Abstract
Background:
Diabetic foot ulcers (DFUs) are a leading cause of disability and morbidity. There is an unmet need for a simple, practical, home method to detect DFUs early and remotely monitor their healing.
Method:
We developed a simple, inexpensive, smartphone-based, “Foot Selfie” system that enables patients to photograph the plantar surface of their feet without assistance and transmit images to a remote server. In a pilot study, patients from a limb-salvage clinic were asked to image their feet daily for six months and to evaluate the system by questionnaire at five time points. Transmitted results were reviewed weekly.
Results:
Fifteen patients (10 male) used the system after approximately 5 minutes of instruction. Participants uploaded images on a median of 76% of eligible study days. The system captured and transmitted diagnostic quality images of the entire plantar surface of both feet, permitting clinical-management decisions on a remote basis. We monitored 12 active wounds and 39 pre-ulcerative lesions (five wounds and 13 pre-ulcerative lesions at study outset); we observed healing of seven wounds and reversal of 20 pre-ulcerative lesions. Participants rated the system as useful, empowering, and preferable to their previous methods of foot screening.
Conclusions:
With minimal training, patients transmitted diagnostic-quality images from home on most days, allowing clinicians to review serial images. This system permits inexpensive home foot screening and monitoring of DFUs. Further studies are needed to determine whether it can reduce morbidity of DFUs and/or the associated cost of care. Artificial intelligence integration could improve scalability.
Keywords: remote patient monitoring, home imaging, foot selfie, wound, diabetic foot ulcer, mobile health
Introduction
In patients with diabetes mellitus, foot ulcers are a leading cause of disability and morbidity, including amputations. Diabetic foot ulcers (DFUs) have a high rate of recurrence and impose a crushing economic burden on health care systems.1,2 Ulcerations vary in degree of severity; large amounts of tissue loss and the presence of infection can significantly increase the cost of treatment and are associated with higher mortality.3,4 Prevention and early identification of ulcerations drastically improve outcomes and quality of life for patients. Early diagnosis of DFUs is hampered by diabetic peripheral neuropathy. Patients with neuropathy lack the “gift of pain” and are often unaware of injury to their feet. Additionally, patients with diabetes who are elderly, inflexible, or have visual impairment such as retinopathy are often unable to examine their feet. These factors also impede self-monitoring of ulcer progression or healing. Currently, patients have limited self-imaging options such as hand mirrors which can be difficult to manipulate and provide no clinical feedback. Patients are often reliant on caretakers or family members to help monitor their feet, but this is not always possible on a regular basis and many patients wish to be more independent.
These considerations illuminate the unmet need for a simple, practical method for early detection and monitoring of DFUs. We created a novel, inexpensive, easy-to-use, smartphone-based system that allows patients to screen their feet at home and providers to monitor these images. 5 We describe this system and report its performance in a pilot study of patients with or at risk for DFUs.
Methods
“Foot Selfie” System
Apparatus
The Foot Selfie apparatus consists of three parts: a heel platform, a base, and a phone holder. Components for this system were designed in CAD software (Fusion 360) and 3D printed. The base is situated on the bottom of the heel platform and holds the phone holder (Figure 1). For imaging, the base extends from the heel platform via two telescoping rods (Figure 2).
Figure 1.

Compact apparatus front view (left) and side view (right). Heel platform (black), base (red), and phone holder (white). Red, green, and blue circular stickers were placed on the anterior part of the heel platform’s frustum to give each image color and size standardization markers to aid in image processing and analysis.
Figure 2.

Apparatus with base and phone holder extended for use oblique view (left) and side view (right).
Smartphone app
The Foot Selfie smartphone app (iOS, Android) allows users to take voice-activated photos, view them, and upload them to a HIPAA-compliant storage server (AWS S3) for review by health care providers. The app sends patients a reminder notification to image their feet if more than 24 hours has passed since their last upload. Participants downloaded the app onto their smartphone from either the Apple or Google Play app store.
