Abstract
To address the ongoing shortage of organs available for replacement, xenotransplantation of hearts, corneas, skin, and kidneys has been attempted. However, a major obstacle facing xenotransplants is rejection due to a cycle of immune reactions to the graft. Both adaptive and innate immune systems contribute to this cycle, in which natural killer cells, macrophages, and T-cells play a significant role. While advancements in the field of genetic editing can circumvent some of these obstacles, biomarkers to identify and predict xenograft rejection remain to be standardized. Several T-cell markers, such as CD3, CD4, and CD8, are useful in both the diagnosis and prediction of xenograft rejection. Furthermore, an increase in the levels of various circulating DNA markers and microRNAs is also predictive of xenograft rejection. In this review, we summarize recent findings on the advancements in xenotransplantation, with a focus on pig-to-human, the role of immunity in xenograft rejection, and its biomarkers.
Keywords: xenotransplantation, immune rejection, diagnostic biomarkers, predictive biomarkers, genetic editing, xenoantigens, tolerance induction
Introduction
The improved life expectancy of humans over the past decades has increased the prevalence of a growing number of chronic diseases1. The increasing application of organ transplantation, the last resort and definitive treatment for end-stage organ failure, has resulted in a disparity in the supply and demand for such organs1. Therefore, xenotransplantation has become an appealing solution to overcome this obstacle2. The U.S. Food and Drug Administration defines xenotransplantation as “any procedure that involves the transplantation, implantation or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source, or (b) human body fluids, cells, tissues or organs that have had ex vivo contact with live nonhuman animal cells, tissues, or organs”3. Currently, xenotransplant use has been reported mainly for the kidneys, hearts, livers, skin, and corneas4.
Pigs are the species of choice to harvest organs for xenotransplantation, as they have anatomically similar organs to humans and are suitable for genetic modification5. They are highly bred and often consumed, clearing the way for the ethical decision to use pig organs to treat human diseases. Although the genetic discrepancies between humans and pigs are greater than those of primates, the use of primate organs is not sustainable for ethical reasons and because most primates are considered endangered5. Furthermore, primate organs have a substantial chance of carrying viruses that can infect humans5. Hence, genetic engineering techniques have been developed to decrease porcine and human genetic dissimilarities1, paving the road for the usage of pig organs for xenotransplants. Indeed, recent studies described two successful cases of kidney transplants from pigs in brain-dead patients6, and another reported a successful case of a heart transplant from a pig to a human7. These breakthroughs marked a great milestone for the field of xenotransplantation.
The main obstacle facing xenotransplants is immunological reactions. Although the mechanism behind hyperacute rejection (HAR) in the xenograft is well defined, the mechanisms of acute cellular rejection are not fully understood2. Identifying the mechanisms behind cellular rejection in xenotransplantation may be the key to the longer survival of xenotransplanted organs. Furthermore, unlike allotransplantation, there is a lack of data on standardized predictive and diagnostic markers of xenotransplantation8, which could permit close monitoring of xenografts9. In this article, we will briefly review the history of xenotransplantation, xenoantigens presenting as obstacles, and genetic modifications to overcome these obstacles. Lastly, we will highlight the role of cellular immunity activated in response to xenotransplantation and describe the immune markers used to predict and detect xenograft rejection.
A Brief History of Xenotransplantation
In the 17th century, the first reported case of xenotransplantation (and blood transfusion) to humans was conducted by Jean-Baptiste Denis who transfused the blood of a lamb into a 15-year-old male suffering from a fever10. Denis subsequently continued to transfuse blood from lambs and calves but with variable outcomes, so the French and English Parliaments banned transfusions for several years to come10.
In 1838, Sharp-Kissam performed the first corneal transplantation by implanting a pig cornea into the eye of a 35-year-old man11. In the 19th century, scientists began using skin xenografts from various animals, such as pigs, sheep, frogs, pigeons, and chickens, as biologic dressings12 and bovine embryonic skin grafts as skin dressing13.
In the 20th century, Voronoff attempted to “rejuvenate” elderly men by conducting several chimpanzee and baboon testicular transplants14 purportedly thereby elevating energy levels in patients. In the 1960s, Reemtsma carried out 13 chimpanzee-to-human kidney xenotransplants, most of which failed within 4–8 weeks due to rejection or infections, except for one that lasted for 9 months with no signs of rejection on autopsy15.
The first cardiac xenotransplantation was performed in 1964 by Hardy with a chimpanzee heart, which was too small and failed within a couple of hours14. During the same era, Starzl performed the first reported liver xenotransplants with limited success. However, after the introduction of tacrolimus (a potent immunosuppressor), he performed two baboon-to-human liver xenotransplants, with one patient surviving 70 days14,16. The rising incidence of type-1 diabetes and the similarities between pig and human insulin motivated contemplation of the benefit of islet xenotransplantation14. Thus, in 1993, Groth et al.17 conducted the first pig-to-human islet xenotransplant but identified no clinical benefit.
Xenoantigens and Genetic Modifications
Initial attempts of porcine-to-human xenotransplants were hindered by the production of antibodies against the α-galactose-1,3-galactose (αGal) antigen18. Approximately 1% of naturally occurring human antibodies are directed against the αGal epitope and are responsible for HAR of pig organs perfused with human blood18. The discovery of the αGal epitope in pigs led to testing its expression in various animal species. In 1988, Galili et al.19 demonstrated that the anti-Gal antibody binds to various nucleated cells of non-primate mammals, prosimians, and New World monkeys, whereas fibroblasts of humans, apes, and Old-World monkeys indicated no αGal expression.
Advancements in the field of genomic editing have then led to the development of genetically modified pigs to overcome immune rejection1, most notably the heterozygous αGal-knockout (GKO) pigs in 2002 and homozygous GKO pigs in 200320. The elimination of αGal increased the survival of pig hearts in baboons for 2–6 months and prevented HAR21, but was insufficient to completely evade the immune system6, leading to the identification of two additional non-Gal epitopes as targets of antibodies: NeuGc and SDa22,23. These antibodies might have played a key role in the rejection of kidney xenotransplant from Gal-depleted pigs to humans6. Adams et al.24 found that elimination of both Gal and SDa genes extended graft survival to up to 435 days in pig-to-primate transplants. Collectively, Gal, NeuGc, and SDa antibodies constitute more than 95% of antibodies formed against pig cells22,25 and may present as major obstacles to the advancement of clinical xenotransplantation.
