Abstract
Background:
Volatile anesthetic consumption can be reduced by minimizing excessive fresh gas flows (FGF). Currently, it is unknown whether decision support tools embedded within commercial electronic health record systems can be successfully adopted to achieve long-term reductions in FGF rates. The authors describe the implementation of an electronic health record-based clinical decision support tool aimed at reducing FGF and evaluate the effectiveness of this intervention in achieving sustained reductions in FGF rates and volatile anesthetic consumption.
Methods:
On August 29, 2018 we implemented a decision support tool within the Epic Anesthesia Information Management System (AIMS) to alert providers of high FGF (> 0.7 L/min for desflurane, >1 L/min for sevoflurane) during maintenance anesthesia. July 22, 2015 to July 10, 2018 served as our baseline period prior to the intervention. The intervention period spanned from August 29, 2018 to December 31, 2019. Our primary outcomes were mean FGF (L/min) and volatile agent consumption (mL/MAC-hour). Because simple comparison of two time periods may result in false conclusions due to underlying trends independent of the intervention, we performed segmented regression of the interrupted time series to assess the change in level at the start of the intervention and the differences in slopes before and after the intervention. The analysis was also adjusted for potential confounding variables. Data included 44,899 cases using sevoflurane pre-intervention with 26,911 cases post-intervention, and 17,472 cases using desflurane with 1,185 cases post-intervention.
Results:
Segmented regression of the interrupted times series demonstrated a decrease in mean FGF by 0.6 L/min (95% CI: 0.6 to 0.6 L/min, P<0.0001) for sevoflurane and 0.2 L/min (95% CI: 0.2 to 0.3 L/min, P<0.0001) for desflurane immediately after implementation of the intervention. For sevoflurane, mL/MAC-hour decreased by 3.8 mL/MAC-hr (95% CI: 3.6–4.1 mL/MAC-hr, P<0.0001) after implementation of the intervention and decreased by 4.1 mL/MAC-hr (95% CI: 2.6–5.6 mL/MAC-hr, P<0.0001) for desflurane. Slopes for both FGF and ml/MAC-hour in the post-intervention period were statistically less negative than the pre-intervention slopes (P<0.0001 for sevoflurane and P<0.01 for desflurane).
Conclusions:
A commercial AIMS-based decision support tool can be adopted to change provider FGF management patterns and reduce volatile anesthetic consumption in a sustainable fashion.
Introduction
Volatile anesthetics are recognized greenhouse gases (GHG), accounting for 5% of hospital-related GHG emissions, yet they are among the most commonly used anesthetic agents.1–3 These anesthetic gases also account for a major portion of anesthetic drug expense, contributing to the rising costs of healthcare.3–6 Common use of fresh gas flow (FGF) rates ≥ 2 L/min for sevoflurane during maintenance phase anesthesia is largely based on safety information that is no longer relevant. The recommended lower sevoflurane FGF limit was originally 2 L/min due to a potential interaction with CO2 absorbents and the production of a nephrotoxic byproduct (Compound A). However, no evidence of human toxicity has been reported, and modern CO2 absorbents such as DragerSorb Free and Amsorb Plus reduce Compound A production to negligible amounts even at minimal FGF.7
Multiple studies have demonstrated that reducing FGF can safely minimize wasted volatile agent, limiting atmospheric contamination and reducing associated costs.2,4,6,8–10 Previous efforts to reduce FGF have largely focused on education, policy changes, and individualized feedback. While these strategies initially achieve moderate reductions, over time FGF rates tend to return to baseline.8,10–12
Clinical decision support (CDS) systems are resources that can encourage behavioral change. Anesthesia-specific CDS has been shown to improve quality measures, such as guideline adherence for postoperative nausea/vomiting prophylaxis and timely administration of perioperative antibiotics.13–18 In fact, Nair et al. reduced the FGF rates of inhaled anesthetic agents in the short-term using a CDS tool, Smart Anesthesia Manager.19 However, the majority of CDS described in the literature, including the Smart Anesthesia Manager, rely on customized proprietary anesthesia information management systems (AIMS)15–19 and require considerable infrastructure, skill and effort to implement and maintain.20 Additionally, these platforms are generally not portable across different AIMS, and therefore, remain inaccessible to other institutions.21,22 Currently, it is unknown whether CDS embedded within existing and widely adopted electronic health record systems can be successfully and seamlessly implemented to achieve sustained reductions in FGF rates and volatile agent consumption.
We aimed to reduce volatile anesthetic waste by lowering FGF through a method that is sustainable and transferrable to other institutions. We hypothesized that a real-time decision support tool integrated within an existing commercially-available AIMS would lead to sustained reduction of volatile anesthetic agent use by prompting providers to lower FGF rates during the maintenance phase of general anesthesia. Our institution uses the Epic AIMS (Epic, Verona, WI), which is widely used across the United States in both academic and non-academic settings. In this study, we describe the use of intrinsic Epic AIMS features to implement CDS rules that encourage clinicians to decrease FGF and evaluate the effectiveness of this intervention in sustainably reducing FGF rates and volatile anesthetic consumption using segmented regression analysis of an interrupted time series.
Materials and Methods
Data Source
This study was approved by the University of California San Francisco Institutional Review Board (#19–28183). The requirement for written informed consent was waived by the IRB. Data were obtained from our anesthesia information system database. Our institution uses the Epic electronic health record, including the Epic AIMS. This system acquires and records specified hemodynamic and device data from patient monitors and anesthesia machines at one-minute intervals. We enhanced our AIMS to capture additional data from our GE Carescape monitors and GE Aisys anesthesia machines (General Electric Corporation), including, minute-by-minute fresh gas flows (L/min) of oxygen, air, and nitrous oxide, and total fresh gas flow. Additional recorded variables included set vaporizer values (volume-percent) of sevoflurane, isoflurane, and desflurane, and cumulative consumption for each (mL). The three consumption variables are reset by the anesthesia machine at the start of each case. Our GE Aisys machines capture both FGF values and volatile anesthetic data. However, some non-operating room locations use machines such as the GE Aestiva, which does not report FGF nor set or cumulative values for the volatile anesthetics.
Study Design and Study Sample
In this observational cohort study, we used an interrupted time series analysis to assess whether implementation of the decision support intervention was associated with reductions in FGF and volatile anesthetic consumption during the maintenance phase of anesthesia (period from procedure start to procedure end).
