Abstract
Background:
Assessing the prognosis preoperatively in patients with upper tract urothelial carcinoma (UTUC) remains a challenge for urologists. Gross hematuria (GH) and flank pain (FP) are the 2 most common and easily perceived symptoms of UTUC. Therefore, we aimed to investigate the prognostic values of GH and FP in patients with UTUC after undergoing radical nephroureterectomy (RNU).
Methods:
This article retrospectively analyzed 179 patients with UTUC who underwent RNU and examined the associations between the FP, GH, and long-term survival. After dividing patients into 4 subgroups (presenting as GH without FP, FP without GH, no FP and GH, FP with GH), we focused on the prognostic values of the 4 subgroups using univariate and multivariate analyses. We then proposed a risk stratification model for UTUC based on the independent prognostic factors for cancer-specific survival (CSS) with external validation (146 additional UTUC patients formed the validation cohort).
Results:
Patients with FP had worse oncological outcomes than those without FP (P < .05). After dividing the 179 patients into 4 subgroups, the “FP without GH” subgroup suffered the worst oncological outcomes (P < .001). The Cox multivariate regression analysis showed that “FP without GH” (P < .001), tumor multifocality (P = .005), and pathological stage (P = .004) were independent prognostic factors for CSS. Good performance of the risk stratification model was achieved in both the training and external validation cohorts.
Conclusion:
The presence of “flank pain without gross hematuria” was one of the independent risk factors of CSS and OS besides the pathological stage and tumor multifocality. To our knowledge, this is the first study that adding complaint to risk stratification model in UTUC.
Keywords: Upper tract urothelial carcinoma, hematuria, flank pain, prognosis, risk assessment
Introduction
Upper tract urothelial carcinoma (UTUC), which includes renal pelvis and ureter cancer, is a rare but very aggressive urothelial malignancy. The 5-year cancer-specific survival (CSS) rates are <50% in patients with pT2/T3 tumors and <10% in patients with pT4 and metastatic UTUC.1,2 Although new diagnostic methods and treatments of UTUC continue to be developed, the 5-year CSS of UTUC has not improved significantly in the past 20 years.3 Up to now, open radical nephroureterectomy (RNU) with bladder cuff excision is still the standard surgical method for UTUC,4 with kidney-sparing surgery (KSS) being an optional approach in some selective cases.5 Therefore, accurate preoperative risk identification is paramount in the appropriate choice of treatment.
However, assessing the prognosis preoperatively in patients with UTUC remains a challenge for urologists. Traditional imaging examination and ureteroscopy lack accuracy.6-8 Most of the strong predictors such as the pathological grade, pathological stage, and lymph node status9,10 are obtained postoperatively. Studies on preoperative predictors revolve around age,11 tumor location, tumor size, multifocality,12-15 and hydronephrosis,16-18 while few studies are about symptoms or complaints. For instance, Yeh et al19 indicated that only symptomatic hydronephrosis was one of independent prognostic factors for long-term survival; Raman et al20 demonstrated that patients with local symptoms (including flank/lumbar pain, hematuria, or palpable mass) did not have worse oncological outcomes compared to patients who discovered UTUC accidentally. Gross hematuria (GH) and flank pain (FP) are the 2 most common and easily perceived symptoms of UTUC, so we aimed to explore the role of FP and GH in predicting long-term survival and hoped that it would guide risk stratification and surgical decisions for UTUC in the future.
Materials and Methods
Patient selection and data collection
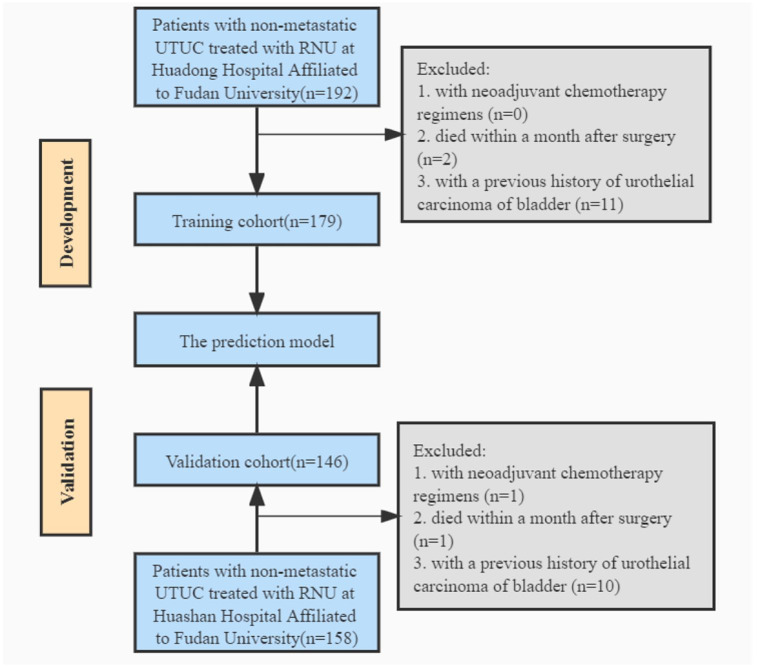
From January 1999 to December 2020, 192 patients diagnosed with non-metastatic UTUC and treated with RNU in Huadong Hospital Affiliated to Fudan University were studied retrospectively, with institutional ethics committee approval. In addition, we included consecutive patients with localized UTUC treated with RNU at Huashan Hospital Affiliated to Fudan University between November 2009 and January 2017 in the validation cohort. Figure 1 provides an overview of the patient selection process. Eventually, 179 and 146 patients were included in the development cohort and validation cohort, respectively. The parameters were collected from their medical records or by phone, including sex, age, body mass index (BMI), smoking history, Chinese herb use, tumor characteristics, surgical approach, adjuvant chemotherapy, and oncological outcomes.
