Abstract
Background:
Despite advancements in diabetes technologies, disparities remain with respect to diabetes device use in youth with type 1 diabetes (T1D). We compared sociodemographic, diabetes, and psychosocial characteristics associated with device (pump and continuous glucose monitor [CGM]) use in 13- to 17-year-old teens with T1D.
Materials/Methods:
Data were derived from a multicenter clinical trial to optimize self-care and glycemic control in teens with T1D. We categorized teens as pump users versus non-users and CGM users versus non-users based on their diabetes device usage. Chi-square and t-tests compared characteristics according to device use.
Results:
The sample comprised 301 teens (50% female) with baseline mean ± SD age 15.0 ± 1.3 years, T1D duration 6.5 ± 3.7 years, and HbA1c 8.5 ± 1.1% (69 ± 12 mmol/mol). Two-thirds (65%) were pump users, and 27% were CGM users. Pump users and CGM users (vs. non-users) were more likely to have a family annual household income ≥$150,000, private health insurance, and a parent with a college education (all P < .001). Pump users and CGM users (vs. non-users) also performed more frequent daily blood glucose (BG) checks (both P < .001) and reported more diabetes self-care behaviors (both P < .05). Pump users were less likely to have baseline HbA1c ≥9% (75 mmol/mol) (P = .005) and to report fewer depressive symptoms (P = .02) than pump non-users. Parents of both CGM and pump users reported a higher quality of life in their youth (P < .05).
Conclusion:
There were many sociodemographic, diabetes-specific, and psychosocial factors associated with device use. Modifiable factors can serve as the target for clinical interventions; youth with non-modifiable factors can receive extra support to overcome potential barriers to device use.
Keywords: adolescents, continuous glucose monitoring, diabetes devices, disparities, insulin pump, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is a chronic illness that requires daily management to prevent complications. 1 It is well-documented that adolescents with T1D are vulnerable to sub-optimal T1D self-management and sub-optimal glycemic outcomes.2,3 Insulin pumps and continuous glucose monitors (CGMs) can help adolescents with T1D achieve better glycemic control. CGM use has also been associated with improved quality of life, and insulin pumps can decrease rates of both diabetic ketoacidosis (DKA) and severe hypoglycemia.4-6 Given the recognized benefits of diabetes technologies, increased uptake of insulin pumps and CGMs has been reported internationally.3,7,8 However, device usage is not universal. Lack of universal device use aimed at helping to achieve glycemic targets raises concerns about potential health disparities due to sociodemographic factors, personal behavioral barriers, and implicit provider biases that may be associated with reduced device uptake and durability, especially in vulnerable adolescents with T1D.
Previously published studies have identified characteristics related to the underuse of advanced diabetes technologies in youth. Socioeconomic status (SES) remains a significant contributor to disparities in device uptake.9-12 However, a study by Willi et al. found that disparities continue to exist in diabetes device uptake despite adjusting for SES. 10 While these studies provide insights into potential health disparities, there remains a need to address other possible causes of treatment inequalities, especially those that are modifiable and can be addressed on an individual level. Few studies to date have identified modifiable barriers to diabetes device use that can be mitigated via clinical intervention, such as psychosocial factors and family dynamics.13-15 In addition, existing evidence on psychosocial predictors of diabetes device uptake and use is scarce.
Given that adolescents with T1D are at high risk for challenges with diabetes self-care and suboptimal glycemic control, due in part to the impact of puberty, as well as changes in peer and family relationships, 16 it is essential to understand factors that may be associated with diabetes technology uptake in this population. Furthermore, the choice to adopt diabetes technologies likely requires shared decision-making between adolescents and their parents/guardians (referred to as parents going forward). Therefore, it is important to investigate adolescent and parent attitudes regarding diabetes technology uptake. The purpose of this study was to identify characteristics associated with the use of insulin pumps and CGM devices in a diverse sample of adolescents with T1D.
Methods
The present study is a secondary analysis of a randomized controlled trial conducted at Joslin Diabetes Center and Texas Children’s Hospital to optimize self-care behaviors and glycemic control in teens with T1D through behavioral and text messaging invention.17,18 In the primary analysis, neither the behavioral intervention, the text messaging intervention, nor the combination of the 2 significantly improved HbA1c from baseline.17,18 Thus, in the current analyses, the data from all intervention groups are analyzed together.
Eligibility criteria included ages 13 to 17 years, diabetes duration of ≥6 months, daily insulin dose ≥0.5 unit/kg, HbA1c 6.5% to 11.0% (48-97 mmol/mol), and fluency in English. Exclusion criteria included any significant developmental, cognitive, or medical conditions that would interfere with study participation. Institutional review boards at both sites approved the study protocol. Eligible teens/parents provided written informed assent/consent, respectively, before beginning any study procedures.
