Abstract
Background:
There is conflicting evidence on the effect of exercise on systemic insulin concentrations in adults with type 1 diabetes.
Methods:
This prospective single-arm study examined the effect of exercise on systemic insulin degludec (IDeg) concentrations. The study involved 15 male adults with type 1 diabetes (age 30.7 ± 8.0 years, HbA1c 6.9 ± 0.7%) on stable IDeg regimen. Blood samples were collected every 15 minutes at rest, during 60 minutes of cycling (66% VO2max) and until 90 minutes after exercise termination. IDeg concentrations were quantified using high-resolution mass-spectrometry and analyzed applying generalized estimation equations.
Results:
Compared to baseline, systemic IDeg increased during exercise over time (P < .001), with the highest concentrations observed toward the end of the 60-minute exercise (17.9% and 17.6% above baseline after 45 minutes and 60 minutes, respectively). IDeg levels remained elevated until the end of the experiment (14% above baseline at 90 minutes after exercise termination, P < .001).
Conclusions:
A single bout of aerobic exercise increases systemic IDeg exposure in adults on a stable basal IDeg regimen. This finding may have important implications for future hypoglycemia mitigation strategies around physical exercise in IDeg-treated patients.
Keywords: exercise, high-resolution mass spectrometry, insulin degludec, type 1 diabetes
Introduction
Physical exercise in people with type 1 diabetes increases the risk of hypoglycemia due to their inability to adapt systemic insulin commensurate with lower requirements. 1 While guidelines generally recommend reducing insulin doses in the context of exercise, this is not easily feasible in patients treated with ultra-long-acting insulin analogues. Additionally, exercise per se was suggested to induce higher systemic availability of exogenous insulin although data are inconclusive and scarce, especially for long-acting insulin analogues. 2 Insulin degludec (IDeg) is a modern ultra-long-acting insulin, with a flat and stable action profile.3,4 There are currently limited data on the effects of exercise on IDeg concentrations, and so far results were based on immunoassays with limited specificity for IDeg.5,6 However, robust quantification of insulin concentrations during exercise conditions will improve our understanding of exercise-associated metabolism, thereby informing treatment recommendations related to exercise, in particular prediction models and algorithms. 7
We, therefore, applied a highly specific mass-spectrometric assay 8 with the objective to explore the effect of exercise on systemic IDeg concentrations in adults with type 1 diabetes.
Methods
Study Participants and Design
Male adults with type 1 diabetes (duration ≥2 years) on stable basal IDeg regimen (Tresiba U-100, Novo Nordisk A/S, Bagsværd, Denmark) for ≥3 months were recruited. Further inclusion criteria were age 18-45 years, HbA1c ≤ 8.0% (64 mmol/mol), and regular exercise (≥30 minutes of exercise ≥3/week). Exclusion criteria are reported elsewhere. 9 Study participants engaged in an exercise intervention in a fasted state as reported previously. 9 Written informed consent was obtained by all study participants, and the protocol was approved by the Ethics Committee Bern (Approval number 2018-02070) and registered on Clinicaltrials.gov (NCT03497260).
Sample Collection and Exercise Intervention
Participants attended the research facility in the morning after an overnight fast having injected their usual IDeg dose. Blood samples were collected from an intravenous cannula in the antecubital vein before (T-30, T-15, T0), during (T15, T30, T45, T60), and after (T90, T150) exercise. A 60-minute continuous exercise at moderate intensity (average oxygen consumption during the exercise period was 66 ± 6% of VO2max) was performed on a cycle ergometer (see Kosinski et al 9 for details). Before and after exercise, participants remained in upright sitting position. Room temperature was kept between 24°C and 26°C with ambient humidity ranging between 20% and 50%. Blood was drawn into pre-chilled EDTA tubes, centrifuged immediately at 4°C and plasma was stored at -80°C until analysis.
Quantification of IDeg
Quantification of IDeg was performed using a highly specific assay combining immunopurification with liquid chromatography high-resolution mass spectrometry (LC-HRMS) 8 . Methodological details are provided in the supplementary appendix.
