Abstract
IGF-1 and GLP-1 receptors are essential in all tissues, facilitating defense by upregulating anabolic processes. They are abundantly distributed throughout the central nervous system, promoting neuronal proliferation, survival, and differentiation. IGF-1/GLP-1 is a growth factor that stimulates neurons' development, reorganization, myelination, and survival. In primary and secondary brain injury, the IGF-1/GLP-1 receptors are impaired, resulting in further neuro complications such as cerebral tissue degradation, neuroinflammation, oxidative stress, and atrophy. Intracerebral hemorrhage (ICH) is a severe condition caused by a stroke for which there is currently no effective treatment. While some pre-clinical studies and medications are being developed as symptomatic therapies in clinical trials, there are specific pharmacological implications for improving post-operative conditions in patients with intensive treatment. Identifying the underlying molecular process and recognizing the worsening situation can assist researchers in developing effective therapeutic solutions to prevent post-hemorrhagic symptoms and the associated neural dysfunctions. As a result, in the current review, we have addressed the manifestations of the disease that are aggravated by the downregulation of IGF-1 and GLP-1 receptors, which can lead to ICH or other neurodegenerative disorders. Our review summarizes that IGF-1/GLP-1 activators may be useful for treating ICH and its related neurodegeneration.
Keywords: Intracerebral hemorrhage, IGF-1, GLP-1, Immune dysregulation, Neuroprotection
Graphical abstract
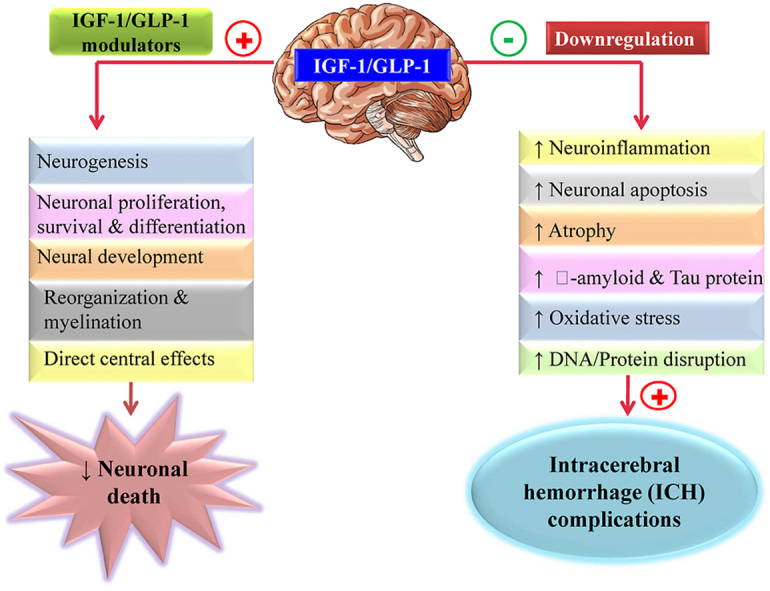
Highlights
-
•
IGF-1/GLP-1 signalling is essential for neuronal cell survival, proliferation, and neurogenesis.
-
•
Cerebral hemorrhage is the most common cause of neuronal death.
-
•
IGF-1/GLP-1 downregulation has been linked to neurological dysfunctions.
-
•
Activation of IGF-1/GLP-1 receptor may play a key role in neuronal protection.
1. Introduction
Intracerebral hemorrhage is characterized as a traumatic, sudden onset of severe headache, impaired level of consciousness, or critical neurological deficiency that results in blood accumulation within the brain (Siddiqui et al., 2021; Syme et al., 2005). The incidence rate of ICH is 24.6 per 100,000 individuals and is linked with a high mortality rate (Poon et al., 2016). Females over the age of 80 had significantly higher numbers across all time points and showed a sustained and increasing gap in 2017 (Krishnamurthi et al., 2020). Using the 45–54 year age group of intracerebral hemorrhage patients as a reference, incidence ratios increased from 0.10 for people under 45 years to 9.6 for people over 85 years (Van Asch et al., 2010) (see Fig. 1).
Fig. 1.
Age-wise mortality incidences in brain hemorrhages.
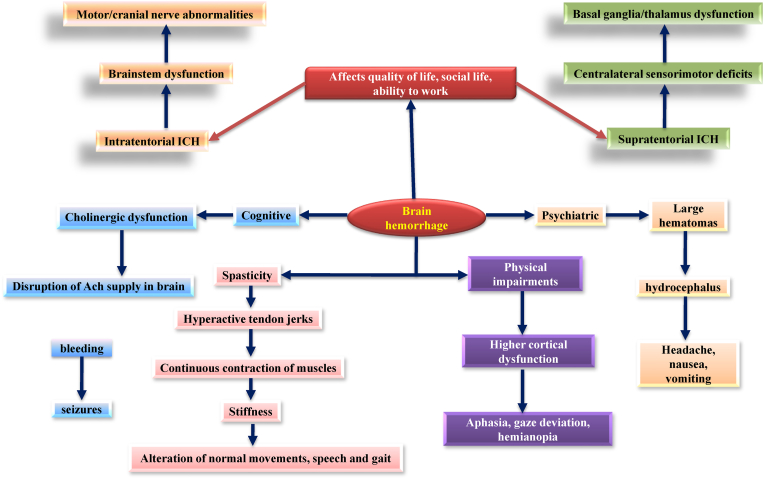
Intraventricular hemorrhage with hydrocephalus, hematoma (HMT) enlargement, seizures, perihematomal edema, venous thromboembolic events, nausea, pneumonia, increased blood pressure, and high glycemic index are all possible causes of death from ICH (Bhattathiri et al., 2006; Steiner et al., 2006). The Lobar hemorrhage causes severe cortical dysfunction, resulting in aphasia, irregular vision, cognitive disability, anisocoria (unequal pupils), seizures/coma, and hemianopia (An et al., 2017). Headache is more common in patients with severe hematomas and is frequently associated with head enlargement and hydrocephalus (Aguilar and Brott, 2011; Wallmark et al., 2014). Eclampsia, opioid misuse, increased systolic BP, trauma, arteriovenous malformation, smoking, low cholesterol, diabetes, and increased alcohol consumption are the leading causes of ICH (Kurth et al., 2003). It could also be due to increased blood flow in the brain, aneurysm burst, altered homeostasis, or vein flow obstruction (Celikbilek et al., 2013; Go et al., 2014; O'donnell et al., 2010).
ICH has the highest stroke mortality rate, with less than 40% of ICH patients attempting to recover stably and safely (Meretoja et al., 2012; Irwin et al., 2011). ICH, in general, affects the basal ganglia, cerebral hemispheres, cerebellum, thalamus, and brainstem (mainly pons) (Qureshi et al., 2001). Mature hematomas are brownish due to two essential haemoglobin (Hb) pigments, haematoidine and haemosiderin. ICH results in massive swelling and brain injuries resulting from pus produced by thrombin and other coagulation end products (Chakrabarty and Shivane, 2008) (see Fig. 2).
Fig. 2.
Clinical presentation during the progression of brain hemorrhage.
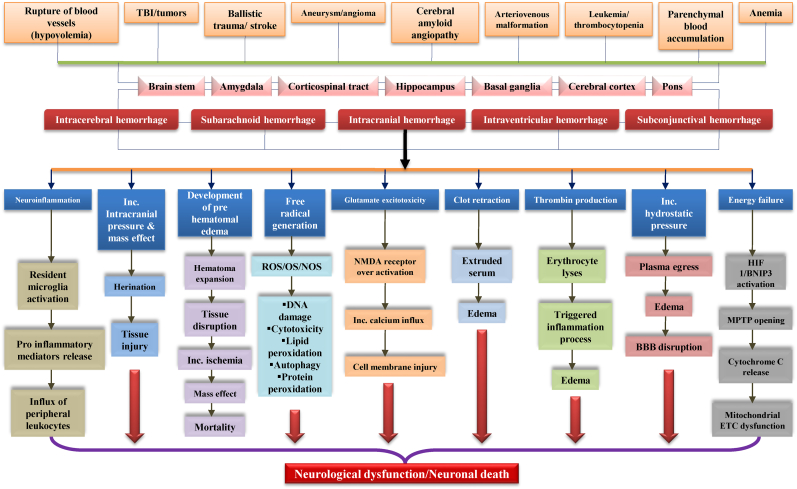
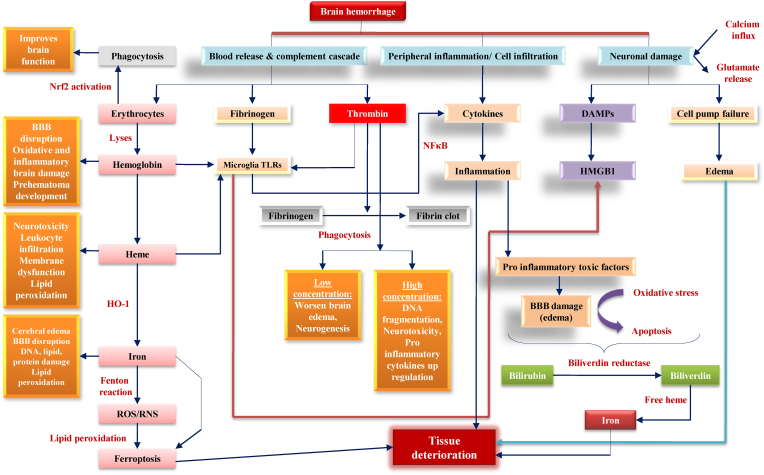
Fig. 3 describes the etiopathological hallmarks responsible for primary brain injury, and Fig. 4 represents the consequences of secondary brain injury due to intracerebral hemorrhage.
