Abstract
The function of the rorf2 gene located on the locus of enterocyte effacement (LEE) pathogenicity island of enteropathogenic Escherichia coli (EPEC) has not been described. We report that rorf2 encodes a novel protein, named EspG, which is secreted by the type III secretory system and which is translocated into host epithelial cells. EspG is homologous with Shigella flexneri protein VirA, and the cloned espG (rorf2) gene can rescue invasion in a Shigella virA mutant, indicating that these proteins are functionally equivalent in Shigella. An EPEC espG mutant had no apparent defects in in vitro assays of virulence phenotypes, but a rabbit diarrheagenic E. coli strain carrying a mutant espG showed diminished intestinal colonization and yet diarrheal attack rates similar to those of the wild type. A second EspG homolog, Orf3, is encoded on the EspC pathogenicity islet. The cloned orf3 gene could also rescue invasion in a Shigella virA mutant, but an EPEC espG orf3 double mutant was not diminished in any tested in vitro assays for EPEC virulence factors. Our results indicate that EspG plays an accessory but as yet undefined role in EPEC virulence that may involve intestinal colonization.
Enteropathogenic Escherichia coli (EPEC) is the most common bacterial cause of diarrhea in infants (21). EPEC is a member of a group of organisms that share the locus of enterocyte effacement (LEE) pathogenicity island (18), which mediates the formation of attaching and effacing lesions on host epithelial cells and which is central to the pathogenic potential of these organisms (10, 18). The LEE contains genes encoding an outer membrane protein (intimin), a type III secretion system (Esc, Sep, Ces), several type III system-secreted Esp proteins, the translocated intimin receptor (Tir), and 18 open reading frames of undetermined function (6).
Type III secretion in EPEC is believed to involve a bacterial membrane complex of Esc/Sep proteins upon which is assembled an extracellular filament of polymerized EspA (10, 16). EspB and EspD proteins are believed to form a pore in the host membrane at the distal end of the EspA filament (11, 28). Together, these function to translocate proteins directly from the bacterial cytoplasm into the host via the EspA filament. Type III system-secreted proteins EspA, -B, and -D are therefore part of the translocation apparatus, although additional roles for these proteins are still possible. Several effector proteins are translocated via the type III pathway into the cell in order to perform functions inside the host cell. Effector proteins are also encoded by the LEE and include Tir (13) (also called EspE by Diebel et al. [3]), EspF (19), and the recently described Map (Orf19) (14).
Analysis of the LEE sequence suggests that it may encode a fourth type III system-secreted effector protein. Gene rorf2 encodes a protein with significant homology to VirA, a type III system-secreted effector protein produced by Shigella flexneri and enteroinvasive E. coli (27). VirA has an accessory role in invasion, although its exact function and mechanism are unclear (4, 27). virA does not affect the expression of IpaB, -C, or -D (27) or entry into cells (4), although virA mutants were recovered at about 20% of wild-type levels after invasion in one assay (27). Several lines of evidence imply that VirA is important in later stages of infection, such as intracellular persistence and spreading. virA expression is induced upon cellular entry or shortly after (4), and virA mutants are strongly attenuated in plaque formation in both CaCo-2 (4) and MK2 cells (27). The picture in vivo appears to be more complex, as virA mutants are highly attenuated in the Serény test but fully virulent in rabbit ileal loops (27).
We now demonstrate that rorf2 encodes a type III system-secreted protein with possible effector functions and have renamed this protein EspG, for E. coli secreted protein G.
MATERIALS AND METHODS
Construction of bacterial strains and plasmids.
Strains and plasmids are listed in Table 1, and construction is described below.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| E2348/69 | E. coli O127:H7 EPEC strain E2348/69 | 17 |
| E2348/69 espG | E2348/69 espG::pJP5603 | This study |
| E2348/69 espG(pCVD453) | E2348/69 espG::pJP5603(pCVD453) | This study |
| E2348/69 escN(pCVD453) | E2348/69 escN::aphA3(pCVD453) | 12; this study |
| E2348/69 espG orf3 | E2348/69 espG::pJP5603 orf3::pJP5608 | This study |
| 85-170 | EHEC O157:H7 | 12 |
| HS | Normal flora E. coli strain | 12 |
| 83/39 | REPEC | 1 |
| SE1090 | REPEC 83/39 espG::pJP5603 | This study |
| S. flexneri | ||
| YSH6000 | Wild-type S. flexneri | 27 |
| virA mutant | S. flexneri N1945 | 27 |
| virA(pEspG) mutant | S. flexneri N1945(pCVD453::Tp) | This study |
| virA(pOrf3) mutant | S. flexneri N1945(pTB101:Orf3) | This study |
| virA(pVirA) mutant | S. flexneri N1945(pKU002) | 27 |
| Inv mutant | S. flexneri 2a cured of invasion plasmid | 22 |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen |
| Plasmids | ||
| pCVD453 | 3.1-kb LEE fragment in pSPORT (Apr) | 19 |
| pEspG | 3.1-kb LEE fragment from pCVD453 and Tpr cassette in pBluescript SK (Apr Tpr) | This study |
| pOrf3 | Orf3 in pTB101 (Tpr) | This study |
| pQE30::His6EspG | N-terminal MRGSHis6 fusion to EspG (Apr) | This study |
| pJP5603 | oriR6K mobRP4 lacZ::MCS kan (Kmr) | 23 |
| pJP5608 | oriR6K mobRP4 lacZ::MCS tet (Tcr) | 23 |
Apr, resistance to 100 μg of ampicillin/ml; Tpr, resistance to 50 μg of trimethoprim/ml; Kmr, resistance to 25 μg of kanamycin/ml; Tcr, resistance to 15 μg of tetracycline/ml.
