Abstract
Introduction
Carotid endarterectomy (CEA) for symptomatic stenosis reduces further stroke risk. Post-CEA haematoma increases the risk of complications including stroke. There are few studies considering protocols aimed at reducing post-CEA haematoma rates. Presented are the outcomes of a protocol developed to reduce this surgical complication.
Method
The protocol was implemented in 112 consecutive CEA. It involves stepwise additional measures to ensure haemostasis before wound closure. Attention to bleeding points is followed by light compression for 10 min. Protamine is then given if haemostasis has not been achieved. If after 20 min the problem persists Tranexamic acid is given. Following a further 20 min if haemostasis is not yet achieved a platelet transfusion is undertaken. Haematoma rates, return to theatre for post-operative haematoma and other complications were compared with 100 consecutive pre-protocol introduction CEA cases.
Results
Of 112 CEA patients, 19 received protamine, 8 protamine and tranexamic acid. One case required platelet transfusion. Neck haematoma rate fell from 10 to 3 cases (P = .02, OR: 0.25 [95% CI .07-.94]), of which returned to theatre for haematoma evacuation fell from 6 to 1 case (P = .03, OR: 0.14 [95% CI .02-1.19]). 30 day stroke and death rate reduced from 5% to 1.8% (P = .11, OR: 0.35 [95% CI .07-1.82]).
Conclusion
The stepwise haemostasis intraoperative protocol can reduce post-CEA haematoma rates.
Keywords: carotid endarterectomy, haematoma, protocol
Introduction
Stroke is the third leading cause of death in developed nations and the leading cause of long-term disability. Carotid artery stenosis embolization accounts for 25% of ischaemic strokes. Carotid endarterectomy (CEA), a procedure where the atherosclerotic plaque is surgically removed following a minor or non-disabling stroke, is part of the standard care to reduce the risk of further stroke in selected patients.1 Post operative wound haematoma increases the risk of infection and wound breakdown and is associated with higher rates of stroke, myocardial infarction and death as well as longer stays in hospital.1-6
Reducing haematoma complication rates should improve surgical outcomes. Several studies have investigated the effects of pharmacological and practical interventions aimed at minimising haematoma formation following CEA.2,7-11 However, most studies have considered interventions individually and few have considered several together in a stepwise protocol driven approach.
This study evaluates the introduction of a stepwise haemostasis intra-operative protocol aimed at reducing post-operative haematoma in patients undergoing CEA.
Methods
A prospective cohort outcome study between June 2019 and March 2021 was conducted, following the introduction of the Stepwise Haemostasis Intraoperative Protocol. The outcome data from 112 consecutive operations following the protocol introduction was compared with the 100 consecutive pre-protocol CEA cases undertaken in our unit. Prior to the introduction of the protocol, haemostasis before wound closure did not follow a coordinated stepwise structured management plan.
The protocol is implemented intraoperatively at the end of successful patch closure and before wound closure. It is stepwise and if haemostasis is not achieved with in the time frame intervention the next step is undertaken.
Step 1. It starts with meticulous attention to patch and other bleeding points. Occult venous oozing is sort by performing a Valsalva manoeuvre. This is followed by light wound compression for 10 min.
Step 2. If haemostasis is yet to be achieved 50units of protamine is given to reverse the effects of the 5000 units of unfractionated heparin introduced earlier just before cross clamping. The ACT is taken to confirm there is no underlying clotting disorder, but is not used as a guide to protamine dosage.
Step 3. If after 20 min absolute haemostasis has not been established a gram of Tranexamic acid is given.
Step 4. Following a further 20 min, if haemostasis is still not adequate a platelet transfusion is undertaken. The protocol has been pre-agreed with the haematology department and perioperative discussion with them is unusual. Platelet mapping is undertaken to determine if there is an underlying antiplatelet medication -platelet effect. This is not used as a guide to determine if platelets are transfused.