Instructions for use
Participants use the system while seated. The apparatus contains a handle at the rear of the base for extension from the heel platform. The patient then places the apparatus on the ground in front of them. Next, they open their smartphone app, press the “Take Photos” button, and press the camera button on the subsequent screen. This activates the front-facing camera and microphone, which begins listening for the image-capture commands. The user leans forward to place their smartphone in the phone holder (this could be done before placing the apparatus on the ground), returns to a normal sitting posture, and positions the calcaneus of their first foot on the heel platform.
After verbalizing the image-capture command, the app captures an image of the plantar foot and plays a pre-recorded message instructing the user to switch feet. The user repeats the same process for the second foot which causes the app to play a second pre-recorded message instructing the user to pick up their phone. The app transitions to a new screen displaying both images taken, asks the user to assign left or right to each foot image, and allows them to “flag” an image if they have a concern. Users are also able to tap either image to enlarge it to full screen and zoom in and around the image for self-monitoring. Finally, pressing the upload button sends the images to their health care team. On this screen, users also have the option to retake images if there are any problems (Figure 3).
Figure 3.
System use. (a) User extends base with phone holder from heel platform. (b) User places apparatus on floor. (c) User opens app on phone to home screen. (d) User transitions to Take Photos screen. (e) User places phone in phone holder. (f) User images foot 1. (g) User images foot 2. (h) App transitions to upload screen.
Pilot Study
Patient recruitment
The study was approved by the USC Biomedical Institutional Review Board. Participants were recruited from the USC Keck Limb Preservation Clinic. Consented participants were provided with a Foot Selfie apparatus, either in clinic or via mail due to Covid. Technical support was provided by our authors including Foot Selfie app installation, live demonstration, and utilizing a teach-back method to ensure participant understanding. For participants who received their apparatus via mail, this process was performed via video conference.
Study design
Participants were instructed to image their feet daily. If a participant did not upload images for a week, they were contacted to inquire about any potential clinical or technical causes for delay. Participants completed a study questionnaire during initial device set-up and were contacted via telephone at time points of one week, one month, three months, and six months to complete the remaining questionnaires. Participant images from the previous week were reviewed by study authors weekly during what was colloquially termed “Foot Selfie rounds.” If pre-ulcerative lesions or new tissue loss was identified based on a participant’s images, the patient was contacted for at-home guidance or an in-person clinic appointment.
Image adjudication
For this study, a wound was defined as a break in the epidermis. Discoloration, redness, skin breakdown, pre-ulcerative callus, hemorrhagic calluses, and eschars were considered pre-ulcerative signs. Wounds were considered healed when they re-epithelialized without drainage. Pre-ulcerative signs were considered reversed when they were no longer noticed in three consecutive images.
Data analysis
Descriptive statistics were reported as median and range or mean ± standard deviation. When the questionnaire asked for graded responses on a scale of 1–10, results were reported as median (interquartile range: first quartile and third quartile).
To determine total compliance, the number of days patients took an image was divided by eligible study days. Eligible days were the number of days spent in the study minus any days there was a technical or user issue with the smartphone app preventing proper use, especially inability to log in, and days spent hospitalized.
Results
Patient Description
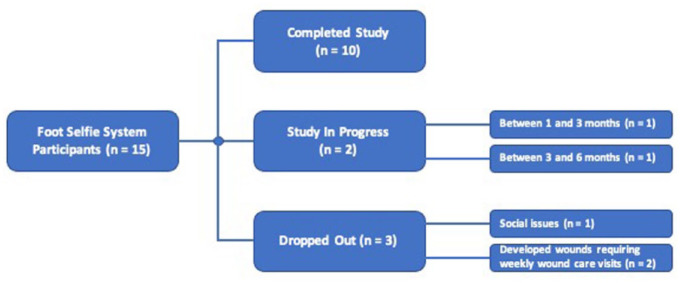
We followed 15 patients for an average of 5.0 months. Both iPhone and Android platforms were used. Instruction for use took approximately 5 minutes for every patient. The median number of times the system needed to be demonstrated before successful teach-back was 1 (range: 1–2, mean: 1.2 ± 0.4). Table 1 summarizes characteristics of study participants. Participants all fit into the category 3 diabetic foot risk category defined as presence of diabetes with a previous history of ulcer or lower extremity amputation, Charcot neuropathy, or chronic venous insufficiency. Figure 4 provides a flow diagram of patient participation.