Fig. 3.
Etiopathological hallmarks responsible for primary brain injury. Various etiopathological causes, such as trauma, stroke, aneurysm, etc., have affected different brain regions. Dysfunction in major brain areas leads to neuronal cell death, which further causes free radical generation, energy failure, hematoma development, etc. Primary brain injury occurs with the final activation of these cascades in the body.
Fig. 4.
Consequences of secondary brain injury due to intracerebral hemorrhage. Another signaling cascade starts in the brain with the onset of primary brain injury, referred to as secondary brain injury. Additional neuro complications occur in the body throughout this phase, such as Nrf2 activation, fibrinogen, and thrombin, further contributing to neuroinflammation, edema, and microglia activation. Finally, these factors cause more severe tissue damage.
Hematomas damage neurons and the early inflammation caused by hematoma plasma proteins is commonly referred to as secondary brain injury (Rajdev and Mehan, 2019; Rajdev et al., 2020). Clotting and the activation of complement cascades cause disruptions of the blood-brain barrier (BBB), resulting in cytotoxicity and edoema. The late onset of edoema is caused by Hb toxicity and the production of free radicals (Fewel et al., 2003). The accumulation of amyloid-beta (A) proteins on the inside surface of arterioles, capillaries, and cortical leptomeningeal arteries cause CAA (Siddiqui et al., 2019). Neurovascular complications include cortical infarction, fibrinoid necrosis, aligned blistering of the vessel walls (double-barrel effect), stenotic lumina, micro-aneurysms, and micro-haemorrhoids (Sahni and Weinberger, 2007). Reactive oxygen species (ROS) are produced in mitochondria as a byproduct of cellular metabolism and oxidative stress (OS) (Chen et al., 2011). A free radical reaction forms lipid peroxide byproducts such as 4-hydroxy-trans-2-non-enal and malondialdehyde (Han et al., 2008; Aronowski and Hall, 2005; Sook et al., 2006). Since BBB can easily traverse HEt detection, it is used to measure in vivo oxygen-free radical production (Swanson, 2006; Ma et al., 2014). The formation of superoxide ion radicals is caused by mitochondrial leakage of one to four percent of all electrons in the electron transport chain, neutralized by antioxidant enzymes (Tang et al., 2005). Mitochondrial failure occurs in hemorrhagic conditions, producing a substantial rise in ROS production (Bedard et al., 2015; Chan and Chan, 2014). This review focuses on multiple approaches and associated therapeutic interventions for early ICH complications and post-hemorrhagic symptoms based on available hypotheses and dogmas. Consequently, we focused our review on these two targets to highlight their neuroprotective contributions to cellular processes and brain functioning.
2. Regulation of IGF-1 signaling in the brain
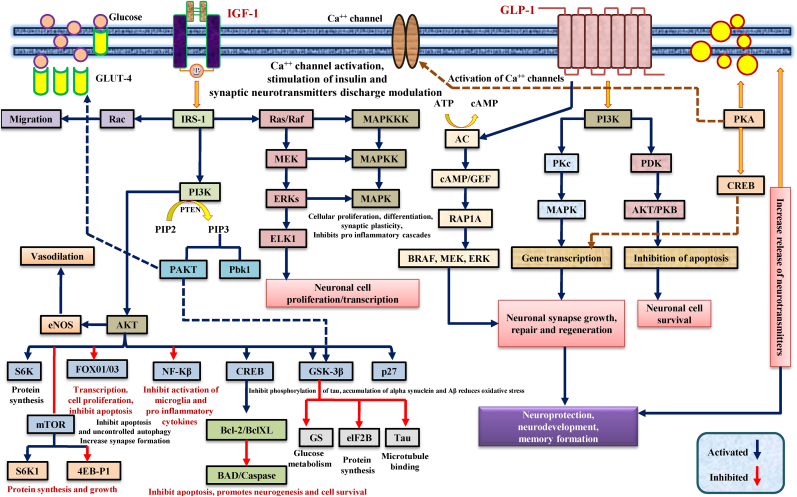
Insulin-like growth factor-1 (IGF-1) is a synthesized polypeptide hormone in the liver. It is found in the olfactory bulb, granule cell layers, cerebellar cortex, dentate gyrus, choroid plexus, hippocampus, amygdala, thalamus, and substantia nigra. Pituitary growth hormone (GH) regulates IGF-1 secretion in the systemic circulation (Laron, 2001; Bentov and Werner, 2006). IGF-1 levels in white matter and cerebrospinal fluid (CSF) are low (Ghazi Sherbaf et al., 2018). Neurons produce IGF-1 under normal physiological conditions, whereas astrocytes produce these trophic factors following local injury. IGF-1 remains bound to high-affinity binding proteins (IGFBPs) in blood circulation. The IGF-1 receptor signaling cascade also includes activation of the MAPK, PI3K, and AKT pathways (Madathil and Saatman, 2015; Ashpole et al., 2015). It promotes neural tissue growth and development by increasing anabolic processes. Fig. 5 illustrates the signaling cascades of the IGF-1/GLP-1 receptors. IGF-1 signaling becomes irregular with age, resulting in age-related alterations in experimental animals. The synthesis of glucose and the migration of GLUT-4 to the neuronal cell membrane were also correlated with the PI3K AKT-mediated IGF-1 signaling to promote glucose absorption into neurons (Holly and Perks, 2006; Muller et al., 2012).
Fig. 5.
Involvement of IGF-1/GLP-1 receptor signaling in various neurocomplications. The IGF-1 signal transduction on the growth axis mainly involves activating two signal transduction chains. The PI3K activation pathway and the MAPK activation pathway transmit mitotic and metabolic signals to the cell nucleus, activating IGF-1 secretion, inducing cell proliferation, differentiation, and inhibiting cell apoptosis. On the other hand, GLP-1R signaling facilitates β-cell glucose metabolism through PI3K-dependent activation of PKC and PDK. The GLP-1 receptor coupled with GLP-1R activates adenyl cyclase when it binds to GLP-1, increasing the cAMP's intracellular level. CAMP-mediated signaling promotes the RAP1A pathway, which, in turn, induces translational activation of BRAF, MEK, ERK, and thus promotes gene transcription, leading to neuroprotection.
Abbreviations: IGF-1 = insulin like growth factor-1; GLP-1 = Glucagon like peptide-1; Ras = Rat sarcoma; Raf = Rapidly Accelerated Fibrosarcoma; IRS-1 = insulin receptor substrate-1; PIP2=Phosphatidylinositol 4,5-bisphosphate; PIP3 = phosphatidylinositol (3,4,5)-trisphosphate; PDK = 3-Phosphoinositide-dependent kinase; PI3k = 3-Phosphoinositide-dependent kinase 1; PKA = protein kinase A; PKB = protein kinase B; Akt = Ak strain transforming; mTOR = mammalian target of rapamycin; ROS = reactive oxygen species; GSK3-β = Glycogen synthase kinase 3-beta; MAPK = mitogen-activated protein kinase; MEK = Mitogen-activated protein kinase kinase; ERK = extracellular signal-regulated protein kinase; CREB = cAMP-response element binding protein; NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells; BCl-2=B-cell lymphoma 2; Bax = Bcl-2 Associated X-protein; Caspase = cysteine-dependent aspartate-directed proteases; AC = adenylyl cyclase; cAMP = cyclic Adenosine MonoPhosphate; GLUT4 = glucose transporter 4; ATP = Adenosine triphosphate; NOS = nitric oxide synthase.
The IGF-1 receptor (IGF-1R) is a membrane-bound tyrosine kinase receptor that regulates the pharmacological activities of IGF-1. When IGF-1 bind to its receptor, it autophosphorylates at several tyrosine sites, phosphorylating the IRS-1 protein sequentially. These phosphorylated tyrosine sites function as docking sites for various intracellular signaling proteins. The complex signaling pathways such as PI3K, MAPK, and AKT are thus activated. IRS-1 binds to PI3K, phosphorylation occurs, and PIP-2 converts to PIP-3, which recruits other proteins to the plasma membrane, including PDK-1 and AKT (Li et al., 2009; Puche and Castilla-Cortázar, 2012). It also binds to two protein kinases, AKT and PDK-1, resulting in AKT initiation and promoting cell growth, maturation, and survival via intracellular protein interactions. AKT phosphorylation regulates the mammalian target of rapamycin (mTOR), enhanced protein synthesis, ribosome development, and pro-apoptotic BAD protein inhibition (Huang et al., 2012). Furthermore, when the PI3K-AKT signal activates the IGF-1R, the GSK-3 inhibitory protein is phosphorylated, resulting in increased glycogen accumulation in neurons (Kreft and Jetz, 2007; Yin et al.,). FOXO-1 has also been inactivated, preventing direct transcription of pro-apoptotic FOXO genes (Junnila et al., 2013). As a result, it was found that PI3K signaling inhibits the pro-apoptotic system through different mechanisms. The olfactory lobe, cerebellum, and hippocampus showed the highest levels of postnatal IGF-1 expression, which then declined after neuronal proliferation (Wrigley et al., 2017). IGF-1 has been shown to stimulate all neural cells (glial cells, astrocytes, neurons, and oligodendrocytes), increase mitochondrial function, and encourage proliferation and differentiation of oligodendrocytes and myelin development survival, and maturation. This mechanism has been confirmed by cultural studies (in-vitro/in-vivo) and experiments with transgenic mice (Chesik et al., 2008; Spielman et al., 2014).
