Abstract
The digital health revolution is transforming the landscape of medicine through innovations in sensor data, software, and wireless communication tools. As one of the most prevalent chronic diseases in the United States, diabetes is particularly impactful as a model disease for which to apply innovation. As with any other newly developed technologies, there are three key questions to consider: 1) How can the technology benefit people with diabetes?, 2) What barriers must be overcome to further advance the technology?, and 3) How will the technology be applied in the future?. In this article, we highlight six areas of innovation that have the potential to reduce the burden of diabetes for individuals living with the condition and their families as well as provide measurable benefits for all stakeholders involved in diabetes care. The six technologies which have the potential to transform diabetes care are (i) telehealth, (ii) incorporation of diabetes digital data into the electronic health record, (iii) qualitative hypoglycemia alarms, (iv) artificial intelligence, (v) cybersecurity of diabetes devices, and (vi) diabetes registries. To be successful, a new digital health technology must be accessible and affordable. Furthermore, the people and communities that would most likely benefit from the technology must be willing to use the innovation in their management of diabetes.
Keywords: artificial intelligence, cybersecurity, diabetes, digital health, electronic health record, hypoglycemia alarms, registries, telehealth
Introduction
Diabetes is a chronic medical condition that affects a significant portion of the United States and has tremendous costs for patients and for the healthcare system. In 2018, there were approximately 34.2 million people in the United States (10.5% of the population) living with diabetes. 1 In 2017, diabetes was estimated to cost the United States approximately $327 billion dollars annually, which increased by 25% between 2012 and 2017. 2 The adoption of digital technologies in healthcare compared to other industries has been challenging, given the need for coordination between many stakeholders including patients, clinicians, engineers, ethicists, payers, and regulatory agencies.3,4 With the abundance of data available to clinicians and researchers, and new technologies being developed, digital health is emerging as a possible way to improve value in healthcare. Digital health has the power to revolutionize diabetes management. 5 The immense amount of diabetes data generated from devices such as continuous glucose monitors (CGMs), insulin pumps, automated insulin delivery systems, and other wearable technologies is overwhelming to process without digital health computing assistance. 6 In this article, we will describe six digital health technologies that will transform diabetes: telehealth, incorporation of diabetes digital data into the electronic health record (EHR), qualitative hypoglycemia alarms, artificial intelligence, cybersecurity of diabetes devices, and diabetes registries.
Telehealth
Benefits of Telehealth
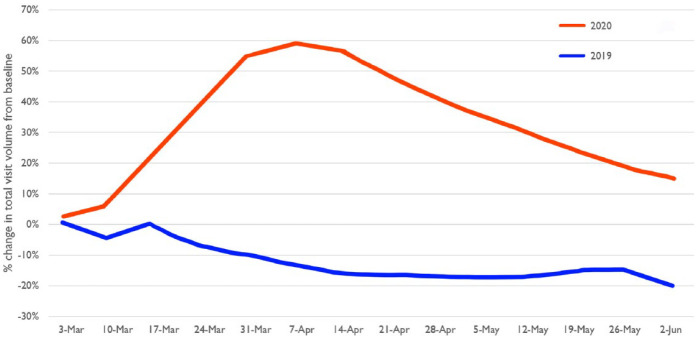
Telehealth is a rapidly progressing field in healthcare delivery, with profound implications on how patients will receive medical care in the future. 7 The US Department of Health and Human Services defines telehealth as the “use of electronic information and telecommunications technologies to support and promote long-distance clinical health care, patient and professional health-related education, and public health and health administration.” 8 Telemedicine refers to services delivered only by physicians whereas telehealth refers to health care services involving all health care professions. 9 In the setting of the COVID-19 pandemic, the use of telehealth has grown substantially (Figure 1), 10 which can be leveraged in management of chronic medical conditions, such as diabetes.
Figure 1.
Percentage change in the total volume of virtual visits from baseline weeks in 2019 and 2020 using the Doctor on Demand telehealth platform. The baseline weeks, which are not shown, were February 25 to March 3, 2019, and February 24 to March 1, 2020. Reproduced from Uscher-Pines L et al 10 under the Creative Commons Attribution License.
Diabetes is a condition that is well-suited to benefit from telehealth, given the need for patient self-management and the use of home medical devices, which both generate and capture data. 11 There is currently a significant shortage of both adult and pediatric endocrinologists. This shortage is expected to worsen in the future. 12 Telehealth may increase access to care for people with diabetes (PwD) who are not otherwise able to see a specialist, particularly in more rural settings.13,14 The use of telehealth has been shown to be statistically significant in lowering hemoglobin A1c in patients with type 1 diabetes (T1D) and type 2 diabetes (T2D) across multiple meta-analyses, although no convincing effect on quality of life, mortality, or hypoglycemia incidence has been shown.15,16 More interactive formats such as web portals and text messaging appear to be effective in lowering hemoglobin A1c. 15
Barriers to Advancing Telehealth
Philosophical and practical barriers limit adoption of telehealth for diabetes. Philosophical barriers include a desire for evidence of benefit prior to implementation and clinical judgment on when it is appropriate to have certain discussions with patients in person rather than through a telehealth modality. Practical barriers include hardware and software compatibilities, integration of telehealth workflow into clinics and EHRs, billing restrictions, and documentation practices. 11 Given the recent increase in the availability of digital health data and telemedicine delivery systems, there is also an increased risk of data breaches. The novel security protections required to reduce the impact of data breaches poses another challenge to the advancement of telehealth. 17
Future Applications of Telehealth
Telehealth offerings will become more widely utilized, and a value-based system will likely determine payment coverage, with focus on improving outcomes and access while limiting cost. 18 Telehealth can implement machine learning (ML), decision support tools, and digital coaching to improve glycemic control. 19 Telehealth will eventually be able to integrate sensors into the EHR. 17 New formats, such as direct-to-consumer telehealth, may increase access and convenience with the potential to decrease costs, which can be done in a synchronous model such as through telephone or videoconferencing, or an asynchronous model, such as through secure messaging and may include audio files or photos.20,21
Incorporation of Diabetes Digital Data into the Electronic Health Record
Benefits of Incorporating Digital Data into the Electronic Health Record
The mandate to incorporate diabetes digital data into all EHRs has reached a major regulatory milestone. The Department of Health and Human Services Office of the National Coordinator (ONC) for Health Information Technology released the 21st Century Cures Act “Final Rule” implementing key provisions to advance interoperability, and seamless access and exchange of electronic health information.22,23 The key technology that this ruling emphasized was the adoption of a modern industry standard application programming interface (API). An API is an interface between unrelated software programs, serving as a bridge and allowing them to communicate. APIs make it simpler and more efficient to develop digital health applications and connect them with EHRs to promote data sharing. This summary covers benefits, barriers, and future opportunities.