Data Collected and Analysis
The patients’ demographics and clinical data were recorded on a standardized form used to collect data for entry into the National Vascular Registry of the Vascular Society of Great Britain and Ireland (www.vsqip.org.uk)12 for which there is national ethical approval. This project was considered to be a quality improvement project and auditing of outcome was accepted by the Hospital as not requiring separate ethical committee approval. The clinical variables recorded included onset of symptoms, dates of neurological/vascular assessment, preoperative antiplatelet/anticoagulation regimen, date of CEA, operating surgeon, type of anaesthetic, use of patch/shunt, protamine/tranexamic acid/intraoperative platelet use. Complications recorded included peri-operative stroke/transient ischaemic attack, post-operative haematoma, other complications such as cardiovascular events and death. Surgical outcomes also included length of hospital stay, postoperative antiplatelet regimen and complications such a nerve injury.
The SPSS Version 21 package was used to calculate P-values, Odds ratio (Exp/Control) and 95% confidence interval. A value of P < .05 was considered significant.
Patients
Patient who had recently (within 14 days) suffered a minor or non disabling ischaemic stroke and had an appropriately sided carotid stenosis (>50% NASCET), following discussion in the neurovascular multidisciplinary meeting underwent CEA. The pre-intervention group included 100 cases (identified as part of the units rolling yearly audit) and post intervention group included 112 cases. All patients were on dual antiplatelet therapy as per the University College London (UCL) stroke unit protocol. The UCL stoke unit protocol involved starting patients who have a non disabling ischaemic stroke on a loading dose of 300 mg of clopidogrel followed by aspirin 75 mg and clopidogrel 75 mg for 30 days. After 30 days patients were dropped down to monotherapy (usually clopidogrel 75 mg). All symptomatic carotid surgery was undertaken during this first 30 days and so patients were taking dual antiplatelet therapy. They were not given while the patient was nil by mouth before surgery, but were restarted the night after surgery.
Patients who underwent symptomatic carotid endarterectomy were felt to have suffered a stroke due to embolization from their carotid plaque. Even if in atrial fibrillation they were not commenced on anticoagulants until after surgery. If they presented to the stroke unit taking formal anticoagulation this was stopped prior to surgery.
Peri-Operative Team
All CEA was performed by 1 of 3 consultant vascular surgeons within 14 days of symptoms onset. A small group of anaesthetic consultants routinely performed these procedures in conjunction with the surgical team. They were re-appraised of the stepwise haemostasis intraoperative protocol prior to starting the procedure as part of the WHO checklist for safer surgery.
Surgery Procedure
The majority of procedures were performed under general anaesthetic with individual surgeon and patient preference guiding the decision (Table 2). Routine invasive blood pressure and cardiac monitoring ensured control of blood pressure fluxes during the procedure with the aim to maintain the perioperative blood pressure at the pre-operative level. Following surgical isolation of the internal carotid artery, 5000iU of intravenous unfractionated heparin were administered prior to carotid artery clamping. A shunt was used for all general anaesthetic cases and for local anaesthetic cases when indicated. After successful plaque endarterectomy, vessel closure involved a bovine pericardial patch being sutured in place.
Table 2.
The Degree of Implementation of the Stepwise Haemostasis Intraoperative Protocol required on completion of 112 CEA.
| Step time point | Haemostatic steps | Number of cases reaching this step |
|---|---|---|
| Patch completion | Ensure patch haemostasis and meticulous attention to other bleeding points | 84 (75%) |