3. Regulation of GLP-1 signaling in the brain
GLP-1 is an endogenous hormone (insulinotropic) that maintains a glucose-insulin balance. Intestinal endocrine L-cells primarily secrete it to initiate glucose-dependent insulin secretion, glucagon secretion blockage, food intake, and gastric clearance (Supeno et al., 2013). GLP-1 is a hormone with trophic effects on neurogenesis, proliferation, and differentiation (Mehan et al., 2022). It also reduces cell death in neurons, beta-cell islets, and fibroblasts (Brooker et al., 2000). The GLP-1 receptor (GLP-1R), a seven-transmembrane GPCR that activates adenyl cyclase (AC), resulting in the production of cyclic adenosine monophosphate, facilitates the physiological function of GLP-1 (cAMP). cAMP stimulates phosphokinase A (PKA), which phosphorylates and inhibits several downstream proteins and anti-apoptotic factors.GLP-1R is found in various tissues, including pancreatic islet cells, blood vessels, lungs, heart, kidneys, neurons, and lymphocytes. They are also expressed on presynaptic neurons, cell bodies, and dendrites (Daftary and Gore, 2005; Lunetta et al., 2012).
GLP-1 is considered down-regulated in ICH and responds via the PI3K and MAPK pathways (Cancedda et al., 2004). GLP-1 is metabolized by DPP-4 in 2–3 min, lowering peripheral glucose levels. DPP-4 (serine protease) explicitly removes dipeptides from the amino terminals of proteins containing proline/alanine residues, inhibiting their function. DPP-4 is a soluble protein that circulates in the blood, penetrates brain tissue, and infiltrates the pancreas and liver (Baggio and Drucker, 2007). GLP-1 analogues such as exendin-4, exendin-XR, liraglutide, albiglutide, dulaglutide, and lixisenatide are DPP-4 insensitive. They can cross the BBB in the same way as GLP-1 (except for albiglutide) and may also bind to GLP-1R (Doyle and Egan, 2001). GLP-1 analogues are neurotrophic, neuroprotective, and memory-enhancing (Brubaker and Drucker, 2004; Li et al., 2005). Exendin-4 has been shown to provide neuroprotection in the SAOS rat model by decreasing the brain region that deteriorated after stroke injury. In experimental rats, intracerebroventricular (ICV) GLP-1 injection was associated with improved memory and recognition and significant neuroprotective effects (Baggio and Drucker, 2007; Hölscher, 2012). GLP-1 has been studied as a potential treatment for neurodegenerative disorders such as Alzheimer's (AD) (During et al., 2003; Cardona-Gomez et al., 2000) and Huntington's disease (HD) (Torres‐Aleman, 2010). GLP-1 stimulates cell formation, maturation, growth, and differentiation while inhibiting apoptosis (Li et al., 2005). GLP-1 has been shown to promote neuritis development in cultured neuronal cells and protect against excitotoxicity and oxidative injury (Campbell and Drucker, 2013). Another study found that mice with higher GLP-1 receptor expression in the hippocampus had better cognitive spatial abilities and neurological performance (Perry and Greig, 2004; Perry and Greig, 2005). Several studies on the IGF-1/GLP-1 receptor have been conducted, and significant findings from trials on drugs acting on these receptors are included in this review in Table 1.
Table 1.
Clinically available IGF-1/GLP-1 receptor modulators in various dysfunctions.
| S.No. | Target modulators | Target involved | Key findings | Therapeutic indications | Current status | Reference |
|---|---|---|---|---|---|---|
| 01. | Demethylasterriquinone B1 (activator) | IGF-1R |
|
|
|
Pitt et al. (2017) |
| 02. | Insulin (human) recombinant expressed in yeast (activator) |
Endogenous peptide agonist | ↓ amino acid (AA)-regulated mTORC1-directed signaling |
|
|
Stretton et al. (2015) |
| 03. | Direct IGF injection (activator) | IGF1R | ↑ prostate cancer cell line PC3 |
|
|
Varkaris et al. (2013) |
| 04. | Somatomedin C (activator) | IGF1R |
|
↑ systemic body growth and growth-promoting effects | NA | Varkaris et al. (2013) |
| 05. | PQ 104 (inhibitor) | IGF-1R | ↑ RPTPβ, AKT Ser473 phosphorylation and VSMC proliferation ↓ IGF-I |
|
|
(Shen et al., 2012; Sanchez-Alavez et al., 2011) |
| 06. | Picropodophyllotoxin (inhibitor) | Selective IGF1R |
↓ MAPK phosphorylation |
|
↓ RMS tumor proliferation by cyclolignan PPP | Tarnowski et al. (2017) |
| 07. | Rapamycin (inhibitor) | mTOR and IGF-1R | ↓ cell growth and survival | ↓ mammalian target of rapamycin (mTOR) signaling |
|
Wan et al. (2007) |
| 08. | Metformin and diazoxide (activator) | IGF-1/Insulin | ↓ insulin/IGF-1 system is beneficial in cancer-bearing animals |
|
|
(Klement and Fink, 2016) |
| 09. | BMS 536924 (inhibitor) | Dual IR/IGF1R | ↑ LAT1 activity via NKCC1 depletion or deletion | ↓ intracellular Na+ concentration by NKCC1 depletion |
|
Demian et al. (2019) |
| 10. | Tirzepatide (activator) | GIP and GLP-1 receptor | ↓ HbA1c (Dose-dependent) | ↑ GIP and GLP-1 receptor signaling in vitro showed glucose-dependent insulin secretion |
|
Frias et al. (2018) |
| 11. | Exenatide (activator) | GLP-1 receptor | ↓ endogenous (hepatic) glucose production | ↓ postprandial hyperglycemia in type 2 diabetes | ↑ energy expenditure and weight loss by neural and metabolic regulation | Cervera et al. (2008) |
| 12. | Liraglutide (activator) | GLP-1 receptor | ↑ GLP1 receptor, glucose concentrations leading to insulin release |
|
↓ risk of a heart attack, stroke, or death in adults | (Drucker et al., 2010) |
| 13. | Lixisenatide (activator) | GLP-1 receptor | ↑ cAMP levels in the brain, neurogenesis in the brain | ↑ neurogenesis and cognition and memory formation in the adult brain |
|
(Hunter and Hölscher, 2012) |
| 14. | Albiglutide (activator) | GLP-1 receptor | ↑ glucose-dependent insulin secretion ↓inappropriately elevated glucagon secretion | ↓ gastric emptying, food intake, blood glucose level |
|
Rosenstock et al. (2009) |
4. Dysregulation of IGF-1 and GLP-1 signaling during ICH
Although IGF-1 and GLP-1 have a primary role in metabolic functions, they are also involved in mitogenic activities and cell differentiation. In several in-vivo and in-vitro experiments, IGF-1 and GLP-1 serve as neuroprotective agents. In ICH, IGF-1/GLP-1 administration facilitates axonal regeneration, improves functional recovery, and improves neuronal transmission. IGF-1/GLP-1 ICV injection significantly reduced neuronal loss in impaired areas due to bleeding following experimental hypoxic ischemia and temporary forebrain ischemia. The neuroprotective and anti-inflammatory properties of IGF-1/GLP-1 suggest that these agents effectively treat patients with stroke conditions. IGF-1/GLP-1 analogues provided significant neuroprotective effects in ischemic/hypoxic conditions (Li et al., 2009).
Several preclinical studies have shown that IGF-1/GLP-1 signaling effectively protects neurons from oxidative stress and reduces inflammatory responses in ischemia and strokes. As a result, it is concluded that IGF-1/GLP-1 is downregulated by a different pathway during hemorrhagic conditions, resulting in neurodegeneration, apoptosis, and necrosis, all of which contribute to neuro complications. Consequently, administering exogenous drugs by targeting such receptors may provide neuroprotective effects.
5. Drug therapy available for the management of intracerebral hemorrhage
Despite significant advances in pre-clinical research, few treatment approaches are effective in clinical trials to manage intracerebral hemorrhage. Most targeted molecular pathways include reducing excitotoxicity and calcium influx, restoring mitochondrial alterations, activating multiple intracellular enzymes, and decreasing free radical formation, nitric oxide production, inflammation necrosis, and apoptosis. However, the ICH has also been compatible with existing standard healthcare facilities in some cases. ICH management is usually supportive to avoid further brain damage and related illnesses. Due to the limited range of treatment options available to ICH patients, effective monitoring is required to enhance protective effects against these complications. There may not be a range of immediate and effective treatments for ICH. However, several studies have shown that certain drugs of different categories remarkably influence the disease through numerous experiments and data, as discussed below.
Table 2 provides an overview of currently available treatment options for the management of intracerebral hemorrhage.
Table 2.
