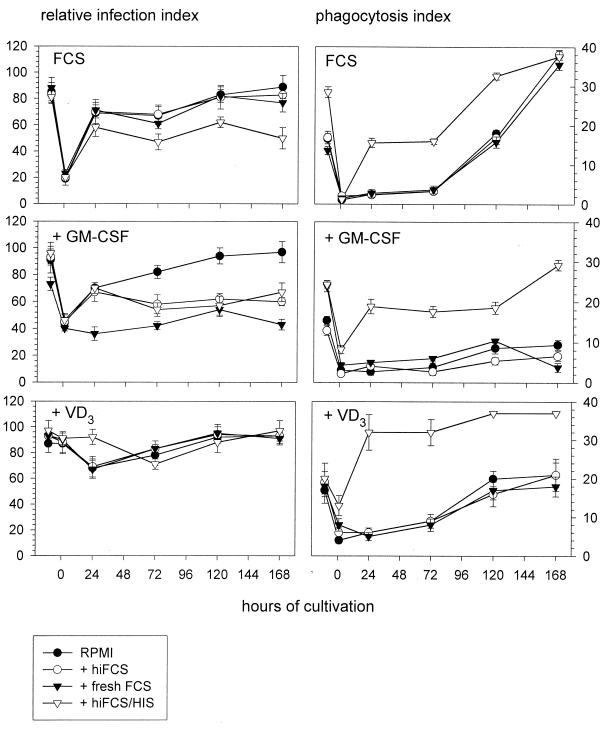
Here, considerable differences with regard to both the incubation medium used and treatment of the host cells became apparent (Fig. 3). The high relative infection index observed with nonadherent PBM was reestablished only by (i) untreated monocytes after 1 day of adherence and in the presence of nonimmune serum, (ii) VD3-treated monocytes after 5 days of adherence for all incubation media, and (iii) GM-CSF-treated monocytes after 5 days of adherence for blank medium. The phagocytic index observed with nonadherent PBM was (i) reestablished by untreated as well as VD3-treated monocytes after 5 days of adherence and in the presence of nonimmune serum and (ii) exceeded by far after 5 (for untreated monocytes), 1 (for VD3-treated monocytes), or 7 (for GM-CSF-treated monocytes) days of adherence in the presence of antiserum. Thus, for antibody-opsonized and nonopsonized bacteria, a reciprocal relation between the relative infection index and the phagocytosis index became apparent: a smaller proportion of monocytes would internalize antibody-opsonized bacteria yet at higher numbers, and a larger proportion of monocytes would internalize nonopsonized bacteria yet at lower numbers.
FIG. 3.
Uptake of differently opsonized brucellae by human monocytes and MDM. Uptake of Brucella was compared for freshly isolated, nonadherent monocytes (pre-0-h time point) and monocytes which were allowed to adhere for 1 h (0-h time point) and kept in culture for up to 7 days (24-, 72-, 120-, and 168-h time points). At each time point indicated, phagocyte aliquots were challenged with GFP-expressing B. suis for 20 min and chased for another 30 min. The effects of different supplements to the monocyte culture medium (10% hiFCS [FCS]), FCS with 500 U of GM-CSF per ml [+ GM-CSF]), or FCS with 100 nM VD3 [+ VD3]) and different challenging media (RPMI alone [RPMI] or RPMI containing either 10% hiFCS [+ hiFCS], fresh FCS [+ fresh FCS], or hiFCS with 5 μl of human anti-Brucella immune serum [+ hiFCS/HIS]) were compared. Infection was quantified by count of monocyte-associated fluorescent bacteria. Results are expressed as means ± standard deviations for duplicate samples from one of two experiments with monocytes from different donors which gave comparable results.
Both the electron microscopic studies and the subsequent experiment suggested that adherent and nonadherent PBM had different kinetics of phagocytosis of Brucella. Thus, we directly addressed the uptake of GFP-expressing B. suis by nonadherent versus adherent PBM. It was found that 81 to 91% of nonadherent PBM were infected, with a mean of 12 to 28 bacteria per cell, depending on the donor and incubation medium used. Upon adherence, both the number of infected PBM and the number of internalized brucellae were significantly reduced (Table 1), suggesting that some formerly phagocytosis-promoting receptors now were engaged in adherence. The presence of heat-inactivated antiserum generally enhanced the uptake of Brucella and gave the highest number of infected cells and the highest phagocytic index, although with pronounced interindividual variations resulting in the differences being statistically nonsignificant. Uptake kinetics were similar in the presence of fresh or heat-inactivated nonimmune serum, however, and in an additional experiment comparable numbers of brucellae (12% ± 2% and 13% ± 3%, respectively) were internalized by CHO cells expressing or not expressing the Mac1 epitope. These results question the contribution of complement or the participation of the human CR3, respectively, in the uptake of Brucella under nonimmune conditions.
TABLE 1.