Plasmid pQE30::His6EspG, which expressed EspG fused to an N-terminal MRGSHis6 tag, was constructed by PwoI PCR amplification of a 2,010-bp fragment region using primers K481 (5′-AGAGATAAAAGGCAGCGGGG-3′) and K603 (5′-CGGTGTAATGCCACAACAGG-3′), digestion with BamHI, and cloning into BamHI/SmaI sites of pQE30. Plasmid pCVD453, which is pSPORT1 containing a 3.1-kb MluI/BglII fragment including espG rorf1 has been previously described (18). Plasmid pEspG, a trimethoprim-resistant (Tpr) variant of pCVD453, was constructed by insertion of a 3.1-kb MluI/BglII fragment from pCVD453 into the EcoRI/SmaI site of pBluescript SK, followed by addition of a 1.8-kb blunt-ended EcoRI/BamHI trimethoprim resistance cassette (isolated from pREG152) to the blunt-ended KpnI site. In this plasmid espG was behind (in order) the trimethoprim resistance gene and the lacZ promoter. pOrf3 was constructed by amplification of the orf3 gene from the EspC pathogenicity island using K1199 (5′-TAGTTCTGCAGTATCAATTCCTCGA-3′) and K1200 (5′-TGGCGTCATGAGTAGCACAACGA-3′), digestion with PstI, and cloning into the PstI/SmaI site of pTB101 (30).
Mutant variants of EPEC E2348/69 and rabbit EPEC (REPEC) 83/39 were constructed using insertional inactivation of espG according to previously described protocols (8). For espG in E2348/69, a fragment internal to espG was amplified from E2348/69 using primers K575 (5′-CCTCGACATGGATCCATAAAGATAGAGC-3′) and K576 (5′-ACCAGATAGGAGAATTCCTCATGATAAATGG-3′) and digested with BamHI and EcoRI, resulting in a 570-bp fragment that was cloned into suicide plasmid pJP5603 (23). The resultant plasmid was introduced into E2348/69 via conjugation. Kanr Nalr transconjugants were examined for loss of the suicide plasmid and insertion into espG using plasmid extraction, PCR, and Southern blotting. Gene disruption was confirmed by Western blotting, showing loss of EspG production. espG was mutated in REPEC strain 83/39 by amplification of a 1,125-bp fragment using K576 and K1375 (5′-TACCTTGGTTGTAGCTTCCTT-3′), which was cloned into pJP5603. The resulting plasmid was recombined into the chromosome of 83/39 using the protocol described above. To mutate orf3 from the EspC pathogenicity island, primers K1863 (5′-AGAGGATCCAGGGGGCTTACGCCAGAA-3′) and K1873 (5′-GAGCGAATTCTAAGCTACTTAGGT-3′) amplified a fragment which was digested with EcoRI and BamHI to generate a 436-bp fragment internal to orf3, which was cloned into pJP5608 (23). Insertion into the chromosomal orf3 gene was achieved using the same protocol as that used for espG above, except that selection was made for Tetr Kanr strains.
EspG secretion and translocation.
Expression of bacterial proteins was examined in supernatants and bacterial fractions which were prepared as previously described (7, 12). Following separation through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, proteins were either stained with Coomassie blue or blotted to polyvinylidene difluoride and Western blotted with monoclonal murine antibodies against the His6 epitope or polyclonal rabbit antibodies against EspG or EPEC secreted proteins as previously described (7, 12).