Overview of clinically available treatment options for the management of intracerebral hemorrhage.
| S.No. | Drug | Therapeutic interventions | Mechanism of action | Clinical status | Reference |
|---|---|---|---|---|---|
| 01. | Magnesium sulfate | ↑ recovery of vital magnesium-dependent cell functions
|
↓ cerebral oxygen metabolism, the synaptic inhibitor | phase II: potent, phase III: in process |
Saver et al. (2004) |
| 02. | Uric acid | ↓ ischemia-induced tyrosine nitration
|
|
phase II: potent, phase III: in process |
(Romanos et al., 2007; Amaro et al., 2007, 2010) |
| 03. | Dapsone |
|
|
clinical pilot trial: potent, phase III: in process |
(Rıos et al., 2004; Kaur et al., 2013) |
| 04. | Cromolyn | ↑ CNS damage in models of ischemic and hemorrhagic stroke by amplifying the inflammatory responses |
|
phase III: in process |
(Strbian et al., 2006, 2007) |
| 05. | Ebselen | ↓ lipid peroxidation and iNOS protein expression in the cerebral cortex |
|
phase III: in process |
Sui et al. (2005) |
| 06. | Statin | ↑ cholesterol independent vasoprotective effects |
|
phase IV: in process |
Prinz et al. (2008) |
| 07. | Cyclosporin A |
|
|
phase II: in process |
Leger et al. (2011) |
| 08. | Clomethiazole |
|
|
non-functional | (Sydserff et al., 2000; Chaulk et al., 2003; Lyden et al., 2002) |
| 09. | Selfotel | ↓ early post-ischemic brain injury in transit focal ischemia |
|
Phase III: non-functional | Davis et al. (2000) |
| 10. | Aptiganel |
|
|
Phase III: non-functional | (Schabitz et al., 2000; Albers et al., 2001) |
| 11. | Fanapanel | ↓ infarct size in a transient middle cerebral artery occlusion in ischemic stroke |
|
non-functional | Walters et al. (2005) |
Abbreviations: IGF-1 = insulin like growth factor-1; GLP-1 = Glucagon like peptide-1; GIP = Gastric inhibitory polypeptide; IGF-1R = Insulin like growth factor-1 receptor; IR=Insulin receptor; AꞵO = amyloid-beta oligomers; mTOR = mammalian target of rapamycin; Akt = Ak strain transforming; GSK3-β = Glycogen synthase kinase 3-beta; MET = Mesenchymal Epithelial Transition; RMS = Rhabdomyosarcoma; VSMC = vascular smooth muscle; LAT1 = Large Amino Acid Transporter 1; iNOS = Inducible nitric oxide synthase; BBB = blood brain barrier; HMG-CoA = β-Hydroxy β-methylglutaryl-Coenzyme A; mTORC1 = mTOR Complex 1.
5.1. Minocycline (MNC)
It is a tetracycline group antibiotic that enters the central nervous system quickly. Several studies have found that MNC has significant neuroprotective effects, including preventing apoptosis and reducing matrix metalloproteinase activity via inflammation inhibition. In ICH rat models, MNC administration reduced cerebral edoema and inflammation while maintaining BBB porosity (Zhao et al., 2011; Shi et al., 2011). The neuroprotective effect of MNC was insignificant in the first three to 4 h following hemorrhage (Szymanska et al., 2006). It is a treatment option for ICH and is currently being evaluated in a clinical trial (CT) for its efficacy (MACH; NCT01805895; 2018) (A pilot study of minocycline in intracerebral hemorrhage patients (MACH); Taken from: https://clinicaltrials.gov/ct2/show/NCT01805895; Accessed on January 7, 2020).
5.2. Albumin (ABM)
In ICH and ischemic stroke, ABM has several neuroprotective effects. ABM therapy improved BBB integrity and neurological functions (Belayev et al., 2005, 2007). Despite the impact of ABM in clinical trials for acute ischemic stroke (ALIAS), the potential effects of ABM in ICH are currently being investigated (ACHIEVE, NCT00990509), which has examined the main results of ABM in ICH patients. The severity of the BBB disruption and the intensity identified in pre-and post-MRI imaging was the key findings of this investigational study (Ginsberg et al., 2011; Rekik et al., 2012).
5.3. Monocytes/macrophages (MCs/MPs)
Monocytes/macrophages (MCs/MPs) have also been shown to repair brain and brain tissue (Sanberg et al., 2010; Sica and Mantovani, 2012). MCs have a neuroprotective effect on the blood cells of the spinal cord. It is divided into M1 and M2 subunits. M1 cells produce pro-inflammatory cytokines, while M2 cells participate in tissue regeneration, angiogenesis, and the inflammatory process (Kigerl et al., 2009; Shechter et al., 2009). M1 was subsequently proven to cause neuronal death, while M2 prevented neuron apoptosis. As a result, MCs revived activity and reduced hematoma size (Hu et al., 2012). Similarly, restoring neurons in brains damaged by lipopolysaccharide due to MC penetration displayed a phenotype associated with cell repair (Jeong et al., 2013).
Mesenchymal stem/stromal cells have been shown to exhibit anti-inflammatory genotypes in clinical settings (Eggenhofer and Hoogduijn, 2012; Walker et al., 2012). Due to various functional modulations, models such as UCB-BM-MNCs8 were replaced by MCs/MPs stroke models. Apoptosis occurs in stem cells replaced, resulting in anti-inflammatory responses and the development of macrophage-derived growth factors such as VEGF, IGF-1, GLP-1, platelet-derived growth factor (PDGF), and erythropoietin. As a result, we believe MCs/MPs are effective therapeutic agents for neural repair and immunoregulation (Lu et al., 2013; Chernykh et al., 2010).
5.4. Deferoxamine mesylate (DM)
Iron is essential in ICH-mediated secondary brain injury (Wagner et al., 2002). Autophagia, OS/HR formation, and excitotoxicity are caused by RBC hemolysis (Nakamura et al., 2004; Chen et al., 2010). In pig and rat models of ICH, DM (iron chelator) was protective against secondary brain damage (Okauchi et al., 2010; Selim, 2009). It possesses anti-phagocytic, anti-apoptotic, anti-inflammatory, and anti-oxidant properties. In particular, hemorrhagic models reduced Hb-induced neurotoxicity and enhanced post-ICH neural defense (Okauchi et al., 2010; Gu et al., 2009). In the Phase I clinical trial, the safety and dose-finding DM study were investigated in ICH patients. The safety and efficacy of various DM formulations have been tested to determine the maximum tolerated dose. A phase II clinical trial with a high amount of DM was also conducted (NCT01662895) (Ji et al., 2009).
5.5. Pioglitazone (PGZ)
Pioglitazone, a peroxisome proliferator-activated-gamma (PPAR gamma) agonist, plays a vital role in the modulation of inflammation and OS and enhances systemic phagocytosis reflux of the hematoma without affecting neighbouring brain cells, and thus may be a practical treatment approach for ICH (Cai et al., 2018; Swanson et al., 2011). In a blind, randomized placebo-controlled CT, the results of an investigation on the safety of PGZ for hematoma resolution in ICH (SHRINC) indicate that PGZ should be evaluated as a possible treatment for ICH in a subsequent phase II/III trial (Gonzales et al., 2020).
5.6. Direct oral anticoagulants (DOACs)
Oral anticoagulants include factor IIa or thrombin inhibitors (dabigatran) and factor Xa inhibitors (betrixaban, apixaban, rivaroxaban, edoxaban). Tix-5, a factor Xa inhibitor, was isolated from tick saliva, while hirudin, a DTI, was isolated from leech saliva (Gonzales et al., 2013; Tanaka-Azevedo et al., 2010). Dabigatran etexilate (Pradaxa), a drug in the DTI family, was the first DOAC-approved drug in the USFDA category. It was quickly followed by rivaroxaban (Xarelto) (approved in July 2011) and apixaban (Eliquis) (approved in December 2012). In contrast, the SFDA approved edoxaban in January 2015 (Savaysa). The FDA approved betrixaban, a new DOAC, in June 2017 (Skelley et al., 2018). DOACs have replaced warfarin as a safer option due to a 50–60% lower ICH risk. DOACs are an appealing alternative to warfarin and other oral anticoagulants due to their ease of administration, rapid onset of action, short half-life, and more predictable pharmacokinetics (Gómez-Outes et al., 2015).
5.7. Clopidogrel and ticlopidine (TCP and CPD)
TCP and CPD are two common pro-drugs that must be metabolized and have been shown to improve platelet function. They are both members of the thienopyridine family (ADP receptor antagonists). Both drugs inhibited platelet accumulation and downregulated AC, resulting in fewer ADP platelet binding sites (Cattaneo, 2007). Thienopyridines have been used to treat platelet function defects, and research has shown that they are particularly antagonistic to P2Y12 (platelet receptor) and have no effect on P2Y1 (Cattaneo, 2007). Platelet agonists such as collagen, thromboxane A2 analogues, and thrombin prevent platelet aggregation (Cattaneo, 2011).
TCP and CPD increase bleeding time through thrombin and shear-induced platelet aggregation. They may also have additional therapeutic effects that result in fibrinogen reduction, which may be associated with hemorrhagic recovery (reduction in plasma and blood viscosity), decreased erythrocyte aggregation, increased nitric oxide production PDTF disruption, and fibronectin synthesis blockage. TCP administration has been related to preventing both moderate and secondary phases of strokes. TCP (500 mg/day) was administered to patients with ischemic stroke and reduced the risk of stroke-related mortality by 12–15 percent (Jeremias and Brown, 2010).