Yet, emerging studies in pigs with Gal, NeuGc, and SDa knockout revealed that transplant-induced coagulopathies also hamper the success of xenotransplantation and that overexpression of human coagulation regulatory proteins in animal donors may resolve this issue1. Therefore, one of the main goals of genetic modulation has become regulating the coagulation dysfunction in graft recipients, such as thrombomodulin (TBM). Porcine TBM fails to successfully interact with human thrombin, leading to a procoagulation state26. Importantly, Miwa et al.27 found that expression of human TBM in porcine aortic endothelial cells successfully regulated coagulation in human plasma and inhibited antibody-induced complement activation. Moreover, antibody therapy combined with expression of human TBM prevents humoral rejection and coagulation dysregulation and increases graft survival beyond 900 days in pig-to-baboon heart transplants28.
Another attractive candidate target for genetic modulation is the endothelial protein-C receptor (EPCR). Although the pig EPCR is compatible with human protein-C26, Iwase et al.29 found a strong positive correlation between the reduction of human platelet aggregation and the expression of human EPCR in pig aortic endothelial cells. Lastly, Wheeler et al.30 showed that expression of human CD39, which hydrolyzes ATP and ADP and prevents thrombus formation, prevented myocardial ischemia/reperfusion injury in transgenic pigs.
Other genetic modifications are also being studied in attempt to target the cellular xenograft rejection (CXR) pathways. For example, due to the incompatibility of human SIRP-α and porcine CD47 (discussed later in the article), Tena et al.31 used porcine hematopoietic cells expressing human CD47, which significantly increased engraftment chimerism in human bone marrow. Expression of human CD47 also led to prolonged survival of pig skin grafts on baboons, with one case showing no signs of acute rejection for 53 days32. In conclusion, genetic modifications are key for the successful transition of xenotransplantation into clinical settings.
Tolerance Induction in Xenotransplantation
Graft recipients require a combination of intensive immunosuppressive therapy, and various attempts to reduce the dose have failed33. Hence, tolerance-inducing strategies are currently under development with the aim of prolonging graft survival times and, eventually, halting immunosuppressive therapy34. Currently, donor thymic transplantation is the most effective method of achieving tolerance in xenotransplantation34. Studies have demonstrated prolonged swine-to-baboon renal graft survival times of more than 6 months after GKO swine kidney and thymus transplantation35,36. In humans, Montgomery et al.6 transplanted GKO swine thymus and kidney into two brain-dead patients; however, the follow-up period was too short for the thymus to assert its effects. Nevertheless, the thymuses were able to revascularize and maintained normal architecture.
Mixed bone marrow chimerism (MBMW), which involves the production of both donor and self hematopoietic stem cells by the recipient after non-myeloablative stem cell transplant regimens, has allowed for allogeneic transplants regardless of HLA barriers34. Although MBMW is successful in pig-to-mouse models, replicating such results has been difficult in pig-to-primate studies34,37. For example, Liang et al.38 demonstrated that only 10% of swine-to-baboon MBMWs resulted in successful engraftment, with failure of engraftment associated with increased anti-non-Gal IgG levels post-transplant. Overall, further studies are needed to determine the effectiveness of thymus transplantation and MBMW in inducing tolerance.
Histological and Systemic Outcomes of Xenograft Rejection
Within minutes to hours of graft transplantation, the xenograft is destroyed by HAR, a process mediated by pre-existing αGal antibodies1. Binding of these antibodies leads to complement pathway activation, which causes lysis of endothelial cells1. Notably, for an unknown reason, the effects of antibody depletion and complement inhibition are generally more effective in heart and kidney transplants than lung and liver transplants39–41. Unlike other rejection types, grafts show no functionality when they undergo HAR39. Histologically, this process is characterized by massive hemorrhage and complement, immunoglobulin, and fibrin deposition39.
Acute humoral xenograft rejection (AHXR), also known as delayed xenograft rejection, can be initiated by naturally occurring αGal antibodies or antibodies formed after sensitization by the graft39. In the latter case, the antibodies may be directed against αGal or non-Gal antigens, such as NeuGc and SDa39. Histologically, this process resembles HAR; however, necrosis and transmural granulocyte infiltration of blood vessels may be present39.
Lastly, CXR may occur after a significant time lag post-xenotransplantation. Contrary to HAR and AHXR, hemorrhage and fibrin and immunoglobulin depositions are not observed. Complement depositions may be seen but are usually of low intensity39. The mechanisms underlying CXR will be described in the next section.
Systemically, three complications characterize xenograft recipients: immune complex diseases, coagulopathies, and infections. Due to the prominent role of antibodies in xenograft rejection, immune complex deposits may be seen in various recipient organs39. After porcine-to-baboon xenotransplant, Holzknecht et al.42 detected deposits of baboon C3 and porcine von Willebrand factor in the spleens and livers of lung recipients. Interestingly, baboons that received porcine hearts and kidneys did not show such depositions. Deposits of rat IgG and IgM have also been found in the glomeruli of recipient rats after a hamster-to-rat liver transplant43.
Given the adverse coagulopathy observed in xenotransplant recipients, thrombotic microangiopathy (TMA) might develop as a fatal complication post-transplant resulting in thrombosis within vessels and ischemic injury1. Briefly, graft recipients rapidly progress into thrombocytopenia, develop schistocytes, and present with high levels of lactate dehydrogenase44. With the progression of TMA, a systemic consumptive coagulopathy may develop leading to recipient death45. However, this issue may be resolved with the rapid excision of the xenograft, inhibiting further consumption of coagulation factors and improving recipient survival45.
Lastly, potential transmission of pathogens is a major concern in xenotransplantation. Swine pathogens can be generally separated into four categories: pathogens that infect healthy humans, pathogens that infect human-transplant recipients, pathogens resembling those of human-transplant recipients, and swine-specific pathogens46. Pathogens of the third category, such as porcine cytomegalovirus (PCMV) and porcine adenovirus, have been associated with syndromic complications in pig and nonhuman primate xenograft recipients46. For example, PCMV is responsible for disseminated intravascular coagulation, hematuria, and reduced graft survival times in pig-to-baboon transplants47,48.