Translocation of EspG into HEp-2 cells was demonstrated using previously described protocols (25, 26). Confluent HEp-2 cell monolayers overlaid with 3 ml of Dulbecco modified Eagle medium (DMEM) were infected with overnight cultures of bacteria, and, after 3 h of incubation at 37°C in 5% CO2, the supernatant was removed and centrifuged to isolate bacteria, which were resuspended in SDS-PAGE loading buffer. The HEp-2 cell monolayer was washed three times with ice-cold phosphate-buffered saline (PBS) supplemented with protease inhibitors (0.2 mg of phenylmethylsulfonyl fluoride/ml and 0.04% aprotinin) and harvested by scraping it and resuspending it in 1 ml of PBS. The HEp-2 cells were centrifuged, and the pellet was resuspended in 100 μl of PBS–1% Triton X-100 and incubated on ice for 30 min, with occasional mixing. The suspension was centrifuged to separate the Triton X-100-soluble fraction, which contains host membrane proteins and translocated bacterial proteins, from the Triton X-100-insoluble fraction containing cytoskeletal elements and the remaining bacteria.
Bacterial persistence.
Intracellular persistence of bacteria was assessed by the method of Anderson et al. (2). Briefly, a confluent HeLa cell monolayer was overlaid with 1 ml of fresh DMEM–10% fetal bovine serum and infected with a PBS suspension of 107 bacteria grown overnight on tryptone-soy agar–0.4% Congo red. After 90 min at 37°C in 5% CO2, wells were washed three times with buffered saline supplemented with 100 μg of gentamicin/ml to kill extracellular bacteria. To estimate total bacterial invasion, cells were washed once with buffered saline and then lysed by the addition of PBS–0.1% Triton X-100 and viable counts were enumerated. To estimate bacterial persistence, the remaining wells were incubated for a further 4 h, lysed, and counted as described above. The experiment was considered valid if wild-type Shigella invaded at more than 5%, if the noninvasive plasmid-cured Shigella strain did not invade, and if the virA mutant invaded at less than 30% of the wild-type level. The ratio of the number of viable intracellular bacteria at 4 h post-gentamicin treatment to the number of intracellular bacteria at the time of gentamicin treatment measures intracellular persistence. To permit measurement of the variation due to strain differences while minimizing variation due to differences between each assay, we calculated persistence as a percentage of the wild-type level in each assay and then grouped these adjusted scores to obtain an average.
Rabbit infection studies.
A rabbit EPEC strain 83/39 espG mutant was tested in the rabbit EPEC infection model described by Adams et al. (1). Bacteria were grown in Penassay broth (Oxoid, Basingstoke, United Kingdom), washed, and resuspended in PBS. Five- to six-week-old rabbits were orally inoculated with 2 ml of sodium bicarbonate and 15 min later with 6 × 106 CFU of the bacterial suspension. Rabbits were monitored for weight gain or loss and diarrhea. Bacterial excretion was determined semiquantitatively by counting suspensions made from rectal swabs, a technique previously determined to accurately reflect actual CFU per 0.01 g of colon contents (unpublished data cited by Adams et al. [1]).
RESULTS AND DISCUSSION
Characterization of EspG.
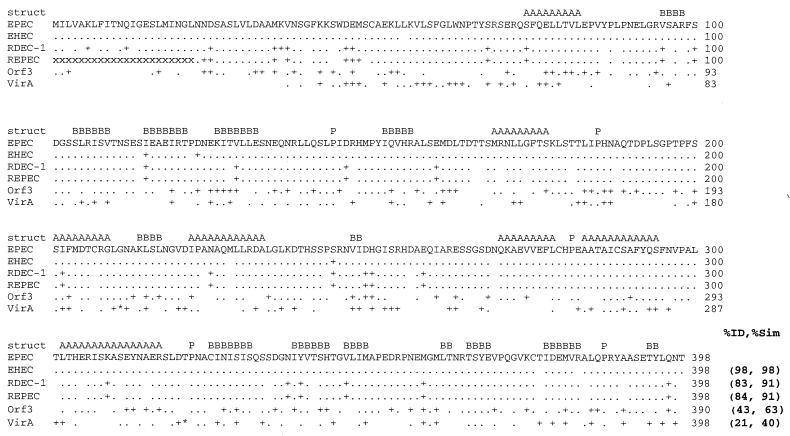
Gene espG (previously rorf2 [6]) is predicted to encode a 44-kDa, 398-amino-acid protein that is highly conserved between the LEE of EPEC strain E2348/69 and other attaching-effacing pathogens including enterohemorrhagic E. coli (EHEC) O157:H7 and rabbit pathogens RDEC-1 and REPEC 83/39 (Fig. 1). The product of espG (rorf2) also has significant homology (21% identity, 40% similarity) over most of its length with VirA, a 400-amino-acid, type III system-secreted effector protein of Shigella (Fig. 1). EspG and VirA were predicted by the Jpred algorithm (http://jura.ebi.ac.uk) to share a number of secondary structural features (Fig. 1). Seven α helices, 14 β sheets, and five prolines are found in similar positions in the secondary structures of both proteins. Many of these structures contain I, L, or V residues that are often associated with protein interactions. Further analysis did not find coiled-coil domains or compelling motifs that could suggest the function. The PSORT algorithms (http://www.psort.nibb.ac.jp/) predicted a cytoplasmic and not membrane location for both proteins. However, these algorithms commonly predict a cytoplasmic location for type III system-secreted proteins as the secretion motif is unique for type III system secretion, is not recognized by normal bacterial secretory pathways, and has not been clearly defined.