The number of diabetes mobile applications has been growing rapidly, with over 100 000 health-related applications on the iOS and Android platforms. 24 However, the lack of a modern API standard across EHRs has limited health information exchange (HIE) between health systems, EHRs, mobile applications, and sensors. The ONC “Final Rule” ruling formalized Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR), a modern, web-based API that is developer-friendly, as the standard. Table 1 presents new developments in HIE for digital diabetes interoperability with EHRs.
Table 1.
New Developments in Health Information Exchange (HIE) for Digital Diabetes Interoperability with EHRs.
|
• New Regulations: 21st Century Cures Act
“Final Rule,” is a set of new regulations to advance
interoperability and support the access, exchange, and use
of electronic health information through standardizing the
application program interface (API) and enacting
“Information blocking regulations.” • Modern API standard: Health Level 7 (HL7) Fast Healthcare Interoperability Resources (FHIR) is a web-based standard which will allow programmers to develop applications with the following benefits to consumers and clinicians: ° Easier access to health information from different EHR platforms. ° More readily available clinical decision support due to more individualized computable data. ° Faster provider-to-provider exchange of information. • Growth of “App Economy” by major private and government companies using FHIR-enabled standards: Apple HealthKit’s clinical record, CMS Blue Button 2.0, and Cerner App Gallery are a few examples. |
A recent Pew Charitable Trust survey highlighted three main use cases for APIs 25 :
Patient-centered access to records: On-demand access to discrete information within the medical records such as clinical notes, test results, and medications.
Clinical decision support (CDS): Individualized, computable data can be algorithmically analyzed through CDS allowing for actionable visualizations.
Provider-to-provider exchange of information: APIs allow diabetes data to be securely and easily shared between different EHRs and health systems, providing real-time aggregation of health data for comprehensive assessments by various members of a diabetes care team.
Barriers to Incorporating Digital Data into the Electronic Health Record
The “Final Rule” by the ONC addresses two major barriers limiting the wide adoption of FHIR-API:
Codifying a non-proprietary API Standard: The ONC support of HL7-FHIR paved the way for industry adoption and further development of security applications and certification kits. 23
Information blocking: Evaluation of EHRs has demonstrated that information blocking is common. In one study, 55% of HIEs reported that EHR vendors at least sometimes engage in information blocking. 26 The ONC “Final Rule” has specific regulation against information blocking for providers, EHR vendors, and health care systems. 27
Future Applications of Incorporating Digital Data into the Electronic Health Record
API standardization will allow for “one-stop shop” markets with turnkey installation that will foster collections of aggregated patient and clinician information streams. Access to readily available large and complex databases residing in an EHR environment will drive innovation in health applications for diabetes patients. Big tech companies have developed FHIR-based “client” apps. For example, Apple developed the Apple HealthKit store. Similarly, the Centers for Medicare & Medicaid Services (CMS) created Blue Button 2.0. We expect that small boutique software companies will develop firmware solutions for incorporating niche datasets into the EHR by directly connecting mobile apps with the EHR and bypassing the need for hospitals to purchase potentially expensive ongoing data-bridging services. We can anticipate rapid growth in the coming year of a robust FHIR-based “app economy” in the health industry.
Qualitative Hypoglycemia Alarms
Benefits of Qualitative Hypoglycemia Alarms
Introduction
Hypoglycemia is a dangerous complication of many diabetes medications. FDA-cleared quantitative devices, such as CGMs, for alarming hypoglycemia are not always sufficiently accurate. Their identification of glycemic states tend to emphasize either sensitivity with poor specificity or vice versa. Qualitative detection of a physiologic response to hypoglycemia is an alternate method for identifying signals indicating the body’s response to hypoglycemia. Since the threshold, below which hypoglycemic symptoms occur, varies among PwD, an individualized personal approach could be based on an individual’s physiology rather than a number, whose significance might vary between people or might not even be analytically accurate. Artificial intelligence (AI) can assemble multiple physiologic data streams from sensors that measure various physiologic responses to hypoglycemia. Any single stream might contribute little to an overall detection of hypoglycemia, but as an aggregated measurement, all this data can be diagnostic.