Effect of adhesion and opsonization on uptake of B. suis by freshly isolated human monocytes from three blood donors
| Parameter | Monocytes | Relative infection index or phagocytosis index after incubation in RPMI medium with:
|
SD | ςxb | P adherencec | |||
|---|---|---|---|---|---|---|---|---|
| RPMI | hiFCS | Fresh FCS | hiHISa | |||||
| Infection | Nonadherent | 86 | 85 | 86 | 86 | 4.47 | 1.58 | 0.0014 |
| Adherent | 18 | 28 | 32 | 38 | 26.16 | 9.25 | ||
| SD | 40.50 | 35.45 | 38.97 | 38.51 | ||||
| ςx | 20.25 | 17.73 | 19.49 | 19.26 | ||||
| P opsonizationc | 0.9754 | |||||||
| Phagocytosis | Nonadherent | 14.3 | 14.7 | 15.9 | 24.7 | 1.76 | 0.63 | 0.0004 |
| Adherent | 1.1 | 1.8 | 2.3 | 3.4 | 8.96 | 3.17 | ||
| SD | 7.85 | 7.67 | 8.45 | 17.25 | ||||
| ςx | 3.93 | 3.83 | 4.22 | 8.62 | ||||
| P opsonization | 0.1669 | |||||||
HIS, human Brucella-specific antiserum.
ςx, standard error.
P, level of significance based on two-way analysis of variance.
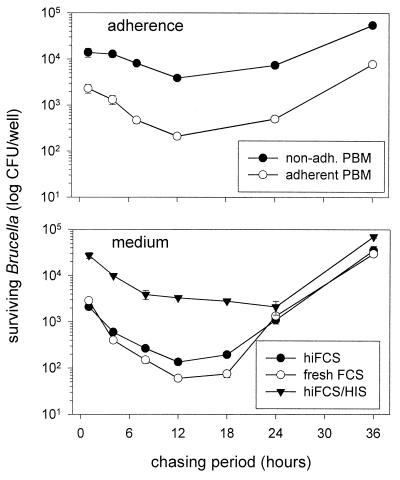
To ascertain whether or not the participation of distinct phagocytosis-promoting receptors influenced the intracellular persistence of Brucella, the number of CFU of bacteria reisolated from infected monocytes was evaluated. It was found that the kinetics of intracellular survival and replication of Brucella were the same regardless of whether the PBM had been adherent or nonadherent during phagocytosis or whether fresh or heat-inactivated nonimmune serum had been used (Fig. 4). The higher numbers of bacteria internalized in the presence of heat-inactivated immune serum, on the other hand, slowly decreased over a chasing period of 24 h until they met the numbers of bacteria internalized in the presence of nonimmune serum (Fig. 4), suggesting that uptake via FcR resulted in compartments which did not kill the bacteria immediately but did not allow persistence either.
FIG. 4.
Intracellular survival of differently opsonized brucellae following uptake by nonadherent and adherent human monocytes. Freshly isolated PBM were challenged with B. suis for 20 min and chased for 1 to 36 h. Viable bacteria reisolated from osmotically lysed PBM were determined by count of CFU. (Top panel) Survival of nonopsonized brucellae internalized by nonadherent (non-adh.) and adherent PBM. (Bottom panel) Survival of brucellae internalized by nonadherent PBM in the presence of either hiFCS, fresh FCS, or 5 μl of human anti-Brucella immune serum per ml in hiFCS (hiFCS/HIS). Results are expressed as means ± standard deviations for duplicate samples from one of three experiments with monocytes from different donors which gave comparable results.
The levels of external and internal Ca2+ influence the uptake and centripetal transport of Brucella.
The pronounced effect of adherence on the uptake of Brucella by PBM suggested the involvement of adhesion receptors such as integrins. Since integrins depend on the presence of external Ca2+ or Mg2+ to stabilize the binding between their α and β subunits, we compared the uptake of nonopsonized Brucella in the presence and absence of external Ca2+ and Mg2+. We also included a virB9 mutant of B. suis in this study, as a graph in a previous paper indicated reduced uptake of this mutant compared to the parental strain (32), which may point to a bacterial ligand.
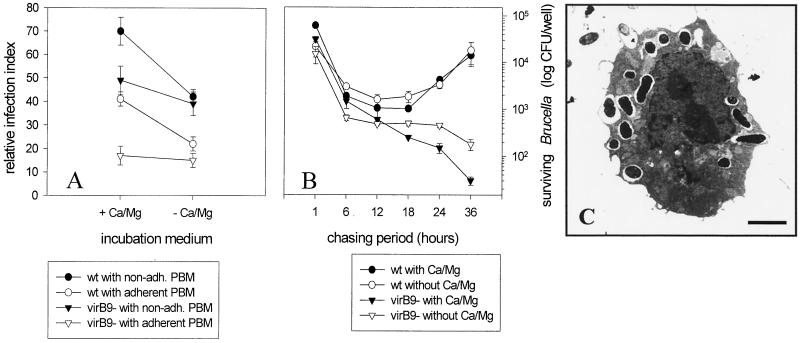
It was found that in the presence of Ca2+ and Mg2+ a higher number of nonadherent PBM than adherent PBM participated in the uptake of both strains, with the wild-type bacteria giving a higher infection index than the virB9 mutant (Fig. 5). In the absence of Ca2+ and Mg2+ the relative differences between nonadherent and adherent PBM were not altered. The number of PBM which phagocytosed wild-type bacteria, however, was greatly reduced, while there was only a slight decrease for the virB9 mutant (Fig. 5). The phagocytic index did not reveal consistent tendencies but varied strongly among the different donors (data not shown). These results indicated that possibly two types of adhesion molecules promoted uptake of Brucella, one being Ca2+ and Mg2+ dependent and the other not, and that both types participate in the uptake of wild-type bacteria but only the latter type participates in the uptake of the virB9 mutant. To ascertain whether these differences would be reflected in different intracellular survival, bacteria were reisolated from monocytes which had been infected in the presence or absence of Ca2+ and Mg2+. Thus, uptake in the presence or absence of Ca2+ and Mg2+ did not influence the subsequent intracellular survival of wild-type Brucella, whereas the decrease in the number of surviving virB9 mutant cells was delayed in the absence of Ca2+ and Mg2+ (Fig. 5).