Swine-specific pathogens, such as porcine endogenous retroviruses (PERVs), are a growing area of concern due to the potential risk of silent transmission and gene alterations46. PERVs integrate themselves within the porcine genome and may be classified as PERV-A, PERV-B, and PERV-C49. PERV-A and PERV-B are present in all pig species, while PERV-C is only present in select species50. Recombinant PERV-A/C, which is characterized by high titer replication, has shown the capability of infecting human cells50. Thus, it is recommended to screen for the presence of PERV-C and only use donor pigs free of the virus50. To date, no literature describes PERVs in preclinical pig-to-primate models and clinical transplantations in humans, yet inactivation of the viruses may be completed using genetic modifications if needed49. In conclusion, it is essential to further study mechanisms that bypass the fatal complications of TMA and consumptive coagulopathy and develop screening assays for potential infective organisms.
Role of Cellular Immunity in Xenogeneic Rejection
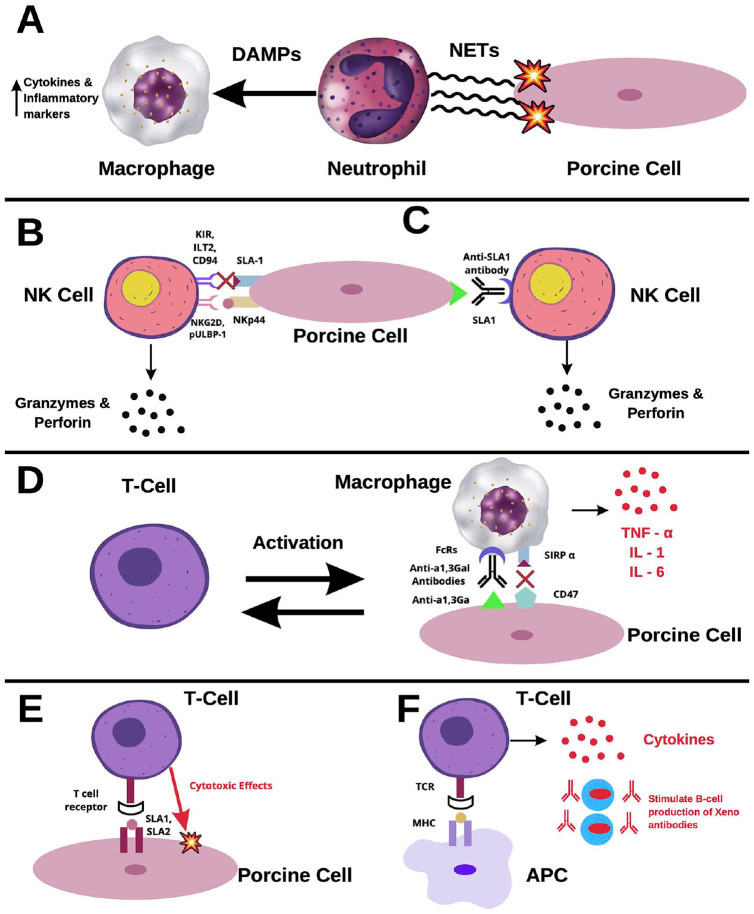
Immune responses after xenotransplantation involve both the innate and immune adaptive systems1. Although the main cells involved in allograft rejection are cytotoxic T-lymphocytes, xenograft reactions activate primarily neutrophils, natural killer (NK) cells, and macrophages51. Neutrophils rapidly infiltrate both cellular and organ grafts52,53. Upon activation, neutrophils release neutrophil extracellular traps (NETs), network structures that induce damage through the generation of reactive oxidative species (ROS), and release of digestive enzymes2,54,55. Furthermore, macrophages recognize NETs as damage-associated molecular patterns (DAMPs) that cause the release of cytokines and inflammatory markers (Fig. 1A)54.
Figure 1.
Cellular rejection in xenotransplants. (A) Neutrophil-mediated rejection. Upon activation, neutrophils release tubular networks known as NETs. NETs induce damage to xenograft cells through ROS and are recognized by macrophages as DAMPs. Binding of DAMPs to macrophages triggers the release of cytokines and inflammatory markers. (B) NK cell-mediated rejection via the direct pathway. Stimulating receptors, such as NKG2D and pULBP-1, bind to NKp44 and an unidentified molecule, respectively. Once activated, NK cells release granzymes and perforin, which induce damage in xenograft cells. The inhibiting receptors, KIR, ILT2, and CD94, do not recognize SLA-1 well in porcine cells. Therefore, there is a lack of inhibitory feedback of NK cells in xenografts. (C) NK cell-mediated rejection via the ADCC pathway. Interactions between FcRs and xenoantibodies lead to activation of NK cells. Among the antibodies recognized by NK cells is anti-SLA1. Binding on NK cells to anti-SLA1 induces the release of granzymes and perforin. (D) Macrophage-mediated rejection. Macrophages recognize anti- α1,3Ga antibodies through FcR, inducing the release of inflammatory cytokines, such as TNF-α, IL-1, and IL-6. Macrophages activate circulating T-cells, which further activate more macrophages. CD47 is an important receptor in the pathway of macrophage inhibition. However, it does not readily recognize its ligand, SIRP-α, in porcine cells, causing ineffective inhibition. (E) T-cell-mediated rejection via the direct pathway. SLA1 and SLA2 bind to T-cell receptors, triggering the release of cytokines and mediating direct cytotoxic effects. (F) T-cell-mediated rejection via the indirect pathway. Recipient antigen-presenting cells (APC) express xenogeneic antigens, activating CD4+ T-cells. Activated T-cells induce a cascade of antibody production and B-cell activation. NET: neutrophil extracellular traps; ROS: reactive oxidative species; DAMP: damage-associated molecular patterns; NK: natural killer; NKG2D: natural killer group-2D; pULBP-1: porcine UL16-binding protein-1; KIR: Killer Ig-like Receptor; ILT2: Ig-like transcript-2; ADCC: antibody-dependent cellular cytotoxicity; TNF-α: tumor necrosis factor; IL: interleukin; SIRP-α: signaling regulatory protein; CD94: cluster of differentiation-94; SLA-1: swine leukocyte antigen-1.