Statistical Analysis
Statistical analysis was performed using R version 4.0.2. 10 Within- and between- participants standard deviation at rest were assessed using a random effects model with participants defining random effects. The exercise-induced increase in insulin concentration was assessed using Generalized Estimation Equation (GEE) modeling assessing the effect of time on relative insulin concentration. Data during exercise were included in the model. Mean relative increases in insulin concentration at the different time points during and after exercise versus mean of pre-exercise values were also assessed using GEE modeling. For all models, a first-order autoregressive correlation structure to account for the repeated measures, assuming a Gaussian error distribution, was used. GEEs were implemented using the R package “geepack”. 11 Results are reported as mean ± SD unless otherwise specified. P values less than .05 were considered statistically significant.
Results
Study Participants and Sample Collection
Samples from 15 participants with type 1 diabetes (see supplementary appendix for characteristics) were quantified for IDeg. Usual daily IDeg dose was 24.1 ± 9.0 units (mean ± SD) and was administered s.c. 8.7 ± 5.3 hours before collection of the first sample. Samples from two subjects were excluded due to substantial measurement interference precluding reliable quantification. Additionally, two samples were excluded due to unsuccessful immunopurification. Consequently, a total of 109 IDeg samples from 13 participants were available for the statistical analysis.
Quantification of IDeg
Plasma IDeg concentration at rest was 2954 ± 1232 pmol/L with a within-participant SD of 64 pmol/L. Concentrations of IDeg increased over time during the 60-minute exercise (0.32%/min, P < .001). The highest values were observed at 45 minutes and 60 minutes of exercise, with increases of 17.9 ± 11.2% (corresponding to an absolute increase of 488 ± 233pmol/L) and 17.6 ± 10.7% (552 ± 454 pmol/L) above baseline (both P < .001), respectively (Supplementary appendix). IDeg levels remained elevated until the end of the experiment (14.0 ± 12.9% and 426 ± 398 pmol/L above baseline 90 minutes after exercise termination, P < .001). Individual IDeg concentration as well as mean IDeg increases relative to resting values for the assessed time points are shown in Figure 1.
Figure 1.
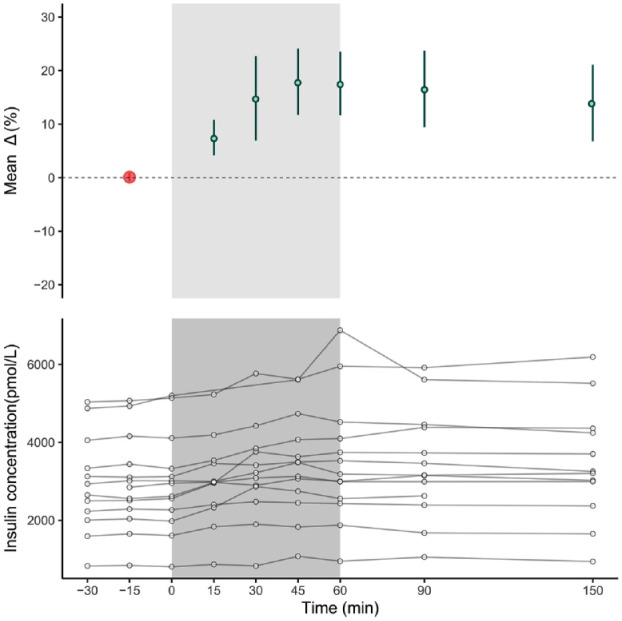
The lower panel shows absolute IDeg plasma concentrations of all individuals. The gray area represents the exercise period. Mean relative change for each time point compared to baseline is shown in the upper panel. Baseline (red dot) is the mean of values measured at -30, -15, and 0 minutes. Error bars represent 95% confidence intervals.
Discussion
In the present study, we assessed the effects of aerobic exercise on IDeg levels in individuals with type 1 diabetes on stable IDeg regimen using a highly specific mass spectrometric IDeg assay. We observed a steady rise in IDeg exposure reaching levels that were 18% above baseline at the end of exercise. These findings not only corroborate the importance of adequate measures to avoid exercise-related hypoglycemia in active individuals with type 1 diabetes (e.g. ingestion of carbohydrates, modification of exercise intensity). Additionally, the data can be integrated into future algorithms to predict exercise-related hypoglycemia.
