Abstract
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Diabetic Foot Consortium (DFC) was established in September 2018 by the NIDDK to build an organization to facilitate the highest quality of clinical research on diabetic foot ulcers (DFUs) that will answer clinically significant questions to improve DFU healing and prevent amputations. The initial focus of the DFC is to develop and validate biomarkers for DFUs that can be used in clinical care and research. The DFC consists of a data coordinating center (DCC) for operational oversight and statistical analysis, clinical sites for participant recruitment and evaluation, and biomarker analysis units (BAUs). The DFC is currently studying biomarkers to predict wound healing and recurrence and is collecting biosamples for future studies through a biorepository. The DFC plans to address the challenges of recruitment and eligibility criteria for DFU clinical trials by taking an approach of “No DFU Patient Goes Unstudied.” In this platform approach, clinical history, DFU outcome, wound imaging, and biologic measurements from a large number of patients will be captured and the in-depth longitudinal data set will be analyzed to develop a computational-based DFU risk factor profile to facilitate scientifically sound clinical trial design. The DFC will expand its platform to include studies of the role of social determinants of health, such as food insecurity, housing instability, limited health literacy, and poor social support. The DFC is starting partnerships with the broad group of stakeholders in the wound care community.
Keywords: biomarkers, clinical network, diabetic foot ulcers, diabetic wound healing
Background
Diabetic foot ulcers (DFUs) are the visible wounds inflicted by years of a multitude of factors, starting with metabolic insults inherent to diabetes. Poor circulation, neuropathy, friction, and trauma to the foot add to the risk for a DFU and lower extremity amputation. The DFUs remain one of the most devastating complications of diabetes due to the high risk of lower extremity amputations, poor quality of life, low physical and social functioning, high mortality, and unacceptably high costs.1-4 The five-year mortality rates after lower extremity amputations may be as high as 50%.3,5 Current standard of care, interpreted and practiced variably across the wound care continuum, leaves approximately 70% of DFUs unhealed, a percentage that has not changed in decades.6,7 The poor healing reflects the comorbidities of vascular disease, neuropathy, and chronic inflammation, as well as the lack of effective therapies. Development of DFU therapies has stalled with the last drug approved in 1984 despite considerable advances in understanding the biology of acute and chronic wounds. Surgical and pressure relief therapies have been proven beneficial interventions for limb salvage but do not forecast or slow disease progression.
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) supports basic and clinical research on diabetic wound healing and sought to improve the translation of discoveries on wound pathology to therapies that would heal DFUs and prevent amputations. The NIDDK convened a panel of experts in the field, who identified a clinical network and DFU biomarkers as two areas of greatest need to advance DFU clinical research. These priorities were incorporated as the goals for the NIDDK Diabetic Foot Consortium (DFC).
Build an Infrastructure to Facilitate the Highest Quality of Clinical Research on DFUs
The DFC is the first multicenter national network dedicated to the study of DFUs. The consortium has attracted the leaders of the field with complementary expertise to select the most promising biomarkers and design protocols that incorporate current site-specific standard of care, outcome measures, and statistical analysis. Implementation of these studies at multiple sites accelerates and optimizes recruitment, a common difficulty in DFU research, and tests the feasibility of biomarker data collection in different clinical settings.
Validate Biomarkers for DFUs That can be Used in Clinical Care and Research
The DFC uses the FDA-NIH definition of a biomarker “as a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. Molecular, histologic, radiographic, or physiologic characteristics are types of biomarkers.” 8 The use of validated biomarkers holds the potential to radically improve treatment and clinical trials for DFUs. Notably, no validated biomarkers for DFU healing or recurrence currently exist. Current treatment strategy includes a tier-based approach in which everyone is treated with standard care at first. If the wound has only limited improvement after several weeks (typically four), more aggressive and expensive therapies are implemented. This “trial and error” approach is costly and ineffective. Thus, developing new biological and/or digital technology markers to predict DFU wound healing or wound recurrence would have a profound impact on millions of patients with diabetes mellitus by (1) aiding the decision-making process in DFU care by identifying those wounds unlikely to respond to standard treatment and require more aggressive therapy; (2) monitoring clinical progression of healing; (3) facilitate testing of new therapies in more homogeneous patient populations; and (4) predicting recurrence to inform prevention and early intervention. Most biological and imaging biomarkers could be obtained at clinic visits, whereas digital biomarkers could be also obtained using biologic sensors that continuously monitor the status of the DFU.