Numerous studies have reported infiltration of NK cells within xenografts, implicating them in xenograft rejection51,56. These cells induce rejection by either direct cytotoxicity or antibody-dependent cellular cytotoxicity (ADCC). The direct pathway is tightly regulated by stimulating and inhibiting receptors. NK-stimulating receptors, such as natural killer group-2D (NKG2D) and porcine UL16-binding protein-1 (pULBP-1), bind to the pig ligand NKp44 and an unidentified molecule, respectively57,58, leading to the release of lytic granules such as granzymes and perforin (Fig. 1B)59. Contrarily, inhibiting receptors, killer Ig-like receptor (KIR), Ig-like transcript-2 (ILT2), and CD94, do not readily recognize swine leukocyte antigen-1 (SLA1), the porcine major histocompatibility complex-1 molecule, dampening NK inhibition in xenografts58. In the ADCC pathway, antibodies deposited on the surface of xenograft cells are recognized by NK cells via interactions with FcRs1. Upon activation, NK cells release granzymes and perforin, leading to apoptosis of the targeted cells. Furthermore, NK cells recognize anti-SLA1 antibodies, activating the ADCC pathway (Fig. 1C)25.
Macrophages have also been implicated in the rejection of cellular grafts and organ grafts60. Peterson et al.61 have shown that xenogeneic αGal is a direct ligand for human monocytes. In addition, immune complexes of porcine cells with xenogeneic antibodies such as anti- αGal antibodies bind to the Fcγ receptor (FcγR) and produce an activation signal62. Once activated, macrophages contribute to a vicious cycle of xenograft destruction, where they are activated by T-cells and, in turn, activate more T-cells63. Furthermore, macrophages induce direct cytotoxicity through the production of cytokines, such as tumor necrosis factor (TNF)-α, interleukin-1 (IL-1), and IL-6 (Fig. 1D)64. Regarding inhibitory feedback, the signaling regulatory protein (SIRP-α)-CD47 pathway is an important regulator of macrophage activity1,65. The CD47 pathway has been shown to regulate the homeostasis of erythrocytes, platelets, and hematopoietic stem cells66. CD47 is recognized by SIRP-a as a “do-not-eat” signal, thus inhibiting phagocytic activity65, a signal utilized by cancer cells to evade immune surveillance. However, Wang et al.67 have reported interspecies incompatibility of CD47 after xenotransplantation, which leads to ineffective inhibition of macrophages.
Like in allograft transplantation, T-cell activation is mediated in xenograft rejection through direct and indirect pathways1,68. Through the direct pathway, interactions between SLA-1 and -2 complexes with T-cell receptors lead to activation of the adaptive immune response against the xenograft (Fig. 1E)1. In the indirect pathway, the presentation of xenogeneic antigens by recipient cells leads to the activation of CD4+ T-cells, instigating a cascade of antibody production and B-cell activation (Fig. 1F)1. Lastly, cytokines produced through this mechanism significantly enhance the cytotoxicity of NK cells and macrophages69.
As mentioned above, B-cells play a role in the rejection of xenografts. In fact, B-cell depletion increased survival time by 8 months after heart transplantation from pigs to baboons, suggesting a significant role of B-cells in xenotransplant rejection, specifically, delayed xenotransplant rejection70. B-cells produce the anti-Gal antibody which targets Gal antigens expressed in pig tissues71 and binds to its antigen, leading to complex formation. Indeed, depletion of the anti-Gal antibody leads to more favorable outcomes, further implicating B-cells in the rejection of xenotransplants71–73. The phenotypic characteristics of anti-Gal antibody-producing subpopulations of B-cells in humans are not clearly identified72. One study has shown that splenic B-cells produce anti-Gal antibodies, whereas peritoneal B-cells do not, although they do express anti-Gal receptors73. Conclusively, both innate and adaptive immune systems play a significant role in xenotransplant rejection.
Biomarkers of Xenograft Rejection
A lack of standardization among methods used to monitor xenograft rejection drives a crucial need to identify markers that can be used to diagnose and predict rejection8. As listed in Table 1, Montgomery et al.6 observed focal C4d deposition at 54 hours post-pig-to-human kidney transplant but no other significant histologic or immunologic indications of antibody-mediated injury. Zhou et al.8 also found that CD68+ macrophages and some CD3+ T-cells infiltrated xenografts in pig-to-mouse models on day 3 after transplantation.
Table 1.
Potential Biomarkers of Xenograft Rejection.
| Biomarker classification | Biomarker | Sample type | Application | Reference |
|---|---|---|---|---|
| Intra-graft biomarkers | C4d | Pig-to-human kidney | Marker of inflammation | 6 |
| CD68 | Pig-mouse models | Identify macrophage infiltration | 8 | |
| CD3 | Pig-mouse models | Identify T-cell infiltration | 8 | |
| NK1.1 and DX5 | Peritoneal mouse cells | Identify NK cell infiltration | 74 | |
| TLR2 mRNA and protein (↑) | Porcine iliac artery endothelial cells | Marker of immune rejection | 75 | |
| CCL2 and CXCL8 (↑) | Porcine cells | Marker of immune rejection | 76 | |
| Serum biomarkers | Non-α-Gal IgM and IgG antibodies (↑) | Pig-to-human kidney | Marker of immune rejection | 6 |
| cpsDNA | Pig-mouse models | Increased levels precede immune rejection | 8 | |
| cfDNA | Pig-to-baboon hearts | Correlates with tissue injury | 77 | |
| ssc-miR-199b | Liver, heart, and lung | Predicts transplant prognosis | 78 | |
| miR-146a (↓) | Mouse-to-rat cardiac models | Marker of immune rejection, potential target of immunotherapy | 79 | |
| miR-155 (↑) | Mouse-to-rat cardiac models | Marker of immune rejection, potential target of immunotherapy | 79 | |
| C3 (↑) | Pig-to-non-human cornea | Increased prior to tissue rejection | 80 |
NK: natural killer; TLR2: toll-like receptor-2; cpsDNA: circulating pig-specific DNA; cfDNA: cell-free DNA; mRNA: messenger ribonucleic acid; CCL2: C–C motif chemokine ligand-2; CXCL8: C-X-C motif chemokine ligand-8; DNA: deoxyribonucleic acid.
