Abstract
This article provides an up-to-date review of technological advances in 3 key areas related to diet monitoring and precision nutrition. First, we review developments in mobile applications, with a focus on food photography and artificial intelligence to facilitate the process of diet monitoring. Second, we review advances in 2 types of wearable and handheld sensors that can potentially be used to fully automate certain aspects of diet logging: physical sensors to detect moments of dietary intake, and chemical sensors to estimate the composition of diets and meals. Finally, we review new programs that can generate personalized/precision nutrition recommendations based on measurements of gut microbiota and continuous glucose monitors with artificial intelligence. The article concludes with a discussion of potential pitfalls of some of these technologies.
Keywords: personalized nutrition, diet monitoring, wearable sensors, machine learning
Introduction
A recent survey examining the consumption of major foods and nutrients among adults aged 25 or older in 195 countries has estimated that improving diet can potentially prevent one in every 5 deaths globally. 1 Using a number of dietary risk factors (eg, diet high in sodium, or low in fiber), the study concluded that poor diet was responsible for more deaths than any other risks globally, including tobacco smoking. 1 An essential step to improve diet is to monitor food intake and eating behaviors. However, conventional methods for monitoring diet are based on self-report measures (eg, food diaries, 24-hour recall), which are problematic. For example, food diaries require manual input, which is burdensome 2 and often leads to low adherence rates. 3 Further, 24-hour records suffer from memory recall, which can lead to severe over and under-reporting. 4 Compounding the problem are the very large inter-individual differences in the response to the same foods, 5 which puts into question the utility of universal dietary recommendations. Thus, there is a need for new techniques that can reduce the burden of monitoring food intake and also allow individuals to personalize their diets to achieve optimum health.
To address these issues, this article provides an overview of current technology in 3 key areas related to precision nutrition, as illustrated in Figure 1: advances in mobile applications for diet logging, new wearable sensors to detect dietary behaviors, and personalized nutrition programs based on analyzing biochemical markers (gut microbiome, blood glucose) through artificial intelligence (AI) techniques. The article concludes with a discussion of potential pitfalls when relying excessively on technology to solve the problems of diet monitoring and personalized nutrition, and other important health problems.
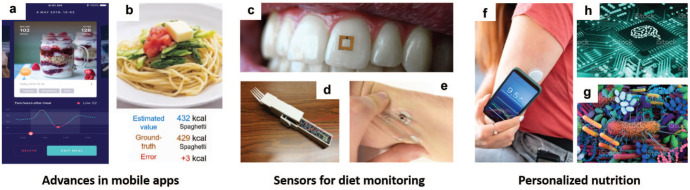
Figure 1.
Overview of the chapter in 3 key areas. advances in mobile apps for diet monitoring, wearable and handheld sensors, and personalized nutrition. (a) Snapshot of the Undermyfork app, 6 which tracks glucose patterns (bottom) and aligns them with food photographs (top). (b) Recognition of foods from photographs. 7 (c) Tooth-mounted sensor (from Tseng et al 8 ). (d) Smart fork utensil. 9 (e) Epidermal sweat sensor (from Sempionatto et al 10 ). Personalized nutrition is achieved by combining (f) continuous glucose monitors, (g) microbiome information and (h) machine learning techniques. (a) Provided, with permission, by Undermyfork, (b) reprinted (adapted) with permission from authors 7 (c) reprinted (adapted) with permission from Tseng et al, 8 (d) reprinted (adapted) with permission from Zhang et al, 9 (e) reprinted (adapted) with permission from Sempionatto et al, 10 Copyright 2020 American Chemical Society, (f) photo credit: iStock.com/AzmanJaka, (g) photo credit: iStock.com/Design Cells, and (h) photo credit: iStock.com/KENGKAT.