DFC Organization
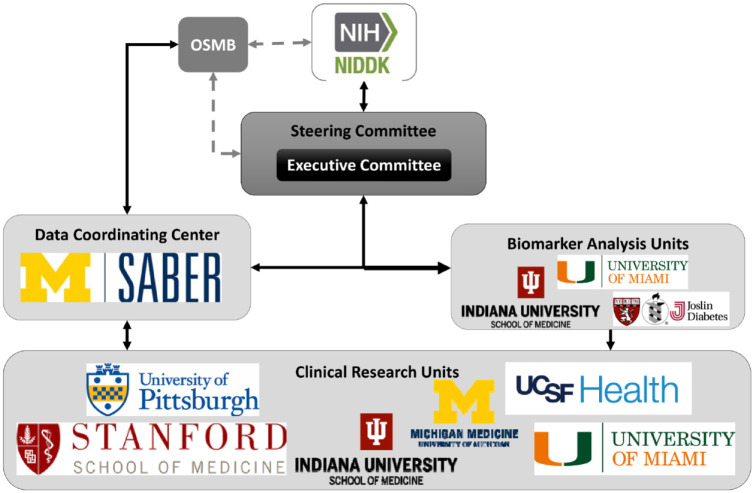
The DFC 9 was funded by the NIDDK in 2018 and created the first multicenter network to study DFUs to improve diabetic wound healing and prevent amputations among the 37 million Americans with diabetes. The current organization of the DFC that consists of a data coordinating center (DCC), six clinical research units (CRUs), and four biomarker analysis units (BAUs) and brings together expertise in the clinical and basic science of wound healing, biostatistics, vascular and plastic surgery, podiatry, dermatology, endocrinology, nursing, and social determinants of health (SDH) (Figure 1).
Figure 1.
Organization and components of the Diabetic Foot Consortium.
The CRUs are responsible for all interactions with participants. They enroll patients who have a recently closed DFU or an open DFU that is being treated with standard care. Following the biomarker protocols, they collect medical histories, biospecimens, and DFU images and perform bedside tests. Biospecimens or biomarker data are sent to the BAUs for laboratory measurements and analysis. The DCC provides operational support and statistical expertise. The DCC and CRUs were selected through Funding Opportunity Announcements, RFA-DK-17-014 and RFA-DK-18-010, which provide more information about their roles in the DFC.
The DFC Steering Committee consists of investigators with a broad range of complementary expertise and includes podiatrists, vascular surgeons, plastic surgeons, endocrinologists, dermatologists, nurses, clinical coordinators, and basic/translational scientists. It develops clinical biomarker protocols through a consensus building process that takes into consideration differences in clinical and research practices at six CRU institutions. Critical discussions are held on the entry criteria to find the balance between narrow eligibility criteria strongly supported by ample pilot data and the likely slower recruitment versus broader eligibility criteria that facilitates recruitment and increases the potential usefulness, but also poses greater risk for biomarker validation. The DCC statisticians worked with the study teams and the DFC Steering Committee to determine sample size to validate the utility of the biomarkers with sufficient power for each study, as well as develop sophisticated analytic approaches. The BAU Study Chairs and the DCC provide oversight on the execution of the protocols and make recommendations to the Steering Committee on protocol improvements. The DFC provides opportunity to support additional projects. The DFC Ancillary Study Committee reviews requests from investigators that want to collaborate through access to biosamples, DFU images or data collected through the Biorepository Protocol (see below) or through collection of new biosamples, DFU images, and/or data. More information about the consortium and Ancillary Study requests can be found at the DFC Web site. 9
DFC Biomarker Studies
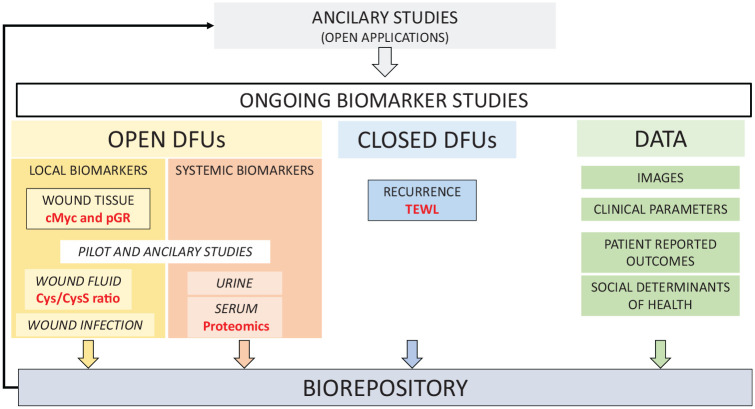
There are several ongoing studies encompassing local and systemic biomarkers for predicting wound closure and recurrence and collecting biosamples and data for future studies (Figure 2). These biomarkers were selected either through (1) a NIDDK Workshop in October 2018 soliciting candidate biomarkers ready for validation in a multicenter study or (2) a NIDDK funding opportunity for early-stage development of DFU biomarkers, RFA-DK-21-001. The DFC considers these biomarkers as only the initial steps within the multitude of potential biomarkers of ulcer and patient characteristics that would provide a comprehensive understanding of chronic diabetic wounds and their healing. In addition to biomarkers, current studies include validated surveys, completed by participants, that address neuropathy signs and symptoms, quality of life, and sleep quality.10-13
Figure 2.