FIG. 1.
Alignment of EspG from EPEC O127: H7 (EPEC) with EspG from EHEC O157: H7 (EHEC), rabbit pathogen RDEC-1, REPEC strain 83/39 (REPEC), Orf3 from the EspC pathogenicity islet, and VirA from S. flexneri. Numbers at the end of the line, amino acid numbers; period, identical amino acids; +, similar but nonidentical amino acids; blank space, nonhomologous amino acids. The DNA sequence encoding the first 22 amino acids (aa) of REPEC VirA is not known, and each of these missing amino acids is indicated with an “x.” Asterisks (within the VirA sequence), areas where VirA contains extra amino acids not found within EspG, including an insertion of 5 aa into the region corresponding to aa 210 and 211 in EspG and a 15-aa insertion between aa 320 and 321 in EspG. The predicted consensus secondary structures (struct) conserved between EspG and VirA are denoted A (α-helix), B (β sheet), and P (proline; indicating a turn). The percent identity and percent similarity (%ID and %Sim, respectively) to the sequence of EPEC EspG are listed at the end of the alignment.
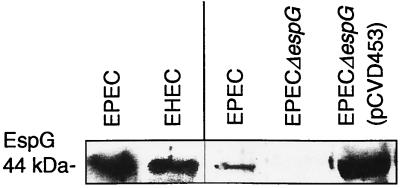
To demonstrate that the espG open reading frame encoded the expected protein product, we first cloned and expressed recombinant EspG. Plasmid pQE30::His6EspG expressed high levels of EspG fused to a hexahistidine tag (His6EspG) in both DH5α and EPEC hosts, observable as a ca. 45-kDa protein in Coomassie blue-stained SDS-PAGE gels and in a Western blot using antibodies directed against the His6 tag. Rabbit antiserum raised against His6EspG recognized 44-kDa proteins in whole-cell lysates of EPEC O127:H7 strain E2348/69 and EHEC O157:H7 strain 85–170 (Fig. 2, lanes 1 and 2) that had been grown in DMEM as previously described (7, 12). EspG expression was not increased by different culture conditions, including the presence of mammalian cells (results not shown). However, we have previously reported that espG transcription and EspG expression are regulated by Ler, the LEE-encoded regulator that activates transcription of many EPEC virulence genes (9).
FIG. 2.
Western blot with anti-EspG antiserum on whole-cell lysates. Exposure times: lanes 1 and 2 (numbering from the left), 3 min; lanes 3 to 5, 15 s. Antiserum recognized the 44-kDa EspG protein in whole-cell lysates of wild-type EPEC E2348/69 (lanes 1 and 3), EHEC (lane 2), and EPEC E2348/69 espG (pCVD453) (lane 5) but not in that of EPEC E2348/69 espG (lane 4). Comparison of relative amounts of EspG produced by EPEC, the espG mutant, and the complemented mutant (lanes 3 to 5) demonstrates that EspG is produced in small amounts by wild-type EPEC and that the complement overproduces EspG.
Characterization of an espG mutant.
An espG mutant variant of E2348/69, E2348/69 espG, produced neither EspG nor a truncated variant of EspG as determined by Western blots using anti-EspG antibodies (Fig. 2). The mutation was complemented by addition of cloned espG on multicopy plasmid pCVD453 (Fig. 2), and the complemented strain, E2348/69 espG (pCVD453), was estimated by densitometry to produce more than 20 times the amount of EspG produced by the wild type, consistent with the increased gene dosage.