Mobile Applications for Diet Monitoring
A major step in reducing the burden of diet monitoring has been the replacement of paper-based journals with smartphone apps. The ubiquity of smartphones makes dieting apps very convenient, since the user does not need to carry around a physical log book or diary. Further, dieting apps provide access to databases containing the nutritional content of a very large number of foods and meals. As an example, one of the most popular dieting apps, MyFitnessPal, 11 has over 11 million food items, though not all of its entries are verified for accuracy (to our knowledge, the largest verified nutrition database is Nutritionix, with nearly 800,000 grocery items and 170,000 restaurant items). Having access to such massive databases can greatly simplify guesswork for users (ie, by providing precise nutritional information of meals) and guide them when
choosing portion sizes and meals. An additional advantage of mobile apps is their ability to scan barcodes for packaged foods, which reduces the need to look up the food in a database or enter food nutrients manually. Finally, dieting apps can also be integrated with external devices, such as smart scales, fitness trackers, and continuous glucose monitors (CGMs) to help users understand the effect of diet and exercise on their weight trends and glucose patterns.
However, written food diaries –whether paper-based or electronic, require a high level of engagement that can lead to fatigue and reduced adherence over time. 12 An alternative that has gained popularity over the past decade are photographic food diaries. 13 Photographic food diaries offer several advantages over written diaries. They can reduce data hording, the situation where the user completes multiple entries at once, typically at the end of each day. Because photographs have to be taken at the point of consumption, they tend to encourage in-the-moment awareness and more accurate recalls (eg, the context in which the meal was eaten, the preparation and makeup of the food, and how much of the food was eaten). In addition, studies with adult and pediatric populations have shown that photographic diaries are preferred to paper diaries, and are easier to use. Further, previous studies have shown that combining images with other forms of information (eg, written, verbal) can increase retention, understanding and future problem solving. 14 An interesting example in this direction is Undermyfork, 15 a diabetes app that combines photo-based food logging with glucose data from CGMs. The app shows food photographs with the corresponding glucose responses, which helps users identify foods that lead to high postprandial glucose, and foods that keep glucose within a more normal range. A further advantage of photo-based food diaries is they can be combined with AI techniques to detect and identify foods, and estimate the nutritional content of foods. 16 An increasing number of commercial apps use these techniques to track nutrition from food photographs, for example, Lose It!, 17 CalorieMama, 18 Snaq, 19 Undermyfork, and several software libraries for food image recognition are available for integration with mobile apps, for example, bite.ai, 20 FoodAI. 21
Sensors for Tracking Eating and Nutrition
In parallel with advances in mobile apps, a number of sensor-based approaches are being developed to automate the process of tracking eating behaviors, thus reducing user burden and increasing measurement accuracy. We organize these various sensing modalities into two broad categories: physical sensors and chemical sensors.
Physical Sensors
Physical sensors have been a popular approach to tracking diet in an automated fashion, 4 either with wearable sensors or smart utensils. Wearable sensors containing inertial measurement units are often used to log food intake by detecting the specific gestures that accompany eating. 22 These gestures could be generic hand-to-mouth movements or more specific actions, including using a specific utensil or even eating with one’s hands directly. 23 While these sensing systems provide accurate results in laboratory settings, accounting for accurate results in real-life environments remains a challenge using only wearable motion sensors, 24 though recently, some success has been found in moving these motion sensors from the wrist to the head and mouth area (eg, jawbone). 25
In order to enhance food intake detection, additional wearable sensors are used, including electromyography (EMG), piezoelectric, and acoustic sensors, to sense the movement of muscles around the jaw and identify chewing and swallowing sounds. EMG sensors attached to eyeglasses are able to detect chewing and swallowing motions through muscle activation. 26 Similarly, a combination of piezoelectric sensors and accelerometers can also track muscle movement to differentiate between eating actions and motions related to talking. 27 As these approaches to combine information across multiple sensors expand, some have even worked on integrating cameras, either within the environment or directly on the body, to help segment the data captured by the wearable sensors. 28 As wearing a large number of sensors may be uncomfortable, physical sensors have also been placed in plates and utensils. These “smart utensils” can detect eating and, if embedded with additional sensors, also to recognize the food and its composition. 29
Chemical Sensors
While physical sensors can be used to detect moments of dietary intake, in most cases they have limited ability to estimate the nutritional content of foods. The latter requires measuring dietary biomarkers that are associated with intake of nutrients. A number of biomarkers have been identified that correlate with intake of various foods, such as fruits and vegetables (eg, vitamin C and carotenoids in blood), sugar (eg, urinary sucrose and fructose), or protein (eg, urinary nitrogen), to mention a few.30,31 Here we focus on dietary biomarkers that can be measured with wearable or handheld sensors.