Specific Products
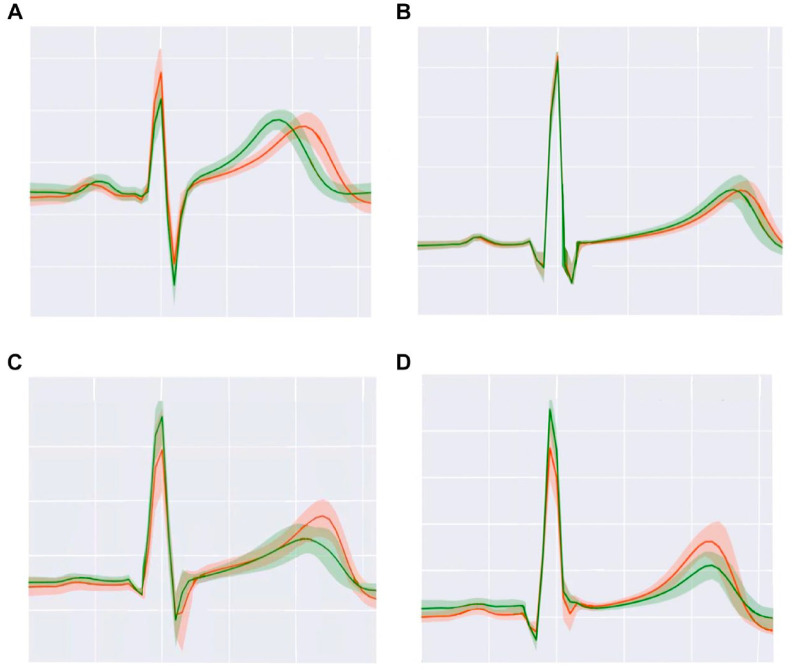
Currently, several classes of products are being developed to serve as qualitative hypoglycemia alarms. Physiologic responses to hypoglycemia that can be measured continuously by wearable sensors include alterations in heart rate, electrocardiogram (EKG) patterns, pulse-wave patterns, electroencephalogram (EEG) patterns, galvanic skin response, skin temperature, and breath volatile organic compounds (VOCs). Commercial galvanic skin response-based devices, such as the Diabalert© (Erpic, Montpellier, France) and Sleep Sentry© (Diabetes Sentry, Fort Worth, TX), claim to detect skin temperature changes, perspiration, and electrodermal activity to diagnose hypoglycemia, but unfortunately, little clinical data supports these claims. Noninvasive EKG wearables, such as the VitalPatch© (VitalConnect, San Jose, CA) not only can quantify changes in heart rate variability when a subject patient with T1D goes from normoglycemia to hypoglycemia, but they can also identify changes in the QT interval (Figure 2A and B) 28 or the amplitude of T waves (Figure 2C and D). 28 EEG devices, such as the minimally invasive 24/7 EEG SubQ© (UNEEG medical, Lynge, Denmark) behind-the-ear implant, can measure brain activity and hypoglycemia-induced decreased cognitive function and alert a wearer if patterns of inadequate glucose delivery to the brain are detected. In T1D, hypoglycemia-related differences in the amplitude spectra of EEG signals during normoglycemia and hypoglycemia can be seen, as illustrated in Figure 3. 29 Thus, EKG and EEG are potentially promising methods for hypoglycemia detection in future qualitative hypoglycemia wearable alarms because they appear to identify altered signals during hypoglycemia, in both hypoglycemia-aware as well as hypoglycemia-unaware PwD. Photoplethysmography, an optical technique to measure the pulse wave pressure in the microvascular bed with each heartbeat, can be affected by sympathetic nervous system tone. This method has been used to qualitatively detect physiological responses to hypoglycaemia. 30 Finally, breathing into a wearable VOC detection device, such as the nano gas AerBetic© (AerBetic, North Birmingham, AL) VOC wristband sensor, can alert a user if one of seven VOCs related to hypoglycemia is detected. 31 Each qualitative detection method can be combined into an aggregate multi-sensor platform that assembles multiple data streams to more accurately predict glycemia.
Figure 2.
Illustration of interindividual differences in EKGs during hypoglycemia (orange lines) compared to normoglycemia (green lines) in 4 subjects with T1D. (A) Subject 1. (B) Subject 2. (C) Subject 3. (D) Subject 4. During hypoglycemia, subjects 1 and 2 have a longer QT interval and subjects 3 and 4 have a higher T wave. Adapted from source: © 2021 Diouri et al 28 Diabetes/Metabolism Research and Reviews published by John Wiley & Sons Ltd. under a CCBY-NC-ND license.
Figure 3.
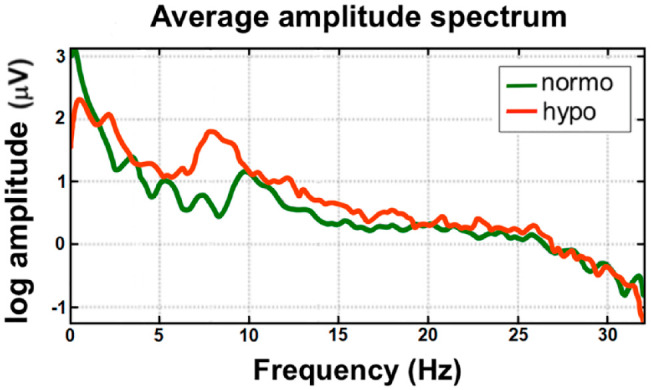
The amplitude spectra of EEG signals during normoglycemia (green line) and hypoglycemia (orange line). Adapted from source: Remvig et al. 29
Barriers to Advancing Qualitative Hypoglycemia Alarms
Hypoglycemia unawareness
Impaired awareness of hypoglycemia is defined as a diminished ability to perceive the onset of acute hypoglycemia. 32 Many of the qualitative methods for detecting hypoglycemia measure a metric related to a sympathetic autonomic response and without it, they might be insensitive. 33 On the other hand, there might still be some detectable subtle changes in various organ systems even during apparent hypoglycemia unawareness.