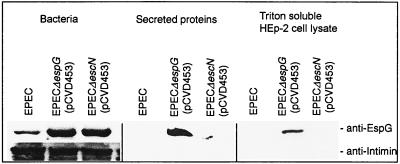
We tested for secretion and translocation of EspG into host epithelial cells by the type III secretory pathway using previously described protocols (25, 26). After a 3-h infection of HEp-2 cells with bacteria, bacteria were removed, and the cells were processed to separate the Triton X-100-soluble fraction, which contains host membrane proteins and translocated bacterial proteins, from the Triton X-100-insoluble fraction containing cytoskeletal elements and the remaining bacteria. EspG was observed in Western blots on the Triton X-100-soluble fraction of HEp-2 cells infected with E2348/69 espG (pCVD453) but was not found in cells infected with E2348/69 escN (pCVD453) (Fig. 3). The Triton X-100-soluble fraction did not contain intimin, indicating that this fraction was free of bacteria. By comparison, the bacterial pellets were determined to contain both intimin and EspG. EspG secretion in the concentrated supernatant of bacteria grown in DMEM was examined using the protocol of Jarvis and Kaper (12). EspG was observed in the supernatant fraction from E2348/69 espG (pCVD453) but not in that from the secretion-defective E2348/69 escN (pCVD453), indicating that EspG found in the supernatant was a result of type III secretion and not a result of an alternative secretion path or bacterial lysis (Fig. 3). Unlike E2348/69 espG (pCVD453), EspG could not be clearly observed in supernatants from wild-type EPEC E2348/69, probably because of the much lower gene dosage. Our data indicate that EspG is secreted and translocated into HEp-2 cells in a manner that is dependent on the type III secretion machinery. It is possible that EspG secretion and translocation by E2348/69 espG (pCVD453) are artifacts of overexpression that do not occur in wild-type EPEC. However, this is unlikely as secretion and translocation were not observed from the escN mutant, which is deficient in type III secretion, despite this strain expressing EspG at the same levels in whole cells as E2348/69 espG (pCVD453).
FIG. 3.
Type III secretion of EspG into supernatants and translocation into HEp-2 cells. Western blots with EspG antiserum indicate that EspG is produced by EPEC E2348/69 espG (pCVD453), EPEC E2348/69 escN (pCVD453), and in lesser amounts by wild-type EPEC E2348/69. EspG is observed in the supernatant and in the Triton X-100-soluble fraction of HEp-2 cells infected with EPEC E2348/69 espG (pCVD453) but not EPEC E2348/69 escN (pCVD453), indicating that secretion and translocation are dependent on type III secretion. Intimin is observed in whole cells but not in the Triton X-100-soluble fraction, indicating that the Triton X-100-soluble fraction is not contaminated with bacteria, and so EspG present in that fraction must be due to translocation and not contamination.
To determine if espG was necessary for type III secretion, the concentrated bacterial culture supernatants from the wild type, E2348/69 espG, and the complement, E2348/69 espG (pCVD453), were examined using Western blots as previously described (7, 12). No differences between these three strains were observed with antisera raised against Tir (7) or another antiserum that recognizes EspABD, EspC, and flagella but that does not recognize EspF, Tir, or EspG (12) (Table 2). In addition, mutation of espG did not affect the formation of the EspA filament (16) (data not shown) (S. Knutton, personal communication). Therefore, these data indicate that espG is unnecessary for type III secretion. As EspG is not part of the type III translocation apparatus but was secreted and translocated, it was reasonable to propose that EspG performs some effector function.
TABLE 2.
Virulence properties of wild-type and mutant EPEC strains
| Strain | Results for assay of:
|
||||
|---|---|---|---|---|---|
| LAa | FASb | Tir, EspABD secretionc | HEp-2 invasion (%)d | Intracellular persistence (%)e | |
| E2348/69 | + | + | + | 100 | 100 |
| E2348/69 espG | + | + | + | 83 | 83 |
| E2348/69 espG (pCVD453) | + | + | + | nt | nt |
| E2348/69 espG orf3 | + | + | ntf | 70 | 70 |
LA, localized adherence.
FAS, fluorescent actin staining (an assay for attaching and effacing lesion formation).
Type III secretion of EspADB and Tir.
Invasion of HEp-2 cells, relative to E2348/69.
Intracellular persistence within HEp-2 cells after invasion, relative to E2348/69.
nt, not tested.
To examine the role of espG in EPEC virulence, we compared wild-type E2348/69, E2348/69 espG, and E2348/69 espG (pCVD453) in various in vitro assays for virulence-associated phenotypes (Table 2). These three strains were indistinguishable in their abilities to form microcolonies on HEp-2 cells in the modified localized-adherence assay (8) and in the 3-h fluorescent-actin staining test (15) for attaching-effacing lesion formation. Wild-type E2348/69 and the espG mutant were equally proficient at decreasing transepithelial resistance across polarized T84-cell monolayers (29) (data not shown) (G. Hecht, personal communication) and caused identical patterns of tyrosine phosphorylation and dephosphorylation in HEp-2 cells after a 3-h infection (data not shown) as described previously (25). These data demonstrated that espG does not affect commonly examined EPEC virulence properties in vitro and indicated the need to examine other phenotypes.
Complementation of a Shigella virA strain.