Figure 1.
Flowchart. RNU indicates radical nephroureterectomy; UTUC, upper tract urothelial carcinoma.
Definitions and follow-up
Smoking history was defined as a consecutive or cumulative smoking time longer than 6 months during the patient’s life time. The definition of exposure to Chinese herbs was cumulative exposure for more than 1 year. Hydronephrotic status was assessed using preoperative imaging reports. The synchronous presence of 2 or more tumors in the upper urinary tract was characterized as tumor multifocality. Tumor location was decided on the dominant lesion based on the tumor grade, stage, or size. The pathological stage was according to the 2017 tumor node metastasis (TNM) classification system and the pathological grade was based on the 2004 World Health Organization grading system.
The evaluation of FP was completed by nurses using Numeric Rating Scales (NRS)21 at admission. The NRS scores range from 0 (“no pain”) to 10 (“worst pain”). All patients were asked to respond to their intensity of overall pain during the past week. The number and the corresponding area of pain were recorded on “Temperature Sheet.” Every pain component would be rated when patients indicated more than one pain. Patients were considered to have UTUC-related FP if their NRS scores at the waist were ⩾1 and the FP occured at the same side of tumor based on imaging or endoscope examination. Patients who took analgesics because of FP were considered to have FP even though their NRS scores were 0 (n = 3). People whose symptoms caused by non-neoplastic diseases such as urolithiasis or benign ureteral stricture were not considered to have FP (n = 2). The definition of GH is blood in the urine that can be observed by the naked eye. Microscopic hematuria (MH) is defined as more than 3 red blood cells/high-power field on urine analysis. Lack of GH includes MH and no hematuria.
Patients were followed at 3 and 6 months after RNU, then every 6 months in the first 5 years, and annually afterward. Evaluations included physical examination, blood tests, urinary cytology, cystoscopy, and computed tomography (CT) scan. Cause of death was determined by the attending physicians, by chart reviews corroborated by death certificates or by death certificates alone. Only patients who had UC listed on the death certificate were considered to have died of UTUC.22 All patients who were coded as dead of cancer had previous disease recurrence.
Statistical analysis
The chi-square test was used to assess the associations between categorical variables while numerical variables were compared using independent samples t-test. The overall survival (OS) and CSS were estimated by the Kaplan-Meier method with a log-rank test. Univariable and multivariable Cox regression analysis were applied in addressing the outcomes after RNU. A risk stratification model was constructed based on the number of risk factors, with external validation. All reported P values are 2-sided, and statistical significance was set at .05. Data analyses were conducted by SPSS version 26.0 (IBM Corporation, USA) and MedCalc version 20.022 (MedCalc Software, Ostend, Belgium).
Results
Patients’ clinicopathological characteristics
The clinicopathological data of training cohort were shown in Table 1. There were 55 (30.7%) patients with FP and 128 (71.5%) patients with GH. Preoperative hydronephrosis was observed in 126 (70.4%) patients. The renal pelvis tumors accounted for 50.8% and multifocal tumors accounted for 23.5%. In terms of pathological features, about 60 percent (56.4%) of the patients had muscle-invasive disease. And 16.2% of tumors were low-grade, 17.3% exhibited lymphovascular invasion (LVI). As for therapy, 118 (65.9%) patients were treated by open RNU while the remaining 61 (34.1%) underwent laparoscopic nephroureterectomy or robot-assisted nephroureterectomy. Fifty-nine (33.0%) patients received adjuvant chemotherapy. The FP was only significantly associated with hydronephrosis (Supplemental Table 1, P = .012), and GH was only significantly associated with renal pelvis tumor (Supplemental Table 1, P = .001).
Table 1.
Clinicopathologic features of the training and validation cohorts.