Given that NK cells are a major type of infiltrating cells identified in xenografts51,56,81, Lin et al.74 used markers such as NK1.1 and DX5 to identify NK cells in pig-to-mouse models. Using a modified ADCC assay, Chen et al.76 found that toll-like receptor-2 (TLR2) mRNA and protein were also upregulated in porcine iliac artery endothelial cells after exposure to human serum. Furthermore, the levels of porcine pro-inflammatory chemokines CCL2 and CXCL8 also increased through a TLR2-mediated pathway76. These findings suggest that blockade of TLR2 may prolong xenograft survival.
Graft biopsies may cause infection, scarring, or induce rejection via immune activation after injury75. Therefore, it is important to identify noninvasive markers of rejection for apply in clinical xenotransplantation. Montgomery et al.6 detected IgM and IgG antibodies directed against non-α-Gal antigens in the serums of pig-to-human kidney transplant patients. Because IgM is confined to the vascular space, its removal via plasmapheresis can, theoretically, be incorporated in future xenotransplantation trials involving humans6.
Circulating DNA is released upon cell death or apoptosis, which are considered classic findings in xenotransplantation8. The release of circulating pig-specific DNA (cpsDNA) reflects the infiltration of immune cells in the graft and precedes the production of anti-pig IgM/IgG antibodies in pig-to-mouse models8. Furthermore, cpsDNA also provided comparable results in monkeys, suggesting potential feasibility in clinical settings8. Similarly, cell-free DNA (cfDNA) levels also correlate with tissue injury in xenograft models77.
While data regarding organ-specific microRNAs (miRNA) in xenotransplant remain limited, they have shown promising usage as biomarkers of rejection78. In a pig model of acute liver failure, recipient plasma levels of various pig-derived miRNAs, including ssc-miR-122, ssc-miR-192, and ssc-miR-124-1, were associated with liver, kidney, and brain injury, respectively82. Most miRNAs are conserved among species, limiting their usage in the field of xenotransplantation78,83. However, some miRNAs, such as the pig-specific ssc-miR-199b, could be useful as they may be differentiated from its human counterpart and are expressed in the liver, heart, and lung78.
One study also observed increased levels of miR-146a and miR-155 in cardiac xenotransplants and evaluated the effect of immunosuppressive treatment on their expression in cardiac xenotransplant models from mouse to rat. Compared to immunosuppressed animals, Zhao et al.79 found a significant decrease in miR-146a levels and increase in miR-155 expression, changes that lead to a pro-inflammatory state in recipients. Notably, miR-146a plays a role in inhibiting inflammatory conditions by targeting various NF-κB pathways84, and miRNA-155 has also been reported as a promoter of TNF-α expression85. Collectively, these findings may provide insight into the potential use of miRNAs as biomarkers and targets of RNA-interfering immunotherapy.
A recent study in nonhuman primates also reported elevated levels of C3 levels in the aqueous humor preceding rejection80. Lastly, high CD4+/CD8+ blood cell ratios are correlated with shorter graft survival times in pig-to-non-human islet transplants86. However, further studies are required to assess the sensitivity and specificity of any proposed markers.
Conclusion
In light of recent organ shortages, xenotransplantation could provide a much-needed solution for patients requiring organ transplants. Historically, the major obstacle facing xenotransplantation from pig sources was the presence of the αGal epitope. However, genetic modulation allowed development of pig models devoid of this epitope. This advancement has prolonged xenograft survival in humans and illuminated other epitopes, such as NeuGc and SDa, which induce immune rejection. Thus, studies aimed at identifying the immune mechanisms that lead to rejection. NK cells, macrophages, and T-cells have been identified as key players in the pivotal role of the immune system in the rejection of xenografts.
Furthermore, the methods used to identify rejection of xenotransplants are based on those used in allotransplantation due to lack of standardization. T-cell markers, such as CD3, CD4, and CD8, seem promising as predictive and diagnostic rejection markers. Markers of cellular injury, such as cpsDNA and cfDNA, have also been identified as early predictive biomarkers of rejection. Various miRNAs have also been recognized as rejection markers and possible targets for the development of new immunotherapy strategies. Lastly, the detection of non-α-Gal IgG and IgM antibodies has recently been used as a marker for pig-to-human kidney transplant rejection. Given the recent advancements in the field, xenotransplantation might ultimately become a viable clinical option. Nonetheless, further progress is needed to overcome the complications of TMA and consumptive coagulopathy. Furthermore, more studies are required to compare various markers and identify a “gold standard” rejection marker in xenotransplantation.
Footnotes
Ethical Approval: This manuscript is a review article and does not involve any ethical issues. All authors reviewed and approved the final version of the manuscript.
Statement of Human and Animal Rights: This study did not involve any human or animal subjects.
Statement of Informed Consent: This article did not involve any human subjects and, thus, informed consent is not applicable.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Lerman is an advisor to AstraZeneca, CureSpec, Butterfly Biosciences, Beren Therapeutics, and Ribocure Pharmaceuticals. The authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly supported by NIH grant numbers: DK120292, DK122734, HL158691, and AG062104.