Biomarker and biorepository research in the Diabetic Foot Consortium.
Development of a Biomarker for Nonhealing DFUs—cMyc and Phosphorylated Glucocorticoid Receptor
The first biomarkers selected for study in the DFC were from the Wound Healing Research Program at the University of Miami (PI Dr Marjana Tomic-Canic), based on promising preliminary data showing that cMyc and phosphorylated glucocorticoid receptor (pGR) were specific and prognostic wound-healing biomarkers. These molecules have strong preclinical evidence and biological role in the wound healing process.14-19 The approach is based on an established premise that tissue samples from DFUs provide a unique resource to assess their pathophysiology. 20 The hypothesis is that edges of nonhealing (open for at least four weeks) chronic wounds contain tissue that is unresponsive to wound-healing stimuli21-23 due to increases in inhibitors of healing. This tissue can be used to assess nonhealing biomarkers as an objective clinical indicator of prognosis.24-26 Both proposed biomarkers, cMyc and pGR, are part of signaling network that plays a major role in the pathophysiology of DFUs and predict wound size reduction at four weeks in a pilot study of DFU patients.14-18 At baseline and four weeks, the CRUs collect and send wound edge tissue samples that are obtained during DFU debridement. The BAU analyzes the sample for tissue quality and the cellular localization and quantification of cMyc and pGR in the epidermal cells. The study will test and validate if a quantification of nuclear cMyc and pGR at the baseline visit will predict whether wounds will heal by 12 weeks with standard care treatment. Standard care treatment is based on published guidelines and includes off-loading with total contact casts or removable cast walkers,27,28 whereas a complete wound closure is defined by skin reepithelialization without drainage or dressing requirements confirmed at two consecutive study visits two weeks apart. 29
Development of a Biomarker for DFU Recurrence—Transepidermal Water Loss Study
The second biomarker selected was from the Comprehensive Wound Center at Indiana University (PI Dr. Chandan K. Sen) based on promising multicenter pilot data showing that high wound transepidermal water loss (TEWL) may be an early indicator of risk for DFU recurrence. Currently, the FDA only recognizes the endpoint of complete wound closure in clinical trials, which is defined as skin reepithelialization with no discharge at two consecutive study visits, two weeks apart.2,29 However, it has become clear that reepithelialization does not guarantee restoration of the skin barrier,30-32 which is a critical determinant of skin function and resistance to infection. An impaired skin barrier can lead to wound recurrence and abnormalities of the repaired skin of DFU patients may underlie the high rates of recidivism in diabetic wounds (30% recur within one year).2,33 The hypothesis is that restoration of skin barrier function, a marker of functional wound closure,34-36 as measured by TEWL at wound closure will predict recurrence in the next 16 weeks. If validated, TEWL levels could change the outcome measure for healing and alert the clinician early on to the need for more aggressive management. The CRUs recruit participants with a recently closed DFU and measure TEWL using a hand-held device (Cortex DermaLab Combo Complete Skin Analyzer System; Cortex Technologies, Hadsund, Denmark) at five specified locations on the healed ulcer and three measurements are obtained on intact skin from same anatomic area on the opposite limb. Measures are obtained at baseline and two weeks later for confirmation of DFU healing. The participants are then contacted weekly by telephone for 16 weeks for the status of the site of the healed DFU and repeat TEWL measurements are performed at week 16, if the DFU remains closed.
