Abstract
Background:
Electrical stimulation (E-Stim) may offer a unique adjunctive treatment to heal complicated diabetic foot ulcers (DFU). Our primary goal is to examine the effectiveness of daily home-based E-Stim therapy to speed-up wound healing.
Methods:
Patients with chronic DFUs and mild to severe peripheral arterial disease (PAD) were recruited and randomized to either control (CG) or intervention (IG) groups. The IG received 1-hour home-based E-Stim therapy on daily basis for 4 weeks (4W). E-Stim was delivered through electrical pads placed above the ankle joint using a bio-electric stimulation technology (BEST®) platform (Tennant Biomodulator® PRO). The CG was provided with an identical but non-functional device for the same period. The primary outcome included wound area reduction at 4W from baseline (BL).
Results:
Thirty-eight patients were recruited and 5 were removed due to non-compliance or infection, leaving 33 participants (IG, n = 16; CG, n =17). At 4W, the IG showed a significant wound area reduction of 22% (BL: 7.4 ± 8.5 cm2 vs 4W: 5.8 ± 8.0 cm2, P = 0.002). Average of wound area was unchanged in the CG (P = 0.982). The self-report adherence to daily home-therapy was 93.9%.
Conclusions:
Daily home-based E-Stim provides early results on the feasibility, acceptability, and effectiveness of E-Stim as an adjunctive therapy to speed up wound healings in patients with chronic DFU and mild to severe PAD.
Keywords: diabetic foot ulcer, electrical stimulation, home-based therapy, skin perfusion, tissue oxygenation, wound healing, wearables, amputation, limb salvage, neuromodulation
Introduction
Electrical stimulation (E-Stim) may offer an effective adjunctive treatment to accelerate wound healing, increase tissue perfusion, and reduce the likelihood of infection in patients with diabetes. 1 E-Stim is broadly utilized for different medical treatments, 2 including diabetic foot ulcers (DFU), yet it is not routinely prescribed as adjunctive therapy for managing wounds. Limited well-performed randomized control trials (RCTs), additional to the absence of level one evidence, have delayed determining application methodology to adopt home-based E-Stim as part of the standard of care.3,4 Consequently, controversy remains on the magnitude of efficacy and the best applicable protocol. 5 A non-invasive, portable, and cost-effective E-Stim device with minimal interference in patients’ daily life would be ideal for performing therapy. 6
Representative barriers for E-Stim application include: (1) Resource intensive. Administration should be supervised 7 especially for compromising areas;8,9 (2) Electrode pads location. E-Stim is often applied near wound beds 5 which requires removing dressings 10 and possibly increasing the likelihood of infection by frequent/inappropriate dressing manipulation.11,12 (3) Inconvenience of daily commutes to the clinic for E-Stim administration; thus, patients’ compliance determines the efficacy of therapy. 1 Only one study provided the practical modality of E-Stim home-based therapy for DFUs. 10 (4) Lack of patient acceptability. Electrode placement or electroshocking sensitivity may cause discomfort. 13
To address these barriers, the present study proposes daily home-based E-Stim adjunctive therapy through electrical adhesive pads applied above the ankle joint to reduce complications from dressing manipulation and further facilitating treatment in a practical, comfortable manner.
Methods
A double-blinded randomized control trial of patients diagnosed with diabetes mellitus type 2 with chronic non-healing wounds was performed. All participants were recruited from the outpatient vascular and endovascular clinic at Baylor St. Luke’s Medical Center (Houston, TX, USA) between March 2019 and March 2020. This study was approved by the local Institutional Review Board (IRB) at Baylor College of Medicine (Houston, TX). All participants read and signed the IRB approved forms of informed consent before initiation of any assessment or data collection. The protocol of the study was registered in clinicaltrials.gov, Identifier: NCT03821675. Inclusion criteria was age between 18 and 85 years old; clinically confirmed with diabetes mellitus type 2; peripheral neuropathy; one or more active chronic DFU (defined as a wound failing to heal after 4 weeks); and willing to maintain E-Stim application. Exclusion criteria was demand-type cardiac pacemaker, implanted defibrillator, or any other implanted electronic device; pregnant or actively lactating women; end-stage renal disease; active wound infection; active Charcot foot; non-ambulatory status; bilateral above or below the knee amputation; active drug/alcohol abuse; dementia or impaired cognitive function; excessive lymphedema; osteomyelitis and/or gangrene; unable to comply with research appointments; wide spread malignancy; systemically immuno-compromising disease; and history of any undercurrent illnesses or conditions that could compromise the safety of the subject according to judgment of a qualified wound specialist.
The demographic and clinical characteristics were collected via electronic medical records. All participants underwent clinical assessments such as Falls Self-Efficacy Scale (FES-I), Center for Epidemiologic Studies Depression (CES-D) scale, Montreal Cognitive Assessment (MoCA), Trauma-Self Frailty Index, and Pittsburgh Sleep Quality Index (PSQI), and Pain Visual Analog Scale (VAS). 14
Patients with DFUs were recruited and randomized (ratio: 1:1) to either control (CG) or intervention (IG) groups through a computer-generated list followed by sequential allocation concealment using sealed envelopes. Participants and care providers were blinded to the group allocation. The IG received E-Stim through electrode adhesive pads placed above ankle level in acupuncture points (KI3, Taixi) 15 of the affected foot using a bio-electric stimulation technology (BEST®) microcurrent platform (Tennant Biomodulator®, Dallas, TX, USA, Figure 1) adjunctive to standard of care (ie, topical mupirocin, calcium alginate, wet to dry dressing). The CG was provided with an identical, non-functional device (placebo) for the same period. E-Stim therapy was administered at home daily for 1 hour for 4 weeks (4W). Participants were monitored and clinically assessed on a weekly basis during the study duration. Compliance was determined by a questionnaire which records daily utilization, time of application, and adverse events.
Figure 1.