The majority of data, including sociodemographic and psychosocial characteristics, were collected at baseline using survey information completed by teens and parents. Diabetes device use was derived from chart review and parent-youth interview every 3 months for 18 months. Blood glucose (BG) monitoring frequency was determined from meter/pump downloads quarterly. This study predated U.S. Food and Drug Administration (FDA) labeling for nonadjunctive CGM use; therefore, participants used BG results for insulin dosing. Blood samples for HbA1c were analyzed centrally (Roche Cobas™, Indianapolis, Indiana) at the Joslin Diabetes Center every 6 months and obtained at each site at the intervening 3-month visit. The results were standardized to the central laboratory. For participants with missing HbA1c values at 18 months (n = 26), the most recent HbA1c value was carried forward for 20 participants, and 6 participants were missing final HbA1c values.
Measures
Youth and parents completed validated psychosocial surveys at baseline. Depressive symptoms were assessed with the validated Center for Epidemiologic Studies Depression Scale (CES-D). 19 Higher scores indicate more depressive symptoms, with scores >15 indicating risk for clinical depression. Diabetes family conflict was measured using the Diabetes Family Conflict Scale. 20 Higher scores indicate more diabetes-specific family conflict. Parent involvement in diabetes management was measured using the Diabetes Family Responsibility Questionnaire. 21 Higher scores indicate more parent involvement in diabetes care. Treatment adherence and diabetes self-care were measured with the Diabetes Management Questionnaire. 22 Higher scores indicate greater adherence. Youth and parent perceived diabetes burden was measured using the pediatric and parent versions of the Problem Areas in Diabetes (PAID) Survey, PAID-Peds 23 and PAID-Parents Revised, 24 respectively. Higher scores indicate more perceived diabetes burden. Youth and parent perception of the youth’s health-related quality of life was measured using the Pediatric Quality of Life Inventory–Generic Core Scales (PedsQL), which incorporates physical and psychosocial functioning. 25 Higher scores indicate the better perceived health-related quality of life.
Participants were categorized based on patterns of insulin pump usage from the initial visit to the final study visit at 18 months (Figure 1):
Figure 1.
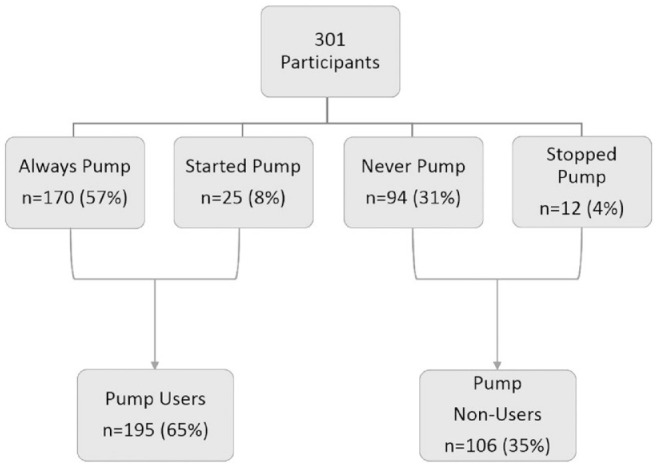
Grouping of participants based on insulin pump usage.
Always pump use : Participants who used an insulin pump throughout the study.
Started pump use : Participants who used injection therapy at initial visit but transitioned to pump therapy during follow-up.
Never pump use : Participants who did not use an insulin pump at all during the study
Stopped pump use : Participants who reported pump therapy at the initial visit but stopped pump use during follow-up.
Participants were categorized based on patterns of CGM usage from the initial visit to the final study visit at 18 months (Figure 2):
Figure 2.
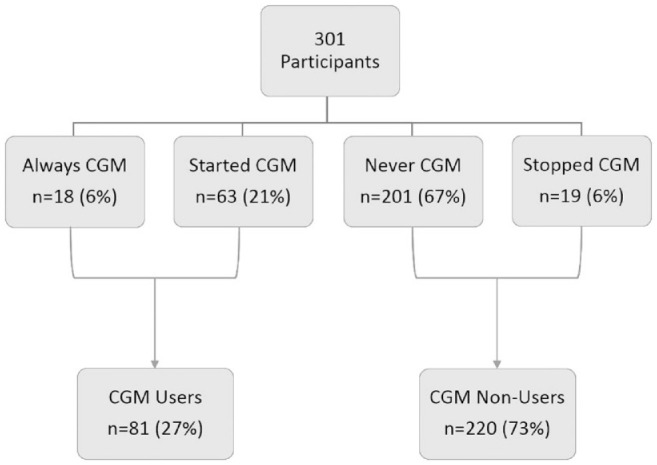
Grouping of participants based on continuous glucose monitor usage.
Always CGM use : Participants who used a CGM throughout the study.
Started CGM use : Participants who initially did not use CGM but transitioned to CGM during follow-up.
Never CGM use: Participants who did not use CGM at all during the study.
Stopped CGM use : Participants who used CGM at the initial visit but stopped CGM use during follow-up.