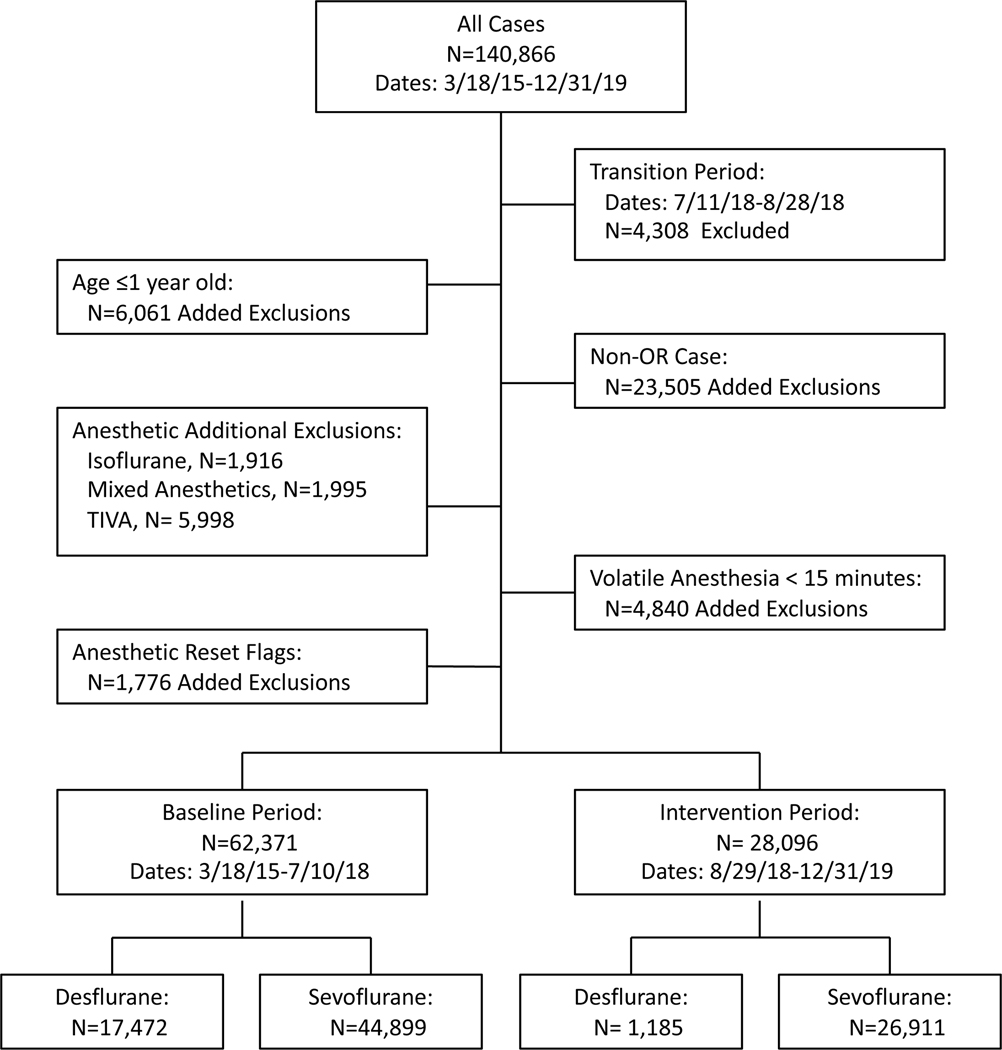
The study sample included 90,467 cases performed under general anesthesia utilizing a volatile anesthetic agent over a total of 51 months spanning July 22, 2015 through December 31, 2019 at our Parnassus, Mission Bay and Mount Zion campuses. Cases that did not use an inhaled agent, cases that used isoflurane as the inhaled agent (< 1.3% of cases), and those that used multiple inhaled agents in the maintenance phase were excluded from the analysis. Cases performed in non-operating room locations were also excluded to avoid data contamination from cases using Aestiva anesthesia machines and from cases involving location changes, which could reset the cumulative amount of volatile anesthetic agent used. Cases during which the GE Aisys machine was reset (i.e., case ended and then restarted) were also excluded as this workflow resets the cumulative inhaled agent count. Cases with patient age less than one year were excluded as this is one of the exclusion criteria for the decision support tool rules. Cases were audited directly in Epic to confirm data. Outliers were also inspected for other potential sources of errors.
Study Variables
Demographic data related to the surgery, patient and anesthesia provider was collected for each case. Minute-by-minute FGF and end-tidal inhaled agent concentration values, cumulative volume of inhaled agent consumption, procedure start time, and procedure end time were also extracted from Epic for each case in the study period.
The primary outcomes of interest were FGF rate (L/min), volatile agent consumption, defined as volume of volatile agent consumed per minimum alveolar concentration-hour (mL/MAC-hr), and volatile agent costs. The time-weighted mean FGF was calculated for each case using each minute FGF value during the maintenance phase. Average FGF for the individual cases were used to calculate mean FGF for all general anesthesia cases. Similarly, for each case, a weighted mean of the end-tidal inhaled agent concentration during the maintenance phase was calculated, and these values were used to estimate the average minimum alveolar concentration (MAC) for the specific agent during the maintenance phase and subsequently the MAC-hours of agent use. The cumulative volume of agent consumption (mL) during the maintenance phase (directly measured by anesthesia machine gas analyzer) was then divided by the MAC-hours to calculate volume of agent used per MAC-hour (mL/MAC-hour), which served as a measure of efficiency in anesthetic gas use. Using pharmacy acquisition costs for sevoflurane and desflurane, the cost per MAC-hour was estimated for each agent. MAC was adjusted for age using published formulas.23
Clinical Decision Support Tool to Reduce FGF Rates: Best Practice Advisory
We designed an intervention to promote and sustain efficient anesthetic gas use through lower FGFs. We set a target of 1 L/min FGF during the maintenance phase of anesthesia when using sevoflurane and 0.7 L/min FGF when using desflurane or isoflurane. These targets were selected to optimize waste reduction while staying within the comfort zone of our providers (based on qualitative assessment of leaders from various subspecialty anesthesia groups) and within safety margins for preventing production of compound A and carbon monoxide. Both sevoflurane and desflurane produce negligible amounts of compound A and carbon monoxide even at an FGF of 0.5 L/min. 7,24
We implemented a clinical decision support tool in the Epic AIMS, to alert anesthesia providers, in real time, of inappropriately high FGF rates during the maintenance phase of anesthesia. The decision support tool was designed using the intraoperative best practice advisory (BPA) functionality, a feature of the Epic AIMS that uses rule-based processing of real-time data, including device values, to fire alerts with various, programmable behaviors. Similar decision support tools using custom or external alerting systems have been described19, however, this BPA was programmed entirely within the commercial Epic AIMS using intrinsic rules-based tools.