Two previous studies measured insulin levels in exercising people with type 1 diabetes with IDeg and rapid-acting insulin on board.5,6 However, in these studies, quantification was performed using immunoassays lacking specificity for IDeg, thereby limiting statements regarding potential exercise-induced changes of IDeg exposure. This is corroborated by the improbably low IDeg concentrations (<50 pmol/L) reported in both studies, unlikely to reflect true plasma IDeg levels which are known to be in the three-to-four-digit picomolar range. 12
Our findings support the hypothesis of a true exercise-induced increase in systemic insulin levels. While results from previous studies were somewhat inconsistent, such heterogeneity may be related to differences in insulin regimen, exercise protocols, and in particular analytical approaches.6,13-15 In contrast, the current results can be deemed robust due to the highly specific mass-spectrometric approach used. Exercise-induced increases in insulin exposure are most likely due to a combination of factors (ie, accelerated insulin absorption, hemoconcentration, decreased insulin clearance) in line with experimental studies using labelled insulin for kinetic calculations. 16 The observed increase in IDeg levels in the present study is fully concordant with a recently proposed pharmacokinetic model predicting that during exercise plasma insulin concentration increases by up to 30%. 14
The observed increase in systemic insulin exposure is likely to substantially contribute to the glucose-lowering effect of exercise and hence risk of exercise-related hypoglycemia. A priori knowledge of exercise-induced higher insulin exposure may therefore trigger pre-emptive actions to avoid hypoglycemia (eg, carbohydrate ingestion, pre-exercise rapid insulin dosing, or glucagon mini-dose applications). Further research is warranted to make quantitative statements regarding the contribution of IDeg exposure to hypoglycemia risk and further develop targeted mitigation strategies.
The duration of our experiment (terminated 90 minutes after the end exercise) does not enable to assess the time for IDeg levels to return to baseline. However, IDeg levels were still substantially increased 90 minutes after the end of exercise (14% compared to baseline), suggesting persistent elevation over hours post exercise, and emphasizing the importance of continued adequate countermeasures and increased awareness to avoid exercise-related hypoglycemia over such time periods.
The strength of this work consists in the application of an accurate and specific analytical method allowing for a robust and direct quantification of IDeg. Still, we acknowledge important limitations such as the small sample size, the lack of a resting control condition, and variability in the timing of administration. Although not proven in the present study, previous work provided evidence of the highly stable pharmacokinetic profile of IDeg under resting conditions, 3 thereby supporting the notion that the observed increases in IDeg concentrations are induced by exercise.
In conclusion, we show that a single bout of aerobic exercise substantially and consistently increases systemic IDeg concentrations in adults with type 1 diabetes treated with IDeg. This finding is important to consider when further optimizing strategies to mitigate the risk of hypoglycemia in exercising people with type 1 diabetes using long-acting insulin analogues.
Supplemental Material
Supplemental material, sj-pdf-1-dst-10.1177_19322968211043915 for Effects of Aerobic Exercise on Systemic Insulin Degludec Concentrations in People With Type 1 Diabetes by David Herzig, Michael Groessl, Mario Álvarez-Martínez, Gemma Reverter-Branchat, Christos T Nakas, Christophe Kosinski, Christoph Stettler and Lia Bally in Journal of Diabetes Science and Technology
Acknowledgments
The authors thank Céline Laesser for her assistance during the study visits, and Michèle Monnard (Department of Anaesthesiology and Pain Medicine, University Hospital Bern) for the support with data management. We are grateful to the Department of Nephrology and Hypertension and Department of Clinical Chemistry, University Hospital of Bern and University of Bern, which supported us with infrastructure for the experiments.
Footnotes
Abbreviations: IDeg, insulin degludec; LC-HRMS, liquid chromatography highresolution mass spectrometry.