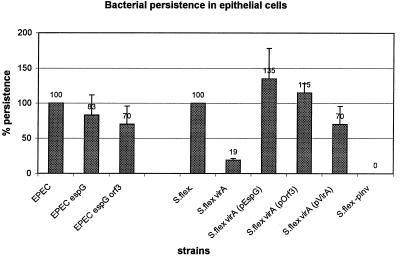
The many similarities between EspG and the homologous VirA suggested that these proteins may have similar functions, and we therefore examined the role of EspG in intracellular invasion and persistence. Intracellular persistence was assessed by the method of Anderson et al. (2). VirA clearly affects Shigella intracellular persistence in this assay, as the virA mutant survived at about one-fifth the level of the wild type (Fig. 4), consistent with the initial findings of Uchiya et al. (27). Shigella intracellular persistence could be restored to nearly wild-type levels by adding cloned virA in trans. When the virA mutant was transformed with plasmid pEspG, it was found that cloned espG from EPEC could restore intracellular persistence to the Shigella virA mutant to levels exceeding those seen with the wild type. Therefore, EspG can functionally substitute for VirA. Although we were able to demonstrate a role for EspG in Shigella, we could not demonstrate a role for EspG in EPEC in this assay. The persistence of EPEC was only weakly attenuated (83% of wild type) by mutation of espG, and this difference was not statistically significant (Fig. 4). A variety of modifications to the assay failed to show any clear difference between the wild type and E2348/69 espG, including altered infection times or times at which persistence was assessed or use of HEp-2 instead of HeLa cells (data not shown).
FIG. 4.
Intracellular persistence of bacteria within HeLa cells as a measure of intracellular survival and/or replication, according to the protocol of Anderson et al. (2), and expressed as percentages of that for wild-type EPEC or S. flexneri (S.flex). The plasmid-free variant of S. flexneri (S.flex-pInv) was used as a negative control. Relative scores are above the bars.
As the previous assay was unable to demonstrate a role for espG in EPEC and as the process of invasion for EPEC is different from that for Shigella, we utilized an alternative assay (24) that was developed for E. coli and that measures the proportion of cell-associated bacteria that are internalized rather than intracellular persistence. Calculating invasion over four triplicate assays, we found that mutation of espG in EPEC did not drastically decrease EPEC invasion (83% of wild type; Table 2), nor did mutation of virA decrease Shigella invasion (results not shown). That EspG is not involved in EPEC invasion is further supported by the observation that EspG is produced by EHEC O157:H7 (Fig. 2), which is noninvasive (5). There are fundamental differences in the invasion process between EPEC and Shigella, and the finding that EspG affects Shigella invasion cannot be readily extrapolated to EPEC.
Role in rabbit infection.
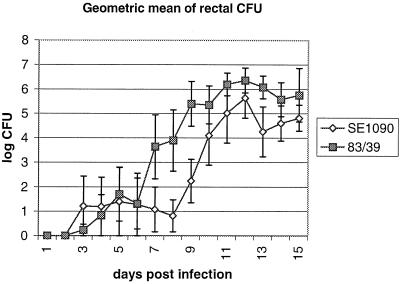
As EspG had no observable effect on EPEC in a variety of in vitro assays, we examined the role of EspG in a natural model of infection. Human EPEC strains do not cause diarrhea in rabbits, but REPEC strains capable of causing diarrhea in rabbits also possess the LEE and produce the attaching and effacing histopathology in rabbit intestines. We constructed a REPEC strain 83/39 espG mutant which is highly virulent in rabbits and compared the virulence of this mutant (SE1090) to that of wild-type 83/39. Of five rabbits inoculated with the wild-type strain, three developed diarrhea including one with severe diarrhea. Four of five rabbits infected with SE1090 developed diarrhea, of which one developed serious diarrhea and was euthanized on day 11. Other gross signs of illness were also similar between the two groups of rabbits, indicating that EspG does not affect gross indices of disease in this model. To examine if the espG mutation resulted in subtle changes in virulence, averages for a number of disease indices were calculated. Weight change is often used in this model to assess the cumulative impact of diarrhea and illness. Rabbits inoculated with wild-type 83/39 gained weight at a slightly lower rate than those infected with SE1090, and 83/39 was slightly more virulent than SE1090 as judged by indices of cumulative diarrheal incidence and severity. However, these differences were not statistically significant (not shown). By contrast, approximately 1 log unit more bacteria were regularly recovered from the rectums of rabbits infected with 83/39 than were recovered from those of rabbits infected with SE1090 (Fig. 5). This difference is suggestive of some accessory role in colonization but is insufficient for a conclusion that EspG has a clear and well-defined effect on virulence. Demers et al. (4) could not find a role for VirA in Shigella infection of rabbits but found that VirA was important for virulence in the Serény test of keratoconjunctivitis in guinea pigs. Perhaps further experimentation in a different animal model may show a more significant role for EspG in the virulence of REPEC.
FIG. 5.
