Abstract
Objective
For the UK Biobank, standardized phenotype codes are associated with patients who have been hospitalized but are missing for many patients who have been treated exclusively in an outpatient setting. We describe a method for phenotype recognition that imputes phenotype codes for all UK Biobank participants.
Materials and Methods
POPDx (Population-based Objective Phenotyping by Deep Extrapolation) is a bilinear machine learning framework for simultaneously estimating the probabilities of 1538 phenotype codes. We extracted phenotypic and health-related information of 392 246 individuals from the UK Biobank for POPDx development and evaluation. A total of 12 803 ICD-10 diagnosis codes of the patients were converted to 1538 phecodes as gold standard labels. The POPDx framework was evaluated and compared to other available methods on automated multiphenotype recognition.
Results
POPDx can predict phenotypes that are rare or even unobserved in training. We demonstrate substantial improvement of automated multiphenotype recognition across 22 disease categories, and its application in identifying key epidemiological features associated with each phenotype.
Conclusions
POPDx helps provide well-defined cohorts for downstream studies. It is a general-purpose method that can be applied to other biobanks with diverse but incomplete data.
Keywords: AI in healthcare, deep learning, rare disease, UK Biobank, machine learning, patient phenotyping
BACKGROUND AND SIGNIFICANCE
Artificial intelligence (AI) allows machines to recognize patterns in electronic patient records (medical notes, laboratory tests, medications, and diagnosis codes). With increasing amounts of data available, machine learning algorithms have enabled healthcare applications, ranging from the detection of pneumonia in frontal chest X-ray images to the identification of heart failures in clinical notes.1,2 There have also been growing efforts to predict clinical events, that is, the automatic prediction of patient phenotypes with data-driven approaches.3,4 However, most studies have focused on a small number (<10) of disease diagnoses (eg, assessing the risks for cardiovascular diseases), and so their general utility is limited.5 Large-scale biobanks with genetic and phenotypic data are a vital source for studying a wide range of diseases. Cohort studies such as UK Biobank support broad multiphenotype research with a range of data including biological samples, physical measures, questionnaires related to sociodemographic conditions, lifestyle and health-related factors, and electronic medical records.6–8 Unfortunately, missing data are common. In the UK Biobank, many individuals who have been treated exclusively on an outpatient basis have missing phenotype labels. To maximize the utility of these data, large-scale patient phenotyping is necessary but expensive, time-consuming, and difficult. Currently, only a subset of conditions has available algorithms for the recognition of unlabeled phenotypes.9–11 These algorithms require extensive task-defined preprocessing and ad hoc feature engineering.7,8,11 A disease recognition system that recognizes multiple phenotypes would be helpful in defining patient cohorts for downstream studies. Recognizing rare phenotypes with small (or nonexistent) training data is a particular challenge, even in large biobanks.
Rare diseases affect about 3.5%–5.9% of people worldwide.12 While predictive models exist for common diseases using carefully curated datasets in sufficient volume to allow statistical characterization, detecting rare or unseen diseases remains difficult.13,14 There is currently no framework that evaluates individual patients for rare and common diseases in parallel. Rare diseases can be associated with noisier data because of inconsistent diagnostic criteria and clinician uncertainty.15 The phenotype-driven approaches to rare diseases therefore typically rely on difficult-to-assemble cohorts. For rare diseases, patient sample sizes follow a long-tailed class distribution. Conventional machine-learning methods typically perform better on the majority class and exhibit poor predictive accuracy on rare disease classes. In recent years, semisupervised and supervised methods have helped improve performance on imbalanced datasets, for example, single-cell annotations to classify cells into cell types and cell states present or absent in the training data.16,17 However, the techniques they employ have not been applied to multiphenotype recognition with heterogeneous patient data. We developed POPDx to associate patients with phenotypes for both common and rare phenotypes. It combines embedded representations of disease features with NLP-based encoding of the text and network-based embedding of the Human Disease Ontology to regularize the disease feature representation. We train POPDx with numerical and categorical data including health records, laboratory tests, individual demographics, lifestyles, and environmental exposures. We compile clinical profiles of 392 246 patients in UK Biobank6 and perform imbalanced learning with 1538 disease and health-related labels. Our phenotype recognition algorithm outperforms the state-of-the-art predictive models. It recognizes a comprehensive set of phenotypes, and makes the following contributions:
It manages missingness, noise, and high dimensionality typical in the electronic health record (EHR) data.
It scales to population-scale sets of patients and phenotypes.
It leverages the Human Disease Ontology to derive an integrated model for 1538 phenotypes that can recognize phenotypes even when there are few or no examples of these phenotypes in the training set.
MATERIALS AND METHODS
The POPDx framework leverages phecode embeddings that are constructed from the Human Disease Ontology covering all the diagnostic codes in UK Biobank and the textual descriptions of the phenotypes18–20 to achieve simultaneous recognition of multiphenotype that outperforms the state-of-the-art models. We assessed our embeddings by computing the dissimilarity of phenotypes within and outside of the disease category. The importance scores of 38 663 features were evaluated to aid the POPDx explainability.