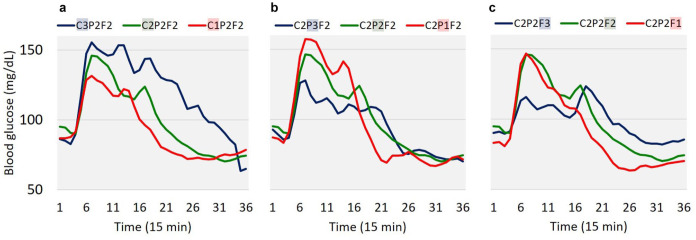
CGMs have gained acceptance to manage type 1 diabetes, but also offer promise for monitoring dietary intake. The mechanism by which CGMs may be used to monitor diet is based on the fact that the change in blood glucose after a meal, also known as the post-prandial glucose response (PPGR), depends on the macronutrients in the meal (eg, carbohydrates, protein, fat, fiber). The major determinant of post-prandial glucose is the amount of carbohydrates, but adding protein, fat, or fiber to a meal generally yields smaller increases and lengthier responses; see Figure 2. This suggest that the shape of the PPGR can be used to recover the macronutrient composition of the meal through the use of machine learning techniques. To test this hypothesis, we recently conducted a study in which 15 healthy participants (not diagnosed with prediabetes or type 2 diabetes, 60-85 years, body mass index 25-35 kg/m2) consumed 9 different meals over the course of 2-3 weeks while wearing a CGM. Each meal had a known but varying amount of CHO (low C1: 42.5 g, medium C2: 85 g, high C3: 170 g), protein (low P1: 15 g, medium P2: 30 g, high P3: 60 g), and fat (low F1: 13 g, medium F2: 26 g, high F3: 52 g). Then, we trained several machine learning models to predict the amount of macronutrients from the PPGRs in a leave-one-participant-out fashion, for example, using data from 14 participants for training and the remaining participant for testing.32,33 The best performing models were able to predict the amount of macronutrients in the meal with a normalized root mean squared error (NRMSE) of 22% for carbohydrates, 50% for protein and 40% for fat, a promising result given the large inter-individual differences in food metabolism 5 and the fact that the models were not customized for each participant.
Figure 2.
PPGRs to mixed meals with carbohydrates (C), protein (P) and fat (F), denoted as CxPxFx, where x represents the amount of each macronutrient in the meal (1: low; 2: medium; 3: high). (a) Average PPGR across subjects as the amount of carbohydrates increases (C1, C2, C3) while the other 2 macronutrients remain fixed (P2, F2). The PPGR becomes more pronounced at higher levels of C. (b) Average PPGR protein increases (P1, P2, P3) while the other 2 macronutrients are fixed (C2, F2). As protein increases, the PPGR decreases, with lower maximum levels and slower return to baseline. (c) Average PPGR as fat increases (F1, F2, F3) while the other 2 macronutrients are fixed (C2, P2). As with protein, as fat increases, the PPGR also decreases, with lower maximum levels and slower return to the baseline (from Das et al. 32 ).