ORCID iDs: Tarek Ziad Arabi  https://orcid.org/0000-0002-6292-7683
https://orcid.org/0000-0002-6292-7683
Lilach O. Lerman  https://orcid.org/0000-0002-3271-3887
https://orcid.org/0000-0002-3271-3887
References
- 1. Lu T, Yang B, Wang R, Qin C. Xenotransplantation: current status in preclinical research. Front Immunol. 2020;10:3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maeda A, Kogata S, Toyama C, Lo PC, Okamatsu C, Yamamoto R, Masahata K, Kamiyama M, Eguchi H, Watanabe M, Nagashima H, et al. The innate cellular immune response in xenotransplantation. Front Immunol. 2022;13:858604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration. Xenotransplantation. 2021. Accessed June 21, 2022. https://www.fda.gov/vaccines-blood-biologics/xenotransplantation
- 4. Cooper DKC, Gaston R, Eckhoff D, Ladowski J, Yamamoto T, Wang L, Iwase H, Hara H, Tector M, Tector AJ. Xenotransplantation—The current status and prospects. Br Med Bull. 2018;125(1): 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Groth CG. The potential advantages of transplanting organs from pig to man: a transplant Surgeon’s view. Indian J Urol. 2007;23(3): 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montgomery RA, Stern JM, Lonze BE, Tatapudi VS, Mangiola M, Wu M, Weldon E, Lawson N, Deterville C, Dieter RA, Sullivan B, et al. Results of two cases of pig-to-human kidney xenotransplantation. N Engl J Med. 2022;386(20): 1889–98. [DOI] [PubMed] [Google Scholar]
- 7. Kuehn BM. First pig-to-human heart transplant marks a milestone in xenotransplantation. Circulation. 2022;145(25): 1870–71. [DOI] [PubMed] [Google Scholar]
- 8. Zhou M, Lu Y, Zhao C, Zhang J, Cooper DKC, Xie C, Song Z, Gao H, Qu Z, Lin S, Deng Y, et al. Circulating pig-specific DNA as a novel biomarker for monitoring xenograft rejection. Xenotransplantation. 2019;26(4): e12522. [DOI] [PubMed] [Google Scholar]
- 9. Chan JL, Mohiuddin MM. Heart xenotransplantation. Curr Opin Organ Transplant. 2017;22(6): 549–54. [DOI] [PubMed] [Google Scholar]
- 10. Roux FA, Saï P, Deschamps JY. Xenotransfusions, past and present. Xenotransplantation. 2007;14(3): 208–16. [DOI] [PubMed] [Google Scholar]
- 11. Snyder C. Richard Sharp Kissam, MD, and ceratoplastice in man. Arch Ophthalmol. 1963;70:870–72. [DOI] [PubMed] [Google Scholar]
- 12. Cooper DKC, Ekser B, Tector AJ. A brief history of clinical xenotransplantation. Int J Surg. 2015;23(Pt B): 205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silvetti AN, Cotton C, Byrne RJ, Berrian JH, Fernandez Menendez A. Preliminary experimental studies of bovine embryo skin grafts. Transplant Bull. 1957;4(1): 25–26. [PubMed] [Google Scholar]
- 14. Cooper DKC. A brief history of cross-species organ transplantation. Proc. 2012;25(1): 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wijkstrom M, Iwase H, Paris W, Hara H, Ezzelarab M, Cooper DKC. Renal xenotransplantation: experimental progress and clinical prospects. Kidney Int. 2017;91(4): 790–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, Zeevi A, Warty V, Kusne S, Demetris AJ, et al. Baboon-to-human liver transplantation. Lancet. 1993;341(8837): 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groth CG, Korsgren O, Tibell A, Tollemar J, Möller E, Bolinder J, Ostman J, Reinholt FP, Hellerström C, Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344(8934):1402–4. [DOI] [PubMed] [Google Scholar]
- 18. Oriol R, Cooper DKC. Major carbohydrate xenotransplantation antigens. Xenotransplantation. 1997:24–32. [Google Scholar]
- 19. Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33): 17755–62. [PubMed] [Google Scholar]
- 20. Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605): 411–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuwaki K, Tseng YL, Dor FJMF, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2004;11:29–31. [DOI] [PubMed] [Google Scholar]
- 22. Byrne G, Ahmad-Villiers S, Du Z, McGregor C. B4GALNT2 and xenotransplantation: a newly appreciated xenogeneic antigen. Xenotransplantation. 2018;25(5): e12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song KH, Kang YJ, Jin UH, Park YI, Kim SM, Seong HH, Hwang S, Yang BS, Im GS, Min KS, Kim JH, et al. Cloning and functional characterization of pig CMP-N-acetylneuraminic acid hydroxylase for the synthesis of N-glycolylneuraminic acid as the xenoantigenic determinant in pig–human xenotransplantation. Biochem J. 2010;427(1): 179–88. [DOI] [PubMed] [Google Scholar]
- 24. Adams AB, Kim SC, Martens GR, Ladowski JM, Estrada JL, Reyes LM, Breeden C, Stephenson A, Eckhoff DE, Tector M, Tector AJ. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg. 2018;268(4): 564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martens GR, Reyes LM, Butler JR, Ladowski JM, Estrada JL, Sidner RA, Eckhoff DE, Tector M, Tector AJ. Humoral Reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation. 2017;101(4): e86–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salvaris EJ, Moran CJ, Roussel JC, Fisicaro N, Robson SC, Cowan PJ. Pig endothelial protein C receptor is functionally compatible with the human protein C pathway. Xenotransplantation. 2020;27(2): e12557. [DOI] [PubMed] [Google Scholar]
- 27. Miwa Y, Yamamoto K, Onishi A, Iwamoto M, Yazaki S, Haneda M, Iwasaki K, Liu D, Ogawa H, Nagasaka T, Uchida K, et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010;17(1): 26–37. [DOI] [PubMed] [Google Scholar]