UK Biobank cohort
We extracted phenotypic and health-related information from the UK Biobank including clinical assessments, lifestyle questionnaires, physical measurements, and electronic medical records. Among approximately 500 000 individuals from the UK Biobank dataset, 392 246 individuals have ICD-10 coded diagnosis information. We binned and applied one-hot encoding to numerical and categorical features, respectively, into 38 663 binary variables. For 1538 diagnostic labels, we map 12 803 International Classification of Diseases Tenth Revision (ICD-10) codes to 1538 phecodes.18–20 ICD billing codes are routinely used to identify patient cohorts from large observational datasets. Cases of multiple ICD codes are often accumulated to define the case or control status of a specific phenotype.20 The use of phecodes is a recognized strategy in clinical research that combines relevant ICD codes into meaningful phenotypes.21 For the repeatability of POPDx to be established, we leveraged a beta version of map from ICD-10 to phecode introduced by Wu et al18 which was validated based on about 84% coverage of the ICD-10 codes in the UK Biobank database. The entire dataset was split into training, validation, and test sets. Instead of dividing the data randomly, we split the individuals to allow experimental evaluation of unseen, rare, and common diseases. We generated a large multilabel patient dataset to contain phecodes that are present or absent in the training dataset. Some phecodes occur zero or a few times to simulate unseen and rare diseases while others are common.
The joint semantic and structure-based embeddings of phenotypes
We relied on both the textual information and hierarchical tree-structure of the phenotypes to compute the joint semantic and structure-based embeddings of phecodes. The phecode embeddings depend on embeddings of the ICD-10 codes which comprise them. We downloaded the hierarchical tree-structured representation of the ICD-10 data from the online showcase of UK Biobank resources. We use the hierarchical relation of the ICD-10 codes to construct an undirected network of phenotypes. There are 19 155 nodes in the network, corresponding to 19 155 codes. Edges are not weighted. We perform a shortest-path graph search and then compute the low-dimensional representation of each diagnostic code by using the singular value decomposition (SVD).22 We thus have a compressed, low-dimensional representation of each ICD-10 code based on the undirected disease network.
The text description of each ICD-10 code is a sentence or a short term that characterizes the meaning of the code. For example, ICD-10 code P29 is described as “Cardiovascular disorders originating in the perinatal period.” We use the pretrained version of the BioBERT23 model which has been widely adopted and very effective for biomedical text mining tasks, to extract a fixed vector of ICD-10 code based on this text. The BERT23,24 model breaks down the textual description of each ICD-10 code into tokens. Then, it adds a special classification token [CLS] at the beginning of each text. The 768-dimensional hidden state embedding of the “[CLS]” token from the last layer is used as the aggregate representation for the ICD-10 code. Finally, we merge the final 2 representations from NLP-mapping and network-based embeddings of disease terms. Given the ICD-10 codes and the vector representation of their textual description, we can calculate the embedding for each phecode by averaging the representations of the ICD-10 codes to which the phecode corresponds. We included 12 803 ICD-10 codes present in our UK Biobank dataset which map to 1538 phecode labels (Figure 1B, C).
Figure 1.
POPDx overview. (A) The flowchart of patient phenotyping in UK Biobank includes raw data extraction, data processing, POPDx development, and result evaluation. After extracted and preprocessed the raw data from UK Biobank, we obtained vectors of features for all patients as shown in the patient data table and a vector of diagnostic labels of phecodes for each patient. We developed POPDx to encode the patient features that are eventually used to perform phenotype recognition. The output of POPDx is a matrix of probabilities which represents the likelihoods of all phecodes for all the patients. We evaluated the accuracy of POPDx by AUROC and AUPRC. (B) The architecture of POPDx is a bilinear model that leverages an embedding matrix of 1538 phecodes. A total number of 38 663 features per patient were input into POPDx. The structure of POPDx includes an input layer, 2 hidden layers each with 150 and 1268 neurons, and a bilinear transformation through an embedding matrix of the phenotypes. The output layer is the probability distribution that this patient is assigned with each phecode label. The predicted labels are either 0 or 1 based on the decision threshold, illustrated as the vertical dashed line. (C) POPDx embeds the phenotypes into low-dimensional space. The hierarchical tree-structured representation of the phenotypes is utilized. For simplicity, we only show 4 categories of diseases. Even if the phecode does not have any patient examples in the training data (686: local infections of skin and subcutaneous tissue), POPDx can leverage its relation to other phecodes (172.11: melanomas of skin, 165.1: cancer of bronchus and lung) in the embedding space. Figure adapted from “Distribution of TRM Cells,” by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates.
Calculating phenotype dissimilarity
We compute 3 measures of phecode dissimilarity based on the disease ontology embedding, the embedding of the associated text, and both together. We measure the distance between embeddings with cosine distance. The distances are calculated as in-group (intra-) and out-group (inter-) distances. The cosine distances between a phenotype and those within the same disease category are considered in-group dissimilarity. The cosine distances between phenotypes of different disease categories are computed as out-group dissimilarity.