Geometric mean of the CFU count from rectal swabs, as a measure of fecal CFU, from rabbits orally inoculated with wild-type REPEC strain 83/39 or espG mutant SE1090. Error bars, standard errors.
A second VirA homolog in EPEC.
A possible explanation for our inability to find a phenotype associated with an EPEC espG mutant could be the presence of a second VirA homolog in EPEC. We have recently identified a second VirA/EspG homolog that is encoded by orf3, which is immediately upstream of espC in the EspC pathogenicity islet (20) (GenBank accession no. AF29706). This gene is found in E2348/69 and other EPEC strains of the EPEC1 evolutionary group but was not detected in rabbit-pathogenic E. coli or other members of the EPEC2 group (20). Orf3 is 42% identical and 62% similar to EspG and 20% identical and 38% similar to VirA (Fig. 1). To determine if the presence of orf3 in EPEC allowed the mutation in espG to be phenotypically silent, an orf3 espG double mutant was constructed. The double mutant was identical to the wild type and the E2348/69 espG mutant in assays for attachment-effacement (fluorescent-actin staining), localized adherence to HEp-2 cells (Table 2), or alteration of transepithelial resistance across a T84 monolayer (data not shown) (G. Hecht, personal communication). Both invasion and intracellular persistence (Fig. 4) of EPEC were only weakly attenuated by the double mutation of espG and orf3 (to 70% of the wild-type value) compared to the mutation of espG alone (83% of the wild-type value) (Table 2). These differences were not statistically significant, although they might suggest a mild cumulative effect associated with loss of espG and then orf3.
To determine if orf3 could affect Shigella invasion, plasmid pOrf3 carrying orf3 was introduced into the S. flexneri virA strain. The complemented strain exhibited intracellular persistence at levels exceeding those for the wild type (Fig. 4), indicating that cloned orf3 was able to complement the virA mutation in Shigella. Therefore, Orf3 is functionally analogous to VirA, at least in a Shigella host background. It is also reasonable to suggest that Orf3 might be secreted by the type III secretion machinery, which would therefore identify it as the first type III system-secreted EPEC protein encoded outside the LEE.
The fact that EPEC produces two orthologous proteins, EspG and Orf3, that function in Shigella analogously to VirA is very interesting. All three proteins are similar at the amino acid level and also share several structural motifs that may be responsible for their shared function, similarities that may aid future research in defining active sites. In the same way that the type III pathway in Shigella secretes VirA, the type III secretory system in EPEC exports EspG. Based on the rescue of the virA mutation, the data further imply that the Shigella type III secretory system can recognize and process the foreign EspG and Orf3 proteins, just as EPEC effector protein Tir can be secreted by Shigella (7). VirA, EspG, and Orf3 appear to affect the intracellular persistence of Shigella rather than initial cell entry, confirming previous studies with VirA (4, 27). However, the precise mechanism or target of these proteins remains unknown, limiting further biochemical characterization. The role of VirA in Shigella also remains unclear, and this protein may have a mostly accessory role in virulence. The role of EspG and Orf3 in EPEC is even less clear.
Concluding remarks.
The role of EspG in attaching-effacing bacteria is compelling but as yet unknown. The espG gene is highly conserved and is found in the LEE of all attaching-effacing pathogens (18). espG expression is activated by virulence gene regulator Ler and produces a type III system-secreted protein that is translocated into epithelial cells. It appears that EspG can act on epithelial cells based on its effect on the intracellular persistence of Shigella. Mutation of espG attenuated gut colonization in a rabbit model of REPEC diarrhea. Finally, strains belonging to the EPEC1 cluster encode another EspG ortholog (Orf3) on a separate pathogenicity island. It would be surprising for an organism to inherit and maintain two orthologous genes within pathogenicity islands if neither gene performs a function. Based on these factors, we speculate that EspG may have an accessory role in virulence. The fact that EspG is normally expressed in very small amounts might suggest that EspG is expressed under specific conditions such as in an intracellular compartment, late in infection, or in some specific intestinal niche. The role of EspG and Orf3 will remain unclear in the absence of any new model to test EspG and Orf3 phenotypes or until the function of VirA is defined.
ACKNOWLEDGMENTS
We acknowledge Gail Hecht and Athanasia Koutsouris for performing the TER assay, Stuart Knutton for examining EspA filament production, Eileen Barry, Richard Anderson, and Zeev Altboum for assistance with invasion assays, Maria Dubois for assistance with pQE30::EspG, and Larissa Nicholls for assistance with the rabbit studies.
This work was supported by grants AI21657 and AI41325 from the National Institutes of Health and by a grant from the Australian National Health and Medical Research Council.