FIG. 5.
Role of Ca2+, monocyte adherence, and the bacterial virB9 gene cluster in uptake and intracellular survival of Brucella. (A and B) Freshly isolated PBM were challenged with nonopsonized GFP-expressing wild-type (wt) B. suis or a virB9 mutant for 20 min and chased for another 30 min. Results are expressed as means ± standard deviations for duplicate samples from one out of three experiments with monocytes from different donors which gave comparable results. (A) Uptake. Counts of fluorescent bacteria internalized by nonadherent (non-adh.) or adherent PBM in the presence (+ Ca/Mg) or absence (− Ca/Mg) of external Ca2+ and Mg2+ are shown. (B) Survival. Counts of CFU of bacteria reisolated from osmotically lysed PBM following uptake in the presence or absence of external Ca2+ and Mg2+ are shown. (C) Electron micrograph of a PBM treated with 2 μM thapsigargin for 5 min before challenge with wt B. suis (15-min pulse, 60-min chase) in the presence of external Ca2+ and Mg2+. Thapsigargin-induced depletion of the internal Ca2+ stores did not affect the uptake of Brucella but did affect the centripetal transport of the phagosomes, as indicated by the predominantly peripheral localization of the phagosomes. The walls of the phagosomes show no tight apposition. Bar, 7 μm.
It could be argued that the inhibitory effect of Ca2+- and Mg2+-free incubation medium on the uptake of Brucella was due to a secondary lowering of the intracellular Ca2+ levels. In order to address this question, adherent PBM were pretreated with 2 μM thapsigargin for 5 min to make sure that the intracellular Ca2+ stores were emptied before being challenged with Brucella in the presence of Ca2+ and Mg2+. The resulting uptake was compared with that of untreated PBM challenged in the presence or absence of Ca2+ and Mg2+. It was found that thapsigargin-induced depletion of internal Ca2+ stores reduced the relative infection index to a lesser extent than did the absence of external Ca2+ and Mg2+ (31% after treatment with thapsigargin, compared to 41% in the presence and 22% in the absence of Ca2+ and Mg2+). In the thapsigargin-treated PBM, however, the majority (87%) of the bacteria were located at the rim and not in the center of the host cells, which was the opposite from the location in untreated PBM in the presence (22%) or absence (18%) of external Ca2+ and Mg2+. To see whether or not this peripheral localization reflected a blockade in the internalization of attached bacteria, the experiment was repeated for electron microscopic investigation. Whereas the absence or presence of external Ca2+ and Mg2+ did not result in any obvious ultrastructural differences, the Brucella-bearing phagosomes in the thapsigargin-treated PBM were located predominantly at the cell periphery. Moreover, the phagosomal wall was not tightly apposed to the engulfed brucellae, and about one out of four Brucella-bearing phagosomes showed fusion with intracellular vesicles (Fig. 5). These results suggest that the presence of external Ca2+ or Mg2+ might be required for some receptor(s) mediating the uptake of Brucella and intracellular Ca2+ for the tight apposition of the phagosomal membrane and the centripetal transport of the phagosomes but not for the fusion of Brucella-bearing phagosomes with intracellular vesicles.
Lysosomotropic amines alter the morphology of Brucella-bearing phagosomes and abolish the intraphagosomal survival of Brucella.