Simultaneous recognition of multiple-phenotypes
Our algorithm (Figure 1B) leverages the text description and ontological relationships of phenotypes to predict novel phenotypes (with no training examples) by relating them to clinically and contextually similar phenotypes. We use a bilinear model to predict the disease type for both seen and unseen phenotypes. Let P be an m by n matrix of input embedding of the patients, where m is the number of patients and n is the number of features. Let Y be an m by c label matrix, where c is the total number of phenotypes. Yij =1 if patient i has a diagnostic label of phenotype j, otherwise Yij=0. c is the total number of phecodes, and the majority of these phenotypes have fewer than 1000 examples in the training data. For example, when a patient is associated with 126 disease labels, the corresponding columns of diseases are ones while the others are all zeros in the label matrix. Let U be a c by h matrix of the low-dimensional representations of disease types, where h is 1268, the dimension of phenotype embedding space. We optimize the following binary cross-entropy loss:
where W1 Rn×q and W2 Rq×h are the parameters that need to be estimated, and q is set to be 150 through parameter tuning. POPDx optimizes the objective function by Adam optimizer. After the optimization, the likelihood of a diagnostic code j presented by a patient with a feature vector p is estimated as
where Lj is the probability that the phenotype j belongs to this patient. L= {L1, L2, …, L1538} is the probability distribution of diagnosis labels for a patient. We use Pytorch,25 Matplotlib,26 and Numpy27 for the experiments.
Feature importance analysis
Scoring the importance of individual features provides some interpretability for model predictions. We use DeepLIFT to compute the importance and relevance of 38 663 features on each of the 1538 phenotypes via a balanced selection of true-positive and true-negative cases. DeepLift is a backpropagation algorithm that measures the contribution of individual features on the output of a neural net for a specific input.28 It computes the differences between the activation of each neuron and their reference activation, where the “reference” is computed based on the selected negative samples. DeepLIFT highlights both positive (supportive) and negative (not supportive) influences on the prediction. The magnitude of the relevance value corresponds to its importance. We implemented DeepLIFT in the framework Captum.29 First, DeepLIFT scores are computed for each feature and for each patient. Then, for each phenotype, the DeepLIFT scores of all the true-positive patient data are averaged to obtain an importance score for each feature. We create a vector of feature importance scores for each phenotype.
RESULTS
Overview of POPDx
Figure 1A summarizes POPDx. First, the raw data are downloaded from UK Biobank. Second, the collected data are transformed into 38 663 patient features and 1538 associated phenotype codes. Third, we apply POPDx to recognize a diverse set of phenotypes, yielding a profile of phenotypes for each patient. Because the training data for many phenotypes are sparse, we introduce the use of ontological relationships to supplement the raw data. In particular, POPDx framework leverages disease ontological relationships (as represented in the Human Disease Ontology) embedded in a low-dimensional space and then projects the high-dimensional features of each patient to the same low-dimensional embedding space by a nonlinear transformation (Figure 1B, C). This has been used in other settings and has been shown to improve classification for classes with zero or few examples.16 The framework encodes the patient data through a bilinear framework with 2 hidden layers of POPDx architecture and a matrix transformation (Figure 1B). The resulting outputs denote the probabilities of each phenotype for each patient. POPDx is written in Python and is made available as an open-source package. Importantly, with a pretrained model, we can recognize 1538 disease phenotypes given an input patient matrix in a few minutes on a GPU.
392 246 individuals selected from UK Biobank cohort
In the UK Biobank, there are about 500 000 participants in total. 392 246 of these individuals have ICD-10 codes in their records. For these patients, we selected 219 604, 86 361, and 86 361 unique patients for training, validation, and testing respectively. The 3 sets have similar basic characteristics (Table 1). Fifty-six percent of individuals are women. The majority of participants are white. Elderly adults dominate the selected cohort with an average age of 71. We assessed 1538 phecode labels extracted from 12 803 ICD-10 codes from the cohort. Diagnostic labels have a long-tail distribution (Figure 2A): nearly 40% of these phecode labels have fewer than 100 positive patients (Figure 2C). Among 392 246 individuals, 377 612 people have fewer than 30 phenotypes (Figure 2B). We integrate 38 663 category-specific features and summarize them into 20 data subgroups as potential risk factors to aid our analysis (Supplementary Table S1).
Table 1.
Basic characteristics of the selected UK Biobank cohort
| Training set | Validation set | Test set | |
|---|---|---|---|
| (N = 219 604) | (N = 86 321) | (N = 86 321) | |
| Race, n (%) | |||
| White | 206 394 (93.985) | 81 646 (94.584) | 81 365 (94.259) |
| Mixed | 1304 (0.594) | 478 (0.553) | 497 (0.576) |
| Asian | 3489 (1.589) | 1394 (1.615) | 1364 (1.580) |
| Black | 4459 (2.030) | 1468 (1.701) | 1621 (1.878) |
| Chinese | 728 (0.332) | 159 (0.184) | 213 (0.247) |
| Other | 2046 (0.932) | 670 (0.776) | 747 (0.865) |
| Unknown | 690 (0.314) | 293 (0.339) | 325 (0.377) |
| Did not answer | 494 (0.225) | 213 (0.246) | 189 (0.219) |
| Sex, n (%) | |||
| Female | 122 353 (55.715) | 47 315 (54.813) | 47 786 (55.358) |
| Male | 97 251 (44.285) | 39 006 (45.187) | 38 535 (44.641) |
| Age, n (%) | |||
| <65 years | 58 988 (59.447) | 16 905 (59.472) | 19 680 (59.449) |
| ≥65 years | 160 616 (74.378) | 69 416 (75.381) | 66 641 (74.946) |
| All | 219 604 (70.367) | 86 321 (72.266) | 86 321 (71.413) |
Figure 2.