Handheld devices are also available to analyze breath biomarkers associated with metabolism. A primary target of these devices are ketones (eg, acetone). During prolonged fasting or carbohydrate restriction, the body resorts to burning fat in order to produce ketones, which are then used as an alternative source of energy instead of glucose. 34 This results in elevated values of ketones in the breath, which can serve as an indicator of whether the body has reached ketosis (ie, the metabolic state where the body generates energy primarily from fat). Several breath ketone meters exist currently in the market, including the Ketonix analyzer, 35 and the Biosense monitor. 36 These devices are aimed at people attempting to lose weight through ketogenic diets, but may also be beneficial for people with diabetes who may be at risk of ketoacidosis (this type of breath analyzers provide a single-point measurement of ketones; for continuous measurement, several recent studies have proposed the development of continuous ketone monitors (CKMs) to measure ketones in interstitial fluid37,38).
Another metabolic biomarker that can be derived from breath analysis is metabolic fuel, a parameter that reflects the body’s fuel preference for energy production (ie, carbohydrates vs. fat). Metabolic fuel is generally estimated as the respiratory exchange ratio (RER), the ratio of CO2 produced during metabolism and oxygen used. But this requires the use of metabolic carts, which are only available in specialized clinics and thus are unsuited for regular use. To address this issue, a hand-held device by Lumen 39 has become available that estimates metabolic fuel by measuring CO2 while the user performs a brief breath maneuver. This information is then used to provide personalized nutrition and exercise recommendations.
Additional dietary biomarkers can be extracted ambulatorily with wearable sensors from other bodily fluids. A primary target of these devices is sweat, since it can be measured at convenient body locations and is ideal for continuous monitoring. A variety of analytes present in sweat may be of interest for metabolic disorders, including various electrolytes, glucose, lactate, ammonia, ethanol, cortisol, and hydration markers.10,40 As an example, Sempionatto et al. 10 developed an epidermal biosensor to track the dynamics of vitamin C in sweat. The device is in the form of a flexible tattoo, and uses iontophoresis stimulation to draw sweat and an enzymatic process for detection. Along the same lines, Yang et al. 41 developed a sweat sensor that can detect uric acid and tyrosine, analytes that are well established for metabolic and nutritional management. For personalized nutrition, another interesting target is saliva, since it can be highly informative of eating behaviors (eg, increase in salivary secretion with eating) and the nutritional composition of the meals. Kim et al. 42 developed a non-invasive mouthguard biosensor that was capable of monitoring lactate continuously during sport activities. However, wearing a large mouthguard is impractical for long studies, so better mounting solutions have also been investigated, such as tooth-mounted sensors. Along these lines, Tseng et al. 8 developed a hydrogel-based sensor that, attached to a tooth, could track glucose, salt and alcohol intake. However, in contrast with CGMs and breath analyzers, which are already available commercially, many of these sweat/saliva sensing devices are still at the research stage.
Technologies for Personalized Nutrition
Finally, we describe how technologies are being used to develop personalized nutrition programs. Here we discuss measurements of gut microbiome (i.e., collection of microorganisms, such as bacteria, viruses and fungi, and their genetic material present in the gastrointestinal tract) and blood glucose to develop personalized nutrition recommendations. In a seminal study on personalized nutrition, Zeevi et al. 5 used CGMs to track the glucose response of 800 participants (healthy and with prediabetes) for 1 week while participants kept detailed records of their diet. The authors then developed a machine-learning model (gradient boosting regression) that could predict the glucose response of a meal for each participant based on individual factors, such as anthropometric variables, blood panels, and gut microbiome. Note that after the machine-learning model is trained, CGMs are no longer needed to make predictions (ie, CGMs only provide the outputs of the model during training). When tested on an independent cohort of 100 participants, the model was able to generate personalized diets that led to improved glucose responses (ie, reduced postprandial hyperglycemia). In a related study, Hall et al. 43 used CGMs to estimate the frequency of hyperglycemia among healthy adults (not previously diagnosed with diabetes). Surprisingly, they found glucose levels that reached prediabetes and diabetes ranges 15% and 2% of the time, respectively, suggesting that glucose dysregulation is more prevalent than commonly assumed.