Development of a DFU Bio- and Data Repository—A Resource of High-Quality Biosamples and Longitudinal Data
An important goal of the DFC is to facilitate clinical and basic research of the DFUs. Therefore, to maximally utilize clinical network and patients enrolled, the DFC established a bio- and data repository. The Biorepository Committee, under the leadership of Dr Brian Schmidt at the University of Michigan, developed a protocol to collect high-quality biological samples (blood, urine, wound tissue, and fluid), DFU images, and data using standardized methods from participants in active DFC protocols. Biorepository samples are collected at two time points during active participation in DFC studies and long-term outcomes are collected by telephone every six months for two years. The data collected as part of these protocols include medical histories, lower extremity examinations, DFU treatments, patient-reported outcomes, and wound images. Such longitudinal collection of both biological materials and clinical data is unique for the field of DFUs and creates a major research resource. The biorepository is intentionally “future proof” and will provide material for metagenomics, next-generation sequencing, and single-cell transcriptomics, as well as development of biomarkers based on wound images. Biosamples and data will be available through the DFC Central Laboratory and eventually through the NIDDK Central Repository.
Patient-Reported Outcomes
Several patient-centered outcomes are currently assessed using a variety of self-reported validated surveys including the Michigan Neuropathy Screening Instrument (MNSI), a validated instrument to assess neuropathic symptoms and pain; SF-12 that assesses the impact of health on everyday life; the Diabetes Foot Ulcer Scale that assess the impact of foot ulcers on treatments on quality of life; and PROMIS 4a (sleep quality).9-12
Early-stage development of DFU biomarkers
Basic wound healing research has made considerable progress in understanding many pathways that contribute to the poor healing of DFUs and provide additional opportunities for biomarker development. These studies, often using animal models, have revealed many potential biomarker and therapeutic targets, but further advancement and effective translation requires (1) proof-of-concept studies of human DFUs that encompass skin and microbiome changes from diabetes, the unique features of plantar skin and foot pressure, and the inflammation of chronic wounds; and (2) the development and validation of the biomarker measurement methods with performance characteristics that are acceptable in terms of sensitivity, specificity, accuracy, and precision. The DFC is supporting the early translation of DFU biomarkers through funding by NIDDK and the Special Diabetes Program for Type 1 Diabetes Research, RFA-DK-21-001. A collaboration between the wound care programs at Beth-Israel Deaconess and Joslin Diabetes Center (PIs Dr Aristides Veves and Dr Monika Niewczas) will use a proteomics approach to determine whether a panel of serum proteins can predict poor DFU healing. High-throughput approaches have been shown successful in identifying candidate biomarkers and underlying mechanisms in the research by Drs Veves and Niewczas laboratories.37,38 The Comprehensive Wound Center at Indiana University Health (PI Dr Sashwati Roy) will test whether a low ratio of cysteine to cystine in wound fluid, as an indicator of tissue redox perturbation, will predict nonhealing of a DFU in 12 weeks.
Future DFC Activities
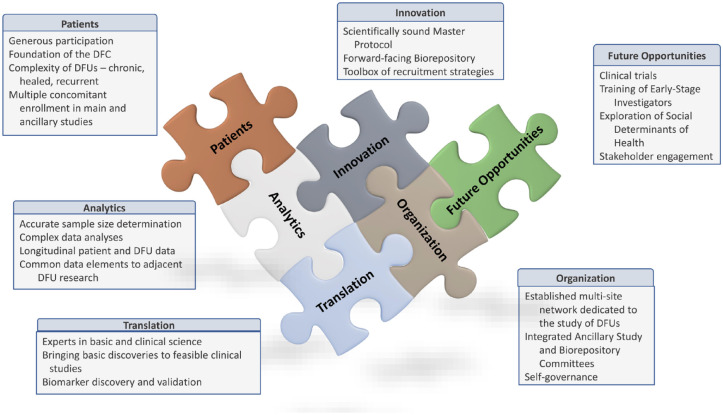
In the future, the DFC plans to leverage its infrastructure to expand its research scope beyond the validation of DFU biomarkers to address significant challenges in DFU research (Figure 3).
Figure 3.
Current and future activities in the Diabetic Foot Consortium.