Table 1.
Characteristics of Study Participants.
| Age | Total (n = 15) |
| Mean ± SD | 57.4 ± 8.7 |
| Min-Max | 39-72 |
| Sex | |
| Male | 10 (66.7%) |
| Female | 5 (33.3%) |
| Ethnicity | |
| Caucasian | 9 (60. 0%) |
| Hispanic | 4 (26.7%) |
| Black | 1 (6.7%) |
| Asian | 1 (6.7%) |
| ADA Diabetic Foot Risk Category | |
| Category 3 | 15 (100.0%) |
| Education | |
| High School or Less | 4 (26.7%) |
| Some College | 3 (20.0%) |
| College or More | 8 (53.3%) |
Abbreviations: ADA, American Diabetes Association.
Figure 4.
Flow diagram shows patient participation throughout study.
Of the two patients who developed wounds requiring weekly care, one developed gangrene on his dorsal hallux not detected by the system until plantar hallux presentation and required amputation. The other sustained a third-degree burn after leaving his foot on a space heater.
Patient Examples
Figure 5 shows a routine daily image reviewed at weekly rounds. This image was taken by a patient who is legally blind from diabetic retinopathy.
Figure 5.
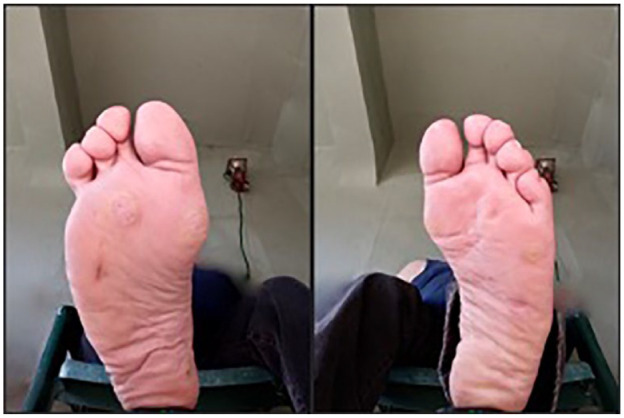
Legally blind patient performs routine imaging.
Ulceration Monitoring
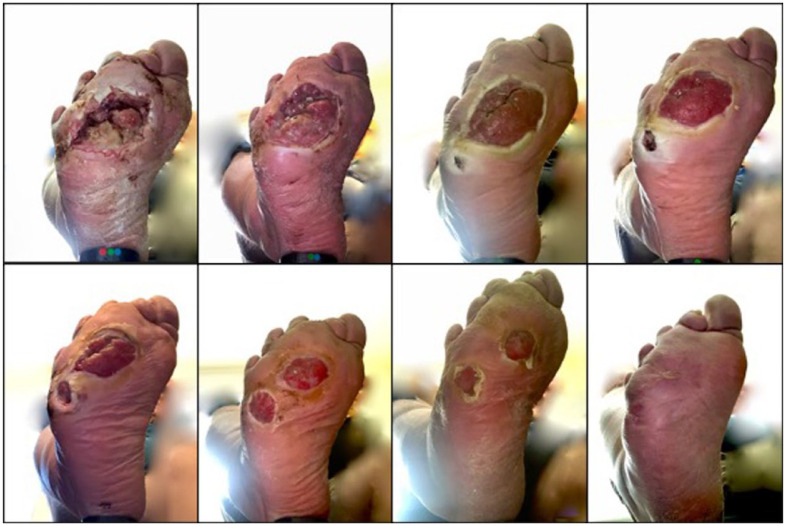
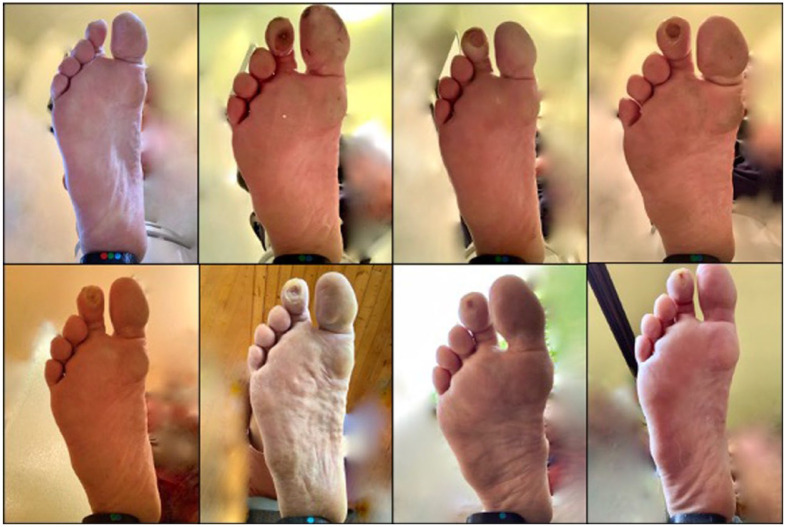
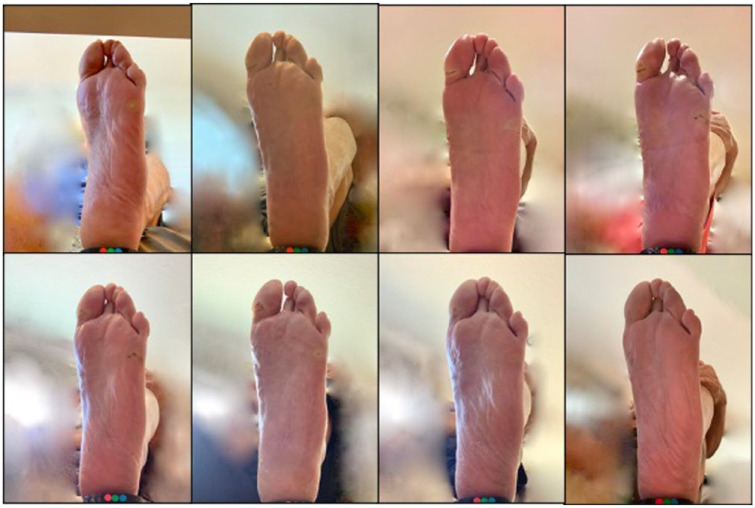
Three patients entered the study with five distinct active wounds and nine patients entered the study with 13 distinct pre-ulcerative lesions. During the study, we identified seven new wounds and 26 new pre-ulcerative lesions. We also observed healing of seven wounds and reversal of 20 pre-ulcerative lesions. Figures 6–8 show examples.