6. Conclusion
ICH has a negative impact on public health and is becoming a significant social and economic burden. Concerns about higher occurrence and mortality rates in low and middle-income countries have been expressed. New therapeutic agents are urgently needed to protect and treat post-hemorrhagic complications. The etiopathological factors linked to ICH are concerning because they are poorly understood. Several studies on the downregulation of IGF-1/GLP-1 signaling in ICH have already been published.
Nonetheless, the review aimed to link the disease to a specific target in the system influenced by the diseased state. Furthermore, the core of this manuscript provides a detailed explanation of the disease and its pathophysiology. Moreover, we attempted to explain the connection between the IGF-1/GLP-1 signaling target and ICH. A range of information is available on clinical trials involving IGF-1 and GLP-1. However, it has been found that there is no specific treatment for ICH other than symptom relief; several medications have been discussed extensively in the review.
As a result of our findings, activation of IGF-1 and GLP-1 may be a potential therapeutic target when combined with existing drug therapy in brain hemorrhage patients with behavioural and neurochemical changes (Shandilya and Mehan, 2021). Antithrombotic agents, antiplatelet agents, thrombolytic agents, calcium channel blockers, AMPA antagonists, and other medications are safer options for hemorrhagic patients. Patients with deep hematomas, increased intracranial pressure, uncontrolled arterial hypertension, and seizures may benefit from healthcare attention. Clinical trials are also required to assess the efficacy of hemostatic treatment. There have also been no reports of specific therapies for improving post-ICH outcomes. To summarise, more detailed research is needed to investigate the role of IGF-1/GLP-1 signaling in brain hemorrhage and develop effective and safe drug therapy.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or non-profit sector.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
Ehraz Mehmood Siddiqui: involved in, Investigation, Writing – original draft, Writing – review & editing, All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used. Sidharth Mehan: has contributed, Conceptualization, Resources, Supervision, Writing – review & editing, All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used. Sonalika Bhalla: Writing – review & editing, involved in revision, scientific editing and literature search, All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used. Ambika Shandilya: Writing – review & editing, involved in revision, scientific editing and literature search, All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Sidharth Mehan reports financial support was provided by Indo Soviet Friendship College of Pharmacy.
Acknowledgments
The authors express their gratitude to Chairman, Mr. Parveen Garg, and Director, Dr. G. D. Gupta, ISF College of Pharmacy, Moga, Punjab, India, for their extraordinary vision and support.
Footnotes
A Peer Review Overview and (sometimes) Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.crneur.2022.100055.
Abbreviations
- ICH
Intracerebral hemorrhage
- IGF-1
Insulin like growth factor-1
- GLP-1
Glucagon like peptide-1
- IVH
Intraventricular hemorrhage
- HMT
Hematoma
- BBB
Blood brain barrier
- RBCs
Red blood cells
- Hb
Hemoglobin
- CAA
Cerebral amyloidal angiopathy
- Aβ
Amyloid beta
- 8-OHdg
8-hydroxy-2-deoxyguanosine
- FOXO
Forkhead box transcription factors
- HEt
Oxidized hydroethidine
- MPTP
Mitochondrial permeability transition pore
- CSF
Cerebrospinal fluid
- GH
Growth hormone
- IGFBPs
IGF-1 binding proteins
- MAPK
Mitogen activated protein kinase
- IRS-1/2
Insulin receptor substrate-1/2
- PDK-1
Phosphoinositide dependent protein kinase-1
- mTOR
Mammalian target of rapamycin
- BAD
BCL2 associated agonist of cell death
- GSK3b
Glycogen synthase 3 b
- IGF-1R
Insulin like growth factor-1 receptor
- GLUT4
Glucose transporter type 4
- GLP-1R
Glucagon like peptide-1 receptor
- GPCR
G protein-coupled receptor
- AC
Adenyl cyclase
- cAMP
Cyclic adenosine monophosphate
- PKA
Protein kinase A
- DPP-4
Dipeptidyl peptidase 4
- AD
Alzheimer's disease
- PD
Parkinson's disease
- HD
Huntington's disease
- Ca
Calcium
- OS
Oxidative stress
- NDD
Neurodegenerative diseases
- NFT
Neuro fibrillary tangles
- LB
Lewy bodies
- DLB
Dementia with lewy bodies
- MSA
Multiple system atrophy
- a-syn
a-synuclein
- ALS
Amyotrophic Lateral Sclerosis
- SNP
Synucleinopathies
- SN
Substantia nigra
- RR
Relative risk
- TBI
Traumatic brain injury
- BDNF
Brain-derived neurotrophic factor
- mHtt
Mutant huntingtin
- DM
Diabetes mellitus
- MMP
Matrix metalloproteinase
- ABM
Albumin
- CT
Clinical trial
- MPs
Macrophages
- MCs
Monocytes
- PIC
Pro inflammatory cytokines
- hUCB-MNCs
Human umbilical cord blood-derived mononuclear cells
- LPS
Lipopolysaccharide
- MSCs
Mesenchymal stem/stromal cells
- UCB- BM-MNC
Sumbilical cord blood-bone marrow-mononuclear cells
- MDGF
Macrophage derived growth factors
- VEGF
Vascular endothelial growth factor
- PDGF
Platelet derived growth factor
- EPO
Erythropoietin
- HR
Hydroxyl radical
- MTD
Maximum tolerated dose
- PGZ
Pioglitazone
- PPARγ
Peroxisome proliferator activated receptor gamma
- DOACs
Direct oral anticoagulants
- DTI
Direct thrombin inhibitor
- USFDA
United States Food and Drug Administration
- PDTF
Platelet dependent tissue factor
- SDDH
Sheng-Di-Da-Huang
- IL-6
Leukocyte interleukin 6
- MMPs
Matrix metalloproteinases
- iNOS
Inducible nitric oxide synthase
- COX-2
Cyclooxygenase-2
- IL-1β
Interleukin-1 beta
- TNF-α:
Tumor necrosis factor-alpha
Appendix A. Peer Review Overview and Supplementary data
A Peer Review Overview and (sometimes) Supplementary data associated with this article:
References
- A pilot study of minocycline in intracerebral hemorrhage patients (MACH); Taken from: https://clinicaltrials.gov/ct2/show/NCT01805895; Accessed on 7th January, 2020.
- Aguilar M.I., Brott T.G. Update in intracerebral hemorrhage. The Neurohospitalist. 2011 Jul;1(3):148–159. doi: 10.1177/1941875211409050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers G.W., Goldstein L.B., Hall D., Lesko L.M. Aptiganel Acute Stroke Investigators. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA. 2001 Dec 5;286(21):2673–2682. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- Amaro S., Soy D., Obach V., Cervera A., Planas A.M., Chamorro A. A pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke. 2007 Jul 1;38(7):2173–2175. doi: 10.1161/STROKEAHA.106.480699. [DOI] [PubMed] [Google Scholar]
- Amaro S., Cánovas D., Castellanos M., Gállego J., Martí-Fàbregas J., Segura T., Chamorro Á. The URICO-ICTUS study, a phase 3 study of combined treatment with uric acid and rtPA administered intravenously in acute ischaemic stroke patients within the first 4.5 h of onset of symptoms. Int. J. Stroke. 2010 Aug;5(4):325–328. doi: 10.1111/j.1747-4949.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- An S.J., Kim T.J., Yoon B.W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. Journal of stroke. 2017;19(1):3. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J., Hall C.E. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol. Res. 2005 Apr 1;27(3):268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Ashpole N.M., Sanders J.E., Hodges E.L., Yan H., Sonntag W.E. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015 Aug 1;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007 May 1;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Bedard K, Whitehouse S, Jaquet V. Challenges, progresses, and promises for developing future NADPH oxidase therapeutics. 10.1089/ars.2015.6450. [DOI] [PubMed]
- Belayev L., Saul I., Busto R., Danielyan K., Vigdorchik A., Khoutorova L., Ginsberg M.D. Albumin treatment reduces neurological deficit and protects blood–brain barrier integrity after acute intracortical hematoma in the rat. Stroke. 2005 Feb 1;36(2):326–331. doi: 10.1161/01.STR.0000152949.31366.3d. [DOI] [PubMed] [Google Scholar]