Lack of accuracy
Calibration algorithms are required to convert sensor output to a blood glucose value. 28 Improvements in speed and accuracy for the detection of hypoglycemia values are still needed because the algorithms are not easily implemented in real-time with wearables. Currently available noninvasive qualitative devices do not attain the same accuracy as their quantitative sensor counterparts. 28
Future Applications of Qualitative Hypoglycemia Alarms
The signal for any one qualitative method may be small. AI can combine data streams which, individually, are weakly correlated but in combination might be strongly correlated. This is speculated because the data streams related to hypoglycemia determined from wearables are only now being identified. We can expect new wearable devices to accurately integrate qualitative hypoglycemia detection features in future wearable devices.34,35
Artificial Intelligence
Whether we know it or not, many aspects of our daily lives already depend on AI. Defined as the capability of a machine to imitate intelligent human behavior, AI has enabled many of the conveniences we enjoy today. 36 Companies like Facebook and Netflix utilize AI to personalize our news feeds and video recommendations. AI is what checks our spelling when we write messages and powers our interactions with digital voice assistants like Siri, Google Assistant, and Amazon Alexa.
Benefits of Artificial Intelligence
AI-based innovations will become a critical tool for medicine and healthcare. A widely used form of AI is ML. This form of data analysis refers to the development of algorithms that can learn over time to recognize patterns and make predictions without being explicitly programmed.37,38
ML is particularly suitable for clinical applications to diabetes, where it will increasingly be used to predict the risk of developing diabetes, optimize treatments for PwD, and diagnose diabetic complications in their early, treatable stages. ML algorithms have already been used to predict a person’s risk of developing diabetes by analyzing lifestyle activities, physiologic sensor data, and genomic data. 38 ML algorithms have also been developed to assist PwD in their self-management of this disease. ML can be used to individualize glucose targets and insulin-sensitivity calculations for automated insulin delivery systems. ML can predict changes in blood glucose following various glycemic perturbation events, adjust insulin delivery accordingly, and learn from these adjustments to understand which treatments are most effective for their unique users. 39
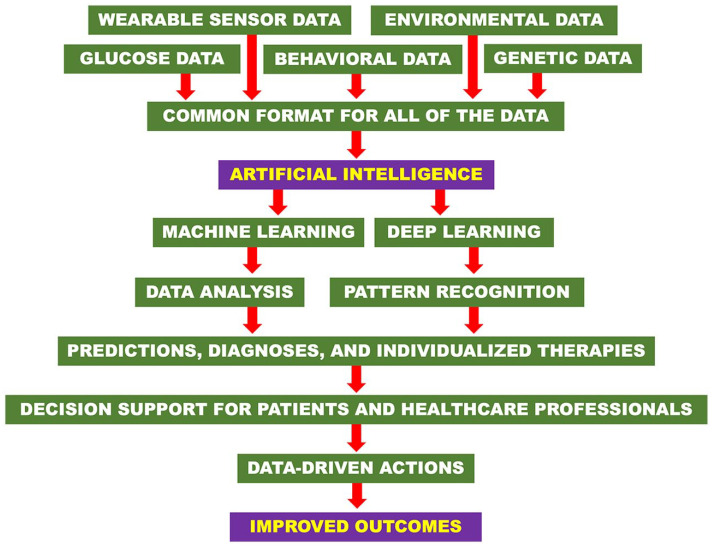
Deep learning (DL) is a subset of ML, which compared to classical ML, relies on more complex algorithms called artificial neural networks (ANNs) to imitate the way a human brain processes data and recognizes patterns. DL is more powerful than ML and has been adapted to diagnose long-term, resource-intensive complications of diabetes, such as diabetic retinopathy (DR) and diabetic macular edema (DME). The ANNs used in DL methods can screen images provided by PwD for abnormal retinal morphology (eg, hemorrhages, microaneurysms, exudates, and neovascularization) and diagnose DR and DME. 39 ANNs self-learn from inputted data without the need for specific decision criteria. Therefore, current limitations in our understanding of DR-related retinal abnormalities will not restrict the ability of DL algorithms to predict outcomes and suggest interventions before the opthalmic disorders advance to vision loss. 40 By detecting patterns that may indicate development of DR or DME, DL-based retinal screening can expedite referrals for specialized opthalmic care and improve patient quality-of-life through timely intervention of DR, which is the leading cause of blindness in PwD. 40 The steps in applying AI in the form of ML and DL to achieve data-driven actions and improved outcomes are illustrated in Figure 4.
Figure 4.
Steps to clinical application of AI-based technologies and how they result in data-driven actions and improved outcomes.
Barriers to Advancing Artificial Intelligence
Despite the potential for AI-based technologies to revolutionize diabetes care, three main barriers must be overcome. First, the high technical ability and clinical expertise required to develop and periodically refine AI used in diabetes care may discourage developers from including the advanced features that may be most useful for physicians and patients alike. Second, developers will have to ensure that AI technologies are affordable and equally accessible before the technology becomes widespread among PwD. 39 Third, the data required for ML-algorithm function is not easily accessible. Many medical devices and mobile apps today record different measurements (eg, blood glucose, and blood ketones) required for ML-algorithms to function, however, they each typically record their measurements in proprietary formats that cannot be used by AI. The lack of a common format or data standards for each parameter makes it difficult to incorporate this information into the EHR, where it can then be accessed by AI. 41 Much information is lost this way, making it difficult to build up a substantial database where ML-algorithms can train and refine their prediction accuracy and decision support. 41 This siloing effect may jeopardize the potential development of ML-algorithms.