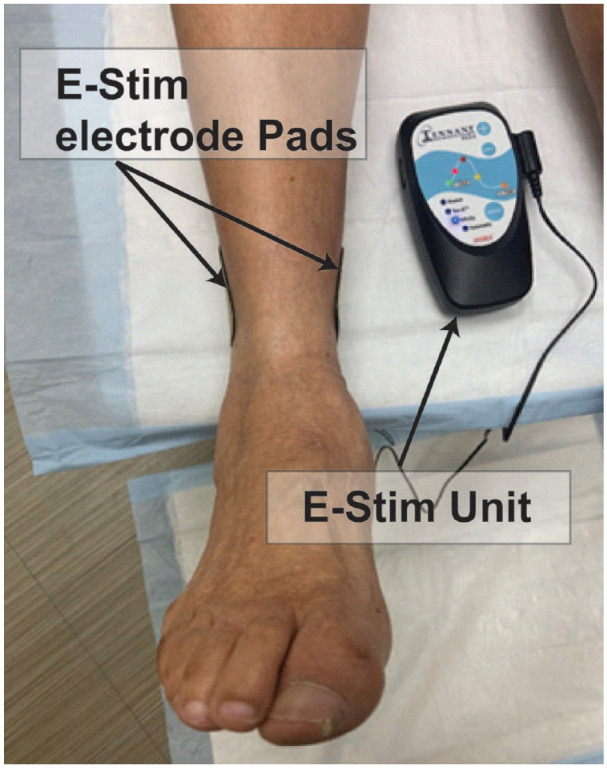
Participants received E-Stim through electrode adhesive pads placed above the level of the ankle in acupuncture points of the affected foot using a bio-electric stimulation technology® (BEST) microcurrent platform (Tennant Biomodulator®). The E-Stim was active in the intervention group and non-functional in the control group.
E-Stim therapy was delivered by an interactive high voltage pulsed alternative current (HVPAC) in the shape of an asymmetrical damped sinusoidal waveform as described in their products’ manual. 16 The waveform's electrical characteristics behave in relation to tissue response (ie, change in skin impedance) until the electrical conductivity stops changing (closed-loop E-Stim). Damping refers to the magnitude of the sinusoidal waveform, which gradually reduces to zero in order to form an asymmetrical biphasic pulsed current allowing muscle relaxation and avoiding muscle fatigue during therapy. 14 All patients underwent E-Stim application with a setting between 150 and 250 Volts. The intensity level has been previously FDA-cleared to cause no harm to the patients for transcutaneous electric nerve stimulation for pain relief.14,17
The primary outcomes were changes at 4W compared to baseline (BL) in wound area and depth as well as the speed of wound healing (defined as percentage change at 4W from BL in wound volume and depth). These outcomes were objectively assessed using a validated non-invasive 3D camera (Silhouette®, Aranz Medical, Christchurch, NZ) that detects length, width, depth, and percentage of weekly wound area reduction by automatic tracing the wound perimeter. Another primary outcome was skin perfusion pressure (SPP) at BL and 4W and changes in SPP at 4W compared to BL (∆SPP). SPP is defined as the minimum blood pressure required for restoring capillary flow after applying controlled occlusion. 18 Prior studies demonstrated SPP as a strong predictor of wound healing particularly among those with limb ischemia. 19 To measure SPP, an FDA-approved SensiLase Pad-IQ SPP device (CorVascular Diagnostics, LLC, MN, USA) was used. Cuffs were placed around the proximal gastrocnemius in order to obtain low extremity SPP values at each time point. The secondary outcome included BL and 4W tissue oxygen saturation (SatO2) and changes in SatO2 at 4W compared to BL (∆SatO2). SatO2 is a parameter demonstrated to be associated with wound outcomes particularly among patients with chronic wounds. 20 For SatO2, a validated non-invasive near-infrared (NIR) camera (Snapshot NIR, KENT Imaging Inc., Calgary, AB, Can) that detects an approximate value of SatO2 level in superficial tissue was used by tracing the wound perimeter in real-time. To reduce the bias of wound size and SatO2 measurements, all wounds were traced in its internal border, leaving out skin layers using an identical tracing technique for all subjects per weekly follow-up visit. Another secondary outcome was changes in plantar sensation at 2 weeks (2W) compared to BL assessed by vibration perception threshold (VPT) using a Horwell Neurothesiometer (Scientific Laboratory Supplies, Nottingham, U.K.) placed on the heel of the injured foot. Device acceptability was assessed using a technology acceptance model (TAM) 21 questionnaire tailored for the purpose of this study (Table 1).
Table 1.
Device Acceptability Survey Developed Based on Technology Acceptance Model to Evaluate Perceived Usefulness, Perceived Ease of use, and Attitude Toward Using the E-Stim at Home.
| # | TAM Item | Strongly agree (7) | Agree (6) | Somewhat agree (5) | Neutral (4) | Somewhat disagree (3) | Disagree (2) | Strongly disagree (1) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Perceived usefulness | I felt less pain during the day | |||||||
| 2 | I felt less pain during the night | ||||||||
| 3 | My foot felt less swollen | ||||||||
| 4 | I feel my wound is healing faster by using the device | ||||||||
| 5 | I would use the device again | ||||||||
| 6 | Caused me physical pain | ||||||||
| 7 | Perceived ease of use | The pads were easy to put on and off | |||||||
| 8 | The device was easy to use | ||||||||
| 9 | Attitude toward using | My doctor was happy with the results of my wound healing progress | |||||||
| 10 | I would recommend to a friend |
A Likert scale to score each response was utilized. Except for item #6, each answer was scored on a 7 to 1 scale, respectively; from strongly agree to strongly disagree. For item #6 a similar score on reversed order was provided.
The sample size was estimated based on our prior double-blinded RCT in which effectiveness of plantar E-Stim was examined in patients with diabetic neuropathy (n = 28). 22 In this study, there was a significant improvement with large effect size (Cohen effect size, d = 0.99) among those with an ankle brachial index (ABI) > 1.2. Because E-Stim has shown to improve vascular health as means of wound healing and neuropathy outcomes,1,4,23 we anticipated a similar effect in our trial. By assuming a statistical power of 80%, alpha of 5%, and two-tailed t-test, the minimum sample size required to observe change in primary outcomes is estimated to be 18 subjects per group. Assuming 10% dropout, we targeted to recruit 40 eligible participants, but halted at 38 eligible subjects in March 2020 due to the COVID-19 pandemic.