| All patients | Training cohort | Validation cohort | P value | |
|---|---|---|---|---|
| Variable | n = 325 | n = 179 | n = 146 | |
| Sex, n (%) | .030 | |||
| Male | 197 (60.6) | 99 (55.3) | 98 (67.1) | |
| Female | 128 (39.4) | 80 (44.7) | 48 (32.9) | |
| Median age (range) | 67 (32-88) | 68 (32-88) | 67 (35-87) | .084 |
| Body mass index, n (%) | .902 | |||
| <18.5 | 15 (4.6) | 8 (4.5) | 7 (4.8) | |
| 18.5-24 | 172 (52.9) | 93 (52.0) | 79 (54.1) | |
| ⩾24 | 138 (42.5) | 78 (43.6) | 60 (41.1) | |
| Hydronephrosis, n (%) | .249 | |||
| Yes | 220 (67.7) | 126 (70.4) | 94 (64.4) | |
| No | 105 (32.3) | 53 (29.6) | 52 (35.6) | |
| Smoking history, n (%) | .764 | |||
| Yes | 60 (18.5) | 32 (17.9) | 28 (19.2) | |
| No | 265 (81.5) | 147 (82.1) | 118 (80.8) | |
| Herbal usage, n (%) | .994 | |||
| Yes | 20 (6.2) | 11 (6.1) | 9 (6.2) | |
| No | 305 (93.8) | 168 (93.9) | 137 (93.8) | |
| Flank pain, n (%) | .052 | |||
| Yes | 115 (35.4) | 55 (30.7) | 60 (41.1) | |
| No | 210 (64.6) | 124 (69.3) | 86 (58.9) | |
| Hematuria, n (%) | .667 | |||
| Gross hematuria | 226 (69.5) | 128 (71.5) | 98 (67.1) | |
| Microscopic hematuria | 46 (14.2) | 23 (12.8) | 23 (15.8) | |
| No hematuria | 53 (16.3) | 28 (15.6) | 25 (17.1) | |
| Tumor location, n (%) | .881 | |||
| Renal pelvis | 164 (50.5) | 91 (50.8) | 73 (50.0) | |
| Ureter | 161 (49.5) | 88 (49.2) | 73 (50.0) | |
| Tumor size, n (%) | .735 | |||
| ⩾3 cm | 177 (54.5) | 99 (55.3) | 78 (53.4) | |
| <3 cm | 148 (45.5) | 80 (44.7) | 68 (46.6) | |
| Multifocality, n (%) | .855 | |||
| Yes | 75 (23.1) | 42 (23.5) | 33 (22.6) | |
| No | 250 (76.9) | 137 (76.5) | 113 (77.4) | |
| Pathologic stage, n (%) | .079 | |||
| pT (a-is-1) | 139 (42.8) | 78 (43.6) | 61 (41.8) | |
| T2 | 59 (18.2) | 24 (13.4) | 35 (24.0) | |
| T3 | 115 (35.4) | 69 (38.5) | 46 (31.5) | |
| T4 | 12 (3.7) | 8 (4.5) | 4 (2.7) | |
| Pathologic grade, n (%) | .010 | |||
| Low | 70 (21.5) | 29 (16.2) | 41 (28.1) | |
| High | 255 (78.5) | 150 (83.8) | 105 (71.9) | |
| Lymph node status, n (%) | .088 | |||
| pN0 | 16 (4.9) | 12 (6.7) | 4 (2.7) | |
| pNx | 297 (91.4) | 163 (91.1) | 134 (91.8) | |
| pN+ | 12 (3.7) | 4 (2.2) | 8 (5.5) | |
| Lymphovascular invasion, n (%) | .104 | |||
| Yes | 67 (20.6) | 31 (17.3) | 36 (24.7) | |
| No | 258 (79.4) | 148 (82.7) | 110 (75.3) | |
| Surgical approach, n (%) | .001 | |||
| Open | 183 (56.3) | 118 (65.9) | 65 (44.5) | |
| Laparoscopic | 131 (40.3) | 56 (31.3) | 75 (51.4) | |
| Robot-assisted, n (%) | 11 (3.4) | 5 (2.8) | 5 (4.1) | |
| Adjuvant chemotherapy, n (%) | .709 | |||
| Yes | 110 (33.8) | 59 (33.0) | 51 (34.9) | |
| No | 215 (66.2) | 120 (67.0) | 95 (65.1) |
Statistically significant data (the P-value is less than 0.05) are indicated in bold.
There were statistical significant differences in sex (P = .030), pathological grade (P = .010), and surgical approach (P = .001) between the training cohort and validation cohort due to the patient heterogeneities in different medical institutions (Table 1).
Predictors of CSS and OS
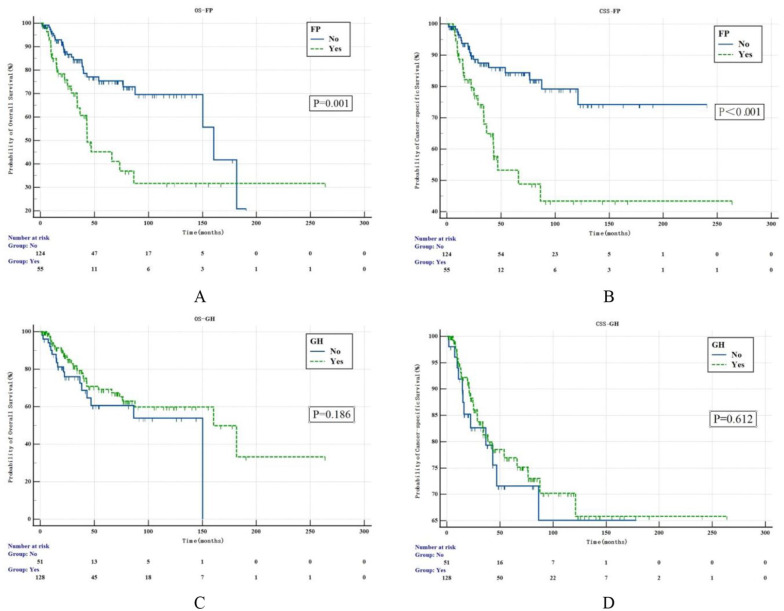
Over a median follow-up period of 47 months, upper tract urothelial cancer death occurred in 38 (21.2%) patients, while 13 (7.3%) cases died of other causes. Thirty-nine (21.8%) patients developed recurrent bladder cancer. Kaplan-Meier survival curves related to FP and GH were shown in Figure 2. Patients with FP had worse 5-year OS (47.2% vs 81.2%, P = .001) and CSS (50.2% vs 83.9%, P < .001) rates than those without FP, but patients with GH and without GH had similar outcomes (74.1% vs 66.4%, P = .186; 76.1% vs 69.5%, P = .612).
Figure 2.
Kaplan-Meier estimates for OS (A) and CSS (B) of UTUC stratified by the presence/absence of flank pain (FP). Kaplan-Meier estimates for OS (C) and CSS (D) of UTUC stratified by the presence/absence of gross hematuria (GH). CSS indicates cancer-specific survival; FP, flank pain; GH, gross hematuria; OS, overall survival; UTUC, upper tract urothelial carcinoma.