“No DFU Patient Goes Unstudied”—A Model to Solve the Critical Challenges of Eligibility Criteria and Recruitment in DFU Research
Execution of high-quality clinical trials for DFUs is difficult with recruitment, 6 a well-recognized challenge of any clinical study related to DFU, due, in part, to investigators with unrealistic enrollment expectations, DFU patients with variable and complex comorbidities,39,40 and ulcers with heterogeneous biology and pathology.20,23,41 The DFC faced enrollment challenges during the implementation of the two biomarker studies, from the COVID-19 pandemic but also many patients are screened but few enroll. In an attempt to make accurate comparisons in more homogeneous populations, protocols often exclude the more complicated and high-risk patients. We have determined that these patients may hold the key to unlocking the etiologies of DFU pathology and triggered the rise of the “No DFU Patient Goes Unstudied” concept, which is an opportunity to capture clinical, outcome, biologic, and other data from the large number of patients excluded from enrollment in our initial biomarker studies. This concept will allow all willing patients with a DFU to participate in a platform model of protocols being performed by the DFC, regardless of their individual wound or patient characteristics. These protocols may include biomarker studies, longitudinal registries, patient-centered outcomes, and others to maximize the data accrued from all DFU patients clinically seen at each of the CRUs.
The DFC plans to explore the use of artificial intelligence and advanced learning methods on the rich complex data on this broad population of participants. In addition to the clinical data, the DFC can incorporate newly developed DFC biomarker discovery data and ‘omics data from biospecimens to analyze factors that are associated with good clinical outcomes and develop a computational-based DFU risk factor profile. The DFC can take that knowledge to design scientifically sound clinical trials using master protocols in a platform approach to simultaneously study multiple-target therapies or a single-target therapy in a precision-medicine approach that maximizes the enrollment of DFU patients. The goal is to take “No DFU Patient Goes Unstudied” to “A Study Protocol for Every DFU Patient.”
Social Determinants of Health and Their Effect on DFU Healing and Recurrence
Patients who suffer from a DFU face numerous social, emotional, and instrumental challenges to achieve DFU healing. While it is known that the risks of DFU and associated limb amputation increase with age, tobacco use, vascular disease, peripheral neuropathy, and duration of diabetes, the extent to which social and environmental contextual factors (“SDH”) present at the individual level are determinants of either failure to heal or recurrence of a previously healed DFU is not known. This represents a significant gap in knowledge, with implications for our understanding of (1) the prevention of DFUs, (2) risk stratification of DFUs, (3) intervention strategies to promote healing, and (4) interventions to prevent recurrence and, ultimately, amputation. Insofar, as the prevalence of type 2 diabetes and its complications are much greater among certain racial and ethnic subgroups in the United States (African American, Hispanic, American Indian, and specific Asian subgroups), determining the extent to which differential DFU outcomes by race and ethnicity can be explained by SDH represents a critical step in our efforts to reduce the “mega-disparities” observed.
The DFC will investigate the extent to which the four domains of food insecurity, housing instability, limited health literacy, and poor social support are associated with nonhealing DFU or DFU recurrence. The DFC is well suited to begin work in this critical domain because of the extensive demographic, clinical, and quality-of-life data and biomarker measurements collected during the studies. Knowledge of the effect of SDH will aid the DFC in a comprehensive understanding of the healing or recurrence potential for each DFU patient.
Training and leadership opportunities for early-stage investigators
Wound care is an emerging medical specialty that is not currently supported by a well-developed residency or fellowship training program. The clinical treatment of complex wounds, such as DFUs, requires multidisciplinary collaboration among surgeons, endocrinologists. dermatologists, infectious disease specialists, podiatrists, nurses, physical therapists, and social workers. There are more than 1000 wound care centers, community-based and academic, in the United States that are administered by wound care management companies. 42 Despite this complexity, there are few formal training opportunities in either the clinical practice of wound care or the research underpinnings of the field. The DFC can provide an opportunity to bridge this gap and develop the next generation of both clinician-scientists and researchers to foster improvement in wound care. 43 Currently, young clinicians and researchers are involved and participate in the clinical and organizational activities of the DFC. In the future, the DFC would like to expand these opportunities to include pilot research studies and a multidisciplinary training program.