- 28. Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, Clark T, Lewis BG, Hoyt RF, Eckhaus M, Pierson RN, Belli AJ, Wolf E, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iwase H, Ekser B, Hara H, Phelps C, Ayares D, Cooper DK, Ezzelarab MB. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2014;21(1): 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wheeler DG, Joseph ME, Mahamud SD, Aurand WL, Mohler PJ, Pompili VJ, Dwyer KM, Nottle MB, Harrison SJ, d’Apice AJ, Robson SC, et al. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol. 2012;52(5): 958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tena A, Kurtz J, Leonard DA, Dobrinsky JR, Terlouw SL, Mtango N, Verstegen J, Germana S, Mallard C, Arn JS, Sachs DH, et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of Pig-to-human hematopoietic cell transplantation. Am J Transplant. 2014;14(12): 2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tena AA, Sachs DH, Mallard C, Yang YG, Tasaki M, Farkash E, Rosales IA, Colvin RB, Leonard DA, Hawley RJ. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation. 2017;101(2): 316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamada K, Sykes M, Sachs DH. Tolerance in xenotransplantation. Curr Opin Organ Transplant. 2017;22(6): 522–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eisenson DL, Hisadome Y, Yamada K. Progress in xenotransplantation: immunologic barriers, advances in gene editing, and successful tolerance induction strategies in pig-to-primate transplantation. Front Immunol. 2022;13: 899657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamada K, Ariyoshi Y, Pomposelli T, Sekijima M. Co-transplantation of vascularized thymic graft with kidney in pig-to-nonhuman primates for the induction of tolerance across xenogeneic barriers. Methods Mol Biol. 2020;2110:151–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1): 32–34. [DOI] [PubMed] [Google Scholar]
- 37. Ohdan H, Yang YG, Shimizu A, Swenson KG, Sykes M. Mixed chimerism induced without lethal conditioning prevents T cell- and anti-Gal alpha 1,3Gal-mediated graft rejection. J Clin Invest. 1999;104(3): 281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liang F, Wamala I, Scalea J, Tena A, Cormack T, Pratts S, Duran-Struuck R, Elias N, Hertl M, Huang CA, Sachs DH. Increased levels of anti-non-Gal IgG following pig-to-baboon bone marrow transplantation correlate with failure of engraftment. Xenotransplantation. 2013;20(6): 458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schuurman HJ, Cheng J, Lam T. Pathology of xenograft rejection: a commentary. Xenotransplantation. 2003;10(4): 293–99. [DOI] [PubMed] [Google Scholar]
- 40. Zhang JP, Blum MG, Chang AC, Shyr Y, Blair KS, Awwad M, Pierson RN, 3rd. Immunohistologic evaluation of mechanisms mediating hyperacute lung rejection, and the effect of treatment with K76-COOH, FUT-175, and anti-Gal column immunoadsorption. Xenotransplantation. 1999;6(4): 249–61. [DOI] [PubMed] [Google Scholar]
- 41. Meyer zu, Vilsendorf A, Link C, Jörns A, Nagel E, Köhl J. Preconditioning with the prostacyclin analog epoprostenol and cobra venom factor prevents reperfusion injury and hyperacute rejection in discordant liver xenotransplantation. Xenotransplantation. 2001;8(1): 41–47. [DOI] [PubMed] [Google Scholar]
- 42. Holzknecht ZE, Coombes S, Blocher BA, Plummer TB, Bustos M, Lau CL, Davis RD, Platt JL. immune complex formation after xenotransplantation: evidence of Type III as well as Type II immune reactions provide clues to pathophysiology. Am J Pathol. 2001;158(2): 627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tandin A, Miki T, Metes D, Lee Y, Kovscek AM, Subbotin V, Fung JJ, Valdivia LA. Immune complex disease in immunosuppressed rat recipients of hamster liver xenografts. Transplant Proc. 2001;33(1–2): 778–78. [DOI] [PubMed] [Google Scholar]
- 44. Bühler L, Basker M, Alwayn IPJ, Goepfert C, Kitamura H, Kawai T, Gojo S, Kozlowski T, Ierino FL, Awwad M, Sachs DH, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70(9): 1323–31. [DOI] [PubMed] [Google Scholar]
- 45. Cooper DK, Bottino R. Recent advances in understanding xenotransplantation: implications for the clinic. Expert Rev Clin Immunol. 2015;11(12): 1379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fishman JA. Infectious disease risks in xenotransplantation. Am J Transplant. 2018;18(8): 1857–64. [DOI] [PubMed] [Google Scholar]
- 47. Denner J. Reduction of the survival time of pig xenotransplants by porcine cytomegalovirus. Virol J. 2018;15(1): 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueller NJ, Barth RN, Yamamoto S, Kitamura H, Patience C, Yamada K, Cooper DK, Sachs DH, Kaur A, Fishman JA. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J Virol. 2002;76(10): 4734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Niu D, Wei H-J, Lin L, George H, Wang T, Lee I-H, Zhao H-Y, Wang Y, Kan Y, Shrock E, Lesha E, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357(6357): 1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Denner J. Recombinant porcine endogenous retroviruses (PERV-A/C): a new risk for xenotransplantation? Arch Virol. 2008;153(8): 1421–26. [DOI] [PubMed] [Google Scholar]
- 51. Lin Y, Vandeputte M, Waer M. Natural killer cell- and macrophage-mediated rejection of concordant xenografts in the absence of T and B cell responses. J Immunol. 1997;158(12): 5658–67. [PubMed] [Google Scholar]
- 52. Al-Mohanna FA, Collison KS, Allen SP, Stern D, Yacoub MH. Naive neutrophils and xenotransplantation. Lancet. 1996;348(9036): 1246–46. [DOI] [PubMed] [Google Scholar]
- 53. Shimizu A, Yamada K, Robson SC, Sachs DH, Colvin RB. Pathologic characteristics of transplanted kidney xenografts. J Am Soc Nephrol. 2012;23(2): 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uchida Y, Freitas MCS, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation. 2010;89(9): 1050–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kashir J, Ambia AR, Shafqat A, Sajid MR, AlKattan K, Yaqinuddin A. Scientific premise for the involvement of neutrophil extracellular traps (NETs) in vaccine-induced thrombotic thrombocytopenia (VITT). J Leukoc Biol. 2022;111(3): 725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen D, Weber M, Lechler R, Dorling A. NK-cell-dependent acute xenograft rejection in the mouse heart-to-rat model. Xenotransplantation. 2006;13(5): 408–14. [DOI] [PubMed] [Google Scholar]
- 57. Lilienfeld BG, Garcia-Borges C, Crew MD, Seebach JD. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J Immunol. 2006;177(4): 2146–52. [DOI] [PubMed] [Google Scholar]