Diagnostic label statistics. (A) The UK Biobank has a long-tailed distribution of phecode labels. The x-axis is the number of patients per phecode label and y-axis is the number of labels. Most of the phecode labels have fewer than 1000 patients in the UK Biobank. (B) The patients in UK Biobank are associated with multiple phenotype labels. The x-axis is the groups of phecode counts and log-scale y-axis is the number of patients. The majority of the patients have fewer than 30 phecodes labels. (C) The phenotypes are categorized based on the number of training samples. The exploded pie chart shows the relative abundance of phecodes based on the number of patients in training.
Phecode embeddings reflect disease similarity
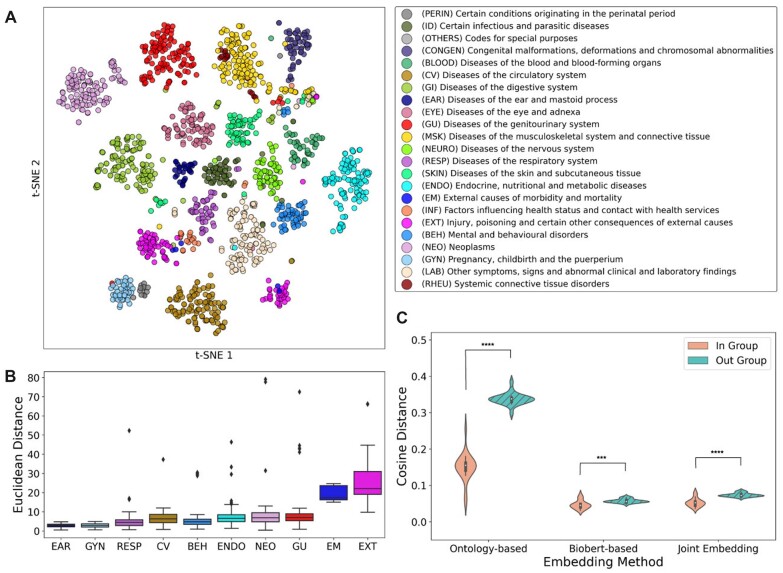
Since POPDx addresses rare and unobserved diagnostic codes based on their textual description and the ontological relationship to common diseases, its performance relies on high-quality embeddings of phenotypes. For that reason, we verified that diagnostic codes that are direct neighbors in the graph of Human Disease Ontology are also close in our low-dimensional embedding space. We compared 3 types of phenotype similarities: the disease ontology structure-based similarity, the text-based similarity, and joint semantic and structure-based similarity (Materials and Methods). We assessed the phenotype embeddings using direct neighborhood and nondirect neighborhood proximity. We first observe the average cosine distance of direct neighbors in the disease ontology graph is 0.15 ± 0.04, which is 69.70% higher than that of the text-based embeddings (0.05 ± 0.01), while the average cosine distance of k-hop neighbors (0.34 ± 0.02) in the disease ontology graph is 83.02% higher than those of the text-based embeddings (0.06 ± 0.01) (Figure 3B). The average cosine distances of joint embeddings in the same disease-type neighborhood and k-hop neighborhood are 0.05 ± 0.01 and 0.07 ± 0.01 respectively (Figure 3C). POPDx incorporates the joint semantic and topology-preserving embeddings under the principle that the unobserved or unseen phenotypes in training can borrow information from other disorders based on their shared characteristics.
Figure 3.
Phenotype embeddings. (A) The t-SNE plot shows the segregation of phenotypes into different disease categories using the joint structure and semantic embedding method. The legend associates a color to each phenotype category based on the hierarchical tree-structure of ICD-10. (B) The Euclidean distance of phecodes to the cluster center of each disease category in the t-SNE plot. (C) The similarity analysis of phenotype groups embedded with 3 different methods is presented as cosine differences within groups and between groups.
Dimension reduction via t-distributed stochastic neighbor embedding (t-SNE)30 on the joint semantic and structure-based embeddings reveals distinct disease groups (Figure 3A). The joint embeddings of phecodes via a biomedical domain-specific pretrained language model23 and canonical classification of the diagnoses present disjoint clusters in the low-dimensional embedding space (Figure 3A, Supplementary Figure S4A, B). Whereas diseases of most organ systems (Figure 3A, B) are independently clustered in latent space (for example, Diseases of the ear and mastoid process, Diseases of the eye and adnexa, Diseases of the circulatory system), some categories of diagnostic codes do not form a clear cluster but intermix (eg, External causes of morbidity and mortality, Injury, poisoning and certain other consequences of external causes). To quantify the similarity of the phecodes within and outside of the disease category, we measure the 2 types of dissimilarity of each disease class (Supplementary Figure S2). The phenotype codes for pregnancy, childbirth, and the puerperium have the highest similarity with a mean in-group cosine distance of 0.04 ± 0.01, in contrast with diseases of the musculoskeletal system and connective tissue that have the lowest in-group similarity with a mean in-group cosine distance of (0.08 ± 0.06). The relationships are visualized in the dendrograms (Supplementary Figure S3) which present the hierarchical relationship between our phenotypic embeddings for different disease categories. For example, phenotypes for the infectious and parasitic diseases (Supplementary Figure S3A) form correlated groupings in the topological space, such as 41.1 and 41.2 (staphylococcus and streptococcus infections).