Continuous data were reported with mean ± standard deviation (SD). Categorical data were presented as count (%). Shapiro-Wilk test was conducted to identify the normal distribution of continuous variables. One-way ANOVA for normally distributed variables or Mann-Whitney U test for non-normally distributed variables was used to estimate differences of mean between control and intervention groups. χ2 test was used to determine significant level between the groups for categorical variables. The effect size was measured using OR and 95% CI for categorical variables, and Cohen’s d to discriminate the difference of primary and secondary outcomes between groups. Outcome changes were assessed over time compared to BL within (ie, time effect) and between (ie, time×group effect) groups using linear mixed model for normally distributed outcome and generalized estimating equations for non-normally distributed outcome with adjustment for potential confounders. Least significant difference method was used for multiple pairwise comparisons. Pearson and Spearman correlation analysis were performed to assess the relationship between wound or demographics/clinical characteristics, and vascular outcomes. The level of statistical significance was set at P < .050. To detect changes in perfusion in individuals with marked limb ischemia, a sub-analysis of patients with moderate to severe peripheral arterial disease (PAD) (ABI < 0.8 or > 1.4 at BL) 24 was performed. To detect associated risk comorbidities influencing wound healing in those healers (defined as ≥50% of area reduction) 25 vs non healers under E-stim, a sub-analysis in the IG was also performed. All results were performed using IBM SPSS Statistics version 27 (IBM, Armonk, NY, USA) and MATLAB Version R2018b (The MathWorks, Natick, MA, USA).
Results
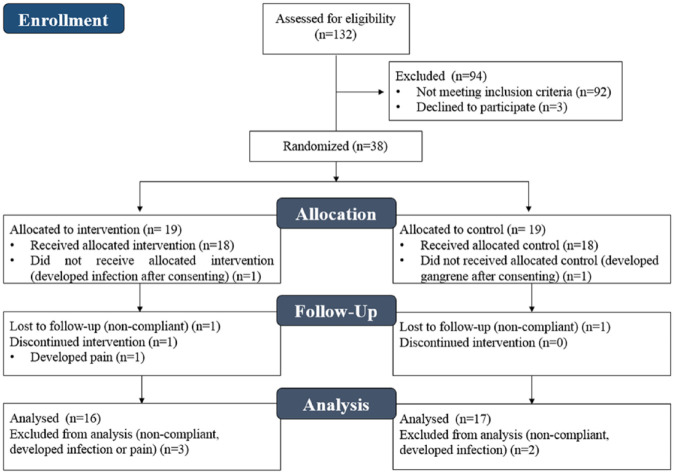
The progress and study phases are illustrated in the Consort Flow Diagram (Figure 2). Thirty-eight patients satisfied inclusion and exclusion criteria, and 33 (IG, n = 16; CG, n = 17) completed all study milestones and were included for data analysis. Demographics and clinical characteristics of these participants are summarized in Table 2. Demographics (age, BMI, and gender) and clinical characteristics (CKD, PAD, WIfI stage, HbA1c, etc.) showed no significant correlation with wound healing outcomes (P >0.05). In the IG, there were no significant differences (P >0.05) for demographics and clinical characteristics between healers and non-healers. Subsequently, 2 patients had their wound completely healed before study termination (early end-point); however, 6 (37.5%) patients had wound relapse after the study period in a mean time of 141.5 ± 52.0 days. No deaths or limb loss were reported during the study period.
Figure 2.
Consort flow diagram.
Table 2.
Overall Baseline Demographics and Clinical Characteristics in the 2 Groups.
| Intervention group (n = 16) | Control group (n = 17) | P-value | OR (95% CI) | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 65.1 ± 13.8 | 61.4 ± 11.2 | 0.400 | – |
| Male (no.) | 10 (62.5%) | 11 (64.7%) | 0.895 | 0.909 (0.220-3.758) |
| BMI (kg/m2) | 32.5 ± 9.2 | 29.2 ± 4.8 | 0.194 | – |
| Obesity (BMI ≥ 30) | 9 (56.3%) | 7 (41.2%) | 0.387 | 1.837 (0.461-7.312) |
| Clinical characteristics | ||||
| DM type 2 | 16 (100%) | 17 (100%) | 1.000 | – |
| HbA1C (% mmol/mol) | 8.0 ± 2.0 | 8.1 ± 1.5 | 0.791 | – |
| HbA1C >9 | 3/15 (20.0%) | 4/17 (23.5%) | 0.810 | 0.813 (0.150-4.404) |
| CKD <stage 5 (no.) | 5 (31.3%) | 8 (47.1%) | 0.353 | 0.511 (0.123-2.122) |
| DLP | 7 (43.8%) | 6 (35.3%) | 0.619 | 1.426 (0.351-5.793) |
| CAD | 5 (31.3%) | 5 (29.4%) | 0.909 | 1.091 (0.247-4.817) |
| CHF | 4 (25.0%) | 3 (17.6%) | 0.606 | 1.556 (0.289-8.379) |
| Anemia | 2 (12.5%) | 6 (35.3%) | 0.127 | 0.262 (0.044-1.560) |
| PAD | 9 (56.3%) | 9 (52.9%) | 0.849 | 1.143 (0.290-4.507) |
| TSFI (score) | 0.26 ± 0.13 | 0.24 ± 0.11 | 0.678 | – |
| Frail (no.) | 5/13 (38.5%) | 4/15 (26.7%) | 0.505 | 1.719 (0.347-8.508) |
| Prev. minor amp | 11 (68.8%) | 13 (76.5%) | 0.619 | 0.677 (0.145-3.159) |
| Prev. recanalization | 7 (43.8%) | 7 (41.2%) | 0.881 | 1.111 (0.279-4.423) |
| <180 days | 5 (31.3%) | 4 (23.5%) | 0.619 | 1.477 (0.317-6.895) |
| >180 days | 2 (12.5%) | 2 (11.8%) | 0.948 | 1.071 (0.132-8.670) |
| Daily prescribed meds (no.) | 9.81 ± 5.6 | 11.5 ± 5.3 | 0.389 | – |
| Wound characteristics | ||||
| WIfI stage | 0.509 | – | ||
| Stage 3/4 | 2 (12.5) | 1 (5.9) | ||
| Stage 1/2 | 14 (87.5) | 16 (94.1) | ||
| Wound area (cm2) | 7.4±8.5 | 3.1 ± 5.6 | 0.035 | – |
| Wound depth (mm) | 3.0 ± 2.1 | 3.2 ± 2.4 | 0.825 | – |
| WBC (no.) | 14/15 (93.3%) | 15/17 (88.2%) | 0.164 | – |