Rules encoded within the BPA included the FGF limits, the “look-back” time (the amount of time FGF must be above the set threshold before triggering the alert), and the actions that occur when a vaporizer is turned on and the FGF is above threshold for the defined look-back time. Initially, the look-back time was programmed to be three minutes. FGF thresholds were set at 0.7 L/min for desflurane and isoflurane and 1 L/min for sevoflurane during the maintenance phase of anesthesia. The BPA alert displayed as a pop-up modal dialog window that forced anesthesia providers to either accept the alert, pause it for 10 minutes (“snooze”), or indicate a reason why the alert should not fire again for the case (e.g., circuit leak requiring high FGF) (Supplemental Digital Content Figure 1).
The Epic intraoperative BPAs evaluate rules at each “refresh,” occurring every minute. The FGF BPA rules are as follows (example for Sevoflurane is shown:
The sevoflurane vaporizer is on (set concentration is greater than 0).
The minimum total fresh gas flow exceeds 1 L/min for the past 3 minutes.
There is no FGF BPA override documented.
There is a Procedure Start.
There is no Procedure Stop.
The patient’s age is ≥ 1 year old.
If 1 AND 2 AND 3 AND 4 AND 5 AND 6 are all true, then the BPA fires, displaying as an indicator or alert in the corner of the screen. The user may pause the notification for 10 minutes or silence the BPA for the duration of the case by documenting a reason.
Study Timeline
Baseline Period
To examine our baseline FGF patterns and inhaled agent use in the baseline period, we queried Epic data from July 22, 2015 to July 10, 2018, which served as our baseline period.
Intervention Roll-out
On July 11, 2018 anesthesia providers were notified of the new BPA. On August 29, 2018 the BPA was activated, and alerts were functional in all anesthesia locations with GE Aysis anesthesia machines at the University of California San Francisco Medical Center.
The BPA was programmed to be active during the time period from the start of the procedure (i.e., surgical incision) until the end of the procedure. Based on qualitative feedback from providers, the look-back time was changed from 3 minutes to 5 minutes on September 12, 2018 in an effort to reduce alert fatigue.25,26 On October 10, 2018 the BPA method of communication to the user, or “channel,” was revised to further reduce alert burden and interruptions to provider workflow. In lieu of a modal dialog box, the BPA alert was modified to display as a less intrusive indicator appearing in the corner of the screen, which would not interrupt clinical documentation and other interactions with the AIMS (Supplemental Digital Content Figure 2). The provider could still access the full dialog box contents by clicking on the advisory alert. The final set of decision rules for the BPA is detailed in Table 1. The BPA rules and rule properties are described further in Supplemental Digital Content Document 1.
Table 1.
Decision Rules for Fresh Gas Flow Best Practice Advisory
| Agent | Rule | Inclusion Criteria (Rule triggered only if all the following criteria are met) | Exclusion Criteria (Rule not triggered if any of the following criteria are met) |
|---|---|---|---|
|
| |||
| Sevoflurane | If FGF > 1.0 l/min, notify provider to reduce FGF to 1.0 l/min | • Set agent is sevoflurane • Set agent concentration is > 0 • Minimum total gas flow > 1 l/min for past 5 minutes • Procedure start has occurred, but procedure stop has not occurred • Patient age ≥ 1 year |
• Set agent concentration is 0 • Set agent is not sevoflurane • Total gas flow ≤ 1 l/min • Procedure has not started • Procedure has ended • Patient age < 1 year • Documentation that low FGF cannot be maintained • Alert “snoozed” for 10 minutes |
| Desflurane | If FGF > 0.7 l/min, notify provider to reduce FGF to 0.7 l/min | • Set agent is desflurane • Set agent concentration is > 0 • Minimum total gas flow > 0.7 l/min for past five minutes • Procedure start has occurred, but procedure stop has not occurred • Patient age ≥ 1 year |
• Set agent concentration is 0 • Set agent is not desflurane • Total gas flow ≤ 0.7 l/min • Procedure has not started • Procedure has ended • Patient age < 1 year • Documentation that low FGF cannot be maintained • Alert “snoozed” for 10 minutes |
| Isoflurane | If FGF > 0.7 l/min, notify provider to reduce FGF to 0.7 l/min | • Set agent is isoflurane • Set agent concentration is > 0 • Minimum total gas flow > 0.7 l/min for past five minutes • Procedure start has occurred, but procedure stop has not occurred • Patient age ≥ 1 year |
• Set agent concentration is 0 • Set agent is not isoflurane • Total gas flow ≤ 0.7 l/min • Procedure has not started • Procedure has ended • Patient age < 1 year • Documentation that low FGF cannot be maintained • Alert “snoozed” for 10 minutes |
Rules were executed every 5 minutes.
FGF, Fresh gas flow
Intervention Period
The BPA was activated on August 29, 2018 and the intervention period was defined from August 29, 2018 to December 31, 2019. Apart from the early enhancements detailed above, the BPA decision rules and behavior remained active and constant for the duration of the intervention period.
Statistical Analysis
Baseline variables are reported with standardized differences, which are the difference in means or proportions divided by the standard deviation. Established guidelines were used to interpret the magnitude of difference or imbalance: 0.2 = small, 0.5 = medium, 0.8 = large.27
Distributions of data were visualized using histograms. The Shapiro-Wilk test was used to estimate whether continuous data were normally distributed. While data were not statistically normally distributed, continuous data are displayed as mean ± SD or 95% confidence intervals to more easily compare to prior literature, and data were not excessively skewed. All data were weighted according to the duration of data relevant for the variable. For example, cases with FGF data twice as long were weighted accordingly. Data were summarized by the two time periods (baseline period and intervention period) for tables, and monthly for figures. The time between announcement of the BPA tool and BPA activation was defined as a transition period (July 11, 2018 to August 28, 2018) and excluded from the analysis.
Due to the before-after study design, a segmented regression for interrupted time series analysis was performed on the relevant outcomes using the methodology described by Mascha et al.28 Because the key dates were in the middle of the month, date was used as the time interval instead of month. The segmented regression estimates the trend or slope during the first period (July 22, 2015 to July 10, 2018), the difference in slope between the first and second time period (August 29, 2018 to December 31, 2019), and the level change at the start of the intervention. The analysis was performed separately for sevoflurane and desflurane, for the two key variables fresh gas flow (FGF) and mL/MAC-hour.