Improved disease recognition for unseen, rare, and common phenotypes
To evaluate the performance of POPDx on different phenotypes, we categorize phenotypes according to the number of instances in the training set (Figure 4). Most diagnostic labels (98.6%) have fewer than 10 000 patient samples (Figure 2C). POPDx (Figure 4, Supplementary Figure S6) yields AUROC (Area Under the Receiver Operating Characteristic Curve) scores of 0.71 and 0.74 for phenotypes with 0-10 and 10-100 training samples. The AUROC and AUPRC (Area Under the Precision-Recall Curve) scores improve with training size. We also investigated performance for phenotypes with no patient sample in the training dataset. For these, POPDx achieves an AUROC score of 0.68 and an AUPRC score of 0.24, which are 74% and 218% higher than those of the logistic regression baseline. A sampling ratio of positive to negative patients of 1:10 is consistently used to report the AUROC and AUPRC of all the experiments.
Figure 4.
POPDx can recognize phenotypes that are not present in the training set. Bar plots comparing POPDx and other methods in terms of (A) AUROC and (B) AUPRC on the test set. POPDx presents competitive performance across all the groups of phenotypes.
Across different disease categories of phenotypes, we investigate how well POPDx outperforms the baseline of logistic regression based on AUROC and AUPRC scores (Figure 5A, B). We achieve an AUROC of 0.81 and an AUPRC of 0.37 with 131 phenotypes for Diseases of the circulatory system (Table 2). We outperform the random forest and logistic regression for increases in AUROC and AUPRC scores by 0.16 and 0.15. We compared POPDx with other strategies for phenotype embedding (Figure 4, Supplementary Figure S4). The joint semantic and structure-based embedding method achieves the best performance compared to NLP-based and ontology-based frameworks. Interestingly, for phenotypes that have fewer than 100 patient samples, the NLP-based and ontology-based frameworks improve the AUROC and AUPRC scores compared to those of random forest and logistic regression. To assess the ability of POPDx to work with even larger sets of phenotypes, we applied patient phenotyping across 12 803 ICD-10 diagnostic codes (almost 8 times more codes than with phecodes) with the same dataset. POPDx detects diagnostic labels for both rare and common codes with competitive AUROC scores (Supplementary Figure S5).
Figure 5.
POPDx improves disease recognition compared with logistic regression across 22 disease categories. (A) The AUROC scores for all the disease categories are substantially improved compared to the logistic regression (LR) baseline. The x-axis is the improvement of AUROC score by POPDx. The y-axis represents different disease categories. (B) The AUPRC scores for all the disease categories are substantially improved compared to the logistic regression (LR) baseline. The x-axis is the improvement of AUPRC score by POPDx. The y-axis represents different disease categories.
Table 2.
AUROC and AUPRC scores of different disease categories
| Disease category | AUROC |
AUPRC |
||
|---|---|---|---|---|
| (Abbrev.) | Mean | SD | Mean | SD |
| (PERIN) Certain conditions originating in the perinatal period | 0.6859 | 0.1669 | 0.2606 | 0.2253 |
| (ID) Certain infectious and parasitic diseases | 0.7215 | 0.1106 | 0.2793 | 0.1318 |
| (CONGEN) Congenital malformations, deformations, and chromosomal abnormalities | 0.6403 | 0.1686 | 0.1953 | 0.1156 |
| (BLOOD) Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 0.7520 | 0.0649 | 0.2851 | 0.1105 |
| (CV) Diseases of the circulatory system | 0.8141 | 0.0725 | 0.3695 | 0.1238 |
| (GI) Diseases of the digestive system | 0.7913 | 0.0887 | 0.3621 | 0.1495 |
| (EAR) Diseases of the ear and mastoid process | 0.7561 | 0.1007 | 0.3179 | 0.1598 |
| (EYE) Diseases of the eye and adnexa | 0.7642 | 0.0762 | 0.3132 | 0.1103 |
| (GU) Diseases of the genitourinary system | 0.7902 | 0.0765 | 0.3252 | 0.1177 |
| (MSK) Diseases of the musculoskeletal system and connective tissue | 0.7423 | 0.0982 | 0.2819 | 0.1285 |
| (NEURO) Diseases of the nervous system | 0.7560 | 0.0849 | 0.3232 | 0.1592 |
| (RESP) Diseases of the respiratory system | 0.8113 | 0.0700 | 0.3886 | 0.1434 |
| (SKIN) Diseases of the skin and subcutaneous tissue | 0.6995 | 0.1149 | 0.2439 | 0.1392 |
| (ENDO) Endocrine, nutritional, and metabolic diseases | 0.7633 | 0.1135 | 0.3234 | 0.1366 |
| External causes of morbidity and mortality | 0.7226 | 0.0979 | 0.2934 | 0.1053 |
| Factors influencing health status and contact with health services | 0.7591 | 0.0960 | 0.3077 | 0.1427 |
| (EXT) Injury, poisoning, and certain other consequences of external causes | 0.7378 | 0.0657 | 0.2684 | 0.0930 |
| (BEH) Mental and behavioral disorders | 0.7656 | 0.0859 | 0.3175 | 0.1452 |
| (NEO) Neoplasms | 0.7571 | 0.0826 | 0.3028 | 0.1208 |
| (GYN) Pregnancy, childbirth, and the puerperium | 0.9282 | 0.0856 | 0.6344 | 0.1879 |
| (LAB) Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified | 0.7375 | 0.0779 | 0.2609 | 0.0903 |
| (RHEU) Systemic connective tissue disorders | 0.7334 | 0.0915 | 0.2730 | 0.1231 |
Explaining output of POPDx
We obtained feature relevance scores over 1538 phenotypes using DeepLIFT (Materials and Methods). For individual predictions, we visualize the features with a polar plot where the radial values represent their contribution scores. Because of the high dimensionality of patient features in our dataset, all the 38 663 variables are organized into 20 data subgroups that summarize different categories of UK Biobank data (Supplementary Table S1). For the phenotype atherosclerosis (440.0 Diseases of arteries, arterioles, and capillaries), Figure 6A shows a polar chart for 3 true-positive patients, who share a similar pattern of feature importance. In particular, features from medical history, education and employment status, and physical measures are important. Figure 6B shows 5 categories of diseases for which we computed the max value of the importance scores of the selected data subgroups. Features in the lifestyle subgroup are critical for the recognition of endocrine, nutritional, and metabolic diseases. Patients’ medical history is important for the recognition of phenotypes originating in the perinatal period. For the external causes of morbidity and mortality, the features related to mental health status are essential.