A number of companies have emerged that seek to provide personalized recommendations of diet intake to improve glucose control and weight loss. As an example, the company DayTwo 44 measures gut microbiome to provide nutrition recommendations using the machine-learning model developed in the study by Zeevi et al. 5 The company Thryve 45 also uses gut microbiome measurements to customize probiotics and food recommendations to improve health. The gut microbiome company Viome 46 conducted a study that tracked the glycemic response of 550 adults for up to 2 weeks, while they consumed a set of standardized meals carefully designed to cover a broad range of proportions of carbohydrates, proteins, fats and fiber. 47 Then, they built a multilevel mixed-effects regression model to predict PPGRs. This allowed the authors to quantify (for the first time) the relative influence of meal composition, anthropometric, gut microbiome and lifestyle variables to postprandial glucose. Based on a proprietary analysis of the gut microbiome, Viome also makes recommendations about the likely positive, neutral, or negative impact that certain dietary choices will have on individual’s health. Note that these companies do not require CGM use: nutrition recommendations are based on information from the gut microbiome and other individual factors; as noted earlier, CGMs are only needed to build the machine-learning model.
Alternatively, the company NutriSense 48 relies on CGMs to develop personalized nutrition recommendations. NutriSense combines CGMs with a smartphone application that integrates diet logging and physical activity, and allows the user to interact with nutritionists that provide recommendations to improve health through a balance of food, exercise and rest. While CGMs are primarily prescribed for people with diabetes (PwD), this is (to our knowledge) the first company to provide CGMs and integrate them with an application for personal health. Other companies in this space also exist, for example, Signos, 49 Levels, 50 but at the time of this writing they appear to be in the early-access stage. These nascent companies and technologies are laying the groundwork for personalized nutrition and health, through improved logging of meals through intelligent smartphone applications and CGMs, and through individualized reporting and recommendation with the measurement of the gut microbiome.
Discussion
We have reviewed a number of technologies and digital tools that can significantly reduce the burden of dietary monitoring, compared to traditional methods that require users to look up the nutritional content of foods in a calorie book and then manually enter the information in a log book. We have also reviewed innovative personalized-nutrition approaches that overcome the limitations of universal dietary recommendations by modeling the unique metabolism of each individual through machine learning techniques. These advances in precision nutrition can be invaluable tools in the fight against diabetes and other metabolic diseases, if used properly. Accordingly, and in the interest of providing a balanced treatment of this field, we wish to close this article by highlighting some potential pitfalls of these tools, and also highlight the need to engage behavior-modification researchers to help design interventions with the highest likelihood of adherence and lifestyle modification.
With diet monitoring tools, the hope is that reducing burden will result in increased adherence and eventually better clinical outcomes (eg, weight loss, glucose control). However, there is a well-established “law of attrition” 51 in eHealth trials, which tend to experience significantly higher dropout rates than drug trials. Thus, it seems likely that adherence to dietary monitoring tools will decrease with time, no matter how low-burden the tool is. A further issue is whether full automation of diet monitoring (ie, no burden) is desirable, as it may prevent users from developing the in-the-moment awareness that comes with food logging. 12 As an example, Turner-McGrievy et al 52 conducted a study (DIET) where participants were randomly assigned to 2 different diet-monitoring methods, a standard diet tracking app (high burden), and a wearable bite tracking device (low burden). After 6 months, participants in the high-burden group lost significantly more weight (−6.8 ± 0.8 kg) than those in the low-burden group (−3.0±0.8 kg; p < 0.001). In a follow up study (2SMART), the authors compared the standard diet tracking app (high burden) against a photo-based app (low burden). 53 At 6 weeks and at 6 months, both apps were equally effective in reducing weight. However, weight loss was correlated with adherence only for the high-burden app (ie, counting calories more often was associated with more weight loss, but taking more food photographs was not). Further, at 6 weeks participants in the 2 low-burden groups (bite-tracking device in DIET, photo-based app in 2SMART) found it more “difficult to remember to use [their] assigned diet tracking device on a regular basis” than participants in the high-burden condition. Thus, there appears to be a tradeoff between developing tools that reduce user burden and allowing the users to form the critical habit of monitoring their diet. 54
Some concerns have also been raised about personalized- or precision-nutrition programs that rely on CGMs. In a recent study, Howard et al 55 asked participants to wear 2 CGM devices simultaneously (Dexcom G4 Platinum, Abbott Freestyle Libre Pro) for 28 days while they consumed ad libitum meals. Then, meals for each participant were ranked according to their corresponding post-prandial glucose (eg, from low to high). Surprisingly, the authors found a low degree of concordance between the meal rankings obtained from the 2 CGM devices. While some of these discrepancies could be explained by the fact that the 2 CGMs were placed at different anatomical locations (upper arm for Abbott, lower abdomen for Dexcom), this result raises important questions about the effectiveness of personalized dietary recommendations based on CGM measurements that are imprecise.