On univariable analyses, the presence of FP (P < .001), tumor multifocality (P < .001), high-grade tumors (P = .009), pT ⩾ 2 (P < .001), LVI (P = .012), and history of adjuvant chemotherapy (P = .005) were significantly correlated with CSS (Table 2). The Cox multivariate regression analysis revealed that the presence of FP (hazard ratio [HR] = 2.951, 95% confidence interval [CI]: 1.548-5.627, P = .001), tumor multifocality (HR = 2.621, 95% CI: 1.344-5.112, P = .005), and pathological stage (HR = 3.621, 95% CI 1.570-8.350) were independent prognostic factors for CSS in UTUC patients who underwent RNU (Table 2). The risk factors for OS in the multivariate analysis were identical to those for CSS (Supplemental Table 2).
Table 2.
Univariable and multivariable Cox regression analyses predicting CSS in 179 patients who underwent RNU for UTUC.
| Univariable | Multivariable | ||
|---|---|---|---|
| P value | P value | HR (95% CI) | |
| Age (⩽68 vs >68) | .811 | – | – |
| Sex (male vs female) | .331 | – | – |
| Body mass index | .609 | – | – |
| 18.5-24 vs <18.5 | .456 | – | – |
| ⩾24 vs <18.5 | .746 | – | – |
| Smoking history (yes vs no) | .390 | – | – |
| Herbal usage (yes vs no) | .469 | – | – |
| FP (yes vs no) | <.001 | .001 | 2.951 (1.548-5.627) |
| GH (yes vs no) | .612 | – | – |
| Hydronephrosis (yes vs no) | .054 | – | – |
| Tumor location (renal pelvis vs ureter) | .214 | – | – |
| Tumor size (⩾3 cm vs <3 cm) | .336 | – | – |
| Multifocality (yes vs no) | <.001 | .005 | 2.621 (1.344-5.112) |
| Pathologic grade (high vs low) | .009 | .231 | – |
| Pathologic stage (pT ⩾ 2 vs pT[a-is-1]) | <.001 | .003 | 3.621 (1.570-8.350) |
| Lymphovascular invasion (yes vs no) | .012 | .071 | – |
| Adjuvant chemotherapy (yes vs no) | .005 | .069 | – |
Abbreviations: CI, confidence interval; HR, hazard ratio; CSS, cancer-specific survival; FP, flank pain; GH, Gross hematuria; RNU, radical nephroureterectomy; UTUC, upper tract urothelial carcinoma. Statistically significant data (the P-value is less than 0.05) are indicated in bold.
Subgroup analyses
Previous study showed opposite prognostic effects between FP/symptomatic hydronephrosis and hematuria on prognosis.19 To remove the counteraction between the 2 variables and identify their respective prognostic values, we divided the patients into four subgroups (GH without FP, FP without GH, no FP and GH, and FP with GH). Ninety-three (52.0%), 20 (11.2%), 30 (16.8%), and 36 (20.1%) patients presented with GH without FP, FP without GH, no FP and GH, and FP with GH, respectively. The group “FP without GH” was significantly linked with ureteral cancer (Table 3, P = .031), pT ⩾ 3 (Table 3, P = .008), and LVI (Table 3, P = .016).
Table 3.
The Association between patients’ clinicopathological characteristics and “FP without GH.”
| Variable | Patients | FP without GH | GH without FP | P value |
|---|---|---|---|---|
| Sex, n (%) | .510 | |||
| Male | 66 | 13 (65.0) | 53 (57.0) | |
| Female | 47 | 7 (35.0) | 40 (43.0) | |
| Age, n (%) | .592 | |||
| <68 | 57 | 9 (45.0) | 48 (51.6) | |
| ⩾68 | 56 | 11 (55.0) | 45 (48.4) | |
| Body mass index, n (%) | .404 | |||
| <18.5 | 5 | 2 (10.0) | 3 (3.2) | |
| 18.5-24 | 56 | 9 (45.0) | 47 (50.5) | |
| ⩾24 | 52 | 9 (45.0) | 43 (46.2) | |
| Smoking history, n (%) | .858 | |||
| Yes | 21 | 4 (20.0) | 17 (18.3) | |
| No | 92 | 16 (80.0) | 76 (81.7) | |
| Herbal usage, n (%) | .689 | |||
| Yes | 8 | 1 (5.0) | 7 (7.5) | |
| No | 105 | 19 (95.0) | 86 (92.5) | |
| Hydronephrosis, n (%) | .074 | |||
| Yes | 77 | 17 (85.0) | 60 (64.5) | |
| No | 36 | 3 (15.0) | 33 (35.5) | |
| Tumor location, n (%) | .031 | |||
| Renal pelvis | 64 | 7 (35.0) | 57 (61.3) | |
| Ureter | 49 | 13 (65.0) | 36 (38.7) | |
| Tumor size, n (%) | .552 | |||
| ⩾3 cm | 61 | 12 (60.0) | 49 (52.7) | |
| <3 cm | 52 | 8 (40.0) | 44 (47.3) | |
| Multifocality, n (%) | .980 | |||
| Yes | 28 | 5 (25.0) | 23 (24.7) | |
| No | 85 | 15 (75.0) | 70 (75.3) | |
| Pathologic stage, n (%) | .008 | |||
| pT < 3 | 64 | 6 (30.0) | 58 (62.4) | |
| pT ⩾ 3 | 49 | 14 (70.0) | 35 (37.6) | |
| Pathologic grade, n (%) | .229 | |||
| Low | 15 | 1 (5.0) | 14 (15.1) | |
| High | 98 | 19 (95.0) | 79 (84.9) | |
| Lymph node status, n (%) | .151 | |||
| pN0 | 7 | 3 (15.0) | 4 (4.3) | |
| pNx | 103 | 17 (85.0) | 86 (92.5) | |
| pN+ | 3 | 0 (0.0) | 3 (3.2) | |
| Lymphovascular invasion, n (%) | .016 | |||
| Yes | 23 | 8 (40.0) | 15 (16.1) | |
| No | 90 | 12 (60.0) | 78 (83.9) |
Abbreviations: FP, flank pain; GH, Gross hematuria. Statistically significant data (the P-value is less than 0.05) are indicated in bold.