Figure 6.
Healing process of patient with post-surgical wound is monitored. Series progresses chronologically from top left to bottom right.
Figure 7.
Patient develops wound on right second toe and is monitored through study conclusion. Series progresses chronologically from top left to bottom right.
Figure 8.
Bleeding into calluses on left hallux and fifth metatarsal is monitored throughout study duration. Series progresses chronologically from top left to bottom right.
Adherence to Daily Foot Selfies
Participants uploaded images on a median of 76% of eligible days (range: 18%-94%). All but one participant imaged their feet at least 50% of eligible days. At study entry, this legally blind, dialysis-dependent patient indicated he would only image his feet weekly. The remaining patients uploaded images on a median of 77% of eligible days (range: 54%-94%).
The most common issues with compliance included hospitalizations, bandaged feet, travel, and inability to log into the app due to forgotten username and password. In total, five patients were hospitalized during their time in the study, only one for foot wound issues.
Participant attitudes toward daily foot imaging throughout the duration of the study are reported in Tables 2 and 3. Participant acceptance of daily imaging increased from 7 of 15 (47%) at one week, to 11 of 15 (73%) at one month, 13 of 13 (100%) at three months, and 8 of 10 (80%) at six months. Most participants reported that they would image their feet daily or every other day if using the system outside of the study.