Anesthesia provider was defined according to case staffing. Anesthesia attendings working alone meant that no resident or certified registered nurse anesthetist (CRNA) ever participated in the case. Since the standardized difference demonstrated some change in staffing patterns during the two time periods, segmented regression analysis was repeated adjusting for important confounders, which included staffing, patient gender, case length, patient age and hospital location.
We aimed to include at least one year of data in both the baseline and BPA intervention periods, since short time periods are more susceptible to confounding. Based on case load trends, we anticipated over 10,000 cases before and after the intervention. A drop in FGF of 0.1 L/min represented a minimum level of clinically significant change. Even with a standard deviation of 1 L/min, a power analysis indicated 99% power to detect a decrease in FGF of 0.1 L/min at a 0.05 level of significance. With the focus on the change in FGF after implementation of the intervention, and not the slopes, we performed simulations of segmented regressions based on the variability of our data and the 0.1 L/min change. The simulations, which included different slopes and used variability even greater than our data, still showed >99% power to detect a decrease in FGF of 0.1 L/min at a 0.05 level of significance.
Data were analyzed using Stata 14.2 (StataCorp, College Station, TX). Segmented regression analysis of the interrupted time series was performed using the “itsa” package.29 P<0.05 was considered statistically significant.
Results
We identified a total of 140,866 general anesthetics, of which 90,467 met inclusion criteria (Figure 1). Apart from distribution of inhaled agent used and provider categorization, demographic variables in the baseline and intervention cohorts were very similar (Table 2).
Figure 1:
Flow chart of excluded cases and groups defined by time period and inhaled anesthetic agent. TIVA, total intravenous anesthesia.
Table 2.
Provider, patient and case information for analyzed data
| Baseline | Intervention | Standardized Difference | |
|---|---|---|---|
|
| |||
| n | 62,371 (68.9%) | 28,096 (31.1%) | |
| Provider | 0.20 | ||
| Resident | 16,183 (48.3%) | 6,099 (35.7%) | |
| CRNA | 10,318 (30.8%) | 6,713 (39.3%) | |
| Attending Alone | 5,860 (17.5%) | 3,504 (20.5%) | |
| Dual Attendings | 58 (0.2%) | 31 (0.2%) | |
| Resident/CRNA | 1,060 (3.2%) | 726 (4.3%) | |
| Patient Age (years) | 48 ± 23 | 49 ± 23 | 0.04 |
| Pediatric vs. Adult Patient | 0.04 | ||
| Age≥18 | 52,150 (83.6%) | 23,885 (85.0%) | |
| Age<18 | 10,221 (16.4%) | 4,211 (15.0%) | |
| Patient Gender | 0.01 | ||
| Female | 31,093 (49.9%) | 14,203 (50.6%) | |
| Male | 31,265 (50.1%) | 13,873 (49.4%) | |
| Anesthetic Agent | 0.68 | ||
| Desflurane | 17,472 (28.0%) | 1,185 (4.2%) | |
| Sevoflurane | 44,899 (72.0%) | 26,911 (95.8%) | |
| Case Length (hours) | 3.3 ± 2.1 | 3.4 ± 2.3 | 0.03 |
| Short or Long Case | 0.01 | ||
| <2 hours | 20,343 (32.6%) | 9,029 (32.1%) | |
| ≥2 hours | 42,028 (67.4%) | 19,067 (67.9%) | |
| Hospital Site | 0.00 | ||
| Parnassus | 33,541 (53.8%) | 15,043 (53.5%) | |
| Mission Bay | 22,059 (35.4%) | 10,130 (36.1%) | |
| Mount Zion | 6,771 (10.9%) | 2,923 (10.4%) | |
Data are mean ± SD, or n(%)
CRNA, certified registered nurse anesthetist
Standardized difference is the difference in means or proportions divided by the standard deviation and calculates potential imbalance in groups; 0.2 = small, 0.5 = medium, 0.8 = large imbalance.
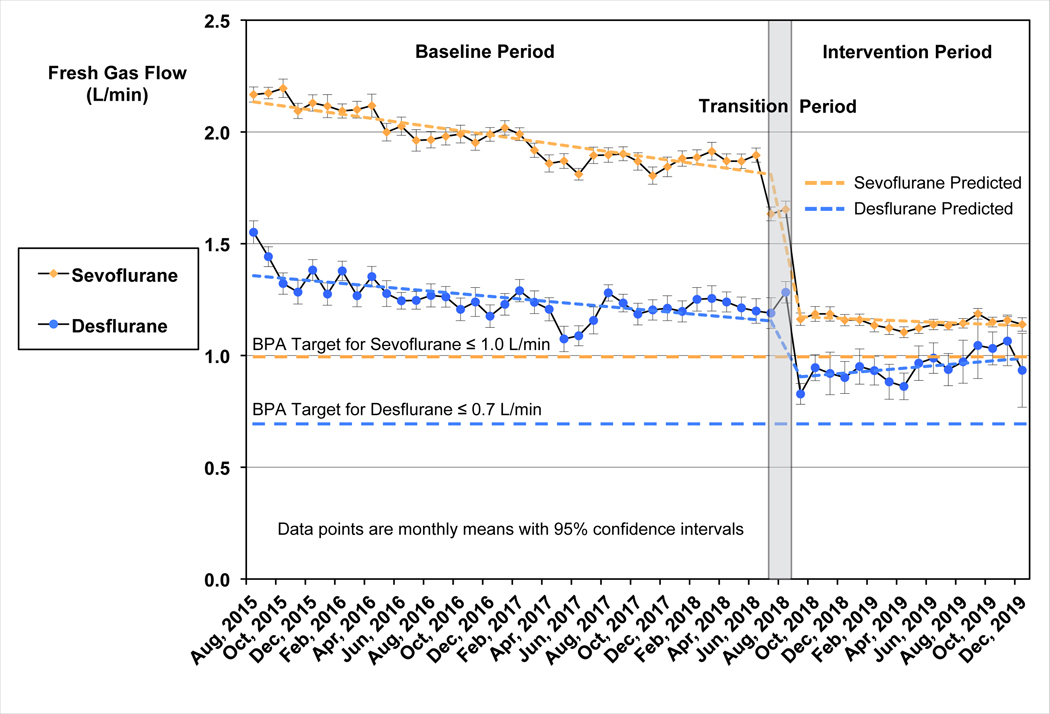
The segmented regression analysis demonstrated significant reductions in FGF and volumes of anesthetic agent used for both sevoflurane and desflurane (Figure 2). Both sevoflurane and desflurane showed a downward trend in mean FGF during the baseline period with slopes significantly different from zero (P < 0.0001). Immediately following implementation of the BPA intervention, mean FGF decreased by 0.6 L/min (95% CI: 0.6 to 0.6 L/min) for sevoflurane and 0.2 L/min (95% CI: 0.2 to 0.3 L/min) for desflurane, both P < 0.0001. Sevoflurane continued to show a small but statistically significant downward trend in mean FGF during the intervention period (P=0.02).