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina). Descriptive data are presented as means ± standard deviation for continuous variables and frequencies or proportions for categorical variables. Statistical analyses included t-tests and chi-square tests. For sociodemographic and diabetes characteristics, P < .01 were considered statistically significant to adjust for multiple comparisons. Given the exploratory nature of the psychosocial characteristics, significance was defined as P < .05 for these comparisons.
Results
Study Sample and Group Allocations
The sample was comprised of 301 teens (50% female) with a mean (± SD) age of 15.0 ± 1.3 years, T1D duration of 6.5 ± 3.7 years, and HbA1c of 8.5 ± 1.1% (69 mmol/mol) at baseline. Only 5% of the sample achieved the recommended HbA1c goal of <7% (53 mmol/mol). 26
The majority of youth (57%, n = 170) utilized insulin pump therapy for the entire study period, while 8% (n = 25) transitioned to pump therapy. Given the similarities between these 2 groups, they were combined, yielding 195 pump users (65%). About one-third (31%, n = 94) never used pump, while 4% (n = 12) transitioned from pump to injection therapy during the study period. Therefore, these 2 groups were combined to form the pump non-users group (35%, n = 106).
CGM users comprised 6% (n = 18) of the participants, while 21% (n = 63) started CGM use during the study period. These 2 groups were similar across all characteristics and were therefore combined to form the CGM users group (n = 81, 27% of the sample). The majority (n = 201, 67%) of participants never used CGM during the study, while 6% (n = 19) stopped using CGM during the study period; the combination of these 2 groups was 73% (n = 220) of the sample. There were no differences in demographic, diabetes, or psychosocial characteristics between never CGM group and stopped CGM group.
Comparisons of Pump Users vs. Pump Non-Users
Demographic and clinical characteristics of pump users versus pump non-users are shown in Table 1. When compared with the pump non-users, pump users were more likely to be non-Hispanic white (83% vs. 61%, P = .0001), have an annual family household income ≥$150,000 (34% vs. 19%, P = .0003), utilize private health insurance (92% vs. 75%, P < .0001), and have a parent with a college education or higher (75% vs. 58%, P = .002). In addition, pump users performed more daily BG monitoring (4.8 ± 1.8 vs. 3.9 ± 2.0, P < .001), had lower percent daily basal insulin (46% vs. 50%, P = .008), and were less likely to have HbA1c ≥9% (75 mmol/mol) at initial study visit (25% vs. 43%, P = .005).
Table 1.
Demographic and Clinical Characteristics of Pump Users vs. Pump Non-Users.
| Pump users | Pump non-users | P-value | |
|---|---|---|---|
| (n = 195, 65%) | (n = 106, 35%) | ||
| Age (years) | 14.9 ± 1.3 | 15.1 ± 1.3 | .4 |
| Diabetes duration (years) | 6.6 ± 3.8 | 6.4 ± 3.5 | .8 |
| Sex (% female) | 51% | 49% | .7 |
| Race/ethnicity (% non-Hispanic white) | 83% | 61% | .0001 |
| Family structure (% 2-parent) | 88% | 78% | .03 |
| Annual household income ≥$150,000 (%) | 34% | 19% | .0003 |
| Parental employment status (% full-time) | 63% | 68% | .7 |
| Health insurance (% private) | 92% | 75% | <.0001 |
| Parental education (% college or higher) | 75% | 58% | .002 |
| Presence of co-morbid conditions (%) | 44% | 59% | .01 |
| Body mass index z-score (Standard Deviation Score) | 0.73 ± 0.8 | 0.97 ± 0.8 | .01 |
| Percent basal insulin (% of total daily dose) | 46% | 50% | .008 |
| Blood glucose monitoring (times/day) | 4.8 ± 1.8 | 3.9 ± 2.0 | <.0001 |
| HbA1c at initial visit (≥9%) | 25% | 43% | .005 |
| HbA1c at final visit (≥9%) | 32% | 49% | .01 |
| Change in HbA1c from initial visit to final visit (%) | 0.15 ± 1.1 | 0.32 ± 1.1 | .2 |
Bold font indicates statistical significance (P < 0.01).