Figure 6.
POPDx explainability across different disease categories. (A) Feature relevance profile for phenotype 440.0. The polar plots summarize feature importance for 3 true-positive patients with atherosclerosis. The polar plots on the right represent the zoomed-in region as highlighted in red to aid the eye. The legend associates a line color to a feature subgroup. Radial value in the polar plots is feature importance magnitude from DeepLIFT. All features are analyzed into different subgroups to show the consistency of the POPDx interpretability. (B) Additive contributions of individual features (medical history, education and employment, lifestyle, eye, physical measures, mental health shown here) to the outputs of POPDx. Each color is associated with a disease category which is consistent with the colors of disease categories in panel C. X-axis specifies the importance value of the corresponding feature subgroups (higher importance to the right). Y-axis is 5 disease categories sampled based on the median importance values from DeepLIFT. (C) A horizontal bar plot of the number of phecodes in the disease categories. The colors in (B) and (C) are consistently used to represent different categories of phenotypes.
DISCUSSION
As large databases of patient records become available for research, associating precise phenotypes with patients becomes critical. Our method comprehensively and simultaneously scores hundreds to thousands of phenotypes. Automated phenotyping of a patient, even for a single disease, faces 2 central challenges: variations in the syntax and semantics of health records (different electronic systems, lack of standards for interoperability among hospitals, etc.), and patient-to-patient variability in the clinical manifestations of the diseases.31,32 Most existing computational approaches to phenotype recognition are built by hand and model a small number of clinical, pathological, and laboratory attributes of patients.33,34 These methods do not easily generalize to cover the whole disease ontology.35,36 In addition, no methods using machine learning have been able to recognize phenotypes for which there are new or no training examples in the UK Biobank. Our integrated analysis allows us to make some progress in recognizing such examples.
The UK Biobank cohort is a long-tailed dataset with heavy class imbalance (Figure 2A). POPDx provides robust and scalable recognition of phenotypes; it performs quite well on common phenotypes for which there are many examples, and gracefully degrades its performance down to phenotypes with zero examples. The ROC curves (Supplementary Figure S6) display the trade-off between sensitivity and specificity and can be helpful when considering the cut-off threshold to identify the diseased cohorts. The AUPRC (Figure 4) is prevalence dependent and less optimistic when the phenotype prevalence is low. Our framework significantly improved the AUPRC for unseen and rare phenotypes (<10 cases in training) by 218% and 151% compared to the logistic regression model. If a clinical team wants to identify patients for a phenotype with very low prevalence, our model on average doubles the ability to find the positive cases in the UK Biobank. When high specificity is desired over sensitivity in a clinical setting, more expert filtering can be used to detect the false positives. Our model provides a better starting point for the clinicians. This is encouraging since POPDx makes rare disease imputation feasible. Our method takes advantage of the non-linear correlation structure of the patient features to assign multiple diagnostic labels. In contrast, conventional machine-learning methods such as random forest are unable to recognize phenotypes that are not present in the training dataset, inevitably limiting their applications in the recognition of new phenotypes. Table 2 shows that the mean and standard deviation (SD) of AUROC and AUPRC are similar for certain disease categories (eg, PERIN and CONGEN). This might imply that some of the phenotypes in those disease categories are close in the embedding space and exist on overlapping patients. In the future, we can explore other aspects of phenotyping such as disease comorbidity to explain specific patterns we have observed in this study.
Our framework incorporates structural knowledge of the disease ontology by embedding disease relationships in a low-dimensional space. The recognition of unseen and rare phenotypes is enabled by explicitly providing information about the network relationships of these phenotypes to others which are well-represented in our data set. This phenotype ontology embedding preserves proximity relations, so two representations of nearby phenotypes are embedded in similar locations. In addition to the disease ontology, our method leverages semantic information about phenotypes by including textual information (embedded by BioBERT23) that provides further context for the phenotype and its distance from other phenotypes. In addition to the widely used BioBERT, other BERT models trained on slightly different biomedical data such as PubMedBERT,37 BioClinicalBERT,38 and ClinicalBERT38 can also serve our objective well. The POPDx makes it simple to train with different types of phenotype embeddings. Either the NLP-based or ontology-based embedding provides meaningful correlations of the phenotypes which can be demonstrated by their competitive performance in recognizing uncommon conditions. The combination of structure-based representations from the disease ontology with the contextual embeddings of phenotype text descriptions provides complementary information. The t-SNE representations (Figure 3A) of the joint embeddings demonstrate that our method preserves the separations of major disease types.