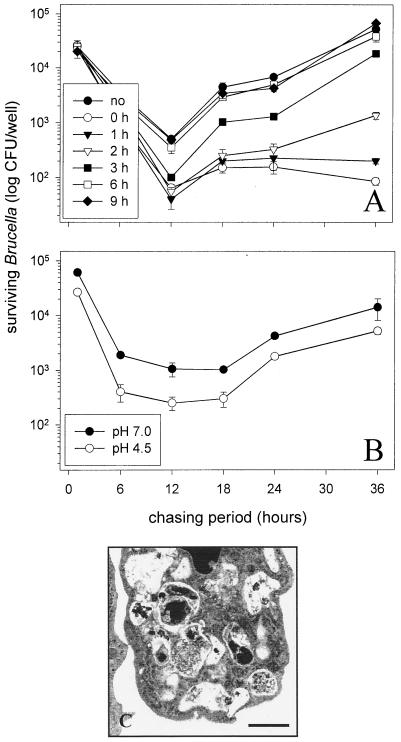
In a recent publication it was concluded that an acidic intraphagosomal pH is essential for the intracellular survival of Brucella, based upon the observation that the lysosomotropic weak base NH4Cl abolished the intracellular survival of Brucella in J774 cells when added after the first hour but not after 7 h of chase (37). We were interested to (i) see whether or not acidic shock of brucellae would increase their subsequent intracellular survival, (ii) narrow down the critical period of application of NH4Cl, and (iii) determine the consequences of this effect for the ultrastructure of the phagosomes. Thus, we exposed brucellae to a pH of 4.5 during 4 h prior to infection. However, this acidic pretreatment reduced the subsequent intracellular survival of brucellae (Fig. 6), suggesting that additional factors apart from an acidic pH may contribute to the intraphagosomal survival of internalized brucellae. When 30 mM NH4Cl was added to infected PBM either immediately when starting the chase or up to 2 h afterwards, the number of CFU was drastically reduced, whereas later applications gradually approached the results for untreated PBM (Fig. 6). With regard to the ultrastructure of Brucella-bearing phagosomes in the presence of NH4Cl, almost no TP were observed in NH4Cl-treated PBM but there were abundant SP containing degraded bacteria (Fig. 6), supporting the view from our other experiments that internalized brucellae survive in TP and are killed in SP.
FIG. 6.
Effect of ammonium chloride (A) and acidic shock (B) on the intracellular survival of Brucella in human monocytes. Freshly isolated PBM were challenged with nonopsonized B. suis for 20 min and chased for 1 to 36 h. Viable bacteria reisolated from osmotically lysed monocytes were determined by count of CFU. Results are expressed as means ± standard deviations for duplicate samples from one out of three experiments with monocytes from different donors which gave comparable results. (A) Compared is the survival of B. suis in the absence (no) or presence of 30 mM ammonium chloride added either immediately (0 h) at the onset of chase or up to 9 h later. (B) Compared is the survival of B. suis kept at either neutral or acidic pH for 4 h prior to infection. (C) Electron micrograph of a Brucella-infected monocyte chased for 8 h in the continuous presence of ammonium chloride. All Brucella-bearing phagosomes are spacious, and the internalized bacteria are degraded. Bar, 1 μm.
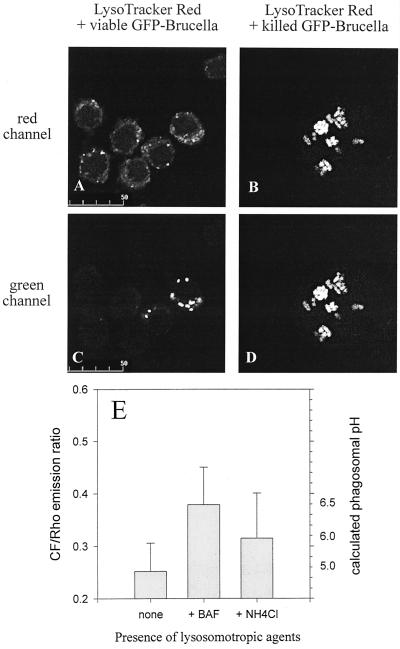
In the next step, using fluorescent probes, we wanted to ensure that NH4Cl indeed caused a rise in the intraphagosomal pH of Brucella-bearing phagosomes. Methodological problems with orange autofluorescence in the PBM forced us to perform the experiments with J774 cells instead. Two approaches were used. One was based on the infection of J774 cells with CF- and Rho-labeled Brucella, which allows for pH measurement. Alternatively, J774 cells were first infected with GFP-expressing Brucella and subsequently incubated with the acidophilic reagent LysoTracker Red, which gives qualitative evidence of an acidic pH in a compartment when both signals colocalize. The use of live or heat-killed CF- and Rho-labeled Brucella revealed that NH4Cl indeed raised the intraphagosomal pH of Brucella-bearing phagosomes to almost neutral, regardless of the viability of the bacteria used for infection (Fig. 7). In contrast, 100 nM LysoTracker Red colocalized with Brucella-bearing phagosomes only when heat-killed and not viable Brucella had been used for infection of the J774 cells. Raising the concentration of LysoTracker Red to 1 μM (10 to 20 times more than the recommended working concentration successfully used in other systems) still resulted in only weak and variable colocalization with internalized viable Brucella (Fig. 7), suggesting that viable brucellae managed to restrict the accessibility of their phagosomes to this lysosomotropic probe. This conclusion is supported by the fact that similar difficulties were faced when trying to reproduce the effect of NH4Cl on Brucella with another lysosomotropic amine with a higher molecular weight, chloroquine, which was effective only at a much higher working concentration than successfully used in other systems (data not shown).
FIG. 7.