| After 10 min of light compression and haemostasis not achieved | Protamine given | 19 (17%) |
| 20 min post protamine and haemostasis not achieved | 1 g Tranexamic acid given | 8 (7%) |
| 20 min post tranexamic acid and haemostasis not achieved | Platelet transfusion given | 1 (1%) |
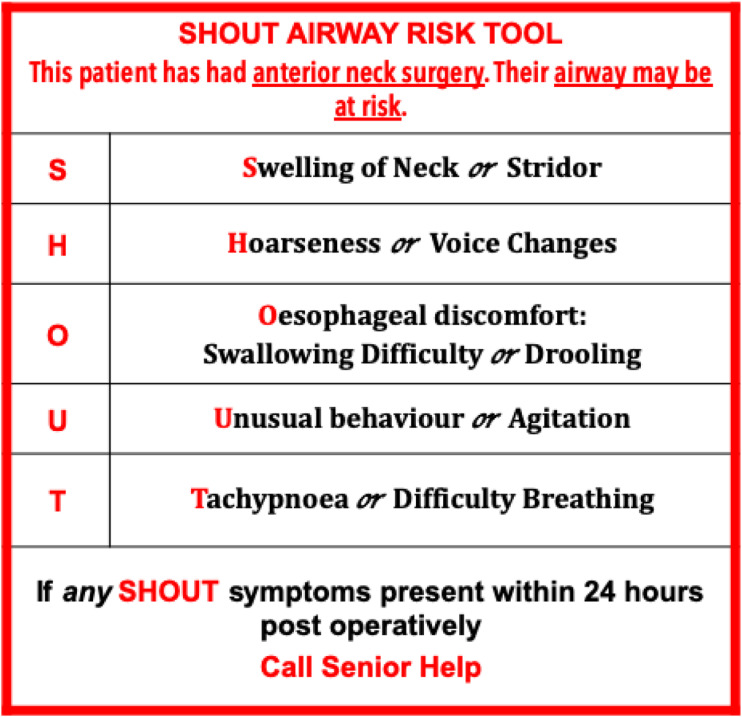
The stepwise haemostasis intraoperative protocol was then implemented. Haemostatic aides such as Surgicel Fibrillar (Johnson and Johnson) was also used routinely to facilitate haemostasis. A drain was placed during wound closure in all cases. Patients were extubated immediately at the end of the procedure and monitored in a high dependency unit. Blood pressure was tightly controlled during the postoperative period with intravenous agent and patients monitored carefully for complications in particular for potential neck haematoma using the “SHOUT” protocol (Figure 1).13
Figure 1.
The SHOUT Protocol used to alert the ward staff to the possibility of a post-carotid endarterectomy neck haematoma.13
Results
Patient demographics (Table 1) were similar between the 2 groups apart from there being more cases undertaken under local anaesthetic in the pre-protocol group. The stepwise haemostasis intraoperative protocol needed to be implemented in 25% of patients (Table 2). 17% required protamine alone, 7% required protamine and tranexamic acid and 1% received a platelet transfusion. Since implementation of the stepwise haemostasis pathway, there was a reduction in haematoma rates, from 10% in pre-intervention group to 2.6% in post intervention group (P = .02, OR: 0.25 [95% CI .07-.94]) (Table 3). Return to theatre rates for haematoma improved, from 6% in pre-intervention group to 0.9% in post-intervention group (P = .03, OR: 0.14 [95% CI .02-1.19]) (Table 3). Initial stroke and TIA rate fell from 9% to 3% (P = .03, OR: 0.28 [95% CI .07-1.06]), and the 30 day stroke and death rate dropped from 5% in pre-intervention group to 1.8% in post intervention group (Table 3) which was not significant (P = .11, OR: 0.35 [95% CI .07-1.82]). There was no improvement in the post operative length of stay following the introduction of the protocol (33% v 41% P = .15, OR: 1.34 [95% CI .77-2.38]).
Table 1.
Patient Demographics.
| Pre-protocol (Mar 2017-Jun 2019) | Post-protocol (Jun 2019-Mar 2021) | |
|---|---|---|
| Patient details | ||
| Number | 100 | 112 |
| Age (median, range) | 73 years (49-93) | 78 y (44-85) |
| Sex (female: Male) | 70:30 | 70:42 |
| Median event to surgery time | 11 days | 10 days |
| Procedure | ||
| Anaesthetic GA/LA | 73:27 | 112:0 |
| Closure with bovine pericardial patch | 100% | 100% |
Table 3.
Surgical Outcomes Following Introduction of the Stepwise Haemostasis Pathway.
| Pre-protocol | Post-protocol | P value, OR [95% CI) | |
|---|---|---|---|
| Haematoma rate | 10 (10%) | 3 (2.6%) | P = .02, OR: 0.25 (95% CI .07-.94) |
| Return to theatre | 6 (6%) | 1 (.9%) | P = .03, OR: 0.14 (95% CI .02-1.19) |
| Perioperative TIA/stroke rate | 9 (9%) | 3 (2.7%) | P = .03, OR: 0.28 (95% CI .07-1.06) |
| 30 d stroke and death rate | 5 (5%) | 2 (1.8%) | P = .11, OR: 0.35 (95% CI .07-1.82) |
| Significant cranial nerve damage (30 d) | 1 (1%) | 0 | P = .29 |
| Acute coronary syndrome | 2 (2%) | 1 (1%) | P = .25, OR: 0.44 (95% CI .04-4.94) |
| Post operation length of stay (>2 d) | 33 (33%) | 46 (41%) | P = .15, OR: 1.34 (95% CI .77-2.38) |
Discussion
The reported prevalence of postoperative CEA haematoma varies from 3.4% to 12%.2-6 The 2019 UK National vascular registry reported a 2.2% return to theatre rates for post-operative CEA haematoma.12 Our pre-protocol post CEA haematoma rate (10%) and associated return to theatre rate (6%) were in the upper range of these values. Following the introduction of the step-wise intra-operative haemostasis protocol there was a reduced haematoma rate (2.6%) and a reduced return to theatre rate (.9%). Initial stroke and TIA rate fell, but the 30 day stroke and mortality rate, as well as the length of stay rate was not altered.