Acknowledgments
None
Footnotes
Abbreviations: CGM, continuous glucose monitor; AI, artificial intelligence; EMG, electromyography; PPGR, post-prandial glucose response; NRMSE, normalized root mean squared error; PwD, people with diabetes; CKM, continuous ketone monitor.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NSF Engineering Research Center for Precise Advanced Technologies and Health Systems for Underserved Populations (PATHS-UP; Award #1648451) and NSF-IIS award #2014475.
ORCID iD: Bobak J. Mortazavi  https://orcid.org/0000-0002-2655-2095
https://orcid.org/0000-0002-2655-2095
References
- 1. Neuhouser ML. The importance of healthy dietary patterns in chronic disease prevention. Nutr Res. 2019;70:3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Epstein DA, Cordeiro F, Fogarty J, Hsieh G, Munson SA. Crumbs: lightweight daily food challenges to promote engagement and mindfulness. Proc SIGCHI Conf Hum Factor Comput Syst. 2016;2016:5632-5644. doi: 10.1145/2858036.2858044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shay LE, Seibert D, Watts D, Sbrocco T, Pagliara C. Adherence and weight loss outcomes associated with food-exercise diary preference in a military weight management program. Eat Behav. 2009;10(4):220-227. doi: 10.1016/j.eatbeh.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell BM, Alam R, Alshurafa N, et al. Automatic, wearable-based, in-field eating detection approaches for public health research: a scoping review. NPJ Digit Med. 2020;3(1):38. doi: 10.1038/s41746-020-0246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079-1094. doi: 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 6. Undermyfork - diabetes food diary. Accessed August 18, 2021. https://undermyfork.com
- 7. Ege T, Yanai K. Image-based food calorie estimation using knowledge on food categories, ingredients and cooking directions. In: Proceedings of the on Thematic Workshops of ACM Multimedia 2017. Association for Computing Machinery; 2017:367-375. [Google Scholar]
- 8. Tseng P, Napier B, Garbarini L, Kaplan DL, Omenetto FG. Functional, RF-trilayer sensors for tooth-mounted, wireless monitoring of the oral cavity and food consumption. Adv Mater. 2018;30(18):e1703257. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Zheng H, Rempel S, et al. A smart utensil for detecting food pick-up gesture and amount while eating. In: Proceedings of the 11th Augmented Human International Conference, Winnipeg, Manitoba, Canada. Association for Computing Machinery; 2020. [Google Scholar]
- 10. Sempionatto JR, Khorshed AA, Ahmed A, et al. Epidermal enzymatic biosensors for sweat vitamin C: toward personalized nutrition. ACS Sens. 2020;5(6):1804-1813. [DOI] [PubMed] [Google Scholar]
- 11. MyFitnessPal. Accessed August 18, 2021. https://www.myfitnesspal.com
- 12. Cordeiro F, Bales E, Cherry E, Fogarty J. Rethinking the mobile food journal: exploring opportunities for lightweight photo-based capture. In: Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems, Seoul, Republic of Korea. Association for Computing Machinery; 2015:3207-3216. [Google Scholar]
- 13. Zepeda L, Deal D. Think before you eat: photographic food diaries as intervention tools to change dietary decision making and attitudes. Int J Consum Stud. 2008;32(6):692-698. doi: 10.1111/j.1470-6431.2008.00725.x [DOI] [Google Scholar]
- 14. Ehrmann BJ, Anderson RM, Piatt GA, et al. Digital photography as an educational food logging tool in obese patients with type 2 diabetes: lessons learned from a randomized, crossover pilot trial. Diabetes Educ. 2014;40(1):89-99. doi: 10.1177/0145721713508826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. HAPI.com . Enjoy your food with HAPIfork. Accessed August 18, 2021. https://www.hapilabs.com/product/hapifork
- 16. Min W, Jiang S, Liu L, Rui Y, Jain R. A survey on food computing. ACM Computing Surveys (CSUR). 2019;52(5):1-36. [Google Scholar]
- 17. Snap itTM - Lose It!. Accessed August 18, 2021. https://www.loseit.com/snapit