- Belayev L., Obenaus A., Zhao W., Saul I., Busto R., Wu C., Vigdorchik A., Lin B., Ginsberg M.D. Experimental intracerebral hematoma in the rat: characterization by sequential magnetic resonance imaging, behavior, and histopathology. Effect of albumin therapy. Brain Res. 2007 Jul 9;1157:146–155. doi: 10.1016/j.brainres.2007.04.077. [DOI] [PubMed] [Google Scholar]
- Bentov I., Werner H. Academic Press; 2006 Jan 1. pp. 1385–1392. (Insulin-like growth factor 1. InHandbook of Biologically Active Peptides). [DOI] [Google Scholar]
- Bhattathiri P.S., Gregson B., Prasad K.S., Mendelow A.D. Brain Edema. Springer; Vienna: 2006. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial; pp. 65–68. XIII. [DOI] [PubMed] [Google Scholar]
- Brooker G.J., Kalloniatis M., Russo V.C., Murphy M., Werther G.A., Bartlett P.F. Endogenous IGF‐1 regulates the neuronal differentiation of adult stem cells. J. Neurosci. Res. 2000 Feb 1;59(3):332–341. doi: 10.1002/(SICI)1097-4547(20000201)59:3<332::AID-JNR6>3.0.CO. 2-2. [DOI] [PubMed] [Google Scholar]
- Brubaker P.L., Drucker D.J. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004 Jun 1;145(6):2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- Cai M., Yu Z., Zhang W., Yang L., Xiang J., Zhang J., Zhang Z., Wu T., Li X., Fu M., Bao X. Sheng-Di-Da-Huang decoction inhibited inflammation expressed in microglia after intracerebral hemorrhage in rats. Evid. base Compl. Alternative Med. 2018 doi: 10.1155/2018/6470534. 2018 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metabol. 2013 Jun 4;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Cancedda L., Putignano E., Sale A., Viegi A., Berardi N., Maffei L. Acceleration of visual system development by environmental enrichment. J. Neurosci. 2004 May 19;24(20):4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gomez G.P., DonCarlos L., Garcia-Segura L.M. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000 Aug 23;99(4):751–760. doi: 10.1016/S0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. Platelet P2 receptors: old and new targets for antithrombotic drugs. Expet Rev. Cardiovasc. Ther. 2007 Jan 1;5(1):45–55. doi: 10.1586/14779072.5.1.45. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. The platelet P2Y12 receptor for adenosine diphosphate: congenital and drug-induced defects. Blood. 2011 Feb 17;117(7):2102–2112. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]
- Celikbilek A., Goksel B.K., Zararsiz G., Benli S. Spontaneous intra-cerebral hemorrhage: a retrospective study of risk factors and outcome in a Turkish population. J. Neurosci. Rural Pract. 2013 Jul;4(3):271. doi: 10.4103/0976-3147.118770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera A., Wajcberg E., Sriwijitkamol A., Fernandez M., Zuo P., Triplitt C., Musi N., DeFronzo R.A., Cersosimo E. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2008 May;294(5):E846–E852. doi: 10.1152/ajpendo.00030.2008. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A., Shivane A. Pathology of intracerebral hemorrhage. ACNR. 2008;8(1):20–21. doi: 10.1152/ajpendo.00030.2008. [DOI] [Google Scholar]
- Chan S.H., Chan J.Y. Brain stem NOS and ROS in neural mechanisms of hypertension. Antioxidants Redox Signal. 2014 Jan 1;20(1):146–163. doi: 10.1089/ars.2013.5230. [DOI] [PubMed] [Google Scholar]
- Chaulk D., Wells J., Evans S., Jackson D., Corbett D. Long-term effects of clomethiazole in a model of global ischemia. Exp. Neurol. 2003 Aug 1;182(2):476–482. doi: 10.1016/S0014-4886(03)00121-3. [DOI] [PubMed] [Google Scholar]
- Chen M., Awe O.O., Chen-Roetling J., Regan R.F. Iron regulatory protein-2 knockout increases perihematomal ferritin expression and cell viability after intracerebral hemorrhage. Brain Res. 2010 Jun 14;1337:95–103. doi: 10.1016/j.brainres.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yoshioka H., Kim G.S., Jung J.E., Okami N., Sakata H., Maier C.M., Narasimhan P., Goeders C.E., Chan P.H. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants Redox Signal. 2011 Apr 15;14(8):1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernykh E.R., Shevela E.Y., Sakhno L.V., Tikhonova M.A., Petrovsky Y.L., Ostanin A.A. The generation and properties of human M2-like macrophages: potential candidates for CNS repair. Cell Ther. Transplant. 2010;2(6) e-000080. https://doi.org/10.3205/ctt-2010-en-000080.01. [Google Scholar]
- Chesik D., De Keyser J., Wilczak N. Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J. Mol. Neurosci. 2008 May 1;35(1):81. doi: 10.1007/s12031-008-9041-2. [DOI] [PubMed] [Google Scholar]
- Daftary S.S., Gore A.C. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp. Biol. Med. 2005 May;230(5):292–306. doi: 10.1177/153537020523000503. [DOI] [PubMed] [Google Scholar]
- Davis S.M., Lees K.R., Albers G.W., Diener H.C., Markabi S., Karlsson G., Norris J. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke. 2000 Feb;31(2):347–354. doi: 10.1161/01.STR.31.2.347. [DOI] [PubMed] [Google Scholar]
- Demian W.L., Persaud A., Jiang C., É Coyaud, Liu S., Kapus A., Kafri R., Raught B., Rotin D. The ion transporter NKCC1 links cell volume to cell mass regulation by suppressing mTORC1. Cell Rep. 2019 May 7;27(6):1886–1896. doi: 10.1016/j.celrep.2019.04.034. [DOI] [PubMed] [Google Scholar]
- Doyle M.E., Egan J.M. Glucagon-like peptide-1. Recent Prog. Horm. Res. 2001;56:377–399. doi: 10.1210/rp.56.1.377. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Dritselis A, Kirkpatrick P. Liraglutide. 10.1038/nrd3148. [DOI] [PubMed]
- During M.J., Cao L., Zuzga D.S., Francis J.S., Fitzsimons H.L., Jiao X., Bland R.J., Klugmann M., Banks W.A., Drucker D.J., Haile C.N. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003 Sep;9(9):1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Eggenhofer E., Hoogduijn M.J. Mesenchymal stem cell-educated macrophages. Transplant. Res. 2012 Dec 1;1(1):12. doi: 10.1186/2047-1440-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewel M.E., Thompson B.G., Hoff J.T. Spontaneous intracerebral hemorrhage: a review. Neurosurg. Focus. 2003 Oct 1;15(4):1–6. doi: 10.3171/foc.2003.15.4.0. [DOI] [PubMed] [Google Scholar]
- Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C., Urva S., Gimeno R.E., Milicevic Z., Robins D., Haupt A. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018 Nov 17;392(10160):2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- Ghazi Sherbaf F., Mohajer B., Ashraf-Ganjouei A., Mojtahed Zadeh M., Javinani A., Sanjari Moghaddam H., et al. Serum insulin-like growth factor-1 in Parkinson's disease; study of cerebrospinal fluid biomarkers and white matter microstructure. Front. Endocrinol. 2018;608 doi: 10.3389/fendo.2018.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M.D., Palesch Y.Y., Martin R.H., Hill M.D., Moy C.S., Waldman B.D., Yeatts S.D., Tamariz D., Ryckborst K., Alias Investigators The albumin in acute stroke (ALIAS) multicenter clinical trial: safety analysis of part 1 and rationale and design of part 2. Stroke. 2011 Jan;42(1):119–127. doi: 10.1161/STROKEAHA.110.596072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014 Jan 21;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- Gómez-Outes A., Lecumberri R., Suárez-Gea M.L., Terleira-Fernández A.I., Monreal M., Vargas-Castrillón E. Case fatality rates of recurrent thromboembolism and bleeding in patients receiving direct oral anticoagulants for the initial and extended treatment of venous thromboembolism: a systematic review. J. Cardiovasc. Pharmacol. Therapeut. 2015 Sep;20(5):490–500. doi: 10.1177/1074248415575154. [DOI] [PubMed] [Google Scholar]
- Gonzales N, Sangha N, Cai C, Hasan KM, Ayish D, Olowu A, Sline MR, Pandurengan R, Bowry R, Hossain MM, Choi E. The Safety of Pioglitazone for Hematoma Resolution in IntraCerebral Hemorrhage (SHRINC): A Randomised, Blinded, Phase 2, Safety Trial.