Conclusion
The DFC is in its early stages as a national network for the clinical study of DFUs. Several biomarkers are currently being studied and more are in the planning phases. In addition, a pathway to support additional investigator-initiated, ancillary studies is in place. The DFC has the potential to change the clinical trial paradigm for DFU therapies through a holistic understanding of DFU patients and their wounds. Advances in diabetic wound healing will occur with alignment of interests of the many stakeholders in DFU care including patients, caregivers, clinicians, payors, pharmaceutical and device businesses, and regulatory agencies. The role of the DFC is to engage stakeholders (academic, community, and industry) to support high-quality clinical research that will aid this alignment of interests by producing scientifically sound results that inform clinical care, policy decisions, and therapy development.
Acknowledgments
We thank the DFU patients who have generously given of their time to participate in DFC studies. The Diabetic Foot Consortium: University of Michigan Data Coordinating Center: Cathie Spino, Peter Song, Katy Clark; Indiana University: Chandan K. Sen, Gayle Gordillo, Sashwati Roy, Mithun Sinha and Lava Timsina; University of Michigan: Rodica Pop-Busui, Brian M. Schmidt, Crystal Holmes, Aaron Burant, Aimee Katona, Catherine Martin, Nicole Baker, Katherine Gallagher, Jonathan Gryak, Kayvan Najarian, Cynthia Plunkett, Brittany Williams, Iulia Dobrin, Sejal Gunarantnam; Stanford University: Geoffrey Gurtner; University of California, San Francisco: Michael Conte, Dean Schillinger, Alexander Reyzelman, Monara Dini; University of Miami: Robert Kirsner, Marjana Tomic-Canic, Hadar Lev-Tov; University of Pittsburgh: Peter Rubin; NIDDK: Henry Burch and Teresa Jones.
Footnotes
Abbreviations: BAU, biomarker analysis unit; CRU, clinical research unit; DCC, data coordinating center; DFC, Diabetic Foot Consortium; DFU, diabetic foot ulcer; MNSI, Michigan Neuropathy Screening Instrument; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; OSMB, Observational Safety and Monitoring Board; pGR, phosphorylated glucocorticoid receptor; PI, Principal Investigator; SDH, social determinants of health; TEWL, transepidermal water loss.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NIDDK through grants U24 DK122927, U01 DK119083, U01 DK119100, U01 DK119094, U01 DK119085, U01 119102, U01 DK119099, R61 DK131915 and R61 DK131909 and the Special Diabetes Program for Type 1 Diabetes Research.
ORCID iDs: Teresa L.Z. Jones  https://orcid.org/0000-0001-8278-2322
https://orcid.org/0000-0001-8278-2322
Rodica Pop-Busui  https://orcid.org/0000-0002-2042-1350
https://orcid.org/0000-0002-2042-1350
Brian M. Schmidt  https://orcid.org/0000-0002-2561-9243
https://orcid.org/0000-0002-2561-9243
References
- 1. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. [DOI] [PubMed] [Google Scholar]
- 3. Microvascular complications and foot care: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S124-S138. [DOI] [PubMed] [Google Scholar]
- 4. Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514-1523. [DOI] [PubMed] [Google Scholar]
- 5. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651-658. [DOI] [PubMed] [Google Scholar]
- 6. Bolton LL. Quality randomized clinical trials of topical diabetic foot ulcer healing agents. Adv Wound Care (New Rochelle). 2016;5(3):137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fife CE, Eckert KA, Carter MJ. Publicly reported wound healing rates: the fantasy and the reality. Adv Wound Care (New Rochelle). 2018;7(3):77-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. https://www.ncbi.nlm.nih.gov/books/NBK326791/. Accessed 12 July 2022.
- 9. http://diabeticfootconsortium.org/. Accessed 12 July 2022.