Figure 2:
Interrupted time series analysis: Monthly mean FGF rates for sevoflurane and desflurane. Fresh gas flow data are shown as monthly means with 95% confidence intervals. Sevoflurane is shown in yellow as indicated, and desflurane in blue. Target fresh gas flow levels for the Best Practice Advisory are shown for each agent (≤1.0 L/minute for Sevoflurane and ≤0.7 L/minute for Desflurane) with horizontal lines. The baseline period was from July 22, 2015 to July 10, 2018. The intervention period began August 29, 2018. The transition period (July 11, 2018 to August 28, 2018), where data were excluded for statistical analysis, is indicated by the gray shaded rectangle. The predicted FGF lines are shown for each agent, derived from the interrupted time series analysis. FGF showed a statistically significant downward trend for both sevoflurane and desflurane during the baseline period, P<0.0001. The decrease in FGF at the beginning of the intervention period was also statistically significant (P<0.0001). For sevoflurane this decrease was 0.6 L/min (95% CI: 0.6 L/min to 0.6 L/min); for desflurane the decrease was 0.2 L/min (95% CI: 0.2 L/min to 0.3 L/min). Sevoflurane FGF continued to show a small but statistically significant downward trend during the intervention period (P=0.02), while no trend was observed for desflurane. FGF, fresh gas flow, BPA, best practice advisory.
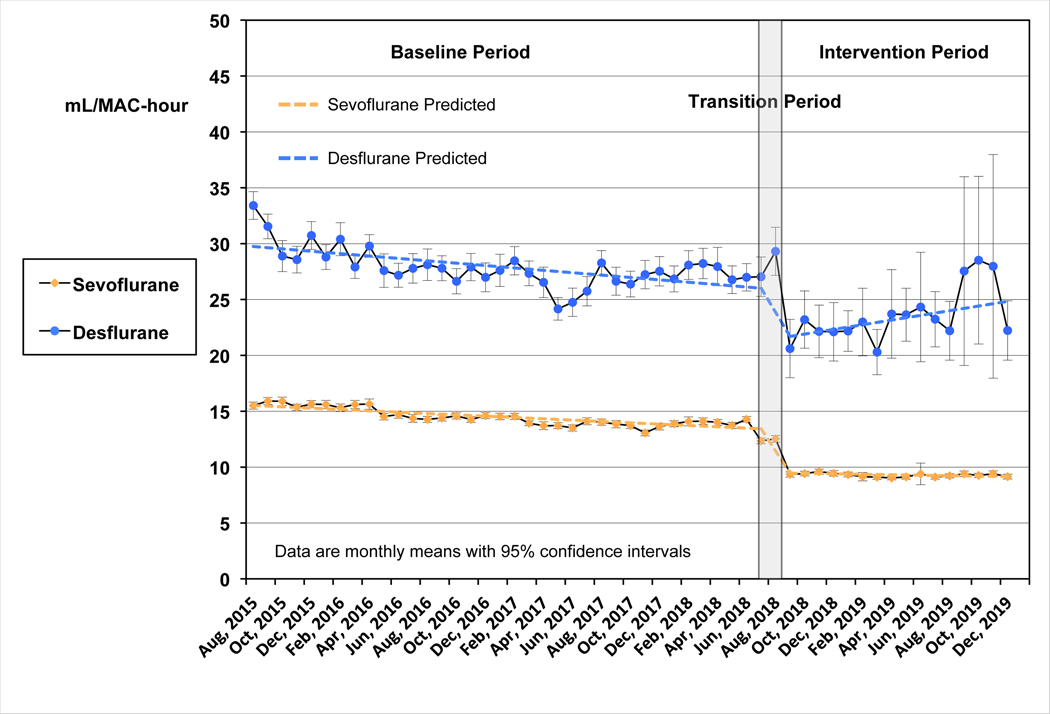
When we plotted the consumption of sevoflurane and desflurane, both inhaled agents showed a statistically significant downward trend in the mean mL/MAC-hour used during the baseline period (Figure 3). However, immediately after initiation of the BPA intervention, mean consumption decreased by 3.8 mL/MAC-hours (95% CI: 3.6 to 4.1) for sevoflurane and 4.1 mL/MAC-hour (95% CI: 2.6 to 5.6) for desflurane. Sevoflurane continued to demonstrate a small but statistically significant downward trend in mean ml/MAC-hour during the intervention period (P=0.02), while a small upward trend for desflurane was not statistically significant (P=0.06). Slopes for both FGF and mL/MAC-hour in the post-intervention period were statistically less negative than the pre-intervention slopes (P<0.0001 for sevoflurane and P<0.01 for desflurane).
Figure 3:
Interrupted time series analysis: Monthly mean mL/MAC-hour for sevoflurane and desflurane. Data for mL/MAC-hour are shown as monthly means with 95% confidence intervals. Sevoflurane is shown in yellow as indicated, and desflurane in blue. The baseline period was from July 22, 2015 to July 10, 2018. The intervention period began August 29, 2018. The transition period (July 11, 2018 to August 28, 2018), where data were excluded for statistical analysis, is indicated by the gray shaded rectangle. The predicted data lines are shown for each agent, derived from the interrupted time series analysis. The mL/MAC-hour showed a statistically significant downward trend for both sevoflurane and desflurane during the baseline period, P<0.0001. The drop in anesthetic agent use at the intervention was also statistically significant (P<0.0001). For sevoflurane this decrease was 3.8 mL/MAC-hours (95% CI: 3.6 to 4.1); for desflurane the decrease was 4.1 mL/MAC-hour (95% CI: 2.6 to 5.6). Sevoflurane use continued to show a small but statistically significant downward trend during the intervention period (P=0.02), while a small upward trend for desflurane was not statistically significant (P=0.06). MAC, mean alveolar concentration.
Standardized difference suggested a change in provider staffing pattern between the baseline and intervention time periods. Based on this observation, we performed a stratified analysis by provider staffing category, which demonstrated that mean FGF rates and volatile agent consumption dropped similarly and significantly for all provider staffing combinations (data not shown). We also added potential confounding variables, including provider, hospital location, patient age, gender and case length directly into the segmented regression model. Reductions in FGF and volatile agent consumption were not altered within the significant figures reported by adjusting for other variables.