In our study, we assume that the collected ICD-10 codes and the associated phecode membership can be reliable representations of the underlying health state of the individuals in the UK Biobank resource. The faithfulness of diagnosis code can be compromised by several sources of errors. The complexities of the ICD coding system and a short time available for clinicians to match the patients with all the ICD-10 codes may cause inappropriate coding and variations in judgments.39,40 Preferably, POPDx could be validated against a true “gold standard” manually contributed by the physicians. However, this is not practicable given the constraints inherent in the data source.
In clinical research, phenotype labels such as ICD-10 codes and phecodes enable an initial selection of patient cohorts.41 We anticipate that POPDx will allow researchers to assemble patient cohorts beyond ICD-10-based search strategy, addressing the challenges of rare diseases and incomprehensive recognition of common phenotypes. With reliable identification of patients with phenotypes, we can use the genotype information present in the UK Biobank to seek genetic associations. We can also use known genetic associations between phenotypes to add an additional element to our embedding to help with phenotype recognition (ie, a third element to our embedding in addition to the text and ontology structure). Our results demonstrate that our model’s DeepLIFT feature relevance scores28 can offer some insights to explain the assignment of 1538 phenotypes. Our results with DeepLIFT show promise but they may not provide sufficiently clear justification for the reasons a feature contributes to a phenotype. For example, chest pain felt outside physical activity has a high importance score for the phecode 335.0 (hereditary/degenerative nervous conditions). While this may be reasonable, the mechanistic connection between these concepts is not clear.
The algorithm is implemented in Python (https://github.com/luyang-ai4med/POPDx).
CONCLUSIONS
The POPDx framework was developed for multiphenotype recognition with heterogeneous patient data in the UK Biobank. Our model outperforms other existing methods on recognizing a comprehensive set of nonexistent, rare, and common phenotypes in training. While we demonstrate our framework on UK Biobank, the model can be applied to any biobank-related records.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Stanford sherlock cluster for high-performance computing (HPC) and access to the long-term data storage through the OAK research filesystem and compute nodes (GPUs and CPUs).
CONFLICT OF INTEREST STATEMENT
None declared.
Contributor Information
Lu Yang, Department of Bioengineering, Stanford University, Stanford, CA, USA.
Sheng Wang, Paul G. Allen School of Computer Science and Engineering, University of Washington, Seattle, WA, USA.
Russ B Altman, Department of Bioengineering, Stanford University, Stanford, CA, USA; Department of Genetics, Stanford University, Stanford, CA, USA; Department of Medicine, Stanford University, Stanford, CA, USA.
FUNDING
The National Institutes of Health grant number GM102365 and Chan-Zuckerberg initiative. Agilent and the Chan-Zuckerberg Biohub to LY.
AUTHOR CONTRIBUTIONS
LY extracted data, carried out the experiments, and conducted the analyses. LY, SW, and RBA conceived the original idea. RBA supervised the project. All authors revised and approved the final manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
DATA AVAILABILITY
This research has been conducted using data from UK Biobank, a major biomedical database. The data used are anonymized and available from the UK Biobank through approved access (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access).
REFERENCES
- 1. Rajpurkar P, Irvin J, Ball RL, et al. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med 2018; 15: e1002686. [DOI] [PMC free article] [PubMed]
- 2. Ford E, Carroll JA, Smith HE, et al. Extracting information from the text of electronic medical records to improve case detection: a systematic review. J Am Med Inform Assoc 2016; 23 (5): 1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol 2019; 103 (2): 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LaPierre N, Ju CJ-T, Zhou G, et al. MetaPheno: a critical evaluation of deep learning and machine learning in metagenome-based disease prediction. Methods 2019; 166: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krittanawong C, Zhang H, Wang Z, et al. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol 2017; 69 (21): 2657–64. [DOI] [PubMed] [Google Scholar]
- 6. Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018; 562 (7726): 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao C, Choi E, Sun J.. Opportunities and challenges in developing deep learning models using electronic health records data: a systematic review. J Am Med Inform Assoc 2018; 25 (10): 1419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shickel B, Tighe PJ, Bihorac A, et al. Deep EHR: a survey of recent advances in deep learning techniques for electronic health record (EHR) analysis. IEEE J Biomed Health Inform 2018; 22 (5): 1589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Zhao H, Zhang S, et al. A novel deep neural network model for multi-label chronic disease prediction. Front Genet 2019; 10: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tafa Z, Pervetica N, Karahoda B. An intelligent system for diabetes prediction. In: Proceedings of the 4th Mediterranean Conference on Embedded Computing (MECO); IEEE Explore; June 2015: 378–82; Budva, Montenegro. [Google Scholar]
- 11. Huang M-J, Chen M-Y, Lee S-C.. Integrating data mining with case-based reasoning for chronic diseases prognosis and diagnosis. Expert Syst Appl 2007; 32 (3): 856–67. [Google Scholar]