| None | 13/14 (92.9) | 11/15 (73.3%) | ||
| Rare-few | 1/14 (7.1%) | 4/15 (26.7%) | ||
| Mod-abund | 0/14 (0%) | 0/15 (0%) | ||
| Vascular & Neuropathic characteristics | ||||
| SPP (mmHg) | 71.0 ± 12.2 | 78.1 ± 18.6 | 0.211 | – |
| Tissue O2 (SatO2 %) | 69.3 ± 21.3 | 73.1 ± 13.5 | 0.971 | – |
| VPT (Volts) | 25.6 ± 17.2 | 25.9 ± 17.8 | 0.975 | – |
| Peripheral neuropathy, VPT>25 Volts (no.) |
7/15 (46.7%) | 6/13 (46.2%) | 0.978 | 1.021 (0.230-4.526) |
| ABI (ratio) | 0.8 ± 0.3 | 0.9 ± 0.5 | 0.677 | – |
| Moderate-severe PAD, ABI<0.8 or >1.40 (no.) |
6/12 (50%) | 6/13 (46.2%) | 0.848 | 1.167 (0.242-5.616) |
| Patient self-report metrics | ||||
| Concern for fall (FES-I score) | 28.8 ± 11.9 | 22.4 ± 4.9 | 0.287 | – |
| High concern, FES-I≥ 28, (no.) |
5/14 (35.7%) | 3/16 (18.8%) | 0.295 | 2.407 (0.456-12.720) |
| Depression (CES-D score) | 9.9 ± 6.4 | 5.3 ± 5.5 | 0.048 | – |
| Depressed, CES-D≥ 16 (no.) |
3/15 (20.0%) | 0/16 (0.0%) | 0.060 | – |
| Cognitive function (MoCA score) | 23.2 ± 4.4 | 24.7 ± 4.3 | 0.302 | – |
| Cognitive impaired, MoCA≤ 25,(no.) |
11/16 (73.3%) | 8/17 (50%) | 0.183 | 2.750 (0.610-12.407) |
| Sleep deprivation (PSQI score) |
7.7 ± 5.4 | 8.6 ± 6.2 | 0.683 | – |
| Clinical insomnia, PSQI>5 (no.) |
9/15 (60%) | 12/17 (70.5%) | 0.755 | 1.250 (0.308-5.072) |
| Pain (VAS, score) | 0.9 ± 1.6 | 2.2 ± 3.2 | 0.428 | – |
Values are presented as mean±standard deviation (SD) or n (%).
Abbreviations. DM, diabetes mellitus; HbA1c, glycated hemoglobin; CKD, chronic kidney disease; DLP, dyslipidemia; CAD, coronary artery disease; CHF, congestive heart failure; PAD, peripheral artery disease; TSFI, trauma specific frailty index; Prev., previous; amp, amputation; WIfI: wound/ischemia/foot infection; SPP, skin perfusion pressure; VPT: vibration perception threshold; ABI, ancle brakial index; WBC: white blood cell count obtained by wound culture; mod, moderate; abund, abundant; FES-I: falls efficacy scale international, CES-D: center for epidemiologic studies depression; MoCA: montreal cognitive assessment; PSQI: pittsburgh sleep quality index; VAS: pain visual analog scale.
Table 3 shows the comparison of primary and secondary outcomes within and between groups. At 4W, the IG showed a statistically significant wound area reduction (5.8 ± 8.0 cm2, P = 0.002, d = 0.20) compared to BL (7.4 ± 8.5 cm2). The wound area in the CG did not significantly reduce between the same time-point comparison (BL 3.1 ± 5.6 cm2; 4W: 3.2 ± 8.7 cm2, P = 0.928, d = 0.01). At 4W, the IG showed a statistically significant wound depth reduction (1.8 ± 1.4 mm, P = 0.020, d = 0.51) compared to BL (3.0 ± 2.1 mm). The wound depth in the CG did not significantly reduce between the same time-point comparison (BL: 3.2 ± 2.4 mm; 4W: 3.3 ± 4.2mm, P=0.865, d=0.04). Moreover, the speed of wound healing was faster in IG but not statistically significant.
Table 3.
Baseline and 4 Weeks Outcome Comparison Within and Between Both Groups.
| IG (n =
16) |
CG (n =
17) |
||||||
|---|---|---|---|---|---|---|---|
| BL | 4W | Time effect P-value (effect size) | BL | 4W | Time effect P-value (effect size) | Time ×Group effect P-value (effect size) | |
| Primary outcomes | |||||||
| Wound | |||||||
| Wound area (cm2) | 7.4 ± 8.5 | 5.8 ± 8.0 | 0.002 (0.20) | 3.1 ± 5.6 | 3.2 ± 8.7 | 0.928 (0.01) | 0.098 |
| Speed of wound healing (%) | 35.1 ± 36.6 | NA | 22.0 ± 58.2 | NA | 0.423 (0.28) | ||
| Daily speed of wound healing (%) | 1.3 ± 1.5 | NA | 0.8 ± 2.0 | NA | 0.440 (0.27) | ||
| Wound depth (mm) | 3.0 ± 2.1 | 1.8 ± 1.4 | 0.020 (0.51) | 3.2 ± 2.4 | 3.3 ± 4.2 | 0.865 (0.04) | 0.167 |
| Speed of wound depth healing (%) | 23.5 ± 52.5 | NA | 0.8 ± 79.2 | NA | 0.372 (0.34) | ||
| Daily speed of wound depth healing (%) | 0.9 ± 2.0 | NA | 0.1 ± 2.6 | NA | 0.349 (0.36) | ||
| Vascular | |||||||
| SPP (mmHg) | 71.0 ± 12.2 | 65.1 ± 19.1 | 0.216 (0.48) | 78.1 ± 18.6 | 72.2 ± 18.1 | 0.249 (0.24) | 0.644 |
| SPP subgroup mod./severe PAD (mmHg) | 76.0 ± 8.4 | 69.2 ± 23.7 | 0.466 (0.47) | 77.0 ± 23.6 | 67.3 ± 11.6 | 0.333 (0.44) | 0.984 |
| Secondary outcomes | |||||||
| Vascular | |||||||
| SatO2 (%) | 69.3 ± 21.3 | 72.3 ± 21.5 | 0.547 (0.15) | 73.1 ± 13.5 | 73.9 ± 12.1 | 0.810 (0.07) | 0.731 |
| SatO2 subgroup mod./severe PAD (%) | 47.3 ± 20.1 | 63.3 ± 22.3 | 0.015 (0.83) | 61.0 ± 13.7 | 70.1 ± 9.9 | 0.087 (0.83) | 0.419 |
| Neuropathic | |||||||
| VPT (Volts) | 25.6 ± 7.2 | 20.2 ± 14.0* | 0.245 (0.16) | 25.9 ± 17.8 | 32.1 ± 9.2* | 0.110 (0.53) | 0.049 |
| VPT subgroup mod./severe PAD (Volts) | 32.1 ± 18.9 | 17.1 ± 10.4* | 0.149 (0.39) | 19.8 ± 21.4 | 31.7 ± 16.5* | 0.099 (0.37) | 0.021 |
Values are presented as mean ± standard deviation (SD) or n (%).