Future Applications of Artificial Intelligence
As current AI-based technologies become more widely used and the infrastructure around AI develops along with it, the wealth of input data available to ML algorithms will further develop and improve both the accuracy and range of predictions and decision support they can provide. While the current use of this technology for analysis of images is limited to diabetic foot ulcers, DR, and DME, ML algorithms will be able to learn from expanded data sets to diagnose many of the other complications of diabetes, such as muscle wasting, skin rashes, and other physical changes from images and databanks. As more and more wearable sensors are deployed and better ML algorithms are developed with improved computing power, the wealth of information about each person will be increasingly understood through data analysis by AI. In the close future, AI-based technologies will revolutionize diabetes care. AI could potentially be used to make every diagnosis of, every prognostic prediction about, and every treatment decision for diabetes.
Cybersecurity of Diabetes Devices
Benefits of Cybersecurity of Diabetes Devices
After COVID-19, the next pandemic the world will face is cybercrime, according to many cybersecurity experts. 42 Monitoring and treatment of diabetes often involves a variety of connected devices such as blood glucose monitors, CGMs, insulin pumps, and other wearable sensors. 43 These devices contain personal patient information that may be transmitted to other devices and execute commands related to medication dosage. Any security threat to these devices could therefore result in device malfunction (such as lack of data integrity or data availability or failure to accurately transmit commands) resulting in potential health risks to the patient.
At various security conferences, researchers have been able to hack nearby insulin pumps, with and even without knowledge of the pump’s ID number, to administer large doses of insulin. 43 Lax cybersecurity of diabetes devices may also be exploited by patients themselves through a method known as do-it-yourself (DIY) hacking in an effort to access additional data stored in their device or visualize data in a different format than the manufacturer provides. 43 Whether the benefits of hacking into one’s own device to expand its capabilities outweigh the risks of potentially degrading its function is a topic of debate. The development and application of cybersecurity standards specific for diabetes devices will foil many hacking attempts and improve safety.
Barriers to Advancing Cybersecurity of Diabetes Devices
Until recently, there has not been a single standard for cybersecurity of wirelessly connected diabetes devices. This may be in part because there has not been much demand from consumers for security measures. Additionally, strengthening cybersecurity may increase the manufacturing costs of products, which is especially pertinent because of the relatively low cost of many diabetes devices. Even if developers want to incorporate secure features into their products after they are already on the market, they face technical challenges of implementing these features. Many of the current diabetes devices are not equipped for firmware or software patches, which can remotely upgrade devices through downloaded updates. Additionally, many diabetes devices function best when they are (as the expression states) “built-in and not bolted on.” This means that cybersecurity measures need to be planned together with other design features when a device is initially developed and not attached onto devices as updates after they have already been deployed.
Future Applications of Cybersecurity of Diabetes Devices
To fill the gap in cybersecurity standards for diabetes devices, Diabetes Technology Society (DTS) developed the DTS Cybersecurity Standard for Connected Diabetes Devices (DTSec) and the DTS Mobile Platform Controlling a Diabetes Device Security and Safety Standard (DTMoSt).44,45 These two standards, whose logos are shown in Figure 5,44,45 together aim to establish performance and assurance recommendations for dedicated controllers and mobile devices that can control diabetes devices.44,45 However, since DTS is not an official standards-developing organization (SDO), DTSec and DTMoSt cannot be recognized by the FDA. Thus, Diabetes Technology Society is now working with two official SDOs, Institute of Electrical and Electronics Engineers (IEEE) Standards Association and Underwriters Laboratories (UL), who are co-managing a project to reformat DTSec and DTMoSt into a standard which will be known as IEEE 2621. 46 The logos of IEEE Standards Association and UL are shown in Figure 6.
Figure 5.

Logos of DTSec and DTMoSt.
Reproduced with permission from Diabetes Technology Society.44,45
Figure 6.

Logos of Institute of Electrical and Electronics Engineers (IEEE) and Underwriters Laboratories (UL).
IEEE 2621 consists of three sections: 2621.1, 2621.2, and 2621.3. 2621.1 provides a framework for security evaluation programs for connected diabetes devices, 2621.2 outlines the security functional recommendations for these devices, and 2621.3 contains recommendations specific for mobile devices. 49 This standard is being developed along with a conformity assessment program that allows manufacturers to demonstrate that their devices adhere to the P2621 standard. 50
Diabetes Registries
Benefits of Diabetes Registries
The value of technology is to help individuals living with diabetes reduce their personal risk of developing the serious, acute, and chronic complications associated with the condition and to improve their quality of life. To achieve this, evidence-based technologies must be equally accessible to and able to capture the outcomes that matter to individuals, their families, and their caregivers. Measuring and comparing outcomes and determining where variation exists is fundamental to eliminating health disparities.