Table 3 summarizes FGF values and anesthetic information between the baseline and intervention periods for sevoflurane and desflurane separately. We include a simple cost estimate showing a decrease of $2.10 (95% CI $2.06-$2.13) for sevoflurane and $3.22 (95%CI $2.74-$3.70) for desflurane per MAC-hour.
Table 3.
Maintenance anesthetic gas information for baseline and intervention periods
| Baseline | Intervention | Difference (95% CI) | |
|---|---|---|---|
|
| |||
| Desflurane | |||
| n cases | 17,472 (93.6%) | 1,185 (6.4%) | |
| Duration (hours) | 2.5 ± 1.9 | 2.3 ± 1.9 | −0.2 (−0.3, −0.1) |
| Fresh Gas Flow (L/minute) | 1.3 ± 0.5 | 0.9 ± 0.4 | −0.3 (−0.4, −0.3) |
| Mean End-tidal Agent Concentration (volume %) | 4.1 ± 1.7 | 3.4 ± 1.7 | −0.7 (−0.8, −0.6) |
| MAC | 0.7 ± 0.3 | 0.6 ± 0.3 | −0.1 (−0.1, −0.1) |
| Total mL/hour | 18.8 ± 11.0 | 12.3 ± 8.3 | −6.5 (−7.1, −5.9) |
| mL/MAC-hour | 28.3 ± 13.4 | 23.0 ± 14.6 | −5.4 (−6.2, −4.6) |
| $/MAC-hour | $17.01 ± $8.05 | $13.79 ± $8.79 | −$3.22 (−$3.70, −$2.74) |
| Sevoflurane | |||
| n cases | 44,899 (62.5%) | 26,911 (37.5%) | |
| Duration (hours) | 2.0 ± 1.7 | 2.1 ± 1.8 | 0.1 (0.1 to 0.1) |
| Fresh Gas Flow (L/minute) | 2.0 ± 0.6 | 1.2 ± 0.5 | −0.8 (−0.8, −0.8) |
| Mean End-tidal Agent Concentration (volume %) | 1.6 ± 0.6 | 1.5 ± 0.5 | −0.1 (−0.1, −0.1) |
| MAC | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.0 (−0.1, 0.0) |
| Total mL/hour | 13.8 ± 6.8 | 8.2 ± 4.8 | −5.5 (−5.6, −5.4) |
| mL/MAC-hour | 14.5 ± 5.3 | 9.3 ± 6.9 | −5.2 (−5.3, −5.1) |
| $/MAC-hour | $5.82 ± $2.11 | $3.72 ± $2.77 | −$2.10 (−$2.13, −$2.06) |
Data are mean ± SD or n(%)
All relevant values are weighted according the length/amount of data.
“Maintenance” is defined as procedure start to procedure end.
CI, confidence interval; MAC, minimum alveolar concentration
Baseline period was from July 22, 2015 to July 10, 2018. Intervention was implemented on August 29, 2018. Data during the transition period (July 11, 2018 to August 28, 2018) were excluded.
Discussion
While there may be rare contraindications to low-flow anesthesia30,31, for most cases, low-flow anesthesia has been demonstrated to be safe, economical, and beneficial to the environment.6,8–10 Higher FGF may be needed in select cases where rapid changes in anesthetic depth are required, or in selected clinical scenarios as recommended by anesthesia machine manufacturers;30,31 however, these instances are rare and do not reflect the needs of standard maintenance anesthesia. In this study, we demonstrated the feasibility and value of implementing real-time CDS within a widely adopted AIMS to change provider behavior and achieve sustainable reductions in FGF rates and volatile agent consumption. Previous studies have relied on pharmacy audits of volatile agent stock and purchasing data, which have limited reliability, to estimate changes in agent consumption associated with reduced FGF rates.19,32 However, we directly measured cumulative volatile agent consumption and provided a more accurate estimate of our intervention’s effect on volatile anesthetic waste and associated costs. Our BPA intervention reduced FGF rates, resulting in more efficient volatile agent use and corresponding annual estimated savings of $123,120 in 2019.
Compared to the highly customized Smart Anesthesia Manager implemented by Nair et al. to prompt providers to lower FGF, our BPA was built using intrinsic features of an electronic health record system from a vendor with a majority share of the US hospital market, and therefore, can be easily disseminated to healthcare systems operating on the same platform. Furthermore, the study by Nair et al. was limited to active intervention periods of 2 months at a time. In contrast, our intervention period of 16 months allowed us to demonstrate the sustainability of the BPA intervention well beyond the initial implementation phase.
Although FGF rates declined significantly for both sevoflurane and desflurane following the intervention, mean FGF rates remained above the FGF limits programmed into the BPA. Several factors may explain this observation. While providers often used the exact target FGF during stable periods of maintenance anesthesia, any period of higher gas flow, such as during an attempt to rapidly increase anesthetic depth, would have raised the overall average. Providers may have also used higher FGF rates to overcome circuit leaks. Furthermore, old beliefs and behaviors may persist since package labels and inserts continue to recommend high FGF in the U.S., despite the lack of evidence to support this guidance.
Notably, we also observed a significant downward slope in FGF rate and volatile anesthetic consumption (mL/MAC-hr) for both sevoflurane and desflurane during the baseline period. This trend likely represents departmental efforts to raise awareness of the environmental harm associated with volatile agent waste as sustainability and climate change issues gained momentum in healthcare. The segmented regression analysis of the interrupted time series takes this trend into account, and separately analyzes the effect of the intervention. The sudden decrease in FGF rate and volatile anesthetic consumption corresponding to activation of the BPA is significantly greater than that expected by the overall trend. These results strongly suggest that the intervention had a significant effect on FGF rates and volatile anesthetic consumption beyond that of growing awareness in the baseline period.
Standardized difference suggested good balance between most key variables during the two time periods. However, there were a few notable exceptions. First, volatile anesthetic agent selection changed significantly between the two time periods, with a lower proportion of cases using desflurane in the intervention period compared to the baseline period. This change in anesthetic use pattern can be attributed to increased provider awareness of the relative environmental impact of desflurane and reduced access to desflurane at one of our hospital sites. Since the time periods were compared for each volatile agent separately, the change in volatile anesthetic use should not explain our overall finding that FGF and inhaled anesthetic consumption decreased during the intervention period compared to baseline. Second, there were differences in provider patterns between the two time periods. During the study period, our institution experienced a significant increase in case volume, which was staffed by CRNAs supervised by an attending anesthesiologist and by anesthesia faculty working alone. However, the drop in FGF and volatile anesthetic consumption was consistent across providers; therefore, the data do not suggest that changes in provider patterns impacted our key findings. Additionally, adjusting the model for multiple potential confounders did not change the size of the reported effect within significant figures.