- 58. Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31: 227–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matter-Reissmann UB, Forte P, Schneider MKJ, Filgueira L, Groscurth P, Seebach JD. Xenogeneic human NK cytotoxicity against porcine endothelial cells is perforin/granzyme B dependent and not inhibited by Bcl-2 overexpression. Xenotransplantation. 2002;9(5): 325–37. [DOI] [PubMed] [Google Scholar]
- 60. Candinas D, Belliveau S, Koyamada N, Miyatake T, Hechenleitner P, Mark W, Bach FH, Hancock WW. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection1,2,3. Transplantation. 1996;62(12): 1920–27. [DOI] [PubMed] [Google Scholar]
- 61. Peterson MD, Jin R, Hyduk S, Duchesneau P, Cybulsky MI, Waddell TK. Monocyte adhesion to xenogeneic endothelium during laminar flow is dependent on α-gal-mediated monocyte activation. J Immunol. 2005;174(12): 8072–81. [DOI] [PubMed] [Google Scholar]
- 62. Fox A, Mountford J, Braakhuis A, Harrison LC. Innate and adaptive immune responses to nonvascular xenografts: evidence that macrophages are direct effectors of xenograft rejection. J Immunol. 2001;166(3): 2133–40. [DOI] [PubMed] [Google Scholar]
- 63. Cadili A, Kneteman N. The role of macrophages in xenograft rejection. Transplant Proc. 2008;40(10): 3289–93. [DOI] [PubMed] [Google Scholar]
- 64. El-Ouaghlidi A, Jahr H, Pfeiffer G, Hering BJ, Brandhorst D, Brandhorst H, Federlin K, Bretzel RG. Cytokine mRNA expression in peripheral blood cells of immunosuppressed human islet transplant recipients. J Mol Med. 1999;77(1): 115–17. [DOI] [PubMed] [Google Scholar]
- 65. Oronsky B, Carter C, Reid T, Brinkhaus F, Knox SJ. Just eat it: a review of CD47 and SIRP-α antagonism. Semin Oncol. 2020;47(2–3): 117–24. [DOI] [PubMed] [Google Scholar]
- 66. Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα immune checkpoint. Immunity. 2020;52(5): 742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang H, Verhalen J, Madariaga ML, Xiang S, Wang S, Lan P, Oldenborg PA, Sykes M, Yang YG. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109(2):836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012;19(1): 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7(7): 519–31. [DOI] [PubMed] [Google Scholar]
- 70. Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Thomas ML, Eckhaus MA, Seavey C, Ayares D, Pierson RN, 3rd, Horvath KA. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12(3): 763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li Q, Shaikh S, Iwase H, Long C, Lee W, Zhang Z, Wang Y, Ayares D, Cooper DKC, Hara H. Carbohydrate antigen expression and anti-pig antibodies in New World capuchin monkeys: relevance to studies of xenotransplantation. Xenotransplantation. 2019;26(3): e12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fischer-Lougheed J, Gregory C, White Z, Shulkin I, Gunthart M, Kearns-Jonker M. Identification of an anti-idiotypic antibody that defines a B-cell subset(s) producing xenoantibodies in primates. Immunology. 2008;123(3): 390–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014;258(1): 241–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin ML, Zhan Y, Nutt SL, Brady J, Wojtasiak M, Brooks AG, Lew AM. NK cells promote peritoneal xenograft rejection through an IFN-γ-dependent mechanism. Xenotransplantation. 2006;13(6): 536–46. [DOI] [PubMed] [Google Scholar]
- 75. Rabbani PS, Rifkin WJ, Kadle RL, Rao N, Diaz-Siso JR, Abdou SA, Rodriguez ED, Ceradini DJ. Noninvasive monitoring of allograft rejection using a novel epidermal sampling technique. Plast Reconstr Surg Glob Open. 2019;7(8): e2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen J, Gao H, Chen L, Wang X, Song Z, Cooper DKC, Qu Z, Cai Z, Mou L. A potential role of TLR2 in xenograft rejection of porcine iliac endothelial cells: an in vitro study. Xenotransplantation. 2019;26(5): e12526. [DOI] [PubMed] [Google Scholar]
- 77. Agbor-Enoh S, Chan JL, Singh A, Tunc I, Gorham S, Zhu J, Pirooznia M, Corcoran PC, Thomas ML, Lewis BGT, Jang MK, et al. Circulating cell-free DNA as a biomarker of tissue injury: assessment in a cardiac xenotransplantation model. J Heart Lung Transplant. 2018;37(8): 967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou M, Hara H, Dai Y, Mou L, Cooper DKC, Wu C, Cai Z. Circulating organ-specific MicroRNAs serve as biomarkers in organ-specific diseases: implications for organ Allo- and Xeno-transplantation. Int J Mol Sci. 2016;17(8): 1232–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao Z, Qi F, Liu T, Fu W. Effect of miR-146a and miR-155 on cardiac xenotransplantation. Exp Ther Med. 2016;12(6): 3972–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Oh JW, Yoon CH, Ryu JS, Kim KP, Kim MK. Proteomics analysis of aqueous humor and rejected graft in pig-to-non-human primate corneal xenotransplantation. Front Immunol. 2022;13: 859929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leventhal JR, Dalmasso AP, Cromwell JW, Platt JL, Manivel CJ, Bolman RM, Matas AJ. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation. 1993;55(4):857–65. [DOI] [PubMed] [Google Scholar]
- 82. Baker LA, Lee KC, Palacios Jimenez C, Alibhai H, Chang YM, Leckie PJ, Mookerjee RP, Davies NA, Andreola F, Jalan R. Circulating microRNAs reveal time course of organ injury in a porcine model of acetaminophen-induced acute liver failure. PLoS ONE. 2015;10(5): e0128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7): 1401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Olivieri F, Prattichizzo F, Giuliani A, Matacchione G, Rippo MR, Sabbatinelli J, Bonafè M. miR-21 and miR-146a: the microRNAs of inflammaging and age-related diseases. Ageing Res Rev. 2021;70:101374–74. [DOI] [PubMed] [Google Scholar]
- 85. Ruggiero T, Trabucchi M, De Santa F, Zupo S, Harfe BD, McManus MT, Rosenfeld MG, Briata P, Gherzi R. LPS induces KH-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. FASEB J. 2009;23(9): 2898–2908. [DOI] [PubMed] [Google Scholar]
- 86. Chung H, Kim HJ, Kim JS, Yoon IH, Min BH, Shin JS, Kim JM, Lee WW, Park CG. CD4+/CD8+ T-cell ratio correlates with the graft fate in pig-to-non-human primate islet xenotransplantation. Xenotransplantation. 2020;27(2): e12562. [DOI] [PMC free article] [PubMed] [Google Scholar]