Measurement of phagosomal pH in Brucella-infected murine macrophage-like cells. (A to D) J774.A1 cells were challenged with antibody-opsonized, viable (A and C) or heat-killed (B and D) GFP-expressing Brucella for 45 min and chased for another 90 min in the presence of the acidophilic reagent LysoTracker Red. Depicted are paired images in the red (A and B) and green (C and D) channels for visualization of LysoTracker Red and GFP-expressing Brucella, respectively. Even at the high concentration of 1 μM used here, LysoTracker Red colocalizes only weakly and inconsistently with viable Brucella but to the full extent with heat-killed Brucella. These features were observed throughout all infected cells in duplicate experiments. (E) J774.A1 cells were challenged with antibody-opsonized, viable CF- and Rho-labeled Brucella for 45 min and chased for another 90 min in the absence or presence of either 100 nM bafilomycin A1 (BAF) or 30 mM ammonium chloride. Results are expressed as means ± standard deviations for four pairs of CF-Rho images of duplicate experiments; the phagosomal pH was calculated from an in situ calibration curve of the CF/Rho emission ratio versus buffers with defined pHs. Heat-killed brucellae gave the same results as viable ones (data not shown).
DISCUSSION
The present study systematically addressed the uptake of virulent B. suis and B. melitensis by human monocytes, MDM, and epithelioid cells and their subsequent intracellular localization. This work proves evidence that brucellae are engulfed via regular zipper-type mechanisms by both professional and nonprofessional phagocytes, resulting in two types of phagosomes: regular SP representing the killing compartment and special TP representing the survival-permitting compartment. Whereas these observations were qualitatively the same for all host cells and experimental conditions investigated, the kinetics of uptake, the receptors involved, and the relative frequency of the survival-permitting compartment varied according to type of host cell and the experimental protocol used for infection. These results likely explain the variety of results obtained in previous studies and argue for great care when comparing results obtained under different experimental conditions.
In our system, Brucella organisms were readily internalized by human monocytes in both the absence and presence of complement and antibodies, and the ultrastructural features for opsonic and nonopsonic uptake were similar. It has long been known that in phagocytosis both the engulfing pseudopods and the phagosomal walls may have more or less continuous contact with the particles and that the engulfing pseudopods may be more or less extended; these two conditions have been classified as FcR- and CR-type or type I and II phagocytosis, respectively, according to the two most prominent opsonins (for a detailed discussion, see references 39 and 40). The present results, however, indicate that (i) these ultrastructural features occur under nonopsonic conditions as well and (ii) discontinuous contact may be seen with extended pseudopods and vice versa, arguing for greater care with this popular classification scheme.
As to the receptors promoting the uptake of Brucella, opsonization with antibodies contributed considerably to the uptake of B. suis. The increased uptake, however, did not result in increased survival, which is in line with results from previous studies (5, 9, 24). FcR-mediated phagocytosis intrinsically leads to the assembly of the NADPH oxidase and generation of reactive oxidative intermediates. Chemiluminescence assays for measuring the overall oxidative burst during uptake of Brucella gave contradictory results (5, 9, 24), whereas the ultracytochemical approaches of the present study and an early publication (21) reveal a rare oxidative activity in Brucella-bearing phagosomes, suggesting that intracellular killing mechanisms other than the oxidative burst mediate the eradication of internalized Brucella.
In contrast to the case for antibodies, the presence of complement did not increase the uptake of Brucella. A major role for complement in the uptake of this pathogen, at least in nonimmune serum, has been questioned before (for a review, see reference 29). Pathogens invading the human body have developed a broad array of methods to avoid recognition or lysis by complement (48). Future studies will have to show which mechanism is used by Brucella to overcome this major effector system of innate immune defense and whether CR-mediated uptake of Brucella depends on the simultaneous presence of complement and Brucella-specific antibodies. Still, Brucella may bind to CR3 directly without complement components acting as bridging opsonins, since this receptor has multiple opsonic and nonopsonic binding sites apart from that for the main opsonic complement fragment iC3b (reviewed in reference 41).
If the reduced bacterial uptake of adherent versus nonadherent monocytes is simply due to a reduced surface area, then there should be (i) a proportional decrease in uptake following adherence, which was not the case, and (ii) no differences between monocytes cultured for various periods of time, which was the case. Thus, a major portion of the nonopsonic receptors used for the uptake of B. suis likely are adhesion molecules of two types, one (possibly an integrin) Ca2+ and Mg2+ dependent and the other not. Nonopsonic entry of B. abortus into bovine macrophages was found to be competitively inhibited by fibronectin, mannan, and lipopolysaccharide (8). Whereas lipopolysaccharide and mannan bind mainly to nonintegrin receptors (4), fibronectin is recognized by several integrins, such as the main vitronectin receptor αVβ3 (10), and all three substances are ligands for CR3 (41). In our hands, expression of the human CR3 on CHO cells did not enhance uptake of B. suis in the presence of fresh or inactivated serum, indicating that this receptor either is not involved at all in the uptake of B. suis or may depend on other factors such as the functional cooperation with CR1 (45).