Limitations
Any before-after design without a concurrent control group is subject to biases, including time-dependent confounding variables, that may explain the observed association between the intervention and outcome. For example, increased awareness of the environmental impact of volatile anesthetic agents among our providers likely explains the downward trends in FGF rate and volatile agent consumption observed prior to the intervention. These trends are accounted for in the segmented regression analysis. Pre-post studies may also be affected by regression to the mean, which can lead to false results from random variations in the outcomes of interest. However, we evaluated long periods both before and after the intervention, therefore it is unlikely that the FGF rate reverted from a random high starting value to the mean value. While we cannot definitively exclude the presence of Hawthorne effect, the segmented regression analysis demonstrates sustained improvements in FGF and volatile agent use over many months, arguing against a significant observation effect. It is possible that over a longer period of time the effect of the intervention may lessen. A longer observation period or follow-up study would be necessary to demonstrate permanent reduction of FGF use.
This was a single-center study conducted in an academic institution that utilizes the Epic AIMS, which limits the generalizability of our findings. Our CDS was programmed using tools specific to the Epic AIMS, however, other commercial AIMS products have similar features that have been used to program rule-based decision support tools. Although not all Epic electronic health record licensing agreements include support for real-time device data use in CDS rules, institutions that purchase this option can also leverage such features to develop CDS tools for a wide variety of indications. Further, increased customer demands for these capabilities may encourage commercial vendors to offer real-time data processing support in standard bundles or with discounted fees. Differences in Epic implementations, equipment, and environment may require limited local IT assistance and additional costs for implementation of this CDS at other institutions. Fortunately, once the required device data are mapped, there should be minimal IT maintenance required.
Other limitations of this study include the possibility that the behavior of our providers may not represent anesthesia practice in non-academic hospitals. Finally, based on the study exclusion criteria, our findings cannot be generalized to patients under one year of age, cases performed in non-operating room locations, cases using isoflurane, nor those using multiple inhaled anesthetic agents. Despite these limitations, this study demonstrates that a decision support tool embedded in a commericial AIMS is a practical intervention to achieve sustained reductions in FGF rates and inhaled anesthetic consumption.
Conclusions
We implemented a commercial AIMS-based clinical decision support tool to change provider FGF management patterns and significantly reduce volatile anesthetic consumption at our institution. Disseminating a similar tool to institutions using Epic or another commercial AIMS product may be a simple and effective way to reduce volatile anesthetic waste, associated costs and greenhouse gas emissions on a larger scale.
Supplementary Material
Key Points Summary.
Question:
Can we adopt decision support tools embedded within a commercial electronic health record system to minimize volatile anesthetic waste?
Findings:
Implementation of a commercial AIMS-based clinical decision support tool led to sustained reductions in fresh gas flow rates and inhaled anesthetic agent consumption.
Meaning:
A commercial AIMS-based clinical decision support tool led to changes in provider FGF management patterns and may be an effective approach to reducing volatile anesthetic waste, associated costs, and greenhouse gas emissions on a larger scale.
Acknowledgements
Carly Deibler, APeX Analyst, Department of Anesthesia and Perioperative Care, University of California, San Francisco Medical Center, San Francisco, CA, USA
Carly Deibler programmed the Epic Best Practice Advisory build and provided technical support.
Financial Disclosures:
Funding for this study was provided solely from departmental sources. CLC receives research funding from UCSF Anesthesia Research Support, the National Institute of Aging (K23 AG072035, PI: Chen), and the UCSF Pepper Center (P30 AG044281 PI: Covinsky).
Glossary of Terms
- AIMS
anesthesia information management system
- BPA
best practice advisory
- CI
confidence interval
- CRNA
certified registered nurse anesthetist
- FGF
fresh gas flow
- MAC
minimum alveolar concentration
Footnotes
Conflicts of Interest: None
Clinical trial number and registry URL: Not applicable
Abbreviated Title: Reducing fresh gas flow with decision support tool
References
- 1.Van Norman GA, Jackson S. The anesthesiologist and global climate change: An ethical obligation to act. Curr Opin Anaesthesiol. 2020;33(4):577–583. doi: 10.1097/ACO.0000000000000887 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: Application to clinical use. Anesth Analg. 2010;111(1):92–98. doi: 10.1213/ANE.0b013e3181e058d7 [doi]. [DOI] [PubMed] [Google Scholar]
- 3.Golembiewski J Economic considerations in the use of inhaled anesthetic agents. Am J Health Syst Pharm. 2010;67(8 Suppl 4):9. doi: 10.2146/ajhp100093 [doi]. [DOI] [PubMed] [Google Scholar]
- 4.Odin I, Feiss P. Low flow and economics of inhalational anaesthesia. Best Pract Res Clin Anaesthesiol. 2005;19(3):399–413. doi: S1521-6896(05)00007-8 [pii]. [DOI] [PubMed] [Google Scholar]
- 5.Euliano TY, van Oostrom JH, van der Aa J. Waste gas monitor reduces wasted volatile anesthetic. J Clin Monit Comput. 1999;15(5):287–293. doi: 10.1023/a:1009963021363 [doi]. [DOI] [PubMed] [Google Scholar]
- 6.Suttner S, Boldt J. Low-flow anaesthesia. does it have potential pharmacoeconomic consequences? Pharmacoeconomics. 2000;17(6):585–590. doi: 10.2165/00019053-200017060-00004 [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Struys MM, Bouche MP, Rolly G, et al. Production of compound A and carbon monoxide in circle systems: An in vitro comparison of two carbon dioxide absorbents. Anaesthesia. 2004;59(6):584–589. doi: ANA3704 [pii]. [DOI] [PubMed] [Google Scholar]