Table 2.
Acceptance of Daily Imaging.
| Participant acceptance | One week (n = 15) | One month (n = 15) | Three months (n = 13) | Six months (n = 10) |
|---|---|---|---|---|
| Too Often | 8 (53%) | 4 (27%) | 0 | 2 (20%) |
| Right Amount | 7 (47%) | 11 (73%) | 13 (100%) | 7 (70%) |
| Not Often Enough | 0 | 0 | 0 | 1 (10%) |
Table 3.
Preferred Foot Selfie System Use.
| Preferred frequency | One week (n = 15) | One month (n = 15) | Three months (n = 13) | Six months (n = 10) |
|---|---|---|---|---|
| Multiple Times Per Day | 0 | 1 (7%) | 1 (8%) | 1 (10%) |
| Daily | 4 (27%) | 3 (20%) | 6 (46%) | 4 (40%) |
| Every Other Day | 7 (47%) | 7 (47%) | 4 (31%) | 3 (30%) |
| Weekly | 3 (20%) | 3 (20%) | 2 (15%) | 1 (10%) |
| Only When I Have a Concern | 1 (7%) | 1 (7%) | 0 | 1 (10%) |
Patient Questionnaire Responses
Table 4 summarizes key responses of study questionnaires. We report important points.
Table 4.
Key Responses to Participant Questionnaire.
| Questions (scale) | 0 day (n = 15) | One week (n = 15) | One month (n = 15) | Three months (n = 13) | Six months (n = 10) |
|---|---|---|---|---|---|
| How easy is using the system for you? (1 = Very difficult; 10 = Extremely Easy) |
10 (9, 10) | — | — | — | — |
| The system is a useful tool in monitoring my
feet. (True/False) |
15/15 | 15/15 | 15/15 | 13/13 | 10/10 |
| How useful does this system seem to you in helping to
prevent foot ulcers? (1 = Not Useful; 10 = Extremely Useful) |
8 (7, 9) | 8 (7, 10) | 9 (8, 10) | 9 (8, 10) | 9.5 (9, 10) |
| Do you feel safer from foot related harm because of this
system? (1 = Not at All; 10 = Significantly Safer) |
— | 8 (7, 10) | 8 (5, 10) | 10 (7, 10) | 9 (6, 10) |
| I like using this system more than previous methods of
viewing the bottom of my feet. (True/False) |
— | 12/15 | 12/15 | 9/13 | 9/10 |
| I like using this system more than having someone else check
my feet for me. (True/False) |
— | 14/15 | 14/15 | 11/13 | 9/10 |
| How concerned are you about developing a foot
wound? (1 = Not at All; 10 = Very concerned) |
9 (5, 10) | 8 (5, 10) | 8 (5, 10) | 7 (3, 9) | 8.5 (5, 10) |
| Using this system, I feel like I have more control with my
foot related issues. (1 = None; 10 = Significantly More) |
— | 9 (5, 10) | 9 (7,10) | 9.5 (8, 10) | 9 (8, 10) |
| I would recommend this system to someone else concerned
about developing foot wounds. (True/False) |
15/15 | 15/15 | 15/15 | 13/13 | 10/10 |
| The positive benefits of using this system outweigh the
negatives. (1 = Not at All; 10 = Absolutely) |
— | 10 (8, 10) | 10 (8, 10) | 10 (10, 10) | 10 (10, 10) |
Results reported as median (IQR first, third) or true responses/total responses.
Ease of Use
Patients found the system easy to use. On a scale from 1 (very difficult) to 10 (extremely easy), they gave it a median score of 10, IQR (9, 10) immediately after learning to use it. The median number of times the system needed to be demonstrated before successful teach-back was 1 (range: 1-2).
Usefulness
All participants at every point answered “True” to the true-false question, “the system is a useful tool in monitoring my feet.” In response to “How useful does this system seem to you in helping to prevent foot ulcers?” on a scale from 1 (not useful) to 10 (extremely useful), the median score at enrollment was 8 (7, 9) and increased throughout the study with a median of 9.5 (9, 10) at six months. When asked if they felt safer from “foot-related harm” on a scale from 1 (not at all) to 10 (significantly safer), the median score at one week was 8 (7, 10) and remained high throughout the study with a median of 9 (6, 10) at six months.