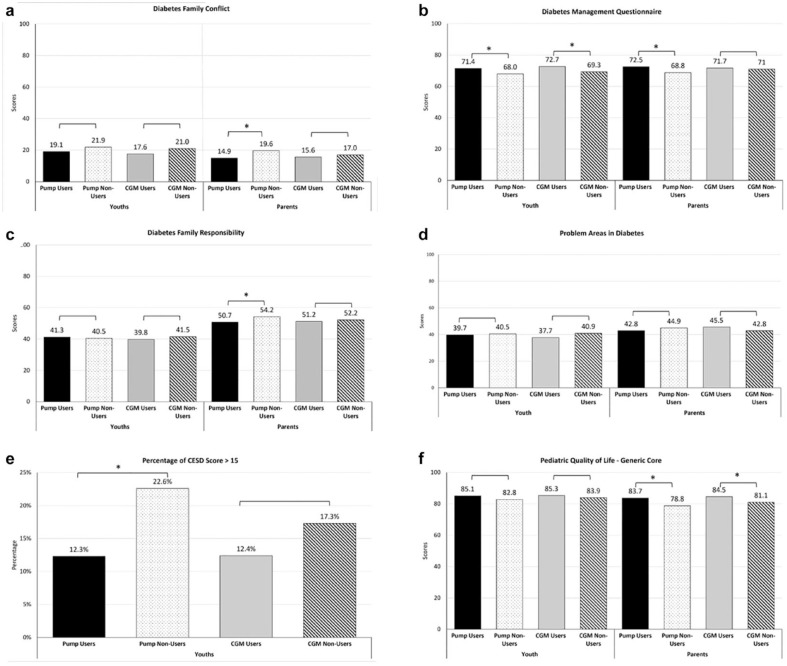
Figure 3 shows the difference between the psychosocial surveys scores of pump users versus pump non-users as well as their parents. Pump users were more likely to self-report higher levels of diabetes self-management (71.4 ± 11.9 vs. 68.0 ± 13.3, P = .03) compared with pump non-users. Pump users compared with pump non-users were less likely to score above 15 on the CESD (12.3% vs. 22.6%, P = .02), reflecting a lower likelihood of having elevated depressive symptomatology. Parents of pump users reported lower levels of diabetes-specific family conflict (14.9 ± 13.4 vs. 19.6 ± 18.2, P = .01), higher perceived diabetes self-management by their teens (72.5 ± 13.3 vs. 68.8 ± 12.7, P = .02), less parental involvement in diabetes care (50.7 ± 12.8 vs. 54.2 ± 14.5, P = .03), and higher proxy report of their child’s general quality of life (83.7 ± 12.4 vs. 78.8 ± 14.2, P = .003) compared with the parents of pump non-users. However, there was no significant difference in diabetes burden between pump users and pump non-users (39.7 ± 23.6 vs. 40.5 ± 24.7, P = .8) nor between the parents of pump users and non-users (42.8 ± 19.0 vs. 44.9 ± 20.0, P = .4). Similarly, self-reported quality of life did not differ between the teen pump users and non-users (85.1 ± 12.5 vs. 82.8 ± 14.3, P = .2).
Figure 3.
Baseline (a) diabetes-specific family conflict, (b) teen self-care, (c) parental involvement in diabetes care, (d) diabetes burden, (e) percentage with depressive symptoms, and (f) quality of life between device (pump, CGM) users and non-users as well as parents of device (pump, CGM) users vs. non-users.
Abbreviation: CGM, continuous glucose monitors.
aReflects significant differences (P-values <.05) between teen users and non-users either by youth-report or parent-report for the particular psychosocial measure.
Comparisons of CGM Users vs. CGM Non-Users
Demographic and clinical characteristics of CGM users versus CGM non-users are shown in Table 2. CGM users compared with CGM non-users were more likely to be non-Hispanic white (88% vs. 70%, P = .009), have a family annual household income ≥$150,000 (44% vs. 23%, P = .0001), a parent with a college education or higher (81% vs. 64%, P = .004), and private health insurance (95% vs. 82%, P = .005). CGM users also performed more frequent daily BG monitoring (5.2 ± 1.9 vs. 4.3 ± 1.9, P = .0002). Figure 3 shows the difference between the psychosocial surveys scores of CGM users versus CGM non-users as well as their parents. CGM users reported higher diabetes self-management compared with CGM non-users (72.7 ± 11.0 vs. 69.3 ± 13.0, P = .03). Parents of CGM users perceived higher quality for their youth compared with CGM non-users (84.5 ± 11.9 vs. 81.1 ± 13.5, P = .04). There was no difference in youth-reported diabetes family involvement (39.8 ± 11.0 vs. 41.5 ± 12.2, P = .3) or diabetes burden (37.7 ± 22.1 vs. 40.9 ± 24.6, P = .3) between the CGM users and CGM non-users. Parent reports of youth adherence (71.7±12.3 vs. 71.0 ± 13.5, P = .7), diabetes burden (45.5 ± 20.4 vs. 42.8 ± 18.8, P = 0.3), and diabetes-specific family conflict (15.6±13.5 vs. 17.0 ± 16.1, P = 0.5) also did not differ between CGM users versus non-users.
Table 2.