Abbreviations: NA: not applicable; SatO2: tissue oxygen saturation; SPP: skin perfusion pressure; VPT: vibration perception threshold.
Values of VPT were reported at week; Effect size values represent Cohen’s d effect size.
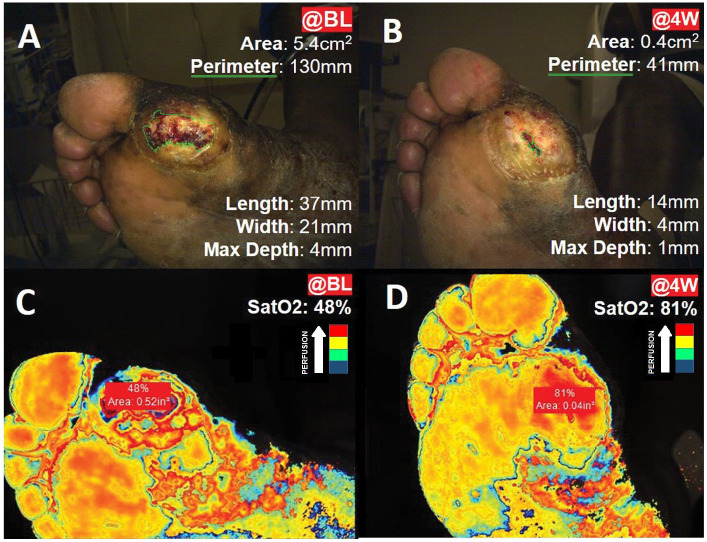
Figure 3 illustrates SatO2 for a typical case in IG at BL and 4W. No group or time effect was observed for changes in SatO2 or SPP in response to E-Stim therapy (Table 3). However, in the IG, a significant time effect with large effect size was observed for SatO2 among patients with moderate to severe PAD (BL: 47.3 ± 20.1% vs 4W: 63.3 ± 22.3%, P=0.015, d=0.83).
Figure 3.
Wound measurements and tissue oxygen saturation (SatO2) in a typical case from the intervention group. Panels A and B represent the wound measurements by 3D Silhouette camera (green outline) at: (A) baseline (@BL); and (B) at 4 weeks (@4W). Panels C and D represent tissue oxygen saturation (SatO2) assessed with near-infrared spectroscopy, where blue color represents the lowest perfusion, and red color represents the highest perfusion: (C) wound SatO2 @BL; (D) wound SatO2 @4W.
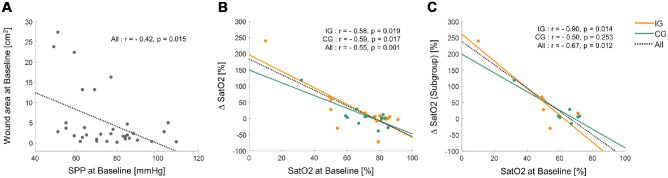
No significant correlation was observed between wound outcomes (area) and vascular metrics (ie, SatO2, SPP), except for BL SPP and wound area (IG: r=−0.44, P = 0.092; CG: r = −0.35, P = 0.173; overall: r = −0.42, P = 0.015, Figure 4A). On the other hand, BL SatO2 showed a significant negative correlation with ∆SatO2 in both groups (IG: r = −0.58, P = 0.019; CG: r = −0.59, P = 0.017; overall: r = −0.55, P = 0.001, Figure 4B). The sub-group of participants with moderate to severe PAD followed the same pattern except for the CG (IG: r = −0.90, P = 0.014; CG: r = −0.50, P = 0.253; overall: r = −0.67, P = 0.012, Figure 4C).
Figure 4.
Significant linear relationship between wound and/or vascular parameters.
Dashed lines represent the linear regression fit to all participants’ data. Solid orange and green lines represent the linear regression fit to individual data in the intervention (IG) and control (CG) groups, respectively. (A) At baseline (BL), overall patients who had lower skin perfusion pressure (SPP) showed greater wound area. (B) Overall patients who had lower wound saturation of oxygen (SatO2) at BL showed greater gain than participants who had higher SatO2 at BL. Additionally, patients who had lower SatO2 at BL in the IG (ie, orange line: slope = −2.5461) showed higher gain in SatO2 than those who had higher SatO2 at BL in the CG (ie, green line: slope = −1.9730). (C) Subgroup of patients with moderate to severe PAD: Overall and IG patients who had lower SatO2 at BL showed greater gain than those who had higher SatO2 at BL.
After 2 weeks of therapy, plantar VPT showed a significant improvement in the IG compared to the CG (58.9%, IG: 20.2 ± 14.0 vs CG: 32.1 ± 9.2 Volts, P = 0.049) as well as for the moderate to severe PAD sub-group (91.2%, IG: 17.1 ± 10.4 vs CG: 32.7 ± 16.5 Volts, P = 0.021).
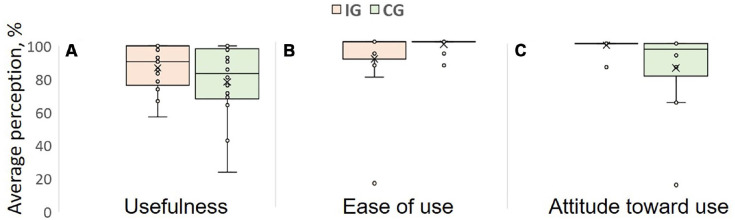
Thirty-one (93.9%) patients self-reported to use E-Stim daily for 1 hour. No functional device problem or related adverse events (ie, skin redness, pain, rash or allergy) were reported from either group. Overall, 81% (IG = 81.2% [13/16]; CG = 82.3% [14/17]) of the patients completed the TAM survey at 4W (Figure 5). Both groups highly graded the perceived ease of use (above 90%) with slightly better scores for the CG (IG = 91.8%; CG = 98.5%). Perceived usefulness and attitude toward using was 11% and 16.07% higher in the IG compared to CG, respectively.
Figure 5.