Randomized clinical trials (RCTs) are no longer the sole source of data that is used to inform decisions while establishing guidelines, regulations, and policies. Real-world data collected from EHRs, insurance claims, pharmacy records, social media, and sensor outputs from devices form real-world evidence (RWE), to supplement RCT-derived information. Benefits of using RWE include the ability to capture additional information usually absent from RCTs, such as data on the social determinants of health which have a major impact on diabetes outcomes. 51 Registries are a valuable source of data that can inform real-world decisions. 52
Barriers to Advancing Diabetes Registries
Although the number of national diabetes registries has increased over recent years, their datasets are often incomplete, with variations in the outcomes collected. The impact of registry data on shaping national policies related to diabetes is also often unclear. 53 In the United States, the T1D exchange provides data from a geographically diverse number of children and adults with T1D across the life span. However, uninsured individuals and adults, who are more likely to be treated in primary care settings are underrepresented in diabetes registries, which limits their potential power. 54 The Diabetes Collaborative Registry, created by a consortium of professional societies, has data on a large number of people with T2D, many of whom are managed in primary care, and includes processes to measure data quality assurance. 55 However, patient-reported outcome measures (PROMs) are not captured. Standardization outcomes to reflect those that matter to PwD and can be used in routine clinical practice to monitor, benchmark, and improve diabetes care have been proposed. Such outcomes include PROMs related to mental well-being, diabetes distress and depression as key domains that should be monitored on a regular basis. 56
Future Applications of Diabetes Registries
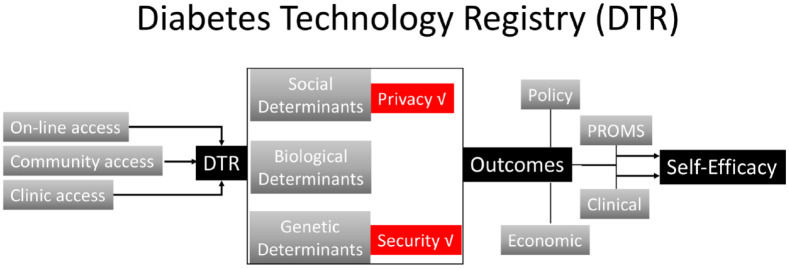
For registries focusing on technologies to support diabetes care, there is a need to agree on processes and outcomes that can help to remove disparities and achieve health equity. At a population level, this requires building infrastructure to create trust in the system, ensure equitable access and enhance self-efficacy for participants providing their personal health information (Figure 7). Traditionally diabetes registries have been clinic-based. The advantage of an online registry related to diabetes technology is that it offers membership to individuals not known to specialist clinics. On the other hand, online registries have the disadvantage of requiring reliable connectivity to the internet with potential bias against older people and individuals from underserved minorities. 57 For underserved communities, concerns over privacy may also be a barrier to engaging with digital tools for capturing registry data requiring community-based initiatives.
Figure 7.
Structure of a Diabetes Technology Registry to develop and maintain trust, improve access, and enhance self-efficacy for participants.
Population-based registries are good at locating problems by finding where in a causal network the trouble truly lies and deciding what actions will work. Thereby, networks can narrow the gap between what is and what ought to be, especially for those facing a disproportionate burden of diabetes in all forms. 58 The diabetes technology community should lead the way.
Conclusion
Many emerging digital software and hardware technologies are being applied to diabetes monitoring and data analysis. The challenge is identifying those technologies which are likely to be successful in the long-term. For any new technology there must be a clear understanding of (a) who the target population is, (b) what success looks like, and (c) who is going to pay for the technology. Further, these three considerations will vary between the different stakeholders involved in diabetes care. The new technologies listed here have great potential to be game-changing, but as always, care needs to be taken to ensure that they are accessible and affordable across different heath systems and populations.
Acknowledgments
We thank Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: API, application programming interface; AI, artificial intelligence; ANN, artificial neural network; CDS, clinical decision support; CGM, continuous glucose monitor; CMS, Centers for Medicare & Medicaid Services; DL, deep learning; DME, diabetic macular edema; DR, diabetic retinopathy; DTS, Diabetes Technology Society; DTSec, DTS Cybersecurity Standard for Connected Diabetes Devices; DTMoSt, DTS Mobile Platform Controlling a Diabetes Device Security and Safety Standard; DIY, do-it-yourself; EEG, electroencephalogram; EHR, Electronic Health Record; EKG, electrocardiogram; FDA, US Food and Drug Administration; FHIR, Fast Healthcare Interoperability Resources; IEEE, Institute of Electrical and Electronics Engineers; ML, machine learning; ONC, Office of the National Coordinator; PROMs, patient-reported outcome measures; PwD, people with diabetes; RCT, randomized clinical trial; RWE, real-world evidence; SDO, standards developing organization; T1D, type 1 diabetes; T2D, type 2 diabetes; UL, Underwriters Laboratories; US, United States; VOC, volatile organic compound.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ANK, W-AL, NYX, KTN, and AD have nothing relevant to disclose. DK reports non-financial support from Abbott Diabetes Care, grants from Lilly and Novo Nordisk, and personal fees from Novo Nordisk and Sanofi outside the submitted work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alexander N. Klonoff  https://orcid.org/0000-0003-0297-6448
https://orcid.org/0000-0003-0297-6448
Wei-An (Andy) Lee  https://orcid.org/0000-0002-2928-7338
https://orcid.org/0000-0002-2928-7338
Nicole Y. Xu  https://orcid.org/0000-0001-9353-8819
https://orcid.org/0000-0001-9353-8819
Kevin T. Nguyen  https://orcid.org/0000-0001-9102-6537
https://orcid.org/0000-0001-9102-6537
Ashley DuBord  https://orcid.org/0000-0002-1478-7065