Demographic and Clinical Characteristics of CGM Users vs. CGM Non-Users.
| CGM users | CGM non-users | P-value | |
|---|---|---|---|
| (n = 81, 27%) | (n = 220, 73%) | ||
| Age (years) | 14.9 ± 1.3 | 15.0 ± 1.3 | .4 |
| Diabetes duration (years) | 6.9 ± 3.9 | 6.4 ± 3.7 | .3 |
| Sex (% female) | 48% | 51% | .6 |
| Race/ethnicity (% non-Hispanic white) | 88% | 70% | .009 |
| Family structure (% 2-parent) | 89% | 83% | .2 |
| Annual household income ≥$150,000 (%) | 44% | 23% | .0001 |
| Parental employment status (% full-time) | 53% | 69% | .005 |
| Health insurance (% private) | 95% | 82% | .005 |
| Parental education (% college or higher) | 81% | 64% | .004 |
| Presence of co-morbid conditions (%) | 44% | 51% | .3 |
| Body Mass Index z-score (Standard Deviation Score) | 0.7 ± 0.7 | 0.9 ± 0.8 | .2 |
| Percent basal insulin (% of total daily dose) | 47% | 48% | .3 |
| Blood glucose monitoring (times/day) | 5.2 ± 1.9 | 4.3 ± 1.9 | .0002 |
| HbA1c at initial visit (≥9%) | 23% | 35% | .2 |
| HbA1c at final visit (≥9%) | 27% | 42% | .03 |
| Change in HbA1c from initial visit to final visit (%) | 0.1 ± 1.1 | 0.2 ± 1.1 | .4 |
Abbreviation: CGM, continuous glucose monitors.
Bold font indicates statistical significance (P < 0.01).
Discussion
This study highlights many distinct sociodemographic, diabetes-specific, and psychosocial factors associated with device use in adolescents with T1D. We identified associations of participant characteristics with diabetes device use in order to identify future opportunities for interventions aimed at increasing device uptake and continued use. In our sample, about two-thirds (65%) of the teens used insulin pump therapy, and 27% used CGM; nearly a quarter (23%) of the sample used both devices. Our discontinuation rates for both pump and CGM were similar to previously published data.12,27 Among the diabetes devices in use, CGM utilization was the lowest as our study predated FDA labeling for nonadjunctive CGM use. There has been a substantial increase in CGM usage in recent years due to CGM’s ability to replace BG monitoring with better-performing devices, nonadjunctive claim, elimination of fingerstick calibration on some devices, and broader insurance coverage. 28 Nonetheless, recent reports continue to highlight disparities in CGM use among youth with T1D. 12
Consistent with other studies, adolescents from families with higher SES status and of non-Hispanic white racial and ethnic backgrounds were more likely to use diabetes devices.10,11,29 We also saw that those utilizing diabetes devices, especially pump users, had more optimal diabetes self-care characteristics than non-users. Previous research has shown that diabetes device users have more favorable HbA1c outcomes, experience lower DKA rates, and demonstrate more frequent self-monitoring of blood glucose.30-32 The glycemic profile was also more favorable among device users compared with non-device users, consistent with the findings from a recent study published from an international network of pediatric diabetes centers. 33 We also did not observe significant differences in the age at onset, duration of diabetes, or gender between device users and device non-users.
Early adopters of advanced diabetes technologies tend to be represented by people with T1D from families with higher education and SES. 34 It is notable that although insulin pump therapy has been a highly accepted mode of insulin delivery for decades while CGM is a more recent addition to diabetes management, the comparisons between device users and non-users were similar. This observation highlights a likely persistent tendency for those with greater knowledge and means to receive diabetes devices, underscoring the need for improved educational outreach and support aimed at increasing access of such devices to more diverse youth with T1D. Such approaches are increasingly important as advanced diabetes technologies, including automated insulin delivery systems, are becoming critical in efforts to optimize glycemic control in people with T1D. Ongoing research is needed to understand the perspectives of multiple stakeholders in these efforts, including prescribers, people with diabetes, families, and payers.
Our study also offers insights into psychosocial factors that may be associated with device use from the teens’ and their parents’ perspectives. Our study showed fewer depressive symptoms and a greater level of self-care in youth on pump therapy. In contrast to our findings, a previous study of adolescents did not identify any difference in depressive symptoms between pump users and non-users. 14 This may reflect different methods used to screen for depression. Our study used the CES-D, which includes perceived mood and level of functioning, while the Patient Health Questionnaire-8, used in the other study, 14 includes more severe criteria for depressive symptoms/depression. It is possible that teens with depressive symptoms are less interested in wearing an insulin pump; it is also possible that the healthcare team may discourage or discontinue insulin pump use among teens with depressive symptoms or depression.
With respect to CGM use, the only difference in psychosocial measures between CGM users and non-users was greater reported diabetes self-care among users. This may reflect the smaller numbers of CGM users and the ongoing need to perform BG monitoring in the nonadjunctive era of these observations. This finding is consistent with a large-scale survey done in 2013 in which 80% of adult CGM users reported that CGM facilitated their diabetes self-management. 35 With rapid advancements in glucose monitoring technology, we expect that the perceived benefits of CGM use will increase, as has been demonstrated in the recently published results from the CGM Intervention in Teens and Young Adults with T1D (CITY) study. 5
Interestingly, in this relatively large sample, we did not observe differences in diabetes burden between device users and non-device users by either youth or parent report. There may likely be a trade-off with the potential for reduced diabetes burden in association with device use that is offset by the potential for increased burden associated with, for example, frequent alerts and alarms from CGM glucose values out of range. 36 It is possible that newer devices, including automated insulin delivery systems, may reduce the diabetes burden; research is ongoing in this area.