Comparison with previous foot-screening methods
The percentage of participants who preferred the Foot Selfie system over their previous method of foot screening was 80% at one week and 90% at six months. Similarly, the percentage who preferred the Foot Selfie system to having somebody else (family member, friend, home nurse) check their feet was 93% at one week and 90% at six months.
Patient empowerment
Participants reported feeling that they had more control with their foot-related issues than before using the Foot Selfie system throughout the study, with a median score at one week of 9 (5, 10) and at six months of 9 (8, 10).
Recommendable
At all timepoints, all users agreed that they would recommend the system to someone else concerned about developing a foot wound. When asked on a scale from 1 (not at all) to 10 (absolutely) if the benefits of using the system outweigh the disadvantages, the median participant score was a 10 throughout all timepoints.
Discussion
DFUs constitute a global public health crisis. A simple, practical, home-based method to monitor foot health of at-risk patients could help increase communication between health care provider and patient while reducing clinic visits and hospitalization.6,7 We developed and validated a novel, easy-to-use, low-cost, smartphone-based system to monitor foot health remotely and evaluated the system’s performance in patients with or at-risk for DFUs. Our principal finding is that the system captured and transmitted diagnostic quality images of the entire plantar surface of both feet, permitting clinical management decisions on a remote basis. Patients found the system easy to operate with minimal instruction, demonstrated high levels of compliance, and indicated high levels of acceptance.
Remote patient imaging of diabetic foot disease was proposed in the 1990s, but technological and financial constraints limited its widespread use. Use of hand mirrors is a common recommendation. 8 The first remote imaging tools were bulky and expensive9,10; they required patients to place their feet on imaging plates which conceal parts of the foot or introduce pressure artifacts that can obscure early signs of inflammation. 11 Recently, smartphones have been proposed for remote imaging of diabetic foot disease. 12 Anthony et al 13 compared weekly smartphone home imaging using hand-held self-photos, photos taken with a selfie stick, and photos taken by another person. However, self-photos are difficult for many patients, selfie sticks may introduce motion artifacts, and imaging by others is impractical for those who live alone. Furthermore, neither of the former methods ensure imaging of the entire plantar surface and none of the methods permit reproducible images.
The Foot Selfie system represents a significant improvement over current clinical practice and previous remote monitoring concepts. Patients were able to use the system effectively after only a brief training period, including one participant who is legally blind. The system ensures screening of the entire plantar foot and does not introduce motion or pressure artifacts. It generates sufficiently reproducible images taken longitudinally so that clinicians can compare images over time. Thus, it permits comparison of images to facilitate detection of pre-ulcerative signs, reducing the severity and complexity of tissue damage upon first presentation, or infected and non-healing wounds, potentially improving patient outcomes and long-term mortality rates.
The custom apparatus is compact, portable, and inexpensive to manufacture. It can be used with any smartphone to provide monitoring for everyone with diabetic foot disease—those who live alone, do not have others check their feet, or with limited access to care. In addition to use in patient homes, the system may be useful in locations high-risk patients visit regularly (eg dialysis clinics) or live and receive care (eg skilled nursing facilities).
Our pilot study was designed to approximate real-world use. Patients used their own smartphones and downloaded the app. They transmitted diagnostic quality images with minimal instruction. To the best of our knowledge, this is the first study in which patients were asked to image their feet daily and transmit images. Furthermore, they expressed satisfaction with the system and felt safer because they used it. The latter observation is consistent with evidence that patients with diabetes fear amputation more than they fear death. 14 The study was conducted during the Covid-19 pandemic, much of it prior to widespread vaccine availability; and most patients were at high risk for severe Covid-19 disease. Because the Foot Selfie system permitted health-care providers to monitor patients’ plantar feet remotely, many patients stopped seeing their participation as a study and started seeing it as an integral clinical service.