Post operative CEA wound haematoma increases local complications such as wound infection, wound breakdown, nerve palsies, fistulae, respiratory obstructions and oesophageal obstruction. Post operative wound haematomas also increase the incidence of stroke, cardiac ischaemic events and length of hospital stay.2-6
Post CEA neck haematoma rates are exacerbated by operating in complex fields (such as high carotid bifurcations, irradiated or redo fields), early post-operative straining and coughing6; postoperative blood pressure fluxes9 and antiplatelet treatment.3,6-8,11 In our unit these have been mitigated for by involving a limited number of experience surgeons and employing a deep extubation from general anaesthesia technique to reduce coughing and straining. Post operatively all CEA patients attend a level 2 high dependency unit for intra-arterial blood pressure control. This is kept within systolic parameters of +/− 20 mmHg of pre-operative levels with intravenous agents. Dual antiplatelet therapy is recommended by the stroke physicians for the first 30 days after a stroke (as per the POINT and CHANCE trials).14,15 This is maintained prior to and on the first night following surgery. Attempts to reduce the post operative haematoma rates therefore focussed on developing a structured step-wise intra-operative haemostasis protocol.
Several studies have aimed to minimise haematoma formation following CEA by investigating the effects of pharmacological and practical interventions. Steps that have been shown to reduce post-CEA haematoma rate and not increase stroke rates included direct pressure,2-4 protamine, a heparin reversal agent,7,8 tranexamic acid, an antifibrinolytic agent that inhibits clot lysis10 use, as well as platelet transfusions.11 The application of these agents has previously been uncoordinated and there are limited studies following a step wise protocol driven approach to achieve haemostasis. In this study we have demonstrated that following a stepwise protocol reduces the haematoma rate (both observed and those requiring a return to theatre).
This study has limitations. Although the data was prospectively collected, the pre-protocol data has been retrospectively analysed. The small size of the study provides limitations.16 The incidence of post-CEA haematoma is not easy to ascertain reliably. Most haematomas which were stable and not requiring intervention are subject to clinician reporting bias. The return to theatre rate for bleeding is, however a definable endpoint. Finally, some studies do show greater intraoperative and postoperative haemodynamic stability in regional anaesthesia use.17-19 In this study the anaesthesia implemented was at the team’s discretion.