- 18. CalorieMama Food AI - Food Image Recognition and Calorie Counter using Deep Learning. Accessed August 18, 2021. https://www.caloriemama.ai
- 19. Snaq | Reveal the Impact of Food on Health. Accessed August 18, 2021. https://www.snaq.io
- 20. bite.ai - Food Recognition API. Accessed August 18, 2021. https://bite.ai/food-recognition
- 21. Foodai - State-of-the-art food image recognition technologies. Accessed August 18, 2021. https://foodai.org
- 22. Kalantarian H, Alshurafa N, Sarrafzadeh M. A survey of diet monitoring technology. IEEE Pervasive Comput. 2017;16(1):57-65. [Google Scholar]
- 23. Junker H, Amft O, Lukowicz P, Tröster G. Gesture spotting with body-worn inertial sensors to detect user activities. Pattern Recognit. 2008;41(6):2010-2024. [Google Scholar]
- 24. Sharma S, Hoover A. The challenge of metrics in automated dietary monitoring as analysis transitions from small data to big data. In: 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Korea (South), 16-19 December, 2020. IEEE; 2020:2647-2653. [Google Scholar]
- 25. San Chun K, Jeong H, Adaimi R, Thomaz E. Eating episode detection with jawbone-mounted inertial sensing. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20-24 July, 2020. IEEE; 2020:4361-4364. [DOI] [PubMed] [Google Scholar]
- 26. Zhang R, Amft O. Monitoring chewing and eating in free-living using smart eyeglasses. IEEE J Biomed Health Inform. 2017;22(1):23-32. [DOI] [PubMed] [Google Scholar]
- 27. Farooq M, Sazonov E. A novel wearable device for food intake and physical activity recognition. Sensors. 2016;16(7):1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doulah A, Ghosh T, Hossain D, Imtiaz MH, Sazonov E. “Automatic ingestion monitor version 2”—a novel wearable device for automatic food intake detection and passive capture of food images. IEEE J Biomed Health Inform. 2020;25:2:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang Q, Yang Z, Zhang Q. Smart-U: smart utensils know what you eat. In: IEEE INFOCOM 2018-IEEE Conference on Computer Communications, Honolulu, HI, 16-19 April, 2018. IEEE; 2018:1439-1447. [Google Scholar]
- 30. Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125(5-6):507-525. doi: 10.1007/s00439-009-0662-5 [DOI] [PubMed] [Google Scholar]
- 31. Hedrick VE, Dietrich AM, Estabrooks PA, Savla J, Serrano E, Davy BM. Dietary biomarkers: advances, limitations and future directions. Nutr J. 2012;11(1):109. doi: 10.1186/1475-2891-11-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Das A, Sajjadi S, Mortazavi B, Chaspari T, et al. A sparse-coding approach to automatic diet monitoring with continuous glucose monitors. In: ICASSP 2021 – 2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Toronto, ON, Canada. IEEE; 2021:2900-2904. [Google Scholar]
- 33. Sajjadi S, Das A, Gutierrez-Osuna R, et al. Towards the development of subject-independent inverse metabolic models. In: ICASSP 2021 – 2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Toronto, ON, Canada. IEEE; 2021:3970-3974. [Google Scholar]
- 34. Suntrup Iii DJ, Ratto TV, Ratto M, McCarter JP. Characterization of a high-resolution breath acetone meter for ketosis monitoring. PeerJ. 2020;8:e9969. doi: 10.7717/peerj.9969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ketonix Breath Ketone Analyzer. Accessed August 18, 2021. https://www.ketonix.com
- 36. Biosense® - The Only Clinically Backed Breath Ketone Monitor. Accessed August 18, 2021. https://mybiosense.com
- 37. Zhang JY, Shang T, Koliwad SK, Klonoff DC. Continuous ketone monitoring: a new paradigm for physiologic monitoring. J Diabetes Sci Technol. 2021;15(4):775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alva S, Castorino K, Cho H, Ou J. Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. J Diabetes Sci Technol. 2021;15(4):768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lumen: Hack your metabolism. Accessed August 18, 2021. https://www.lumen.me.