This study identified the need for an optional dorsal foot imaging feature. One participant developed gangrene initially only on the dorsal hallux. It was not detected until discoloration was identified in the plantar hallux by the Foot Selfie system. The patient required amputation and dropped out of the study.
Future studies will determine optimal intervals between plantar-imaging sessions. We asked patients to image daily, both to enforce their foot-imaging habits and to ensure that the health care team had sufficient data to make good clinical decisions if patients missed sessions. It is likely that optimal imaging frequency will depend on the patient risk profile. Future studies will also be needed to determine if the Foot Selfie system can reduce cost of medical care, the need for in-person clinic visits, and the incidence of severe or infected DFUs.
Ultimately, we envision this system being combined with other imaging and analysis technologies such as machine learning and hyperspectral imaging. The use of machine learning for image classification is well-established; its application to aid in classifying diabetic foot images is already being advanced.15-20 The performance of machine learning depends on the robustness of the training data set. Our pilot study is the first to demonstrate the feasibility of daily acquisition and transmission of diagnostic-quality images to generate such a robust training set. Furthermore, we achieved this result with a simple, inexpensive system that requires minimal patient training. A sensitive machine-learning algorithm would allow clinicians to review only images flagged by the algorithm, reducing the time and cost required for clinician monitoring of diabetic foot disease.
Hyperspectral imaging, imaging outside of the visible wavelengths of light, has been shown to have the potential to identify pre-ulcerous areas of diabetic feet before lesions can be identified with visible light. 21 Currently, hyperspectral imagers are bulky and expensive, but work is underway to make them less expensive and adaptable to smartphones. 22 The Foot Selfie system can serve as an enabling platform for hyperspectral or any other type of imaging. The triad of easy-to-take foot selfies with a hyperspectral overlay and machine learning screening offers promise for remote monitoring of diabetic foot disease.
Conclusions
We describe a novel, inexpensive, easy-to-use, smartphone-based system for remote monitoring of diabetic foot disease. In a pilot study, the system captured and transmitted diagnostic-quality images of the entire plantar surfaces of both feet. With minimal training, patients transmitted images from home on most days, permitting clinicians to review serial images in individual patients. This system, coupled with other technologies, may help increase ulcer-free, hospital-free, and activity-rich patient days for individuals with, or at risk for, diabetic foot ulcers. Based on our preliminary experience, we anticipate wide adoption of the Foot Selfie system.
Footnotes
Abbreviations: DFUs, Diabetic Foot Ulcers.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is partially supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Award Number 1R01124789-01A1 and National Science Foundation (NSF) CNS Award Number 2052578.
ORCID iD: Mark Swerdlow  https://orcid.org/0000-0003-3311-6408
https://orcid.org/0000-0003-3311-6408
References
- 1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DG, Lavery LA, Harkless LB, Van Houtum WH. Amputation and reamputation of the diabetic foot. J Am Podiatr Med Assoc. 1997;87(6):255-259. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skrepnek GH, Mills JL, Sr, Armstrong DG. A diabetic emergency one million feet long: disparities and burdens of illness among diabetic foot ulcer cases within emergency departments in the United States, 2006-2010. PLoS ONE. 2015;10(8):e0134914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swerdlow M. System and method for screening a patient’s foot [US patent]. https://patentimages.storage.googleapis.com/f8/1b/80/557bda00dfd872/US10993655.pdf. Published 2021. Accessed August 9, 2021.
- 6. Rogers LC, Armstrong DG, Capotorto J, et al. Wound center without walls: the new model of providing care during the COVID-19 pandemic. Wounds. 2020;32(7):178-185. https://www.ncbi.nlm.nih.gov/pubmed/32335520. Accessed October 6, 2021. [PMC free article] [PubMed] [Google Scholar]
- 7. Rogers LC, Lavery LA, Joseph WS, Armstrong DG. All feet on deck-the role of podiatry during the COVID-19 pandemic: preventing hospitalizations in an overburdened healthcare system, reducing amputation and death in people with diabetes. J Am Podiatr Med Assoc. Published online March 25, 2020. doi: 10.7547/20-051 [DOI] [PubMed] [Google Scholar]
- 8. Mayo Clinic. Amputation | diabetes: how to protect your feet. https://www.mayoclinic.org/diseases-conditions/diabetes/in-depth/amputation-and-diabetes/art-20048262. Published September 23, 2020. Accessed August 10, 2021.