In our cohort, youth, in general, scored higher in diabetes-specific family conflict than parents. These results are consistent with other studies and suggest that youth may find these family interactions around diabetes care more stressful compared with their parents.37,38 On the other hand, parents are more likely to provide socially desirable responses than teens, who are more prone to display extreme responses. Significantly, the use of pumps and/or CGM devices was not associated with higher diabetes-specific family conflict by teen report, while parents of pump non-users did report higher diabetes-specific family conflict than the parents of pump users. The latter finding may reflect the overall constellation of sociodemographic and diabetes-specific factors among pump non-users, creating a more stressful environment for such families. Several studies have shown that parents usually report positive benefits associated with insulin pump use, such as increased flexibility.39-41 Thus, our findings are consistent with the parents of pump users who report both less diabetes-specific family conflict and higher general quality of life for their youth.
Given that diabetes technology aims to decrease the diabetes burden of self-care for individuals with T1D and improve glycemic outcomes, it is essential to ensure accessibility to these devices and to promote consistent usage. For example, Medtronic’s hybrid closed-loop system was shown to be safe and able to improve glycemic control in the short term 42 ; however, the discontinuation rate was as high as 30% in an observational study. 43 One strength of the present study is that it identifies modifiable diabetes and psychosocial characteristics, such as glucose monitoring frequency, diabetes control, diabetes-specific family conflict, and depressive symptoms, associated with device use in teens with T1D. These modifiable barriers can be addressed individually and serve as the foundation for the future development of tailored interventions to increase uptake and durability of device use in youth with T1D, including automated delivery systems. For example, additional mental health support or the use of a patient decision aid/decision coaching may help teens with diabetes device uptake and use. 44 Other strengths of this study include the relatively large sample size, a wide range of sociodemographic characteristics (e.g., 22% of the participants represented racial and ethnic minorities, many teens were from families of lower SES) and assessments of both teen and parent psychosocial characteristics pertinent to diabetes care.
Limitations of this study include a timeframe that antedated improvements in CGM devices and FDA designation of CGM for nonadjunctive use. Furthermore, this study predates the availability of the newer automated insulin delivery systems, which warrant study regarding use and non-use beyond the initial hybrid closed system noted above. Our study does not include the perceptions of providers, which would be an essential perspective to understand in order to ensure the equitable dissemination of advanced diabetes technologies. Given the exploratory nature of this study, a univariate comparison was used, which could be a limitation. Therefore, future studies should include multivariable analyses. The cost of diabetes devices was not compensated by the study, so it is possible that the cost played a role in the discontinuation of diabetes devices. However, there is generally reasonable insurance reimbursement for diabetes devices for youth with T1D across most payers in the current era. With respect to glycemic control, our study sample included an entry A1c value of 6.5% to 11.0%, while glycemia may vary to a greater extent in the general population of T1D. In clinical practice, there is often no HbA1c or glycemic control criteria for initiating CGM devices, although insulin pumps may be discouraged from individuals with extremely high HbA1c levels for safety concerns. Overall, the use of advanced diabetes technologies generally depends on if and when the family and the teen with diabetes are ready to use such devices, and the healthcare team deems it is safe to do so. Future research can include broader samples of teens with T1D with a wider range of HbA1c levels. Nonetheless, our study provides a broad range of data about diabetes care from both adolescents and their parents, spanning sociodemographic, diabetes-specific, and general psychosocial characteristics.
Conclusion
Our study found many distinct modifiable and non-modifiable sociodemographic (e.g., higher SES), diabetes-specific (eg, more frequent BG monitoring), and psychosocial factors (e.g., less depressive symptoms) associated with device use in adolescents with T1D. More research is needed to overcome modifiable barriers by developing tailored interventions, such as providing more mental health support and designing approaches to offer additional education to those with non-modifiable barriers.
Acknowledgments
We acknowledge the support of the research teams from the Joslin Diabetes Center and Texas Children’s Hospital, as well as all the participating adolescents and families.
Footnotes
Abbreviations: T1D, type 1 diabetes; CGM, continuous glucose monitors, DKA, diabetes ketoacidosis; SES, socioeconomic status; FDA, Food and Drug Administration; CES-D, Center for Epidemiologic Studies Depression Scale, PedsQL, Pediatric Quality of Life Inventory–Generic Core Scales; PAID, problem areas in diabetes; CITY, CGM Intervention in Teens and Young Adults with T1D.