- Gonzales N.R., Shah J., Sangha N., Sosa L., Martinez R., Shen L., Kasam M., Morales M.M., Hossain M.M., Barreto A.D., Savitz S.I. Design of a prospective, dose-escalation study evaluating the safety of pioglitazone for hematoma resolution in intracerebral hemorrhage (SHRINC) Int. J. Stroke. 2013 Jul;8(5):388–396. doi: 10.1111/j.1747-4949.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hua Y., Keep R.F., Morgenstern L.B., Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009 Jun 1;40(6):2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han N., Ding S.J., Wu T., Zhu Y.L. Correlation of free radical level and apoptosis after intracerebral hemorrhage in rats. Neurosci. Bull. 2008 Dec 1;24(6):351–358. doi: 10.1007/s12264-008-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly J., Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83(3–4):154–160. doi: 10.1159/000095523. [DOI] [PubMed] [Google Scholar]
- Hölscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012 Oct 1;26(10):871–882. doi: 10.2165/11635890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hu X., Li P., Guo Y., Wang H., Leak R.K., Chen S., Gao Y., Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012 Nov;43(11):3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Huang P., Zhou C.M., Liu Y.Y., Hu B.H., Chang X., Zhao X.R., Xu X.S., Li Q., Wei X.H., Mao X.W., Wang C.S. Cerebralcare Granule® attenuates blood–brain barrier disruption after middle cerebral artery occlusion in rats. Exp. Neurol. 2012 Oct 1;237(2):453–463. doi: 10.1016/j.expneurol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Hunter K., Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012 Dec 1;13(1):33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin J., Wright P., Reeve P. Temporal trends and clinical characteristics of spontaneous intracerebral haemorrhage in the Waikato region of New Zealand: a hospital-based analysis. NZ Med J. 2011 Nov 4;124:16–25. [PubMed] [Google Scholar]
- Jeong H.K., Ji K.M., Kim J., Jou I., Joe E.H. Repair of astrocytes, blood vessels, and myelin in the injured brain: possible roles of blood monocytes. Mol. Brain. 2013 Dec;6(1):1–6. doi: 10.1186/1756-6606-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremias A., Brown D.L. Elsevier Health Sciences; 2010 May 15. Cardiac Intensive Care E-Book. [Google Scholar]
- Ji S., Kronenberg G., Balkaya M., Färber K., Gertz K., Kettenmann H., Endres M. Acute neuroprotection by pioglitazone after mild brain ischemia without effect on long-term outcome. Exp. Neurol. 2009 Apr 1;216(2):321–328. doi: 10.1016/j.expneurol.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Junnila R.K., List E.O., Berryman D.E., Murrey J.W., Kopchick J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013 Jun;9(6):366. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Prakash A., Medhi B. Drug therapy in stroke: from preclinical to clinical studies. Pharmacology. 2013;92(5–6):324–334. doi: 10.1159/000356320. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009 Oct 28;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Han J Sook, Kopp S.J., Dugan L.L., Diringer M.N. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke. 2006 Oct 1;37(10):2457–2462. doi: 10.1161/01.STR.0000240674.99945.4e. [DOI] [PubMed] [Google Scholar]
- Klement R.J., Fink M.K. Dietary and pharmacological modification of the insulin/IGF-1 system: exploiting the full repertoire against cancer. Oncogenesis. 2016 Feb;5(2):e193. doi: 10.1038/oncsis.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft H., Jetz W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA. 2007 Apr 3;104(14):5925–5930. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthi R.V., Ikeda T., Feigin V.L. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54(2):171–179. doi: 10.1159/000506396. [DOI] [PubMed] [Google Scholar]
- Kurth T., Kase C.S., Berger K., Gaziano J.M., Cook N.R., Buring J.E. Smoking and risk of hemorrhagic stroke in women. Stroke. 2003 Dec 1;34(12):2792–2795. doi: 10.1161/01.STR.0000100165.36466.95. [DOI] [PubMed] [Google Scholar]
- Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol. 2001 Oct;54(5):311. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger P.L., De Paulis D., Branco S., Bonnin P., Couture-Lepetit E., Baud O., Renolleau S., Ovize M., Gharib A., Charriaut-Marlangue C. Evaluation of cyclosporine A in a stroke model in the immature rat brain. Exp. Neurol. 2011 Jul 1;230(1):58–66. doi: 10.1016/j.expneurol.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Li L., El-Kholy W., Rhodes C.J., Brubaker P.L. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005 Jul 1;48(7):1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- Li R., Pourpak A., Morris S.W. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J. Med. Chem. 2009 Aug 27;52(16):4981–5004. doi: 10.1021/jm9002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Fu C., Song L.I., Yao Y., Zhang X., Chen Z., Li Y., Ma G., Shen C. Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int. J. Cardiol. 2013 May 10;165(2):333–340. doi: 10.1016/j.ijcard.2012.03.088. [DOI] [PubMed] [Google Scholar]
- Lunetta C., Serafini M., Prelle A., Magni P., Dozio E., Ruscica M., Sassone J., Colciago C., Moggio M., Corbo M., Silani V. Impaired expression of insulin‐like growth factor‐1 system in skeletal muscle of amyotrophic lateral sclerosis patients. Muscle Nerve. 2012 Feb;45(2):200–208. doi: 10.1002/mus.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P., Shuaib A., Ng K., Levin K., Atkinson R.P., Rajput A., Wechsler L., Ashwood T., Claesson L., Odergren T., Salazar-Grueso E. Clomethiazole acute stroke study in ischemic stroke (CLASS-I) Stroke. 2002;33(1):122–129. doi: 10.1161/hs0102.101478. [DOI] [PubMed] [Google Scholar]
- Ma Q., Chen S., Hu Q., Feng H., Zhang J.H., Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann. Neurol. 2014 Feb;75(2):209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madathil S.K., Saatman K.E. IGF-1/IGF-R signaling in traumatic brain injury: impact on cell survival, neurogenesis, and behavioral outcome. Brain Neurotrauma. 2015 Feb 25:61–78. [PubMed] [Google Scholar]
- Mehan S., Bhalla S., Siddiqui E.M., Sharma N., Shandilya A., Khan A. Potential roles of glucagon-like peptide-1 and its analogues in dementia targeting impaired insulin secretion and neurodegeneration. Degener. Neurol. Neuromuscul. Dis. 2022;12:31. doi: 10.2147/DNND.S247153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meretoja A., Strbian D., Putaala J., Curtze S., Haapaniemi E., Mustanoja S., Sairanen T., Satopää J., Silvennoinen H., Niemelä M., Kaste M. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012 Oct;43(10):2592–2597. doi: 10.1161/STROKEAHA.112.661603. [DOI] [PubMed] [Google Scholar]
- Muller A.P., Fernandez A.M., Haas C., Zimmer E., Portela L.V., Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol. Cell. Neurosci. 2012 Jan 1;49(1):9–12. doi: 10.1016/j.mcn.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Keep R.F., Hua Y., Schallert T., Hoff J.T., Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J. Neurosurg. 2004 Apr 1;100(4):672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- O'donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., Rangarajan S., Islam S., Pais P., McQueen M.J., Mondo C. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010 Jul 10;376(9735):112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Okauchi M., Hua Y., Keep R.F., Morgenstern L.B., Schallert T., Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010 Feb 1;41(2):375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T.A., Greig N.H. A new Alzheimer's disease interventive strategy: GLP-1. Curr. Drug Targets. 2004 Aug 1;5(6):565–571. doi: 10.2174/1389450043345245. [DOI] [PubMed] [Google Scholar]
- Perry T., Greig N.H. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer's disease. Curr. Alzheimer Res. 2005 Jul 1;2(3):377–385. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- Pitt J., Wilcox K.C., Tortelli V., Diniz L.P., Oliveira M.S., Dobbins C., Yu X.W., Nandamuri S., Gomes F.C., DiNunno N., Viola K.L. Neuroprotective astrocyte-derived insulin/IGF-1 stimulate endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol. Biol. Cell. 2017 Jun 21 doi: 10.1091/mbc.e17-06-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon M.T., Bell S.M., Salman R.A.S. Epidemiology of intracerebral haemorrhage. New Insights in Intracerebral Hemorrhage. 2016;37:1–12. [Google Scholar]
- Prinz V., Laufs U., Gertz K., Kronenberg G., Balkaya M., Leithner C., Lindauer U., Endres M. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008 Feb 1;39(2):433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- Puche J.E., Castilla-Cortázar I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 2012 Dec;10(1):1–29. doi: 10.1186/1479-5876-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A.I., Tuhrim S., Broderick J.P., Batjer H.H., Hondo H., Hanley D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001 May 10;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Rajdev K., Mehan S. Neuroprotective methodologies of Co-enzyme Q10 mediated brain hemorrhagic treatment: clinical and pre-clinical findings. CNS Neurol. Disord. - Drug Targets. 2019 Aug 1;18(6):446–465. doi: 10.2174/1871527318666190610101144. [DOI] [PubMed] [Google Scholar]
- Rajdev K., Siddiqui E.M., Jadaun K.S., Mehan S. Neuroprotective potential of solanesol in acombined model of intracerebral and intraventricular hemorrhage in rats. IBRO reports. 2020 Apr 22 doi: 10.1016/j.ibror.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekik I., Allassonnière S., Carpenter T.K., Wardlaw J.M. Medical image analysis methods in MR/CT-imaged acute-subacute ischemic stroke lesion: segmentation, prediction and insights into dynamic evolution simulation models. A critical appraisal. NeuroImage: Clinical. 2012 Jan 1;1(1):164–178. doi: 10.1016/j.nicl.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rıos C., Nader-Kawachi J., Rodriguez-Payán A.J., Nava-Ruiz C. Neuroprotective effect of dapsone in an occlusive model of focal ischemia in rats. Brain Res. 2004 Mar 5;999(2):212–215. doi: 10.1016/j.brainres.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Romanos E., Planas A.M., Amaro S., Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J. Cerebr. Blood Flow Metabol. 2007 Jan;27(1):14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- Rosenstock J., Reusch J., Bush M., Yang F., Stewart M., Albiglutide Study Group Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009 Oct 1;32(10):1880–1886. doi: 10.2337/dc09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni R., Weinberger J. Management of intracerebral hemorrhage. Vasc. Health Risk Manag. 2007 Oct;3(5):701. [PMC free article] [PubMed] [Google Scholar]