Average of 3 technology acceptance model (TAM) items including (A) perceived usefulness, (B) perceived ease of use, (C) attitude toward using. TAM model suggests perceived usefulness is lower in CG leading to lower attitude of use compared to IG
Discussion
E-Stim efficacy has been demonstrated in pre-clinical and human studies during the past 30 years. 26 Wide evidence substantiates E-Stim therapy for wound management, 1 and has been accepted by multiple medical corporations and insurances. 27 However, the transition of this treatment to the clinical setting is still impeded due to many methodological limitations (ie, complex in-hospital use, intermittent application, not applicable to covered wounds).1,5,28 We have addressed these gaps by demonstrating the feasibility, acceptability, and efficacy of daily home-based E-Stim adjunctive therapy placed above the ankle to accelerate wound healing outcomes among people with DFU.
Wound healing has been previously associated with increased tissue perfusion after E-Stim application,29-31 especially in patients with diabetes and PAD.32,33 In our prior cross-sectional study, 14 E-Stim revealed an immediate increase of tissue perfusion parameters (ie, SPP and SatO2) in response to one-hour therapy in DFU patients. 14 In addition, the present RCT involving the same population showed that among assessed demographics and clinical characteristics, only SatO2, as surrogate of skin perfusion, was found to be associated with wound healing outcomes at the end of the study. Since perfusion is a key component for healing processes, this mechanism may enhance the prevalence of epithelialization. 10 Therefore, we speculate that frequency of therapy (ie, 1 hour daily) may have contributed to decrease the wound size and depth in the targeted cohort. It is worth to mention that both groups in our study showed significant magnitude of improvement in SatO2 with time; however, the patients under E-Stim showed a faster healing period with higher gain of SatO2.
Even though immediate lower-extremity skin perfusion is significantly increased by E-Stim in DFU patients, 34 in our study, 4 weeks were not sufficient for the IG to achieve and maintain significant SPP and SatO2 levels as compared to the baseline. Similar randomized controlled trials have shown that in order to reach significant skin oxygenation levels, high dosages of lower-extremity E-Stim therapy (ie, daily 3-8 hours, 10-12 weeks) may be required.10,35 Perhaps other factors such as concomitant disease (ie, calcifications, atherosclerosis) 36 may impede permanent limb perfusion. 37 This effect was reflected in those patients (n = 6, 37.5%) who showed a pattern of wound relapse between 2.6 and 7 months after the termination of our study. Therefore, therapy dosage might be needed to adjust for vascular deficiencies, or in worse cases, revascularization. 38
Another speculation is E-Stim may be more effective to improve tissue perfusion depending on severity of disease. This hypothesis is supported by the significant increase and maintenance of SatO2 in the sub-group of patients with moderate to severe PAD. In addition, only this sub-group showed a significant gain and maintenance of SatO2 at 4 weeks of therapy. Together, both findings suggest that E-Stim therapy may be more effective among those with poorer lower-extremity blood perfusion or poor tissue oxygen supply. These hypotheses need to be validated in future studies.
Diverse modality of E-Stim waveforms (ie, biphasic, monophasic, symmetrical, asymmetrical, etc.) and currents (ie, high, low, direct, pulsed) have all proven to promote healing when applied over wounds.39,40 However, controversy exists in whether which modality is better for DFU.41,42 A recent meta-analysis of RCTs including E-Stim exclusively for DFU management showed inconclusive results due to the different therapeutic schedule between studies. 41 In addition, all studies applied E-Stim over/near the wounds; a known limitation given the fact that this fashion has not been well-established. 43 The present study showed that asymmetrical biphasic pulsed currents with placement of electrical pads above the ankle improve wound healing and magnitude of skin perfusion without the need of removing any dressing from the lesion site. The patient comfort and practicality under this treatment modality led to an overall 95.1% on self-perceived ease of use and a 92.05% on attitude towards device use, supporting the feasibility and acceptability of daily home-based therapy above the ankle level.
The improvement as means of pain, numbness, and wound healing in DFU has been associated with neuropathic changes while being exposed to electrical fields. 44 Studies revealed VPT improvement with E-Stim use in patients with diabetic neuropathy.45-48 Our study showed significant improvement of VPT in the IG compared to the CG at 2 weeks from baseline specifically in patients with moderate to severe PAD. This result suggests a benefit in prompt foot sensation after being exposed to electrical fields, but requires later follow up. Moreover, Schreuder et al. 49 concluded vessel neurologic autoregulation dysfunctionality might be a factor for failed revascularizations in patients with DFUs and history of PAD. In our cohort, 7 (43.8%) patients with history of PAD in the IG were revascularized before E-stim treatment. This sub-group improved in VPT at 2W from BL compared to the CG with a further 33.2% wound reduction suggesting E-Stim plays an important role in nerve function of patients with PAD and failed revascularization.
Conclusion
This study demonstrates feasibility, acceptability, and proof of concept effectiveness of daily home-based of E-Stim adjunctive therapy delivered above the ankle level to improve wound outcomes in patients with chronic DFU. No related adverse events were reported indicating safety of this adjunctive therapy. Additionally, our results revealed a positive contribution of E-Stim to improve tissue oxygen saturation in patients with moderate to severe PAD. Furthermore, results support improvement in plantar sensation in response to E-Stim at 2W. The observation of this study may not be generalizable and should be confirmed in a larger sample size, over longer period of time, and controlling for all potential confounders.
Limitations
The main limitation of this study is small sample size. The initial enrollment plan consisted of 40 subjects based on priori sample size estimation. Due to the declaration of pandemic in March 2020, the recruitment had to stop at 38 subjects. There were 5 dropouts (13%), which is slightly higher than the 10% anticipated in priori estimation. The initial sample size estimation was based on a different study design and thus may be underpowered for the outcomes of this study. Furthermore, while no difference between the groups was observed for major BL potential confounders (ie, age, gender, BMI, HbA1c, vascular parameters), the BL wound area was different between groups. Our exploratory analysis however showed no significant correlation between baseline wound size and wound healing outcomes in either group. Thus, we speculate that the between-group difference in baseline wound size had minimum impact in our analysis. Frequency and duration of therapy was self-reported on weekly basis, which may not be accurate. A device log on reporting the duration of use is recommended as an objective solution to control adherence to therapy. Whereas the duration of therapy in this study was limited to 4W, 75% of IG did not reach to full wound closure within the time frame. Another study is warrant to examine long-term use of E-Stim until complete healing.