Author Contributions: CWC analyzed the data, contributed to data interpretation, and wrote the initial manuscript draft. LJT directed statistical analyses and reviewed/edited the manuscript. LKV researched the data and reviewed/edited the manuscript. BJA researched the data, contributed to data interpretation, and reviewed/edited the manuscript. LML researched data, contributed to data interpretation, and revised/edited the manuscript. LML and CWC are the guarantors of this work, and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All co-authors have reviewed and approved of the manuscript before submission.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (grant numbers R01DK095273, T32DK007260, and P30DK036836); JDRF (grant number 2-SRA-2014-253-M-B); the Katherine Adler Astrove Youth Education Fund; the Maria Griffin Drury Pediatric Fund; and the Eleanor Chesterman Beatson Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of these organizations.
ORCID iDs: Charlotte W. Chen  https://orcid.org/0000-0001-6328-7051
https://orcid.org/0000-0001-6328-7051
Lori M. Laffel  https://orcid.org/0000-0002-9675-3001
https://orcid.org/0000-0002-9675-3001
References
- 1. Diabetes C, Complications Trial Research Group, Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2. Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035-2037. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cemeroglu AP, Stone R, Kleis L, Racine MS, Postellon DC, Wood MA. Use of a real-time continuous glucose monitoring system in children and young adults on insulin pump therapy: patients’ and caregivers’ perception of benefit. Pediatr Diabetes. 2010;11(3):182-187. doi: 10.1111/j.1399-5448.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- 5. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388-2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358-1366. doi: 10.1001/jama.2017.13994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeSalvo DJ, Miller KM, Hermann JM, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271-1275. doi: 10.1111/pedi.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2020;44:133–140. doi: 10.2337/dc20-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Connor MR, Carlin K, Coker T, Zierler B, Pihoker C. Disparities in insulin pump therapy persist in youth with type 1 diabetes despite rising overall pump use rates. J Pediatr Nurs. 2019;44:16-21. doi: 10.1016/j.pedn.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipman TH, Willi SM, Lai CW, Smith JA, Patil O, Hawkes CP. Insulin pump use in children with type 1 diabetes: over a decade of disparities. J Pediatr Nurs. 2020;55:110-115. doi: 10.1016/j.pedn.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 11. Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424-434. doi: 10.1542/peds.2014-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai CW, Lipman TH, Willi SM, Hawkes CP. Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care. 2021;44(1):255-257. doi: 10.2337/dc20-1663. [DOI] [PubMed] [Google Scholar]
- 13. Seereiner S, Neeser K, Weber C, et al. Attitudes towards insulin pump therapy among adolescents and young people. Diabetes Technol Ther. 2010;12(1):89-94. doi: 10.1089/dia.2009.0080. [DOI] [PubMed] [Google Scholar]
- 14. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi: 10.1089/dia.2019.0509. [DOI] [PubMed] [Google Scholar]
- 15. McGill DE, Volkening LK, Butler DA, Harrington KR, Katz ML, Laffel LM. Baseline psychosocial characteristics predict frequency of continuous glucose monitoring in youth with type 1 diabetes. Diabetes Technol Ther. 2018;20(6):434-439. doi: 10.1089/dia.2018.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rebecca K, Snelgrove DEM, Lori MB. Laffel adolescence and emerging adulthood: diabetes in transition. In: Richard IGH, Clive C, Allan F, Barry JG, eds. Textbook of diabetes. 5th ed. John Wiley; 2017:896–908. [Google Scholar]
- 17. McGill DE, Volkening LK, Butler DA, Wasserman RM, Anderson BJ, Laffel LM. Text-message responsiveness to blood glucose monitoring reminders is associated with HbA1c benefit in teenagers with Type 1 diabetes. Diabet Med. 2019;36(5):600-605. doi: 10.1111/dme.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGill DE, Laffel LM, Volkening LK, et al. Text message intervention for teens with type 1 diabetes preserves HbA1c: results of a randomized controlled trial. Diabetes Technol Ther. 2020;22(5):374-382. doi: 10.1089/dia.2019.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts RE, Andrews JA, Lewinsohn PM, Hops H. Assessment of depression in adolescents using the Center for Epidemiologic Studies Depression Scale. Psychol Assess: J Consult Clin Psychol. 1990;2:122-128. [Google Scholar]