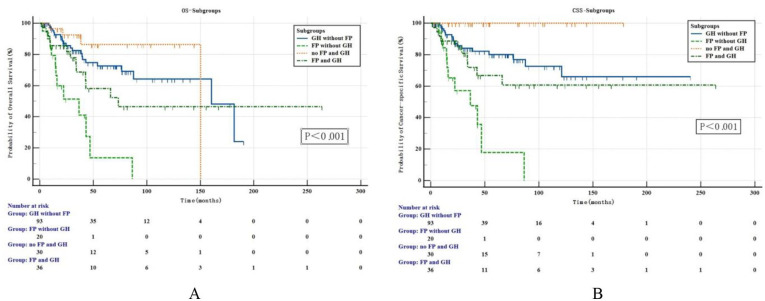
And we discovered that the subgroup “FP without GH” suffered the worst oncological outcomes, and its 5-year OS and CSS rates were 9.64% and 12.0%, which were significantly lower than 76.9% and 79.3% in subgroup “GH without FP,” 85.3% and 100.0% in subgroup “no FP and GH,” and 60.7% and 62.3% in subgroup “FP with GH,” the difference was statistically significant (OS: P < .001, P < .001, P = .012, respectively; CSS: P < .001, P < .001, P = .009, respectively, Figure 3).
Figure 3.
Kaplan-Meier analysis for OS (A) and CSS (B) in 179 patients with UTUC who divided into 4 subgroups by the presence/absence of gross hematuria (GH) and flank pain (FP). CSS indicates cancer-specific survival; FP, flank pain; GH, gross hematuria; OS, overall survival; UTUC, upper tract urothelial carcinoma.
On univariable analyses, the presence of FP without GH was significantly correlated with adverse CSS (P < .001) while the presence of no FP and GH was associated with favorable CSS (P = .012; Table 4). The Cox multivariate regression analysis revealed that the presence of FP without GH (HR = 5.495, 95% CI: 2.423-12.461, P < .001), tumor multifocality (HR = 2.740, 95% CI: 1.355-5.540, P = .005), and pathological stage (HR = 3.744, 95% CI: 1.529-9.168, P = .004) were independent prognostic factors for CSS in UTUC patients (Table 4). The risk factors for OS in the multivariate analysis were identical to those for CSS (Supplemental Table 3).
Table 4.
Univariable and multivariable Cox regression analyses predicting CSS in 179 patients who underwent RNU for UTUC.
| Univariable | Multivariable | ||
|---|---|---|---|
| P value | P value | HR (95% CI) | |
| Age (⩽68 vs >68) | .811 | – | – |
| Sex (male vs female) | .331 | – | – |
| Body mass index | .609 | – | – |
| ⩽18.5-24 vs <18.5 | .456 | – | – |
| ⩾24 vs <18.5 | .746 | – | – |
| Smoking history (yes vs no) | .390 | – | – |
| Herbal usage (yes vs no) | .469 | – | – |
| FP and GH | <.001 | – | – |
| FP without GH vs GH without FP | <.001 | <.001 | 5.495 (2.423-12.461) |
| no FP and GH vs GH without FP | .012 | – | – |
| FP with GH vs GH without FP | .208 | – | – |
| Hydronephrosis (yes vs no) | .054 | – | – |
| Tumor location (renal pelvis vs ureter) | .214 | – | – |
| Tumor size (⩾3 cm vs <3 cm) | .336 | – | – |
| Multifocality (yes vs no) | <.001 | .005 | 2.740 (1.355-5.540) |
| Pathologic grade (high vs low) | .009 | .377 | – |
| Pathologic stage (pT ⩾ 2 vs pT[a-is-1]) | <.001 | .004 | 3.744 (1.529-9.168) |
| Lymphovascular invasion (yes vs no) | .012 | .128 | – |
| Adjuvant chemotherapy (yes vs no) | .005 | .137 | – |
Abbreviations: CI, confidence interval; HR, hazard ratio; CSS, cancer-specific survival; FP, flank pain; GH, Gross hematuria; RNU, radical nephroureterectomy; UTUC, upper tract urothelial carcinoma. Statistically significant data (the P-value is less than 0.05) are indicated in bold.