- 9. Basatneh R, Najafi B, Armstrong DG. Health sensors, smart home devices, and the internet of medical things: an opportunity for dramatic improvement in care for the lower extremity complications of diabetes. J Diabetes Sci Technol. 2018;12(3):577-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boghossian JA, Miller JD, Armstrong DG. Towards extending ulcer-free days in remission in the diabetic foot syndrome. Front Diabetes. 2018;26:210-218. https://www.karger.com/Article/Abstract/480104. Accessed October 6, 2021. [Google Scholar]
- 11. Hazenberg CEVB, Hazenberg CEV, van Baal JG, Manning E, Bril A, Bus SA. The validity and reliability of diagnosing foot ulcers and pre-ulcerative lesions in diabetes using advanced digital photography. Diabetes Technol Ther. 2010;12(12):1011-1017. doi: 10.1089/dia.2010.0088. [DOI] [PubMed] [Google Scholar]
- 12. Ploderer B, Brown R, Seng LSD, Lazzarini PA, van Netten JJ. Promoting self-care of diabetic foot ulcers through a mobile phone app: user-centered design and evaluation. JMIR Diabetes. 2018;3(4):e10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anthony CA, Femino JE, Miller AC, et al. Diabetic foot surveillance using mobile phones and automated software messaging, a randomized observational trial. Iowa Orthop J. 2020;40(1):35-42. [PMC free article] [PubMed] [Google Scholar]
- 14. Wukich DK, Raspovic KM, Suder NC. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot Ankle Spec. 2018;11(1):17-21. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen H, Agu E, Tulu B, et al. Machine learning models for synthesizing actionable care decisions on lower extremity wounds. Smart Health (Amst). 2020;18:100139. doi: 10.1016/j.smhl.2020.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyal M, Yap MH, Reeves ND, Rajbhandari S, Spragg J. Fully convolutional networks for diabetic foot ulcer segmentation. In: 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC). Institute of Electrical and Electronics Engineers; 2017:618-623. doi: 10.1109/SMC.2017.8122675. [DOI] [Google Scholar]
- 17. Wang C, Anisuzzaman DM, Williamson V, et al. Fully automatic wound segmentation with deep convolutional neural networks. Sci Rep. 2020;10(1):21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alzubaidi L, Fadhel MA, Oleiwi SR, Al-Shamma O, Zhang J. DFU_QUTNet: diabetic foot ulcer classification using novel deep convolutional neural network. Multimed Tools Appl. 2020;79(21):15655-15677. [Google Scholar]
- 19. Yap MH, Hachiuma R, Alavi A, et al. Deep learning in diabetic foot ulcers detection: a comprehensive evaluation. arXiv [csCV]. http://arxiv.org/abs/2010.03341. Published 2020. Accessed October 6, 2021. [DOI] [PubMed]
- 20. Goyal M, Reeves ND, Rajbhandari S, Yap MH. Robust methods for real-time diabetic foot ulcer detection and localization on mobile devices. IEEE J Biomed Health Inform. 2019;23(4):1730-1741. [DOI] [PubMed] [Google Scholar]
- 21. Murphy GA, Singh-Moon RP, Mazhar A, Cuccia DJ, Rowe VL, Armstrong DG. Quantifying dermal microcirculatory changes of neuropathic and neuroischemic diabetic foot ulcers using spatial frequency domain imaging: a shade of things to come? BMJ Open Diabetes Res Care. 2020;8(2):e001815. doi: 10.1136/bmjdrc-2020-001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saiko G, Lombardi P, Au Y, Queen D, Armstrong D, Harding K. Hyperspectral imaging in wound care: a systematic review. Int Wound J. 2020;17:1840-1856. doi: 10.1111/iwj.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]