Acknowledgments
We thank Jeffrey Ross, DPM, MD, for patient care and referral of this study. We thank Hector Elizondo-Adamchik, MD, Anmol Momin, BS, Naima Rodriguez, MA, Sogol Golafshan, BS, and Alan Pham, BS, for assisting with data collection and coordination of this research study between involved key investigators.
Footnotes
Abbreviations: ABI, ankle brachial index; BMI, Body mass index; CAD, Coronary artery disease; CES-D, Center for Epidemiologic Studies Depression; CHF, Congestive heart failure; CKD, Chronic kidney disease; DFU, diabetic foot ulcer; DLP, Dyslipidemia; DM, Diabetes mellitus; FES-I, falls efficacy scale international; HbA1c, Glycated hemoglobin; HTN, hypertension; HVPAC, high voltage pulsed alternative current; HVPC, high voltage pulsed current; meds, medications; MoCA, Montreal Cognitive Assessment; PAD, Peripheral artery disease; PSQI, Pittsburgh Sleep Quality Index; SatO2, Tissue Oxygen Saturation; SPP, skin perfusion pressure; TSFI, Trauma specific frailty index; VAS, Pain Visual Analog Scale; VPT, vibration perception threshold; WBC, White blood cell; WIfI, wound, ischemia, foot infection; SOC, standard of care.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by a grant from AVAZZIA Inc., (Dallas, TX, US). The sponsor did not have any role in designing the study, recruiting the participants, analysing the data, or interpretation of results.
ORCID iD: Alejandro Zulbaran-Rojas  https://orcid.org/0000-0002-3663-6882
https://orcid.org/0000-0002-3663-6882
References
- 1. Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle. 2013;4. doi: 10.3402/dfa.v4i0.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson MI. Transcutaneous electrical nerve stimulation (TENS) as an adjunct for pain management in perioperative settings: a critical review. Expert Rev Neurother. 2017;17(10):1013-1027. [DOI] [PubMed] [Google Scholar]
- 3. Ennis WJ, Lee C, Gellada K, Corbiere TF, Koh TJ. Advanced technologies to improve wound healing: electrical stimulation, vibration therapy, and ultrasound-what is the evidence? Plast Reconstr Surg. 2016;138(3 Suppl):94S-104S. [DOI] [PubMed] [Google Scholar]
- 4. Polak A, Franek A, Taradaj J. High-voltage pulsed current electrical stimulation in wound treatment. Adv Wound Care (New Rochelle). 2014;3(2):104-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khouri C, Kotzki S, Roustit M, Blaise S, Gueyffier F, Cracowski JL. Hierarchical evaluation of electrical stimulation protocols for chronic wound healing: an effect size meta-analysis. Wound Repair Regen. 2017;25(5):883-891. [DOI] [PubMed] [Google Scholar]
- 6. Ashrafi M, Alonso-Rasgado T, Baguneid M, Bayat A. The efficacy of electrical stimulation in lower extremity cutaneous wound healing: a systematic review. Exp Dermatol. 2017;26(2):171-178. [DOI] [PubMed] [Google Scholar]
- 7. Proper Usage of Electrical Stimulation. Find-a-code articles [article online]. 2018. Accessed 13 November 2020. Available from https://www.findacode.com/articles/proper-usage-of-electrical-stimulation-32658.html
- 8. Bai H, McCaig CD, Forrester JV, Zhao M. DC electric fields induce distinct preangiogenic responses in microvascular and macrovascular cells. Arterioscler Thromb Vasc Biol. 2004;24(7):1234-1239. [DOI] [PubMed] [Google Scholar]
- 9. Kawasaki L, Mushahwar VK, Ho C, Dukelow SP, Chan LL, Chan KM. The mechanisms and evidence of efficacy of electrical stimulation for healing of pressure ulcer: a systematic review. Wound Repair Regen. 2014;22(2):161-173. [DOI] [PubMed] [Google Scholar]
- 10. Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil. 2001;82(6):721-725. [DOI] [PubMed] [Google Scholar]
- 11. Ud-Din S, Bayat A. Electrical stimulation and cutaneous wound healing: a review of clinical evidence. Healthcare (Basel). 2014;2(4):445-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gianino E, Miller C, Gilmore J. Smart wound dressings for diabetic chronic wounds. Bioengineering (Basel). 2018;5(3):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nussbaum EL, Houghton P, Anthony J, Rennie S, Shay BL, Hoens AM. Neuromuscular electrical stimulation for treatment of muscle impairment: critical review and recommendations for clinical practice. Physiother Can. 2017;69(5):1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zulbaran-Rojas A, Park C, Lepow B, Najafi B. Effectiveness of lower-extremity electrical stimulation to improve skin perfusion. J Am Podiatr Med Assoc. 2021; 20-172. [DOI] [PubMed] [Google Scholar]
- 15. Lei H, Toosizadeh N, Schwenk M, et al. A pilot clinical trial to objectively assess the efficacy of electroacupuncture on gait in patients with Parkinson’s disease using body worn sensors. PLoS One. 2016;11(5):e0155613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Senergy Medical Group, LLC. Tennant Biomodulator-Pro Owner’s Manual. (Ed) 2017, manufactured by Avazzia Inc., DAL, TX, US. [Google Scholar]
- 17. Nair HKR. Microcurrent as an adjunct therapy to accelerate chronic wound healing and reduce patient pain. J Wound Care. 2018;27(5):296-306. [DOI] [PubMed] [Google Scholar]
- 18. Pan X, Chen G, Wu P, Han C, Ho JK. Skin perfusion pressure as a predictor of ischemic wound healing potential. Biomed Rep. 2018;8(4):330-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan X, You C, Chen G, Shao H, Han C, Zhi L. Skin perfusion pressure for the prediction of wound healing in critical limb ischemia: a meta-analysis. Arch Med Sci. 2018;14(3):481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venkatesh V, Davis FD. A Theoretical extension of the technology acceptance model: four longitudinal field studies. Manag Sci. 2000;46(2):186-204. [Google Scholar]
- 22. Najafi B, Talal TK, Grewal GS, Menzies R, Armstrong DG, Lavery LA. Using plantar electrical stimulation to improve postural balance and plantar sensation among patients with diabetic peripheral neuropathy: a randomized double blinded study. J Diabetes Sci Technol. 2017;11(4):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thakral G, La Fontaine J, Kim P, Najafi B, Nichols A, Lavery LA. Treatment options for venous leg ulcers: effectiveness of vascular surgery, bioengineered tissue, and electrical stimulation. Adv Skin Wound Care. 2015;28(4):164-172. [DOI] [PubMed] [Google Scholar]