Risk stratification model
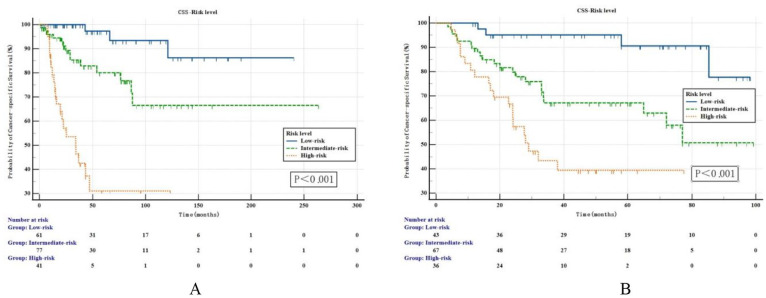
A risk stratification model for UTUC patients was developed based on the independent prognostic factors for CSS in subgroup analyses: patients without independent prognostic risk factor were categorized as the low-risk group; patients with one independent prognostic risk factor were categorized as the intermediate-risk group; patients with 2 or more independent prognostic risk factors were categorized as the high-risk group. The survival outcomes of the training set were shown in Figure 4A. The 3- and 5-year CSS rates were 97.7% and 94.4%, 83.5% and 79.0%, and 43.6% and 25.8% in the low-risk, intermediate-risk, and high-risk groups, respectively. The differences among the 3 groups were significant (P < .001). Similar results were observed in external validation cohort. The 3- and 5-year CSS rates were 92.8% and 89.7%, 66.7% and 63.5%, and 40.8% and 37.2% in the low-risk, intermediate-risk, and high-risk groups, respectively. The differences among the 3 groups were statistically significant based on the log-rank test (P < .001, Figure 4B).
Figure 4.
Kaplan-Meier estimates for CSS stratified by the risk level in the primary cohort (A) and validation cohort (B). CSS indicates cancer-specific survival.
Discussion
Assessing the prognosis preoperatively in patients with UTUC is quite a challenge. Researchers have focused mainly on hydronephrosis in the field of the predictive effect of clinical manifestations in UTUC, but the common symptoms of UTUC are hematuria23,24 and FP.20 Only few researchers have focused on the association between prognosis and hematuria/FP. For example, Inman et al23 revealed that FP or weight loss was a novel adverse predictor of survival in the preoperative model; Ataus et al25 classified the preoperative symptoms into 3 categories: “macroscopic hematuria,” “flank pain,” and “during the follow-up of bladder tumor.” The results showed that the survival time of the “flank pain” group was significantly shorter than the survival time of the other 2 groups. But only the tumor stage was an independent predictor in the Cox multivariate regression, and the authors explained that all patients in the FP group had high-grade tumors and all but one had T3/T4 tumors. However, similar findings were not found in our study. There were no significant differences in the proportion of high-grade and T3/T4 tumors between the FP group and non-FP group, except for hydronephrosis.
Patients with FP suffered a worse prognosis than those without FP, which is consistent with the conclusions of previous studies.23,25 There were no differences in OS and CSS between the GH group and non-GH group. After dividing the 179 patients into 4 subgroups, the subgroup “FP without GH” was significantly associated with adverse pathological characteristics and suffered a worst prognosis. Meanwhile, “FP without GH” was one of the independent predictors of CSS and OS besides the pathological stage and tumor multifocality. Moreover, the hazard ratio of cancer-specific death in people with FP was 2.951 times higher than those without FP. In the subgroup analysis, patients with “FP without GH” had a 5.495 times higher hazard ratio for cancer-specific death than patients with painless GH. Stratification of the chief complaint may help in screening populations with extreme risk of death. Finally, given that the risk stratification systems are mostly based on the clinicopathological characteristics of Western UTUC patients, we developed a simple risk stratification model suitable for Chinese based on the number of risk factors. To our knowledge, this is the first study that added complaint to risk stratification model in UTUC. Our model could help with counseling and identifying potential patients who would benefit from more intensive therapy or closer surveillance.
In this study, we explored the predictive effects of combination of 2 common and easily perceived presenting symptoms in UTUC on long-term survival. We found that the oncological outcomes of patients with “FP without GH” were poorest in all UTUC patients. There are 2 possible explanations: firstly, “FP without GH” probably means that the blockage was in very serious condition because tumor had invaded urinary tract widely at that time. They may present with FP due to ipsilateral hydronephrosis or nerve invasion, but not GH. Our data also showed the subgroup “FP without GH” had a higher percentage of hydronephrosis than the other 3 subgroups. Second, the presence of GH may have a warning effect. Actually, bleeding usually causes anxiety, while mild pain or discomfort may not always be taken seriously. Similarly, Yeh et al19 thought hematuria, which included both GH and MH, had a protective effect. Sadly, GH was not found to be associated with tumor stage and failed to act as a protective factor in our study. After removing the counteraction effect of FP on GH, we did not know whether painless GH was a protective factor, as it was used as a reference variable (because it is UTUC’s most prevalent symptom) in the Cox multivariate analysis. Therefore, further evidence is needed to determine the impact of GH on long-term prognosis.
Unexpectedly, the 5-year CSS rates of the “no FP and GH” subgroup reached 100%. These tumors were detected primarily by physical examination or auxiliary examination required after patient complaints that were not related to UTUC, highlighting the importance of routine physical examination for early diagnosis and treatment. People should be aware of the occurrence of lumbar/flank discomfort and request auxiliary examinations for the early discovery of the potential tumor. Doctors should also enhance awareness and alertness to avoid missed diagnosis in clinical practice.
Our data suggested that the presence of GH was significantly correlated with renal pelvis tumor, which was similar to what were reported in the 2 earlier studies.26,27 The later one26 also revealed that papillary tumors were more likely to develop GH than sassicular tumors because papillary tumor cells or surrounding tissues were easier to shed and bleed. Besides, hydronephrosis was associated with ureteral tumor (61.1% vs 38.9%, P < .001). Raman et al28 suggested that ureteral tumors were likely to be detected earlier because ureters are more prone to obstruction. In contrast, patients with ureteral tumors showed a tendency of muscle-invasive disease at diagnosis in our cohort (64.8% vs 50.5%, P = .054) and one likely theory was that plenty of patients had asymptomatic hydronephrosis. However, patients with pelvic tumors tended to seek treatment at the hospital for GH.