The present study showed that the uptake of Brucella leads to two types of phagosomes, with only one permitting survival. Morphological heterogeneity of Brucella-bearing phagosomes has also been observed in J774 cells before (3), but without consideration of possible functional consequences. In nonprofessional phagocytes, on the other hand, brucellae persist within cisternae of the rough ER (1, 2, 15–17, 46) which are reached quite late (≥24 h postinfection [hpi]) (17) via the phagosomal route. The autophagosome-like structures of B. abortus-bearing phagosomes in HeLa cells at 24 hpi, which are thought to derive from a merging with autophagosomes (34, 35), were not observed in the present study up to 8 hpi. The same applies to the osmiophilic membrane remnants, possibly derived from autophagosomes, described for long-term-infected hamster kidney tissue cultures (25). In a recent study on murine macrophage-like cells, only a minor proportion of intracellular B. abortus organisms colocalized with markers for the ER and autophagosomes (3), further supporting the view that the parasitism-permitting compartments of Brucella are distinct in professional and nonprofessional phagocytes.
Several other intracellular parasites are known to lodge in tight phagosomes, and it was concluded that the tight membrane apposition is crucial for a reduced fusiogenicity of these compartments (14). Despite similar morphology, the molecular characteristics of such tight phagosomes may differ according to the different microbial strategies used to interfere with the organelle trafficking of the host cell (11). Our difficulties in labeling Brucella-bearing phagosomes with chloroquine or LysoTracker Red but not with ammonium chloride indicate that brucellae selectively influence the accessibility of their intracellular compartment. Since these lysosomotropic weak bases have greatly different molecular weights (515.9, 399.25, and 53.49 for chloroquine, LysoTracker Red, and ammonium chloride, respectively), the size of the substrate may be the factor determining access. Still, the extremely narrow intraphagosomal space typical of the tight phagosomes also has to be kept in mind. The formation of tight phagosomes did not depend on bacterial viability or virulence, pointing to the effect of some yet-to-be determined membrane component(s) of Brucella.
Early publications (19, 31) suggested that Brucella inhibited phagosome-lysosome fusion in murine macrophages. More recently (3), it was reported that early Brucella-bearing phagosomes fused neither with early nor with late endosomes, although the procedure used to label early endosomes raises some questions as to the character of these vesicles (for a critical comparison, see reference 38). Fusion between Brucella-bearing phagosomes and early endosomes obviously was not impaired in a recent study on murine macrophages (36) and was a frequent event in our system. However, regarding the drastic decline in the number of surviving bacteria within the first 18 to 24 h of infection, only the few remaining truly nonfusiogenic phagosomes may represent the actual survival-permitting compartments.
Our results show that the Brucella-bearing phagosomes in J774 cells have an acidic pH, which is in line with previous reports (3, 37). Since acidification and fusiogenicity of the phagosome are two differently regulated consecutive steps (6, 47), TP may have an acidic pH and, nevertheless, restricted fusiogenicity. This raises the question of what is more important for permitting the survival of Brucella in this compartment, an acidic pH or the tight apposition of the phagosomal wall. Although the answer is not yet clear, our findings that (i) ammonium chloride leads to both a rise in the intraphagosomal pH and a swelling of the phagosome, (ii) brucellae survive in acidic TP but are killed in acidic SP, and (iii) acidic shock of Brucella prior to phagocytosis does not increase subsequent intracellular survival suggest that an acidic pH alone is not sufficient to establish intracellular infection. Successful parasitism may be determined by the timely synthesis of bacterial factors, and macrophage-specific induction of protein synthesis has been demonstrated for B. abortus (30) and B. suis (27). Moreover, brucellae share type IV secretion and regulatory systems with Agrobacterium tumefaciens, Rhizobium meliloti, and Bordetella pertussis (32, 43). Thus, it is tempting to assume that internalized brucellae sense the intraphagosomal environment and assemble secretion systems in order to establish intracellular parasitism, with the sensing and/or the secretion being dependent on a tight apposition of the phagosomal and bacterial membranes.
ACKNOWLEDGMENTS
We are indebted to Jacques Dornand, Virginie Lafont, Michel Vignes, Safia Ouahrani-Bettache, David O‘Callaghan, and Marina Cinco for providing material and to Hubert Reggio, Philippe Montcourrier, and Philip D’Arcy Hart for helpful comments.
M.G.R. held a “Poste Orange” senior research fellowship from INSERM, and B.R. was supported by the Association pour la Recherche contre le Cancer (grant ARC 5566).