- 24. Stanford Medicine 25. Measuring and understanding the ankle brachial index (ABI). Stanford Medicine [article online]. Accessed 16 November 2020. Available from https://stanfordmedicine25.stanford.edu/the25/ankle-brachial-index.html
- 25. Coerper S, Beckert S, Küper MA, Jekov M, Königsrainer A. Fifty percent area reduction after 4 weeks of treatment is a reliable indicator for healing - - analysis of a single – center cohort of 704 diabetic patients. J Diabetes Complications. 2009;23(1):49-53. [DOI] [PubMed] [Google Scholar]
- 26. Heidland A, Fazeli G, Klassen A, et al. Neuromuscular electrostimulation techniques: historical aspects and current possibilities in treatment of pain and muscle waisting. Clin Nephrol. 2013;79 Suppl 1:S12-S23. [PubMed] [Google Scholar]
- 27. Aetna Inc. Electrical stimulation for chronic ulcers (2020). [article online]. Accessed 16 November 2020. Available from http://www.aetna.com/cpb/medical/data/600_699/0680.html
- 28. Ellul C, Formosa C, Gatt A, Hamadani AA, Armstrong DG. The effectiveness of calf muscle electrostimulation on vascular perfusion and walking capacity in patients living with type 2 diabetes mellitus and peripheral artery disease. Int J Low Extrem Wounds. 2017;16(2):122-128. [DOI] [PubMed] [Google Scholar]
- 29. Feedar JA, Kloth L, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther. 1991;71:639-649. [DOI] [PubMed] [Google Scholar]
- 30. Gault W. Use of low intensity direct current in management of ischemic skin ulcers. Phys Ther. 1976;56:256-269. [DOI] [PubMed] [Google Scholar]
- 31. Lundeberg T, Kjartansson J, Samuelsson U. Effect of electrical nerve stimulation on healing of ischaemic skin flaps. Lancet. 1988;2:712-714. [DOI] [PubMed] [Google Scholar]
- 32. Forst T, Pfutzner A, Bauersachs R, et al. Comparison of the microvascular response to trancutaneous electrical nerve stimulation and postocclusive ischemia in the diabetic foot. J Diabetes Complications. 1997;11:291-297. [DOI] [PubMed] [Google Scholar]
- 33. Peters EJ, Armstrong DG, Wunderlich RP, Bosma J, StacpooleShea S. The benefit of electrical stimulation to enhance perfusion in persons with diabetes mellitus. J Foot Ankle Surg. 1998;37:396-400 [DOI] [PubMed] [Google Scholar]
- 34. Gilcreast DM, Stotts NA, Froelicher ES, Baker LL, Moss KM. Effect of electrical stimulation on foot skin perfusion in persons with or at risk for diabetic foot ulcers. Wound Repair Regen. 1998;6(5):434-441. [DOI] [PubMed] [Google Scholar]
- 35. Clover AJ, McCarthy MJ, Hodgkinson K, Bell PRF, Brindle NPJ. Noninvasive augmentation of microvessel number in patients with peripheral vascular disease. J Vasc Surg. 2003;38(6):1309-1312. [DOI] [PubMed] [Google Scholar]
- 36. Frykber RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015:4(9):560-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamabata S, Shiraishi H, Munechika M, et al. Effects of electrical stimulation therapy on the blood flow in chronic critical limb ischemia patients following regenerative therapy. SAGE Open Med. 2016;4:2050312116660723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montero-Baker M, Zulbaran-Rojas A, Chung J, et al. Endovascular therapy in an “all-comers” risk group for chronic limb-threatening ischemia demonstrates safety and efficacy when compared with the established performance criteria proposed by the society for vascular surgery. Ann Vasc Surg. 2020;67:425-436. [DOI] [PubMed] [Google Scholar]
- 39. Kloth LC. Electrical stimulation technologies for wound healing. Adv Wound Care (New Rochelle). 2014;3(2):81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Girgis B, Duarte JA. High voltage monophasic pulsed current (HVMPC) for stage II-IV pressure ulcer healing. A systematic review and meta-analysis. J Tissue Viability. 2018;27(4):274-284. [DOI] [PubMed] [Google Scholar]
- 41. Chen Z, Chen Z-Y, Liu W-H, Li G-S. Electric stimulation as an effective adjunctive therapy for diabetic foot ulcer: a meta-analysis of randomized controlled trials. Adv Skin Wound Care. 2020;(33)11:608-612 [DOI] [PubMed] [Google Scholar]
- 42. Tiktinsky R, Chen L, Narayan P. Electrotherapy: yesterday, today and tomorrow. Haemophilia. 2010;16(Suppl 5):126-131. [DOI] [PubMed] [Google Scholar]
- 43. Koel G, Houghton PE. Electrostimulation: current status, strength of evidence guidelines, and meta-analysis. Adv Wound Care. 2014;3:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tippett A. Treating peripheral neuropathy. Wounds. 2014;26(3);65-71. [PubMed] [Google Scholar]
- 45. Bosi E, Conti M, Vermigli C, et al. Effectiveness of frequency-modulated electromagnetic neural stimulation in the treatment of painful diabetic neuropathy. Diabetologia. 2005;48(5):817-823. [DOI] [PubMed] [Google Scholar]
- 46. Najafi B, Talal TK, Grewal GS, Menzies R, Armstrong DG, Lavery LA. Using plantar electrical stimulation to improve postural balance and plantar sensation among patients with diabetic peripheral neuropathy: a randomized double blinded study. J Diabetes Sci Technol. 2017;11(4):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Najafi B, Crews RT, Wrobel JS. A novel plantar stimulation technology for improving protective sensation and postural control in patients with diabetic peripheral neuropathy: a double-blinded, randomized study. Gerontology. 2013;59(5):473-480. [DOI] [PubMed] [Google Scholar]
- 48. Thakral G, Kim PJ, LaFontaine J, Menzies R, Najafi B, Lavery LA. Electrical stimulation as an adjunctive treatment of painful and sensory diabetic neuropathy. J Diabetes Sci Technol. 2013;7(5):1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schreuder SM, Nieuwdorp M, Koelemay MJW, Bipat S, Reekers JA. Testing the sympathetic nervous system of the foot has a high predictive value for early amputation in patients with diabetes with a neuroischemic ulcer. BMJ Open Diabetes Res Care. 2018;6(1):e000592. [DOI] [PMC free article] [PubMed] [Google Scholar]