- 8.Weiskopf RB, Eger EI. 2nd Comparing the costs of inhaled anesthetics. Anesthesiology. 1993;79(6):1413–1418. doi: 10.1097/00000542-199312000-00033 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Ryu HG, Lee JH, Lee KK, et al. The effect of low fresh gas flow rate on sevoflurane consumption. Korean J Anesthesiol. 2011;60(2):75–77. doi: 10.4097/kjae.2011.60.2.75 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman JM. Managing fresh gas flow to reduce environmental contamination. Anesth Analg. 2012;114(5):1093–1101. doi: 10.1213/ANE.0b013e31824eee0d [doi]. [DOI] [PubMed] [Google Scholar]
- 11.Hanci V, Yurtlu S, Ayoğlu H, et al. Effect of low-flow anesthesia education on knowledge, attitude and behavior of the anesthesia team. Kaohsiung J Med Sci. 2010;26(8):415–421. doi: 10.1016/S1607-551X(10)70067-X [doi]. [DOI] [PubMed] [Google Scholar]
- 12.Body SC, Fanikos J, DePeiro D, Philip JH, Segal BS. Individualized feedback of volatile agent use reduces fresh gas flow rate, but fails to favorably affect agent choice. Anesthesiology. 1999;90(4):1171–1175. doi: 10.1097/00000542-199904000-00033 [doi]. [DOI] [PubMed] [Google Scholar]
- 13.Epstein RH, Dexter F, Patel N. Influencing anesthesia provider behavior using anesthesia information management system data for near real-time alerts and post hoc reports. Anesth Analg. 2015;121(3):678–692. doi: 10.1213/ANE.0000000000000677 [doi]. [DOI] [PubMed] [Google Scholar]
- 14.Gabel E, Shin J, Hofer I, et al. Digital quality improvement approach reduces the need for rescue antiemetics in high-risk patients: A comparative effectiveness study using interrupted time series and propensity score matching analysis. Anesth Analg. 2019;128(5):867–876. doi: 10.1213/ANE.0000000000003828 [doi]. [DOI] [PubMed] [Google Scholar]
- 15.Kooij FO, Klok T, Hollmann MW, Kal JE. Decision support increases guideline adherence for prescribing postoperative nausea and vomiting prophylaxis. Anesth Analg. 2008;106(3):893–8, table of contents. doi: 10.1213/ane.0b013e31816194fb [doi]. [DOI] [PubMed] [Google Scholar]
- 16.Kooij FO, Vos N, Siebenga P, Klok T, Hollmann MW, Kal JE. Automated reminders decrease postoperative nausea and vomiting incidence in a general surgical population. Br J Anaesth. 2012;108(6):961–965. doi: 10.1093/bja/aes024 [doi]. [DOI] [PubMed] [Google Scholar]
- 17.Wax DB, Beilin Y, Levin M, Chadha N, Krol M, Reich DL. The effect of an interactive visual reminder in an anesthesia information management system on timeliness of prophylactic antibiotic administration. Anesth Analg. 2007;104(6):1462–6, table of contents. doi: 104/6/1462 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.Nair BG, Newman SF, Peterson GN, Wu WY, Schwid HA. Feedback mechanisms including real-time electronic alerts to achieve near 100% timely prophylactic antibiotic administration in surgical cases. Anesth Analg. 2010;111(5):1293–1300. doi: 10.1213/ANE.0b013e3181f46d89 [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Nair BG, Peterson GN, Neradilek MB, Newman SF, Huang EY, Schwid HA. Reducing wastage of inhalation anesthetics using real-time decision support to notify of excessive fresh gas flow. Anesthesiology. 2013;118(4):874–884. doi: 10.1097/ALN.0b013e3182829de0 [doi]. [DOI] [PubMed] [Google Scholar]
- 20.Simpao AF, Tan JM, Lingappan AM, Gálvez JA, Morgan SE, Krall MA. A systematic review of near real-time and point-of-care clinical decision support in anesthesia information management systems. J Clin Monit Comput. 2017;31(5):885–894. doi: 10.1007/s10877-016-9921-x [doi]. [DOI] [PubMed] [Google Scholar]
- 21.Greenes R Clinical decision support: The road to broad adoption. 2nd ed. London, UK: Elsevier; 2014. [Google Scholar]
- 22.Nair BG, Newman SF, Peterson GN, Schwid HA. Smart anesthesia manager™ (SAM)--a real-time decision support system for anesthesia care during surgery. IEEE Trans Biomed Eng. 2013;60(1):207–210. doi: 10.1109/TBME.2012.2205384 [doi]. [DOI] [PubMed] [Google Scholar]
- 23.Lerou JG. Nomogram to estimate age-related MAC. Br J Anaesth. 2004;93(2):288–291. doi: S0007–0912(17)35933–0 [pii]. [DOI] [PubMed] [Google Scholar]
- 24.Keijzer C, Perez RS, de Lange JJ. Compound A and carbon monoxide production from sevoflurane and seven different types of carbon dioxide absorbent in a patient model. Acta Anaesthesiol Scand. 2007;51(1):31–37. doi: AAS1187 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Ancker JS, Edwards A, Nosal S, et al. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):36–8. doi: 10.1186/s12911-017-0430-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGreevey JD, Mallozzi CP, Perkins RM, Shelov E, Schreiber R. Reducing alert burden in electronic health records: State of the art recommendations from four health systems. Appl Clin Inform. 2020;11(1):1–12. doi: 10.1055/s-0039-3402715 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J Statistical power analysis for the behavior sciences. 2nd Edition ed. Hillsdale, NJ, USA: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 28.Mascha EJ, Sessler DI. Segmented regression and difference-in-difference methods: Assessing the impact of systemic changes in health care. Anesth Analg. 2019;129(2):618–633. doi: 10.1213/ANE.0000000000004153 [doi]. [DOI] [PubMed] [Google Scholar]
- 29.Linden A Conducting interrupted time-series analysis for single- and multiple-group comparisons. The Stata Journal. 2015;15(2):480–500. [Google Scholar]
- 30.Honemann C, Bert M. Low-flow, minimal-flow and metabolic flow aneaesthesia. Clinical techniques for use with rebreathing systems. Draeger Corporation. Accessed at www.draeger.com/Products/Content/low-minimal-flow-anaesthesia-bk-9067990-en.pdf Last accessed: Jan 13, 2022. [Google Scholar]
- 31.Baum JA. Low flow anaesthesia with Drager Machines. Drager Corporation. Accessed at www.draeger.com/Library/Content/rsp-curves-and-loops-booklet-9097339-en.pdf. Last accessed: Jan 13, 2022. [Google Scholar]
- 32.Zuegge KL, Bunsen SK, Volz LM, et al. Provider education and vaporizer labeling lead to reduced anesthetic agent purchasing with cost savings and reduced greenhouse gas emissions. Anesth Analg. 2019;128(6):e97–e99. doi: 10.1213/ANE.0000000000003771 [doi]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.