REFERENCES
- 1.Adams L M, Simmons C P, Rezmann L, Strugnell R A, Robins-Browne R M. Identification and characterization of a K88- and CS31A-like operon of a rabbit enteropathogenic Escherichia coli strain which encodes fimbriae involved in the colonization of rabbit intestine. Infect Immun. 1997;65:5222–5230. doi: 10.1128/iai.65.12.5222-5230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson R J, Pasetti M F, Sztein M B, Levine M M, Noriega F R. ΔguaBA attenuated Shigella flexneri 2a strain CVD 1204 as a Shigella vaccine and as a live mucosal delivery system for fragment C of tetanus toxin. Vaccine. 2000;18:2193–2202. doi: 10.1016/s0264-410x(00)00025-6. [DOI] [PubMed] [Google Scholar]
- 3.Deibel C, Krämer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 4.Demers B, Sansonetti P J, Parsot C. Induction of type III secretion in Shigella flexneri is associated with differential control of transcription of genes encoding secreted proteins. EMBO J. 1998;17:2894–2903. doi: 10.1093/emboj/17.10.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnenberg S M, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989;160:452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- 6.Elliott S, Wainwright L A, McDaniel T, MacNamara B, Donnenberg M, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 7.Elliott S J, Hutcheson S W, Dubois M S, Mellies J L, Wainwright L A, Batchelor M, Frankel G, Knutton S, Kaper J B. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol Microbiol. 1999;33:1176–1189. doi: 10.1046/j.1365-2958.1999.01559.x. [DOI] [PubMed] [Google Scholar]
- 8.Elliott S J, Kaper J B. Role of type 1 fimbriae in EPEC infections. Microb Pathog. 1997;23:113–118. doi: 10.1006/mpat.1997.0135. [DOI] [PubMed] [Google Scholar]
- 9.Elliott S J, Sperandio V, Giron J A, Mellies J L, Wainwright L A, Hutcheson S W, McDaniel T K, Kaper J B. The locus of enterocyte efffacement (LEE)-encoded regulator controls the expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 2000;68:6115–6126. doi: 10.1128/iai.68.11.6115-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel G, Phillips A D, Rosenshine I, Dougan G, Kaper J B, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 11.Hartland E L, Daniell S J, Delahay R M, Neves B C, Wallis T, Shaw R K, Hale C, Knutton S, Frankel G. The type III protein translocation system of enteropathogenic Escherichia coli involves EspA-EspB protein interactions. Mol Microbiol. 2000;35:1483–1492. doi: 10.1046/j.1365-2958.2000.01814.x. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis K G, Kaper J B. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 14.Kenny B, Jepson M. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell Microbiol. 2000;2:579–590. doi: 10.1046/j.1462-5822.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutton S, Rosenshine I, Pallen M J, Nisan L, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine M M, Bergquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Sotman S, Rowe B. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are noninvasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 18.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 19.McNamara B P, Donnenberg M S. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol Lett. 1998;166:71–78. doi: 10.1111/j.1574-6968.1998.tb13185.x. [DOI] [PubMed] [Google Scholar]
- 20.Mellies J L, Navarro-Garcia F, Okeke I, Frederickson J, Nataro J P, Kaper J B. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect Immun. 2001;69:315–324. doi: 10.1128/IAI.69.1.315-324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noriega F R, Losonsky G, Wang J Y, Formal S B, Levine M M. Further characterization of ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203 as a mucosal Shigella vaccine and as a live-vector vaccine for delivering antigens of enterotoxigenic Escherichia coli. Infect Immun. 1996;64:23–27. doi: 10.1128/iai.64.1.23-27.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penfold R J, Pemberton J M. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene. 1992;118:145–146. doi: 10.1016/0378-1119(92)90263-o. [DOI] [PubMed] [Google Scholar]
- 24.Robins-Browne R M, Bennett-Wood V. Quantitative assessment of the ability of Escherichia coli to invade cultured animal cells. Microb Pathog. 1992;12:159–164. doi: 10.1016/0882-4010(92)90119-9. [DOI] [PubMed] [Google Scholar]
- 25.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor K A, O'Connell C B, Luther P W, Donnenberg M S. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect Immun. 1998;66:5501–5507. doi: 10.1128/iai.66.11.5501-5507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchiya K I, Tobe T, Komatsu K, Suzuki T, Watarai M, Fukuda I, Yoshikawa M, Sasakawa C. Identification of a novel virulence gene, virA, on the large plasmid of Shigella, involved in invasion and intercellular spreading. Mol Microbiol. 1995;17:241–250. doi: 10.1111/j.1365-2958.1995.mmi_17020241.x. [DOI] [PubMed] [Google Scholar]
- 28.Warawa J, Finlay B B, Kenny B. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect Immun. 1999;67:5538–5540. doi: 10.1128/iai.67.10.5538-5540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuhan R, Koutsouris A, Savkovic S D, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]