- 12. Nguengang Wakap S, Lambert DM, Olry A, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet 2020; 28 (2): 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaefer J, Lehne M, Schepers J, et al. The use of machine learning in rare diseases: a scoping review. Orphanet J Rare Dis 2020; 15 (1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horn W. AI in medicine on its way from knowledge-intensive to data-intensive systems. Artif Intell Med 2001; 23 (1): 5–12. [DOI] [PubMed] [Google Scholar]
- 15. Budych K, Helms TM, Schultz C.. How do patients with rare diseases experience the medical encounter? Exploring role behavior and its impact on patient–physician interaction. Health Policy 2012; 105 (2-3): 154–64. [DOI] [PubMed] [Google Scholar]
- 16. Wang S, Pisco AO, McGeever A, et al. Leveraging the cell ontology to classify unseen cell types. Nat Commun 2021; 12 (1): 5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brbić M, Zitnik M, Wang S, et al. MARS: discovering novel cell types across heterogeneous single-cell experiments. Nat Methods 2020; 17 (12): 1200–6. [DOI] [PubMed] [Google Scholar]
- 18. Wu P, Gifford A, Meng X, et al. Mapping ICD-10 and ICD-10-CM codes to phecodes: workflow development and initial evaluation. JMIR Med Inform 2019; 7 (4): e14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei W-Q, Bastarache LA, Carroll RJ, et al. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLoS One 2017; 12 (7): e0175508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denny JC, Bastarache L, Roden DM.. Phenome-wide association studies as a tool to advance precision medicine. Annu Rev Genomics Hum Genet 2016; 17: 353–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bastarache L. Using phecodes for research with the electronic health record: from PheWAS to PheRS. Annu Rev Biomed Data Sci 2021; 4: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wall ME, Rechtsteiner A, Rocha LM.. Singular value decomposition and principal component analysis. In: Berrar DP, Dubitzky W, Granzow M, eds. A Practical Approach to Microarray Data Analysis. Boston, MA: Springer US; 2003: 91–109. [Google Scholar]
- 23. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics 2020; 36 (4): 1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Devlin J, Chang M-W, Lee K, Toutanova K. BERT: pre-training of deep bidirectional transformers for language understanding. In: Proceedings of the 2019 Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies; June 3–5, 2019; Minneapolis, MN.
- 25. Paszke Gross AA, Massa SA, et al. Pytorch: an imperative style, high-performance deep learning library. Adv Neural Inform Process Syst 2019; 32. [Google Scholar]
- 26. Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng 2007; 9 (3): 90–5. [Google Scholar]
- 27. Van Der Walt S, Colbert SC, Varoquaux G.. The NumPy array: a structure for efficient numerical computation. Comput Sci Eng 2011; 13 (2): 22–30. [Google Scholar]
- 28. Shrikumar A, Greenside P, Kundaje A.. Learning important features through propagating activation differences. In: Precup D, Teh YW, eds. Proceedings of the 34th International Conference on Machine Learning. PMLR; 06–11 Aug 2017: 3145–53. [Google Scholar]
- 29. Model Interpretability for PyTorch using Captum. https://captum.ai/. Accessed September 10, 2022.
- 30. Van der Matten L, Hinton G.. Visualizing data using t-SNE. J Mach Learn Res 2008; 9: 2579–605. [Google Scholar]
- 31. Palmer AC, Sorger PK.. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell 2017; 171 (7): 1678–91.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Middleton B, Bloomrosen M, Dente MA, et al. ; American Medical Informatics Association. Enhancing patient safety and quality of care by improving the usability of electronic health record systems: recommendations from AMIA. J Am Med Inform Assoc 2013; 20 (e1): e2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saranya G, Pravin A.. A comprehensive study on disease risk predictions in machine learning. Int J Elect Comput Eng 2020; 10 (4): 4217. [Google Scholar]
- 34. Long E, Lin H, Liu Z, et al. An artificial intelligence platform for the multihospital collaborative management of congenital cataracts. Nat Biomed Eng 2017; 1 (2): 1–8. [Google Scholar]
- 35. Goh K-I, Cusick ME, Valle D, et al. The human disease network. Proc Natl Acad Sci USA 2007; 104 (21): 8685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schriml LM, Mitraka E, Munro J, et al. Human Disease Ontology 2018 update: classification, content and workflow expansion. Nucleic Acids Res 2019; 47 (D1): D955–D962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gu Y, Tinn R, Cheng H, et al. Domain-specific language model pretraining for biomedical natural language processing. ACM Trans Comput Healthcare 2022; 3 (1): 1–23. [Google Scholar]
- 38. Alsentzer E, Murphy J, Boag W, et al. Publicly available clinical BERT embeddings. In: Proceedings of the 2nd Clinical Natural Language Processing Workshop. Minneapolis, MN: Association for Computational Linguistics; 2019: 72–8. [Google Scholar]
- 39. McKay KM, Apostolopoulos N, Dahrouj M, et al. Assessing the uniformity of uveitis clinical concepts and associated ICD-10 codes across health care systems sharing the same electronic health records system. JAMA Ophthalmol 2021; 139 (8): 887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horsky J, Drucker EA, Ramelson HZ.. Accuracy and completeness of clinical coding using ICD-10 for ambulatory visits. AMIA Annu Symp Proc 2017; 2017: 912–20. [PMC free article] [PubMed] [Google Scholar]
- 41. Boyd AD, Li JJ, Kenost C, et al. Metrics and tools for consistent cohort discovery and financial analyses post-transition to ICD-10-CM. J Am Med Inform Assoc 2015; 22 (3): 730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research has been conducted using data from UK Biobank, a major biomedical database. The data used are anonymized and available from the UK Biobank through approved access (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access).