- 20. Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised Diabetes Family Conflict Scale. Diabetes Care. 2007;30(7):1764-1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vesco AT, Anderson BJ, Laffel LM, Dolan LM, Ingerski LM, Hood KK. Responsibility sharing between adolescents with type 1 diabetes and their caregivers: importance of adolescent perceptions on diabetes management and control. J Pediatr Psychol. 2010;35(10):1168-1177. doi: 10.1093/jpepsy/jsq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta SN, Nansel TR, Volkening LK, Butler DA, Haynie DL, Laffel LM. Validation of a contemporary adherence measure for children with Type 1 diabetes: the Diabetes Management Questionnaire. Diabet Med. 2015;32(9):1232-1238. doi: 10.1111/dme.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markowitz JT, Volkening LK, Butler DA, Laffel LM. Youth-perceived burden of type 1 diabetes: problem areas in diabetes survey-pediatric version (PAID-Peds). J Diabetes Sci Technol. 2015;9(5):1080-1085. doi: 10.1177/1932296815583506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio J, Anderson BJ, Laffel LM. Re—examining a measure of diabetes—related burden in parents of young people with Type 1 diabetes: the Problem Areas in Diabetes Survey—Parent Revised version (PAID-PR). Diabet Med. 2012;29(4):526-530. doi: 10.1111/j.1464-5491.2011.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26(3):631-637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 26. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD clinical practice consensus guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(suppl 27):105-114. doi: 10.1111/pedi.12737. [DOI] [PubMed] [Google Scholar]
- 27. Wong JC, Boyle C, DiMeglio LA, et al. Evaluation of Pump Discontinuation and Associated Factors in the T1D Exchange Clinic Registry. J Diabetes Sci Technol. 2017;11(2):224-232. doi: 10.1177/1932296816663963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prahalad P, Addala A, Buckingham BA, Wilson DM, Maahs DM. Sustained continuous glucose monitor use in low-income youth with type 1 diabetes following insurance coverage supports expansion of continuous glucose monitor coverage for all. Diabetes Technol Ther. 2018;20(9):632-634. doi: 10.1089/dia.2018.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23:306–313. doi: 10.1089/dia.2020.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Commissariat PV, Boyle CT, Miller KM, et al. Insulin pump use in young children with type 1 diabetes: sociodemographic factors and parent-reported barriers. Diabetes Technol Ther. 2017;19(6):363-369. doi: 10.1089/dia.2016.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G, Beck RW, Buckingham B, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947-1953. doi: 10.2337/dc09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Addala A, Maahs DM, Scheinker D, Chertow S, Leverenz B, Prahalad P. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes. 2020;21(7):1301-1309. doi: 10.1111/pedi.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardona-Hernandez R, Schwandt A, Alkandari H, et al. Glycemic outcome associated with insulin pump and glucose sensor use in children and adolescents with type 1 diabetes. Data from the International Pediatric Registry SWEET. Diabetes Care. 2021;44:1176–1184. doi: 10.2337/dc20-1674. [DOI] [PubMed] [Google Scholar]
- 34. Braune K, O’Donnell S, Cleal B, et al. Real-world use of do-it-yourself artificial pancreas systems in children and adolescents with type 1 diabetes: online survey and analysis of self-reported clinical outcomes. JMIR Mhealth Uhealth. 2019;7(7):e14087. doi: 10.2196/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? a survey of current users. Diabetes Technol Ther. 2013;15(4):295-301. doi: 10.1089/dia.2012.0298. [DOI] [PubMed] [Google Scholar]
- 36. Kubiak T, Priesterroth L, Barnard-Kelly KD. Psychosocial aspects of diabetes technology. Diabet Med. 2020;37(3):448-454. doi: 10.1111/dme.14234. PubMed PMID: 31943354 [DOI] [PubMed] [Google Scholar]
- 37. Anderson BJ. Family conflict and diabetes management in youth: clinical lessons from child development and diabetes research. Diabetes Spectrum. 2004;17(1):22-26. doi: 10.2337/diaspect.17.1.22. [DOI] [Google Scholar]
- 38. Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LM. Family conflict, adherence, and glycaemic control in youth with short duration Type 1 diabetes. Diabet Med. 2002;19(8):635-642. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- 39. Alsaleh FM, Smith FJ, Thompson R, Al-Saleh MA, Taylor KM. Insulin pump therapy: impact on the lives of children/young people with diabetes mellitus and their parents. Int J Clin Pharm. 2014;36(5):1023-1030. doi: 10.1007/s11096-014-9990-1. [DOI] [PubMed] [Google Scholar]
- 40. Rankin D, Harden J, Noyes K, Waugh N, Barnard K, Lawton J. Parents’ experiences of managing their child’s diabetes using an insulin pump: a qualitative study. Diabet Med. 2015;32(5):627-634. doi: 10.1111/dme.12683. [DOI] [PubMed] [Google Scholar]
- 41. Sullivan-Bolyai S, Knafl K, Tamborlane W, Grey M. Parents’ reflections on managing their children’s diabetes with insulin pumps. J Nurs Scholarsh. 2004;36(4):316-323. doi: 10.1111/j.1547-5069.2004.04058.x. [DOI] [PubMed] [Google Scholar]
- 42. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. doi: 10.1001/jama.2016.11708. [DOI] [PubMed] [Google Scholar]
- 43. Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21(2):319-327. doi: 10.1111/pedi.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lawson ML, Shephard AL, Feenstra B, Boland L, Sourial N, Stacey D. Decision coaching using a patient decision aid for youth and parents considering insulin delivery methods for type 1 diabetes: a pre/post study. BMC Pediatr. 2020;20(1):1. doi: 10.1186/s12887-019-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]