Author Contributions: LB and CS designed the study. CK was involved in participant recruitment and performance of experiments. LB conceptualized laboratory analysis. GRB and MG performed the sample workup, analytical measurements, and analysis. DH contributed to the collection of the data, analyzed the data, and produced the display items. CN, LB, and CS contributed to the statistical analysis. DH, LB, CS, and MAM, interpreted the findings and wrote the manuscript. All authors critically reviewed the manuscript. LB and CS are the guarantors of this work and take responsibility for the integrity and accuracy of the data.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the UDEM Research Fund, the University of Bern, the Diabetes Center Bern and an ISS (Investigator Sponsored Studies) program of Novo Nordisk A/S, Denmark. MAM received financial support from the Engineering and Physical Sciences Research Council (EPSRC). The supporting entities were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report, and did not impose any restriction regarding the publication of the report.
ORCID iD: David Herzig  https://orcid.org/0000-0003-1028-9445
https://orcid.org/0000-0003-1028-9445
Data availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Basu R, Johnson ML, Kudva YC, Basu A. Exercise, hypoglycemia, and type 1 diabetes. Diabetes Technol Ther. 2014;16(6):331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pitt JP, McCarthy OM, Hoeg-Jensen T, Wellman BM, Bracken RM. Factors influencing insulin absorption around exercise in type 1 diabetes. Front Endocrinol (Lausanne). 2020;11:573275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014;53(9):787-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moser O, Eckstein ML, Mueller A, et al. Reduction in insulin degludec dosing for multiple exercise sessions improves time spent in euglycaemia in people with type 1 diabetes: a randomized crossover trial. Diabetes Obes Metab. 2019;21(2):349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aronson R, Li A, Brown RE, McGaugh S, Riddell MC. Flexible insulin therapy with a hybrid regimen of insulin degludec and continuous subcutaneous insulin infusion with pump suspension before exercise in physically active adults with type 1 diabetes (FIT Untethered): a single-centre, open-label, proof-of-concept, randomised crossover trial. Lancet Diabetes Endocrinol. 2020;8(6):511-523. [DOI] [PubMed] [Google Scholar]
- 7. Reddy R, Resalat N, Wilson LM, Castle JR, El Youssef J, Jacobs PG. Prediction of hypoglycemia during aerobic exercise in adults with type 1 diabetes. J Diabetes Sci Technol. 2019;13(5):919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reverter-Branchat G, Groessl M, Nakas CT, et al. Rapid quantification of insulin degludec by immunopurification combined with liquid chromatography high-resolution mass spectrometry. Anal Bioanal Chem. 2020;412(30):8351-8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kosinski C, Herzig D, Laesser CI, et al. A single load of fructose attenuates the risk of exercise-induced hypoglycemia in adults with type 1 diabetes on ultra-long-acting basal insulin: a randomized, open-label, crossover proof-of-principle study. Diabetes Care. 2020;43(9):2010-2016. [DOI] [PubMed] [Google Scholar]
- 10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 11. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2005;2005:15(2):11. [Google Scholar]
- 12. Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14(10):944-950. [DOI] [PubMed] [Google Scholar]
- 13. Brugnara L, Vinaixa M, Murillo S, et al. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS One. 2012;7(7):e40600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frank S, Jbaily A, Hinshaw L, Basu R, Basu A, Szeri AJ. Modeling the acute effects of exercise on insulin kinetics in type 1 diabetes. J Pharmacokinet Pharmacodyn. 2018;45(6):829-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McAuley SA, Horsburgh JC, Ward GM, et al. Insulin pump basal adjustment for exercise in type 1 diabetes: a randomised crossover study. Diabetologia. 2016;59(8):1636-1644. [DOI] [PubMed] [Google Scholar]
- 16. Ferrannini E, Linde B, Faber O. Effect of bicycle exercise on insulin absorption and subcutaneous blood flow in the normal subject. Clin Physiol. 1982;2(1):59-70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dst-10.1177_19322968211043915 for Effects of Aerobic Exercise on Systemic Insulin Degludec Concentrations in People With Type 1 Diabetes by David Herzig, Michael Groessl, Mario Álvarez-Martínez, Gemma Reverter-Branchat, Christos T Nakas, Christophe Kosinski, Christoph Stettler and Lia Bally in Journal of Diabetes Science and Technology