- 10. Askew RL, Cook KF, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol. 2016;73:103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wukich DK, Sambenedetto TL, Mota NM, Suder NC, Rosario BL. Correlation of SF-36 and SF-12 component scores in patients with diabetic foot disease. J Foot Ankle Surg. 2016;55(4):693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bann CM, Fehnel SE, Gagnon DD. Development and validation of the Diabetic Foot Ulcer Scale-Short Form (DFS-SF). Pharmacoeconomics. 2003;21(17):1277-1290. [DOI] [PubMed] [Google Scholar]
- 13. Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. 2012;29(7):937-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jozic I, Sawaya AP, Pastar I, et al. Pharmacological and genetic inhibition of caveolin-1 promotes epithelialization and wound closure. Mol Ther. 2019;27(11):1992-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jozic I, Vukelic S, Stojadinovic O, et al. Stress signals, mediated by membranous glucocorticoid receptor, activate PLC/PKC/GSK-3β/β-catenin pathway to inhibit wound closure. J Invest Dermatol. 2017;137(5):1144-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sawaya AP, Pastar I, Stojadinovic O, et al. Topical mevastatin promotes wound healing by inhibiting the transcription factor c-Myc via the glucocorticoid receptor and the long non-coding RNA Gas5. J Biol Chem. 2018;293(4):1439-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vukelic S, Stojadinovic O, Pastar I, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286(12):10265-10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stojadinovic O, Lee B, Vouthounis C, et al. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. J Biol Chem. 2007;282(6):4021-4034. [DOI] [PubMed] [Google Scholar]
- 19. Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167(1):59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pastar I, Wong LL, Egger AN, Tomic-Canic M. Descriptive vs mechanistic scientific approach to study wound healing and its inhibition: is there a value of translational research involving human subjects? Exp Dermatol. 2018;27(5):551-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg. 2016;138(3, suppl.):18s-28s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brem H, Stojadinovic O, Diegelmann RF, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13(1-2):30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stojadinovic O, Pastar I, Nusbaum AG, Vukelic S, Krzyzanowska A, Tomic-Canic M. Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound Repair Regen. 2014;22(2):220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stone RC, Stojadinovic O, Rosa AM, et al. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med. 2017;9(371):eaaf8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawaya AP, Stone RC, Brooks SR, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun. 2020;11(1):4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the society for vascular surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2, suppl.):3S-21S [DOI] [PubMed] [Google Scholar]
- 28. American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 12. retinopathy, neuropathy, and foot care: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(suppl 1):S185-S194. [DOI] [PubMed] [Google Scholar]
- 29. United States Department of Health and Human Services, Centers for Disease Control. Guidance for industry: chronic cutaneous ulcer and burn wounds-developing products for treatment. Wound Repair Regen. 2001;9(4):258-268. [DOI] [PubMed] [Google Scholar]
- 30. Barki KG, Das A, Dixith S, et al. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann Surg. 2019;269(4):756-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roy S, Elgharably H, Sinha M, et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol. 2014;233(4):331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roy S, Santra S, Das A, et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann Surg. 2020;271(6):1174-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rankin TM, Miller JD, Gruessner AC, Nickerson DS. Illustration of cost saving implications of lower extremity nerve decompression to prevent recurrence of diabetic foot ulceration. J Diabetes Sci Technol. 2015;9(4):873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rustagi Y, Abouhashem AS, Verma P, et al. Endothelial phospholipase Cγ2 improves outcomes of diabetic ischemic limb rescue following VEGF therapy. Diabetes. 2022;71(5):1149-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sinha M, Ghosh N, Wijesinghe DS, et al. Pseudomonas aeruginosa theft biofilm require host lipids of cutaneous wound [published online ahead of print October 12, 2021]. Ann Surg. doi: 10.1097/SLA.0000000000005252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sen CK, Roy S. The hyperglycemia stranglehold stifles cutaneous epithelial‒mesenchymal plasticity and functional wound closure. J Invest Dermatol. 2021;141(6):1382-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Theocharidis G, Baltzis D, Roustit M, et al. Integrated skin transcriptomics and serum multiplex assays reveal novel mechanisms of wound healing in diabetic foot ulcers. Diabetes. 2020;69(10):2157-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niewczas MA, Pavkov ME, Skupien J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25(5):805-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maderal AD, Vivas AC, Eaglstein WH, Kirsner RS. The FDA and designing clinical trials for chronic cutaneous ulcers. Semin Cell Dev Biol. 2012;23(9):993-999. [DOI] [PubMed] [Google Scholar]
- 40. Nickinson ATO, Bridgwood B, Houghton JSM, et al. A systematic review investigating the identification, causes, and outcomes of delays in the management of chronic limb-threatening ischemia and diabetic foot ulceration. J Vasc Surg. 2020;71(2):669-681. [DOI] [PubMed] [Google Scholar]
- 41. Stojadinovic O, Landon JN, Gordon KA, et al. Quality assessment of tissue specimens for studies of diabetic foot ulcers. Exp Dermatol. 2013;22(3):216-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ennis WJ. Wound care specialization: the current status and future plans to move wound care into the medical community. Adv Wound Care (New Rochelle). 2012;1(5):184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]