Conclusion
Our Stepwise Haemostasis Intraoperative Protocol appears to reduce post-CEA haematoma rates from 10% to 2.6%. Return to theatre rates for bleeding also dropped from 6% to .9%.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Daryll Baker https://orcid.org/0000-0002-0098-405X
References
- 1.Saha SP, Saha S, Vyas KS. Carotid endarterectomy: Current concepts and practice patterns. Int J Angiol. 2015;24(3):223-235. doi: 10.1055/s-0035-1558645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saghir R, Humm G, Rix T. Haematomas after carotid endarterectomy can be reduced by direct pressure to the neck postoperatively. Ann R Coll Surg Engl. 2018;100(7): 580-583. doi: 10.1308/rcsann.2018.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baracchini C, Gruppo M, Mazzalai F, Lorenzetti R, Meneghetti G, Ballotta E. Predictors of neck bleeding after eversion carotid endarterectomy. J Vasc Surg. 2011;54(3):699-705. doi: 10.1016/j.jvs.2011.03.262. [DOI] [PubMed] [Google Scholar]
- 4.Doig D, Turner EL, Dobson J, Featherstone RL, de Borst GJ, Brown MM,et al. Incidence, Impact, and predictors of cranial nerve palsy and haematoma following carotid endarterectomy in the International Carotid Stenting Study. Eur J Vasc Endovasc Surg. 2014;48(5):498-504. doi: 10.1016/j.ejvs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton D, Bryson GL, Sullivan PJ. Risk factors for post carotid endarterectomy hematoma formation. Can J Anesth. 1999;46(7):635-640. doi: 10.1007/BF03013950. [DOI] [PubMed] [Google Scholar]
- 6.Beard JD, Mountney J, Wilkinson JM, Payne A, Dicks J, Mitton D. Prevention of postoperative wound haematomas and hyperperfusion following carotid endarterectomy. Eur J Vasc Endovasc Surg. 2001;21:490-493. doi: 10.1053/ejvs.2001.1366. [DOI] [PubMed] [Google Scholar]
- 7.Newhall KA, Saunders EC, Larson RL, Stone DH, Goodney PP. Use of protamine for anticoagulation during carotid endarterectomy. A meta-analysis. JAMA Surg. 2016;151(3):247-255. doi: 10.1001/jamasurg.2015.359. [DOI] [PubMed] [Google Scholar]
- 8.Kakisis JD, Antonopoulos CN, Moulakakis KG, Schneider F, Geroulakos G, Ricco JB. Protamine reduces bleeding complications without increasing the risk of stroke after carotid endarterectomy: A meta-analysis. Eur J Vasc Endovasc Surg. 2016;52(3):296-307. doi: 10.1016/j.ejvs.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Morales Gisbert SM, Almonacil VAS, García JMZ, Gascó BG, Palonés FJG, Monzón EO. Predictors of cervical bleeding after carotid endarterectomy. Ann Vasc Surg. 2014;28(2):366-374. doi: 10.1016/j.avsg.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Murao S, Nakata H, Roberts I, Yamakawa K. Effect of tranexamic acid on thrombotic events and seizures in bleeding patients: A systematic review and meta-analysis. Crit Care. 2021;25:380-391. doi: 10.1186/s13054-021-03799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiele T, Sumnig A, Hron G, Muller C, Althaus K, Schroeder HW,et al. Platelet transfusion for reversal of dual antiplatelet therapy in patients requiring urgent surgery: A pilot study. International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2012;10(5):968-971. doi: 10.1111/j.1538-7836.2012.04699.x. [DOI] [PubMed] [Google Scholar]
- 12.National Vascular Registry . 2020. https://www.vsqip.org.uk/content/uploads/2020/11/NVR-2020-Annual-Report.pdfp75 Accessed January 12 2022.
- 13.Kyle B, Scott H, Kaur N, Bouras I. SHOUT airway risk after anterior neck surgery: addressing an educational deficit in a large multicentre teaching hospital. BRJ Anaesth. 2018;121(1): e7-e8. [Google Scholar]
- 14.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ,et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225. doi: 10.1056/NEJMoa1800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang DY, Eisert WG. Chance trial. Early short-term dual antiplatelet treatment for stroke prevention. Stroke. 2013;44:3623-3624. doi: 10.1161/STROKEAHA.113.003380. [DOI] [PubMed] [Google Scholar]
- 16.Hackshaw A. Small studies: strengths and limitations. Eur Respir J. 2008;32(5):1141-1143. doi: 10.1183/09031936.00136408. [DOI] [PubMed] [Google Scholar]
- 17.Youssef F, Jenkins MP, Dawson KJ, Berger L, Myint F, Hamilton G. The value of suction wound drain after carotid and femoral artery surgery: A randomised trial using duplex assessment of the volume of post-operative haematoma. Eur J Vasc Endovasc Surg. 2005;29:162-166. doi: 10.1016/j.ejvs.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Shakespeare WA, Lanier WL, Perkins WJ, Pasternak JJ. Airway management in patients who develop neck hematomas after carotid endarterectomy. Anesth Analg. 2010;110(2):588-593. doi: 10.1213/ANE.0b013e3181c85128. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan MK, Baker WH, Littooy FN, Mansour MA, Kang SS. Timing of post-carotid complications: A guide to safe discharge planning. J Vasc Surg. 2001;34(1):13-16. doi: 10.1067/mva.2001.116106. [DOI] [PubMed] [Google Scholar]