- 40. Bariya M, Nyein HYY, Javey A. Wearable sweat sensors. Nat Electron. 2018;1(3):160-171. [Google Scholar]
- 41. Yang Y, Song Y, Bo X, et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat Biotechnol. 2020;38(2):217-224. [DOI] [PubMed] [Google Scholar]
- 42. Kim J, Valdés-Ramírez G, Bandodkar AJ, et al. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst. 2014;139(7):1632-1636. [DOI] [PubMed] [Google Scholar]
- 43. Hall H, Perelman D, Breschi A, et al. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018;16(7):e2005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DayTwo. Accessed August 18, 2021. https://daytwo.com
- 45. Thryve Gut Health - Personalized Probiotics & Microbiome Testing. Accessed August 18, 2021. https://www.thryveinside.com
- 46. Gut Microbiome Testing, Health Supplements & Probiotics | Viome. Accessed August 18, 2021. https://www.viome.com
- 47. Tily H, Perlina A, Patridge E, et al. Gut microbiome activity contributes to individual variation in glycemic response in adults. Preprint. Posted online May 28, 2019. bioRxiv 2019:641019. doi: 10.1101/641019 [DOI] [Google Scholar]
- 48. NutriSense: Unlock Your Body’s Data. Accessed August 18, 2021. https://www.nutrisense.io
- 49. Signos - Continuous Glucose Monitor Device for Weight Loss. Accessed August 18, 2021. https://signos.com
- 50. Levels - Metabolic Fitness Program. Accessed August 18, 2021.https://www.levelshealth.com
- 51. Eysenbach G. The law of attrition. J Med Internet Res. 2005;7(1):e11. doi: 10.2196/jmir.7.1.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turner-McGrievy GM, Boutté A, Crimarco A, et al. Byte by bite: use of a mobile Bite Counter and weekly behavioral challenges to promote weight loss. Smart Health. 2017;3-4:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dunn CG, Turner-McGrievy GM, Wilcox S, Hutto B. Dietary self-monitoring through calorie tracking but not through a digital photography app is associated with significant weight loss: the 2SMART pilot study-a 6-month randomized trial. J Acad Nutr Diet. 2019;119(9):1525-1532. [DOI] [PubMed] [Google Scholar]
- 54. Turner-McGrievy GM, Yang C-H, Monroe C, Pellegrini C, West DS. Is burden always bad? Emerging low-burden approaches to mobile dietary self-monitoring and the role burden plays with engagement. J Technol Behav Sci. 2021;6(3):447-455. doi: 10.1007/s41347-021-00203-9 [DOI] [Google Scholar]
- 55. Howard R, Guo J, Hall KD. Imprecision nutrition? Different simultaneous continuous glucose monitors provide discordant meal rankings for incremental postprandial glucose in subjects without diabetes. Am J Clin Nutr. 2020;112(4):1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]