In terms of preoperative prognostic factors, there is no consensus on the relationship between tumor location, multifocality, and survival rates. We found that the tumor location was not an independent prognostic factor, which is in accordance with the findings of previous large-sample studies.28,29 However, a multicenter study which involves 609 patients concluded that ureteral and multifocal tumors displayed a poorer survival status when compared with renal pelvic tumors.15 They thought dividing multifocal tumors into renal pelvis and ureter tumors according to grade, stage, or the size of the tumors in previous studies could lead to bias. After stratifying pathological parameters, Tai et al13 found that there was no significant relationship between the location and prognosis for tumors ⩽pT2, while the long-term survival rates of pT3 ureteral tumors were significantly lower than pT3 renal pelvis tumors. Park et al30 speculated that renal parenchyma may have a protective effect on renal pelvis tumors. All in all, prospective randomized controlled trials on tumor location and multifocality are required.
This study has some limitations, such as its retrospective nature. In addition, in evaluating the relationship between hematuria and prognosis, we did not distinguish between MH and no hematuria because MH could not be observed by patients. Moreover, since only few patients underwent lymphadenectomy in our institution, the lymph node status was not included in the regression analysis. Finally, it was difficult to standardize the adjuvant chemotherapy dose schedule and operative approaches between the 2 medical institutions. So the results need to be validated by well-designed multi-institution studies.
Conclusion
Flank pain was significantly associated with poor oncological outcomes, but no differences were noted between GH and oncological outcomes. The presence of “flank pain without gross hematuria” was one of the independent risk factors of CSS and OS besides the pathological stage and tumor multifocality in patients with UTUC treated by RNU. Finally, we developed a simple risk stratification model based on the number of risk factors. To our knowledge, this is the first study that adds complaint to risk stratification model in UTUC.
Supplemental Material
Supplemental material, sj-docx-1-onc-10.1177_11795549221147993 for Predictive Value of Flank Pain and Gross Hematuria on Long-Term Survival in Patients With Upper Tract Urothelial Carcinoma Treated by Radical Nephroureterectomy by Ke Ning Sun, Jian Hong Wu, Zhi Hao Chen, Yi Jun He, Yi Ling Chen, Jin Zhong Hu and Lu Sheng in Clinical Medicine Insights: Oncology
Acknowledgments
The authors are grateful for the contribution of linguistic revision performed by Kexin Shi.
Footnotes
Author Contributions: KNS and JHW contributed equally to this work. All authors contributed to the study conception and design; data collection and analysis were performed by KNS, ZHC, YJH, YLC, and JZH. The draft of the manuscript was written by KNS and JHW. LS had primary responsibility for final content. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Commission of Shanghai Municipality (grant no. 18411960700) and Key Specialized Disease of Huadong Hospital (grant no. ZDZB2222).
Ethical Statement: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Huadong Hospital Affiliated to Fudan University (approval no: 20220040). This was a retrospective study, so informed consent was not required by the board.
ORCID iD: Lu Sheng  https://orcid.org/0000-0003-4520-6953
https://orcid.org/0000-0003-4520-6953
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Roupret M, Babjuk M, Burger M, et al. European Association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2021;79:62-79. doi: 10.1016/j.eururo.2020.05.042 [DOI] [PubMed] [Google Scholar]
- 2. Tufano A, Cordua N, Nardone V, et al. Prognostic significance of organ-specific metastases in patients with metastatic upper tract urothelial carcinoma. J Clin Med. 2022;11:5310. doi: 10.3390/jcm11185310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Doeveren T, van der Mark M, van Leeuwen PJ, Boormans JL, Aben KKH. Rising incidence rates and unaltered survival rates for primary upper urinary tract urothelial carcinoma: a Dutch population-based study from 1993 to 2017. BJU Int. 2021;128:343-351. doi: 10.1111/bju.15389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224-1233. doi: 10.1002/cncr.24135 [DOI] [PubMed] [Google Scholar]
- 5. Seisen T, Peyronnet B, Dominguez-Escrig JL, et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol. 2016;70:1052-1068. doi: 10.1016/j.eururo.2016.07.014 [DOI] [PubMed] [Google Scholar]
- 6. Brown GA, Matin SF, Busby JE, et al. Ability of clinical grade to predict final pathologic stage in upper urinary tract transitional cell carcinoma: implications for therapy. Urology. 2007;70:252-256. doi: 10.1016/j.urology.2007.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scolieri M, Paik M, Brown S, Resnick MJU. Limitations of computed tomography in the preoperative staging of upper tract urothelial carcinoma. Urology. 2000;56:930-934. doi: 10.1016/s0090-4295(00)00800-1 [DOI] [PubMed] [Google Scholar]
- 8. Smith AK, Stephenson AJ, Lane BR, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology. 2011;78:82-86. doi: 10.1016/j.urology.2011.02.038 [DOI] [PubMed] [Google Scholar]
- 9. Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100-114. doi: 10.1016/j.eururo.2012.02.030 [DOI] [PubMed] [Google Scholar]