REFERENCES
- 1.Ackermann M, Cheville N, Deyoe B. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet Pathol. 1988;25:28–35. doi: 10.1177/030098588802500104. [DOI] [PubMed] [Google Scholar]
- 2.Anderson T, Cheville N. Ultrastructural morphometric analysis of Brucella abortus-infected trophoblasts in experimental placentitis. Bacterial replication occurs in rough endoplasmic reticulum. Am J Pathol. 1986;124:226–237. [PMC free article] [PubMed] [Google Scholar]
- 3.Arenas G N, Staskevich A S, Aballay A, Mayorga L S. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect Immun. 2000;68:4255–4263. doi: 10.1128/iai.68.7.4255-4263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barclay N, Brown M, Law S, McKnight J, Tomlinson M, van der Merwe P. The leucocyte antigen facts book. 2nd ed. San Diego, Calif: Academic Press; 1997. [Google Scholar]
- 5.Bounous D, Enright F, Gossett K, Berry C. Phagocytosis, killing, and oxidant production by bovine monocyte-derived macrophages upon exposure to Brucella abortus strain 2308. Vet Immunol Immunopathol. 1993;37:243–256. doi: 10.1016/0165-2427(93)90197-c. [DOI] [PubMed] [Google Scholar]
- 6.Bouvier G, Benoliel A, Foa C, Bongrand P. Relationship between phagosome acidification, phagosome-lysosome fusion, and mechanism of particle ingestion. J Leukoc Biol. 1994;55:729–734. doi: 10.1002/jlb.55.6.729. [DOI] [PubMed] [Google Scholar]
- 7.Briggs R, Karnovsky M, Karnovsky M. Cytochemical demonstration of hydrogen peroxide in polymorphonuclear leukocyte phagosomes. J Cell Biol. 1975;64:254–260. doi: 10.1083/jcb.64.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell G, Adams L, Sowa B. Mechanisms of binding of Brucella abortus to mononuclear phagocytes from cows naturally resistant or susceptible to brucellosis. Vet Immunol Immunopathol. 1994;41:295–306. doi: 10.1016/0165-2427(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 9.Caron E, Peyrard T, Köhler S, Cabane S, Liautard J, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect Immun. 1994;62:5267–5274. doi: 10.1128/iai.62.12.5267-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheresh D, Mecham R. Integrins. Molecular and biological responses to the extracellular matrix. San Diego, Calif: Academic Press; 1994. [Google Scholar]
- 11.Clemens D, Lee B, Horwitz M. Deviant expression of rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect Immun. 2000;68:2671–2684. doi: 10.1128/iai.68.5.2671-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Preparation of human mononuclear cell populations and subpopulations. I, Part 7/I. New York, N.Y: Wiley and Sons; 1996. Current protocols in immunology. [Google Scholar]
- 13.Corbel M. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Chastellier C, Thilo L. Phagosome maturation and fusion with lysosomes in relation to surface property and size of the phagocytic particle. Eur J Cell Biol. 1997;74:49–62. [PubMed] [Google Scholar]
- 15.Detilleux P, Cheville N, Deyoe B. Pathogenesis of Brucella abortus in chicken embryos. Vet Pathol. 1988;25:138–146. doi: 10.1177/030098588802500206. [DOI] [PubMed] [Google Scholar]
- 16.Detilleux P, Deyoe B, Cheville N. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Vet Pathol. 1990;27:317–328. doi: 10.1177/030098589002700503. [DOI] [PubMed] [Google Scholar]
- 17.Detilleux P, Deyoe B, Cheville N. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect Immun. 1990;58:2320–2328. doi: 10.1128/iai.58.7.2320-2328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enright F. The pathogenesis and pathobiology of Brucella infection in domestic animals. In: Nielsen K, Duncan J, editors. Animal brucellosis. Boca Raton, Fla: CRC Press; 1990. pp. 301–320. [Google Scholar]
- 19.Frenchick P, Markham R, Cochrane A. Inhibition of phagosome-lysosome fusion in macrophages by soluble extracts of virulent Brucella abortus. Am J Vet Res. 1985;46:332–335. [PubMed] [Google Scholar]
- 20.Garcia-Carrillo C. Laboratory animal models for Brucellosis studies. In: Nielsen K, Duncan J, editors. Animal brucellosis. Boca Raton, Fla: CRC Press; 1990. pp. 423–442. [Google Scholar]
- 21.Gay B, Sanchez-Teff S, Caravano R. Ultrastructural localization of NADPH-oxidase activity in murine peritoneal macrophages during phagocytosis of Brucella. Virchows Arch B. 1984;45:147–155. doi: 10.1007/BF02889861. [DOI] [PubMed] [Google Scholar]
- 22.Glauert A. Fixation, dehydration and embedding of biological specimens. Vol. 3 1975. , part I. Coordinating ed., A. Glauert. North-Holland Publishing Company, Amsterdam, The Netherlands. [Google Scholar]
- 23.Gross A, Spiesser S, Terraza A, Rouot B, Caron E, Dornand J. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect Immun. 1998;66:1309–1316. doi: 10.1128/iai.66.4.1309-1316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmon B, Adams L. Assessment of bovine mammary gland macrophage oxidative burst activity in a chemiluminescence assay. Am J Vet Res. 1987;48:119–125. [PubMed] [Google Scholar]