https://orcid.org/0000-0002-1478-7065
David Kerr  https://orcid.org/0000-0003-1335-1857
https://orcid.org/0000-0003-1335-1857
References
- 1. National Diabetes Statistics Report, 2020 and CDC. Published September 28, 2020. Accessed June 22, 2021. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi: 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heintzman ND. A digital ecosystem of diabetes data and technology: services, systems, and tools enabled by wearables, sensors, and apps. J Diabetes Sci Technol. 2015;10(1):35-41. doi: 10.1177/1932296815622453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coravos A, Goldsack JC, Karlin DR, et al. Digital medicine: a primer on measurement. Digit Biomark. 2019;3(2):31-71. doi: 10.1159/000500413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fagherazzi G, Ravaud P. Digital diabetes: perspectives for diabetes prevention, management and research. Diabetes Metab. 2019;45(4):322-329. doi: 10.1016/j.diabet.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 6. Cahn A, Akirov A, Raz I. Digital health technology and diabetes management. J Diabetes. 2018;10(1):10-17. doi: 10.1111/1753-0407.12606 [DOI] [PubMed] [Google Scholar]
- 7. Dorsey ER, Topol EJ. State of telehealth. Campion EW, ed New Engl J Med. 2016;375(2):154-161. doi: 10.1056/NEJMra1601705 [DOI] [PubMed] [Google Scholar]
- 8. Office for Civil Rights (OCR). 3015-What is telehealth? HHS.gov. Published March 27, 2020. Accessed June 22, 2021. https://www.hhs.gov/hipaa/for-professionals/faq/3015/what-is-telehealth/index.html
- 9. Doraiswamy S, Abraham A, Mamtani R, Cheema S. Use of telehealth during the COVID-19 pandemic: Scoping review. J Med Internet Res. 2020;22(12):e24087. doi: 10.2196/24087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uscher-Pines L, Thompson J, Taylor P, et al. Where virtual care was already a reality: experiences of a nationwide telehealth service provider during the COVID-19 pandemic. J Med Internet Res. 2020;22(12):e22727. doi: 10.2196/22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crossen S, Raymond J, Neinstein A. Top 10 Tips for successfully implementing a diabetes telehealth program. Diabetes Technol Ther. 2020;22(12):920-928. doi: 10.1089/dia.2020.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vigersky RA, Fish L, Hogan P, et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab. 2014;99(9):3112-3121. doi: 10.1210/jc.2014-2257 [DOI] [PubMed] [Google Scholar]
- 13. Vadheim LM, Patch K, Brokaw SM, et al. Telehealth delivery of the diabetes prevention program to rural communities. Behav Med Pract Policy Res. 2017;7(2):286-291. doi: 10.1007/s13142-017-0496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dixon RF, Zisser H, Layne JE, et al. A virtual type 2 diabetes clinic using continuous glucose monitoring and endocrinology visits. J Diabetes Sci Technol. 2020;14(5):908-911. doi: 10.1177/1932296819888662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faruque LI, Wiebe N, Ehteshami-Afshar A, et al. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. 2017;189(9):E341-E364. doi: 10.1503/cmaj.150885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu C, Wu Z, Yang L, et al. Evaluation of the clinical outcomes of telehealth for managing diabetes: a PRISMA-compliant meta-analysis. Medicine. 2018;97(43):e12962. doi: 10.1097/MD.0000000000012962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klonoff DC. Telemedicine for diabetes after the COVID-19 pandemic: we can’t put the toothpaste back in the tube or turn back the clock. J Diabetes Sci Technol. 2020;14(4):741-742. doi: 10.1177/1932296820932958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehrotra A, Bhatia RS, Snoswell CL. Paying for telemedicine after the pandemic. JAMA. 2021;325(5):431-432. doi: 10.1001/jama.2020.25706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kompala T, Neinstein AB. Telehealth in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2021;28(1):21-29. doi: 10.1097/med.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 20. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-To-consumer telehealth may increase access to care but does not decrease spending. Health Aff. 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130 [DOI] [PubMed] [Google Scholar]
- 21. Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7(8):2546-2552. doi: 10.1016/j.jaip.2019.06.027 [DOI] [PubMed] [Google Scholar]
- 22. 21st century cures act: interoperability, information blocking, and the ONC health IT certification program. Federal Register. Published May 1, 2020. Accessed June 22, 2021. https://www.federalregister.gov/documents/2020/05/01/2020-07419/21st-century-cures-act-interoperability-information-blocking-and-the-onc-health-it-certification
- 23. Gordon WJ, Mandl KD. The 21st century cures act: a competitive apps market and the risk of innovation blocking. J Med Internet Res. 2020;22(12):e24824. doi: 10.2196/24824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doupis J, Festas G, Tsilivigos C, Efthymiou V, Kokkinos A. Smartphone-based technology in diabetes management. Diabetes Ther. 2020;11(3):607-619. doi: 10.1007/s13300-020-00768-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Standard technology presents opportunities for medical record data extraction | The Pew Charitable Trusts. Accessed June 22, 2021. https://www.pewtrusts.org/en/research-and-analysis/reports/2021/01/standard-technology-presents-opportunities-for-medical-record-data-extraction
- 26. Everson J, Patel V, Adler-Milstein J. Information blocking remains prevalent at the start of 21st Century Cures Act: results from a survey of health information exchange organizations. J Am Med Inform Assoc. 2021;28(4):727-732. doi: 10.1093/jamia/ocaa323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Information blocking. Accessed June 22, 2021. https://www.healthit.gov/curesrule/final-rule-policy/information-blocking