- 10. Nazzani S, Mazzone E, Preisser F, et al. Rates of lymph node invasion and their impact on cancer specific mortality in upper urinary tract urothelial carcinoma. Eur J Surg Oncol. 2019;45:1238-1245. doi: 10.1016/j.ejso.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 11. Kim H, Jeong C, Kwak C, Kim H, Ku JJO. Association between demographic factors and prognosis in urothelial carcinoma of the upper urinary tract: a systematic review and meta-analysis. Oncotarget. 2017;8:7464-7476. doi: 10.18632/oncotarget.10708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yafi FA, Novara G, Shariat SF, et al. Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: a homogeneous series without perioperative chemotherapy. BJU Int. 2012;110:E7-E13. doi: 10.1111/j.1464-410X.2011.10792.x [DOI] [PubMed] [Google Scholar]
- 13. Tai YS, Chen CH, Huang CY, Tai HC, Wang SM, Pu YS. The effect of tumor location on oncologic outcomes in patients with upper urinary tract urothelial carcinoma stratified by pathologic stage. Urol Oncol. 2016;34:4.e19-4.e25. doi: 10.1016/j.urolonc.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 14. Foerster B, Abufaraj M, Mari A, et al. The performance of tumor size as risk stratification parameter in Upper Tract Urothelial Carcinoma (UTUC). Clin Genitourin Cancer. 2021;19:272.e1-272.e7. doi: 10.1016/j.clgc.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 15. Ouzzane A, Colin P, Xylinas E, et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol. 2011;60:1258-1265. doi: 10.1016/j.eururo.2011.05.049 [DOI] [PubMed] [Google Scholar]
- 16. Fukui T, Kanno T, Kobori G, Moroi S, Yamada H. Preoperative hydronephrosis as a predictor of postnephroureterectomy survival in patients with upper tract urothelial carcinoma: a two-center study in Japan. Int J Clin Oncol. 2019;25:456-463. doi: 10.1007/s10147-019-01535-6 [DOI] [PubMed] [Google Scholar]
- 17. Chung PH, Krabbe LM, Darwish OM, et al. Degree of hydronephrosis predicts adverse pathological features and worse oncologic outcomes in patients with high-grade urothelial carcinoma of the upper urinary tract. Urol Oncol. 2014;32:981-988. doi: 10.1016/j.urolonc.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 18. Bozzini G, Nison L, Colin P, et al. Influence of preoperative hydronephrosis on the outcome of urothelial carcinoma of the upper urinary tract after nephroureterectomy: the results from a multi-institutional French cohort. World J Urol. 2013;31:83-91. doi: 10.1007/s00345-012-0964-4 [DOI] [PubMed] [Google Scholar]
- 19. Yeh HC, Jan HC, Wu WJ, et al. Concurrent preoperative presence of hydronephrosis and flank pain independently predicts worse outcome of upper tract urothelial carcinoma. PLoS ONE. 2015;10:e0139624. doi: 10.1371/journal.pone.0139624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raman JD, Shariat SF, Karakiewicz PI, et al. Does preoperative symptom classification impact prognosis in patients with clinically localized upper-tract urothelial carcinoma managed by radical nephroureterectomy? Urol Oncol. 2011;29:716-723. doi: 10.1016/j.urolonc.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 21. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63:S240-S252. doi: 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 22. Rink M, Fajkovic H, Cha EK, et al. Death certificates are valid for the determination of cause of death in patients with upper and lower tract urothelial carcinoma. Eur Urol. 2012;61:854-855. doi: 10.1016/j.eururo.2011.12.055 [DOI] [PubMed] [Google Scholar]
- 23. Inman BA, Tran VT, Fradet Y, Lacombe L. Carcinoma of the upper urinary tract: predictors of survival and competing causes of mortality. Cancer. 2009;115:2853-2862. doi: 10.1002/cncr.24339 [DOI] [PubMed] [Google Scholar]
- 24. Cowan NC. CT urography for hematuria. Nat Rev Urol. 2012;9:218-226. doi: 10.1038/nrurol.2012.32 [DOI] [PubMed] [Google Scholar]
- 25. Ataus S, Onal B, Tunc B, et al. Factors affecting the survival of patients treated by standard nephroureterectomy for transitional cell carcinoma of the upper urinary tract. Int Urol Nephrol. 2006;38:9-13. doi: 10.1007/s11255-005-3151-3 [DOI] [PubMed] [Google Scholar]
- 26. Fang D, Gong YQ, Singla N, et al. The significance of the initial symptom in Chinese patients with upper tract urothelial carcinoma: regular health examination is still underutilized. Kaohsiung J Med Sci. 2018;34:511-521. doi: 10.1016/j.kjms.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Zhu Z, Zhong S, Xu T, Shen Z. Ureteral tumours showing a worse prognosis than renal pelvis tumours may be attributed to ureteral tumours more likely to have hydronephrosis and less likely to have haematuria. World J Urol. 2013;31:155-160. doi: 10.1007/s00345-012-0885-2 [DOI] [PubMed] [Google Scholar]
- 28. Raman JD, Ng CK, Scherr DS, et al. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol. 2010;57:1072-1079. doi: 10.1016/j.eururo.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 29. Isbarn H, Jeldres C, Shariat SF, et al. Location of the primary tumor is not an independent predictor of cancer specific mortality in patients with upper urinary tract urothelial carcinoma. J Urol. 2009;182:2177-2181. [DOI] [PubMed] [Google Scholar]
- 30. Park J, Ha SH, Min GE, et al. The protective role of renal parenchyma as a barrier to local tumor spread of upper tract transitional cell carcinoma and its impact on patient survival. J Urol. 2009;182:894-899. doi: 10.1016/j.juro.2009.05.040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-onc-10.1177_11795549221147993 for Predictive Value of Flank Pain and Gross Hematuria on Long-Term Survival in Patients With Upper Tract Urothelial Carcinoma Treated by Radical Nephroureterectomy by Ke Ning Sun, Jian Hong Wu, Zhi Hao Chen, Yi Jun He, Yi Ling Chen, Jin Zhong Hu and Lu Sheng in Clinical Medicine Insights: Oncology