- 25.Hatten B, Huang S, Schulze M, Sulkin S. Electron microscopy of tissue culture cells infected with Brucella abortus. J Bacteriol. 1971;108:535–544. doi: 10.1128/jb.108.1.535-544.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karnovsky M. Cytochemistry and reactive oxygen species: a retrospective. Histochemistry. 1994;102:15–27. doi: 10.1007/BF00271045. [DOI] [PubMed] [Google Scholar]
- 27.Köhler S, Ouahrani-Bettache S, Layssac M, Teyssier J, Liautard J. Constitutive and inducible expression of green fluorescent protein in Brucella suis. Infect Immun. 1999;67:6695–6697. doi: 10.1128/iai.67.12.6695-6697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krause A, Burmester G, Rensing A, Schoerner C, Schaible U, Simon M, Herzer P, Kramer M, Wallich R. Cellular immune reactivity to recombinant OspA and flagellin from Borrelia burgdorferi in patients with Lyme borreliosis. Complexity of humoral and cellular immune responses. J Clin Invest. 1992;90:1077–1084. doi: 10.1172/JCI115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liautard J, Gross A, Köhler S. Interactions between professional phagocytes and Brucella spp. Microbiol Semin. 1996;12:197–206. [PubMed] [Google Scholar]
- 30.Lin J, Ficht T. Protein synthesis in Brucella abortus induced during macrophage infection. Infect Immun. 1995;63:1409–1414. doi: 10.1128/iai.63.4.1409-1414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberti J, Caravano R, Roux J. Attempts of quantitative determination of phagosome-lysosome fusion during infection of mouse macrophages by Brucella suis. Ann Immunol. 1981;132D:201–206. [Google Scholar]
- 32.O‘Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli M, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 33.Ouahrani-Bettache S, Porte F, Teyssier J, Liautard J, Köhler S. pBBR1-GFP: a broad-host-range vector for prokaryotic promoter studies. BioTechniques. 1999;26:620–622. doi: 10.2144/99264bm05. [DOI] [PubMed] [Google Scholar]
- 34.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege J, Gorvel J. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizarro-Cerda J, Meresse S, Parton R, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel J. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizarro-Cerda J, Desjardins M, Moreno E, Akira S, Gorvel J. Modulation of endocytosis in nuclear factor IL-6(-/-) macrophages is responsible for a high susceptibility to intracellular bacterial infection. J Immunol. 1999;162:3519–3526. [PubMed] [Google Scholar]
- 37.Porte F, Liautard J, Köhler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabinowitz S, Horstmann H, Gordon S, Griffiths G. Immunocytochemical characterization of the endocytic and phagolysosomal compartments in peritoneal macrophages. J Cell Biol. 1992;116:95–112. doi: 10.1083/jcb.116.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittig G M, Burmester G R, Krause A. Coiling phagocytosis: when the zipper jams, the cup is deformed. Trends Microbiol. 1998;6:384–388. doi: 10.1016/s0966-842x(98)01343-2. [DOI] [PubMed] [Google Scholar]
- 40.Rittig G M, Wilske B, Krause A. Phagocytosis of microorganisms by means of overshooting pseudopods: where do we stand? Microbes Infect. 1999;1:727–735. doi: 10.1016/s1286-4579(99)80074-4. [DOI] [PubMed] [Google Scholar]
- 41.Ross G, Vetvicka V, Thornton B. Analysis of the phagocyte membrane lectin CR3 using fluorescence-labeled polysaccharides and flow cytometry. In: Robinson J, Babcock G, editors. Phagocyte function: a guide for research and clinical evaluation. New York, N.Y: John Wiley & Sons, Inc.; 1998. pp. 1–17. [Google Scholar]
- 42.Sangari F, Aguero J. Molecular basis of Brucella pathogenicity: an update. Microbiol Semin. 1996;12:207–218. [PubMed] [Google Scholar]
- 43.Sola-Landa A, Pizarro-Cerda J, Grillo M, Moreno E, Moriyon I, Blasco J, Gorvel J, Lopez-Goni I. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol Microbiol. 1998;29:125–138. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 44.Strasser J, Newman S, Ciraolo G, Morris R, Howell M, Dean G. Regulation of the macrophage vacuolar ATPase and phagosome-lysosome fusion by Histoplasma capsulatum. J Immunol. 1999;162:6148–6154. [PubMed] [Google Scholar]
- 45.Sutterwala F, Rosenthal L, Mosser D. Cooperation between CR1 (CD35) and CR3 (CD 11b/CD18) in the binding of complement-opsonized particles. J Leukoc Biol. 1996;59:883–890. doi: 10.1002/jlb.59.6.883. [DOI] [PubMed] [Google Scholar]
- 46.Tobias L, Cordes S, Schurig G. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet Pathol. 1993;30:119–129. doi: 10.1177/030098589303000204. [DOI] [PubMed] [Google Scholar]
- 47.Weidner E, Sibley L. Phagocytized intracellular microsporidian blocks phagosome acidification and phagosome-lysosome fusion. J Protozool. 1985;32:311–317. doi: 10.1111/j.1550-7408.1985.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 48.Würzner R. Evasion of pathogens by avoiding recognition or eradication by complement, in part via molecular mimicry. Mol Immunol. 1999;36:249–260. doi: 10.1016/s0161-5890(99)00049-8. [DOI] [PubMed] [Google Scholar]