- 28. Diouri O, Cigler M, Vettoretti M, Mader JK, Choudhary P, Renard E. Hypoglycaemia detection and prediction techniques: a systematic review on the latest developments. Diabetes Metab Res Rev. 2021. Published online March 24, 2021. doi: 10.1002/dmrr.3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Remvig LS, Elsborg R, Sejling A-S, et al. Hypoglycemia-related electroencephalogram changes are independent of gender, age, duration of Diabetes, and awareness status in type 1 Diabetes. J Diabetes Sci Technol. 2012;6(6):1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris ND, Baykouchev SB, Marques JLB, et al. A portable system for monitoring physiological responses to hypoglycaemia. J Med Eng Technol. 1996;20(6):196-202. doi: 10.3109/03091909609008998 [DOI] [PubMed] [Google Scholar]
- 31. Siegel AP, Daneshkhah A, Hardin DS, Shrestha S, Varahramyan K, Agarwal M. Analyzing breath samples of hypoglycemic events in type 1 diabetes patients: towards developing an alternative to diabetes alert dogs. J Breath Res. 2017;11(2):026007. doi: 10.1088/1752-7163/aa6ac6 [DOI] [PubMed] [Google Scholar]
- 32. Frier BM. Impaired awareness of hypoglycaemia. In: Frier BM, Heller SR, McCrimmon RJ. (eds) Hypoglycaemia in clinical diabetes. John Wiley & Sons, Ltd; 2013;114-144. [Google Scholar]
- 33. Howsmon D, Bequette BW. Hypo- and hyperglycemic alarms: devices and algorithms. J Diabetes Sci Technol. 2015;9(5):1126-1137. doi: 10.1177/1932296815583507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Apple patent hints at non-invasive glucose monitoring tech for Apple watch. AppleInsider. Accessed June 22, 2021. https://appleinsider.com/articles/18/08/23/apple-patent-suggests-work-on-non-invasive-glucose-monitoring-tech
- 35. Lovejoy B. Apple watch blood sugar sensor “coming in Series 7” – report. 9to5Mac. Published January 25, 2021. Accessed June 22, 2021. https://9to5mac.com/2021/01/25/apple-watch-blood-sugar-measurement/
- 36. Definition of artificial intelligence. Accessed June 22, 2021. https://www.merriam-webster.com/dictionary/artificial+intelligence
- 37. Rigla M, García-Sáez G, Pons B, Hernando ME. Artificial intelligence methodologies and their application to Diabetes. J Diabetes Sci Technol. 2018;12(2):303-310. doi: 10.1177/1932296817710475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dagliati A, Marini S, Sacchi L, et al. Machine learning methods to predict diabetes complications. J Diabetes Sci Technol. 2018;12(2):295-302. doi: 10.1177/1932296817706375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133(8):895-900. doi: 10.1016/j.amjmed.2020.03.033 [DOI] [PubMed] [Google Scholar]
- 40. Gunasekeran DV, Ting DSW, Tan GSW, Wong TY. Artificial intelligence for diabetic retinopathy screening, prediction and management. Curr Opin Ophthalmol. 2020;31(5):357-365. doi: 10.1097/ICU.0000000000000693 [DOI] [PubMed] [Google Scholar]
- 41. Broome DT, Hilton CB, Mehta N. Policy implications of artificial intelligence and machine learning in diabetes management. Curr Diab Rep. 2020;20(2):5. doi: 10.1007/s11892-020-1287-2 [DOI] [PubMed] [Google Scholar]
- 42. Fisher J. The next pandemic: cybercrime. FleetOwner. Published June 15, 2021. Accessed June 17, 2021. https://www.fleetowner.com/technology/article/21163965/the-next-pandemic-cybercrime
- 43. Klonoff DC. Cybersecurity for connected diabetes devices. J Diabetes Sci Technol. 2015;9(5):1143-1147. doi: 10.1177/1932296815583334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diabetes Technology Society. DTS cybersecurity standard for connected diabetes devices. Accessed June 15, 2021. https://www.diabetestechnology.org/dtsec.shtml
- 45. Diabetes Technology Society. Diabetes Technology Society mobile platform controlling a diabetes device security and safety standard. Accessed June 15, 2021. https://www.diabetestechnology.org/dtmost.shtml
- 46. Yuan S, Fernando A, Klonoff DC. Standards for medical device cybersecurity in 2018. J Diabetes Sci Technol. 2018;12(4):743-746. doi: 10.1177/1932296818763634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Master brand and logos. IEEE brand experience. Accessed August 13, 2021. https://brand-experience.ieee.org/guidelines/master-brand-and-logos/
- 48. UL Empowering Trust®. Accessed June 23, 2021. https://www.ul.com/
- 49. P2621.1 - Standard for wireless diabetes device security assurance evaluation—connected electronic product security evaluation programs. Accessed June 15, 2021. https://standards.ieee.org/project/2621_1.html#Standard
- 50. IEEE P2621 Series of standards. Accessed June 15, 2021. https://standards.ieee.org/products-services/icap/programs/p2621-series-of-standards.html#ConformityAssessmentProgram
- 51. Klonoff DC, Gutierrez A, Fleming A, Kerr D. Real-world evidence should Be used in regulatory decisions about new pharmaceutical and medical device products for Diabetes. J Diabetes Sci Technol. 2019;13(6):995-1000. doi: 10.1177/1932296819839996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. Kumar S, ed PLoS One. 2017;12(9):e0183667. doi: 10.1371/journal.pone.0183667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bak JCG, Serné EH, Kramer MHH, Nieuwdorp M, Verheugt CL. National diabetes registries: do they make a difference? Acta Diabetol. 2021;58(3):267-278. doi: 10.1007/s00592-020-01576-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383-4389. doi: 10.1210/jc.2012-1561 [DOI] [PubMed] [Google Scholar]
- 55. Tinsley LJ, Wong ND, Reusch JEB, et al. Regional differences in the management of cardiovascular risk factors among adults with diabetes: an evaluation of the Diabetes Collaborative registry. J Diabetes Complications. 2020;34(8):107591. doi: 10.1016/j.jdiacomp.2020.107591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nano J, Carinci F, Okunade O, et al. A standard set of person-centred outcomes for diabetes mellitus: results of an international and unified approach. Diabet Med. 2020;37(12):2009-2018. doi: 10.1111/dme.14286 [DOI] [PubMed] [Google Scholar]
- 57. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386-1389. doi: 10.1001/jamainternmed.2020.2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kerr D, Glantz N. Diabetes, like COVID-19, is a wicked problem. Lancet Diabetes Endocrinol. 2020;8(11):873-874. doi: 10.1016/S2213-8587(20)30312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]