- Sanberg P.R., Park D.H., Kuzmin‐Nichols N., Cruz E., Hossne N.A., Jr., Buffolo E., Willing A.E. Monocyte transplantation for neural and cardiovascular ischemia repair. J. Cell Mol. Med. 2010 Mar;14(3):553–563. doi: 10.1111/j.1582-4934.2009.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M., Osborn O., Tabarean I.V., Holmberg K.H., Eberwine J., Kahn C.R., Bartfai T. Insulin-like growth factor 1-mediated hyperthermia involves anterior hypothalamic insulin receptors. J. Biol. Chem. 2011 Apr 29;286(17):14983–14990. doi: 10.1074/jbc.M110.188540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver J.L., Kidwell C., Eckstein M., Starkman S. Prehospital neuroprotective therapy for acute stroke: results of the field administration of stroke therapy–magnesium (FAST–MAG) pilot trial. Stroke. 2004 May 1;35(5):e106–e108. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Li F, Fisher M. The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats. [DOI] [PubMed]
- Selim M. Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke. 2009 Mar 1;40(3_Suppl. l_1):S90–S91. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- Shandilya A., Mehan S. Dysregulation of IGF-1/GLP-1 signaling in the progression of ALS: potential target activators and influences on neurological dysfunctions. Neurol. Sci. 2021 May 21 doi: 10.1007/s10072-021-05328-6. Epub ahead of print. PMID: 34018075. [DOI] [PubMed] [Google Scholar]
- Shechter R., London A., Varol C., Raposo C., Cusimano M., Yovel G., Rolls A., Mack M., Pluchino S., Martino G., Jung S. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009 Jul 28;6(7) doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Xi G., Maile L.A., Wai C., Rosen C.J., Clemmons D.R. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase β and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol. Cell Biol. 2012 Oct 15;32(20):4116–4130. doi: 10.1128/MCB.01011-12. https://doi.org/MCB.01011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Wang Z., Pu J., Wang R., Guo Z., Liu C., Sun J., Gao L., Zhou R. Early Brain Injury or Cerebral Vasospasm. Springer; Vienna: 2011. Changes of blood–brain barrier permeability following intracerebral hemorrhage and the therapeutic effect of minocycline in rats; pp. 61–67. [DOI] [PubMed] [Google Scholar]
- Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012 Mar 1;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui M.A., Mittal P.K., Little B.P., Miller F.H., Akduman E.I., Ali K., Sartaj S., Moreno C.C. Secondary hypertension and complications: diagnosis and role of imaging. Radiographics. 2019 Jul;39(4):1036–1055. doi: 10.1148/rg.2019180184. [DOI] [PubMed] [Google Scholar]
- Siddiqui E.M., Mehan S., Upadhayay S., Khan A., Halawi M., Halawi A.A., Alsaffar R.M. Neuroprotective efficacy of 4-Hydroxyisoleucine in experimentally induced intracerebral hemorrhage. Saudi J. Biol. Sci. 2021;28(11):6417–6431. doi: 10.1016/j.sjbs.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelley J.W., Thomason A.R., Nolen J.C. Betrixaban (bevyxxa): a direct-acting oral anticoagulant factor Xa inhibitor. Pharmacy and Therapeutics. 2018 Feb;43(2):85. [PMC free article] [PubMed] [Google Scholar]
- Spielman L.J., Little J.P., Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J. Neuroimmunol. 2014 Aug 15;273(1–2):8–21. doi: 10.1016/j.jneuroim.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Steiner T., Diringer M.N., Schneider D., Mayer S.A., Begtrup K., Broderick J., Skolnick B.E., Davis S.M. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006 Oct 1;59(4):767–774. doi: 10.1227/01.NEU.0000232837.34992.32. [DOI] [PubMed] [Google Scholar]
- Strbian D., Karjalainen-Lindsberg M.L., Tatlisumak T., Lindsberg P.J. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J. Cerebr. Blood Flow Metabol. 2006 May;26(5):605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- Strbian D., Tatlisumak T., Ramadan U.A., Lindsberg P.J. Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. J. Cerebr. Blood Flow Metabol. 2007 Apr;27(4):795–802. doi: 10.1038/sj.jcbfm.9600387. [DOI] [PubMed] [Google Scholar]
- Stretton C., Hoffmann T.M., Munson M.J., Prescott A., Taylor P.M., Ganley I.G., Hundal H.S. GSK3-mediated raptor phosphorylation supports amino-acid-dependent mTORC1-directed signalling. Biochem. J. 2015 Sep 1;470(2):207–221. doi: 10.1042/BJ20150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui H., Wang W., Wang P.H., Liu L.S. Protective effect of antioxidant ebselen (PZ51) on the cerebral cortex of stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2005 Mar;28(3):249–254. doi: 10.1016/j.transproceed.2013.03.030. [DOI] [PubMed] [Google Scholar]
- Supeno N.E., Pati S., Hadi R.A., Ghani A.R., Mustafa Z., Abdullah J.M., Idris F.M., Han X., Jaafar H. IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells. Int. J. Med. Sci. 2013;10(5):522. doi: 10.7150/ijms.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA. Intracerebral hematoma: beyond the mass lesion. 10.1161/01.STR.0000244060.83388.76. [DOI] [PubMed]
- Swanson C.R., Joers V., Bondarenko V., Brunner K., Simmons H.A., Ziegler T.E., Kemnitz J.W., Johnson J.A., Emborg M.E. The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J. Neuroinflammation. 2011 Dec;8(1):1–4. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydserff S.G., Cross A.J., Murray T.K., Jones J.A., Green A.R. Clomethiazole is neuroprotective in models of global and focal cerebral ischemia when infused at doses producing clinically relevant plasma concentrations. Brain Res. 2000 Apr 17;862(1–2):59–62. doi: 10.1016/S0006-8993(00)02071-0. [DOI] [PubMed] [Google Scholar]
- Syme P.D., Byrne A.W., Chen R., Devenny R., Forbes J.F. Community-based stroke incidence in a scottish population: the scottish borders stroke study. Stroke. 2005 Sep 1;36(9):1837–1843. doi: 10.1161/01.STR.0000177873.82478.1c. [DOI] [PubMed] [Google Scholar]
- Szymanska A., Biernaskie J., Laidley D., Granter-Button S., Corbett D. Minocycline and intracerebral hemorrhage: influence of injury severity and delay to treatment. Exp. Neurol. 2006 Jan 1;197(1):189–196. doi: 10.1016/j.expneurol.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Tanaka-Azevedo A.M., Morais-Zani K., Torquato R.J., Tanaka A.S. Thrombin inhibitors from different animals. J. Biomed. Biotechnol. 2010 doi: 10.1155/2010/641025. 2010 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Liu J., Zhou C., Ostanin D., Grisham M.B., Neil Granger D., Zhang J.H. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J. Neurochem. 2005 Sep;94(5):1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- Tarnowski M., Tkacz M., Zgutka K., Bujak J., Kopytko P., Pawlik A. Picropodophyllin (PPP) is a potent rhabdomyosarcoma growth inhibitor both in vitro and in vivo. BMC Cancer. 2017 Dec;17(1) doi: 10.1186/s12885-017-3495-y. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Aleman I. Toward a comprehensive neurobiology of IGF‐I. Developmental neurobiology. 2010 Apr;70(5):384–396. doi: 10.1002/dneu.20778. [DOI] [PubMed] [Google Scholar]
- Van Asch C.J., Luitse M.J., Rinkel G.J., van der Tweel I., Algra A., Klijn C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010 Feb 1;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- Varkaris A., Gaur S., Parikh N.U., Song J.H., Dayyani F., Jin J.K., Logothetis C.J., Gallick G.E. Ligand‐independent activation of MET through IGF‐1/IGF‐1R signaling. Int. J. Cancer. 2013 Oct 1;133(7):1536–1546. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K.R., Packard B.A., Hall C.L., Smulian A.G., Linke M.J., de Courten-Myers G.M., Packard L.M., Hall N.C. Protein oxidation and heme oxygenase-1 induction in porcine white matter following intracerebral infusions of whole blood or plasma. Dev. Neurosci. 2002;24(2–3):154–160. doi: 10.1159/000065703. [DOI] [PubMed] [Google Scholar]
- Walker P.A., Shah S.K., Jimenez F., Aroom K.R., Harting M.T., Cox C.S., Jr. Bone marrow–derived stromal cell therapy for traumatic brain injury is neuroprotective via stimulation of non-neurologic organ systems. Surgery. 2012 Nov 1;152(5):790–793. doi: 10.1016/j.surg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Wallmark S., Ronne-Engström E., Lundström E. Prevalence of spasticity after aneurysmal subarachnoid haemorrhage. J. Rehabil. Med. 2014 Jan 5;46(1):23–27. doi: 10.2340/16501977-1229. [DOI] [PubMed] [Google Scholar]
- Walters M.R., Kaste M., Lees K.R., Diener H.C., Hommel M., De Keyser J., Steiner H., Versavel M. The AMPA antagonist ZK 200775 in patients with acute ischaemic stroke: a double-blind, multicentre, placebo-controlled safety and tolerability study. Cerebrovasc. Dis. 2005;20(5):304–309. doi: 10.1159/000087929. [DOI] [PubMed] [Google Scholar]
- Wan X., Harkavy B., Shen N., Grohar P., Helman L.J. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007 Mar;26(13):1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- Wrigley S., Arafa D., Tropea D. Insulin-like growth factor 1: at the crossroads of brain development and aging. Front. Cell. Neurosci. 2017 Feb 1;11:14. doi: 10.3389/fncel.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Jiang T, Cadenas E. Metabolic triad in brain aging: mitochondria, insulin/IGF-1 signalling and JNK signalling. 10.1042/BST20120260. [DOI] [PMC free article] [PubMed]
- Zhao F., Hua Y., He Y., Keep R.F., Xi G. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke. 2011 Dec;42(12):3587–3593. doi: 10.1161/STROKEAHA.111.623926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.