Abstract
Objective
To assess the prospective associations of circulating levels of omega 3 polyunsaturated fatty acid (n-3 PUFA) biomarkers (including plant derived α linolenic acid and seafood derived eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid) with incident chronic kidney disease (CKD).
Design
Pooled analysis.
Data sources
A consortium of 19 studies from 12 countries identified up to May 2020.
Study selection
Prospective studies with measured n-3 PUFA biomarker data and incident CKD based on estimated glomerular filtration rate.
Data extraction and synthesis
Each participating cohort conducted de novo analysis with prespecified and consistent exposures, outcomes, covariates, and models. The results were pooled across cohorts using inverse variance weighted meta-analysis.
Main outcome measures
Primary outcome of incident CKD was defined as new onset estimated glomerular filtration rate <60 mL/min/1.73 m2. In a sensitivity analysis, incident CKD was defined as new onset estimated glomerular filtration rate <60 mL/min/1.73 m2 and <75% of baseline rate.
Results
25 570 participants were included in the primary outcome analysis and 4944 (19.3%) developed incident CKD during follow-up (weighted median 11.3 years). In multivariable adjusted models, higher levels of total seafood n-3 PUFAs were associated with a lower incident CKD risk (relative risk per interquintile range 0.92, 95% confidence interval 0.86 to 0.98; P=0.009, I2=9.9%). In categorical analyses, participants with total seafood n-3 PUFA level in the highest fifth had 13% lower risk of incident CKD compared with those in the lowest fifth (0.87, 0.80 to 0.96; P=0.005, I2=0.0%). Plant derived α linolenic acid levels were not associated with incident CKD (1.00, 0.94 to 1.06; P=0.94, I2=5.8%). Similar results were obtained in the sensitivity analysis. The association appeared consistent across subgroups by age (≥60 v <60 years), estimated glomerular filtration rate (60-89 v ≥90 mL/min/1.73 m2), hypertension, diabetes, and coronary heart disease at baseline.
Conclusions
Higher seafood derived n-3 PUFA levels were associated with lower risk of incident CKD, although this association was not found for plant derived n-3 PUFAs. These results support a favourable role for seafood derived n-3 PUFAs in preventing CKD.
Introduction
Chronic kidney disease (CKD) affects about 700 million people worldwide, with an estimated global prevalence of one in 11 in the general population.1 2 Patients with CKD are at higher risk of cardiovascular disease and death3 4 because the condition could eventually progress to kidney failure that severely impacts health and quality of life.5 6 Therefore, there is a need to identify factors that might prevent the onset and progression of CKD.
The consumption of long chain omega 3 polyunsaturated fatty acids (n-3 PUFAs) that are mostly obtained from seafood (including eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA)) confers cardiometabolic benefits.7 Meta-analyses of randomised controlled trials have shown that increased n-3 PUFA intake improved arterial stiffness,8 lowered blood pressure,9 and reduced plasma triglycerides.10 11 Because endothelial dysfunction, hypertension, and dyslipidaemia are CKD risk factors,12 13 14 n-3 PUFAs could protect against the development of CKD.
However, there are important limitations to the observational research addressing this important theory. Firstly, most previous studies assessed self-reported intake of n-3 PUFAs using dietary questionnaires and evaluated associations with CKD in cross sectional analyses, with inconsistent associations with prevalent CKD.15 16 17 18 Only two longitudinal studies assessed self-reported intake of n-3 PUFAs and risk of incident CKD, and also reported inconsistent findings.18 19 Secondly, the use of self-reported n-3 PUFA intake is subject to errors related to misreporting and inaccuracy of food composition databases.20 Circulating n-3 PUFA levels are valid and reliable biomarkers of intake as shown by several randomised controlled trials.20 21 22 23 24 Moreover, biomarkers reflect the dietary intake and also the underlying metabolism, and therefore indicate the bioavailable n-3 PUFA intake; this might explain why n-3 PUFA biomarkers have been reported to be superior to self-reported n-3 PUFA intake in predicting outcomes such as cardiovascular disease and all cause mortality.25 Thirdly, self-reported intake could not distinguish accurately between individual n-3 PUFAs, which might differ in their biological properties and effects on CKD.26 Finally, studies evaluating the association between α linolenic acid (ALA) biomarker level and incident CKD are lacking, even though ALA is the major dietary n-3 PUFA obtained from specific plant foods and oils that has been associated with lower cardiovascular outcome risk.27 28
We pooled cohort studies conducted by different research teams to assess the association of n-3 PUFA biomarkers with incident CKD. This strategy allowed us to more precisely quantify the association with a larger sample size, distinguish between individual n-3 PUFAs, and avoid measurement errors related to self-reported dietary intake.
Methods
Study population
The study was conducted within the Fatty Acids and Outcomes Research Consortium (FORCE) (http://force.nutrition.tufts.edu), a consortium of studies with circulating or adipose tissue fatty acid biomarker measurements and ascertained chronic disease events.28 29 30 31 32 Studies were identified and study investigators were asked to participate based on previous FORCE projects, expert contacts, and literature searches. All prospective studies of adult participants with n-3 PUFA biomarkers measured in blood fractions or adipose tissue and with data on the primary outcome of incident CKD were eligible. A total of 50 potentially relevant studies were identified in May 2020, but 22 (44%) were not eligible because of lack of exposure or outcome data, and 5 (10%) studies were not included because of a limited number of patients with incident CKD (supplementary table S1). Of the remaining 23 cohorts, 19 (83%) participated in this project, while 4 (17%) declined. The study proposal and analytical plan were approved by FORCE, and all participating cohorts have ethics approval with appropriate data sharing agreements, which enabled the current project to be performed. Detailed cohort information is provided in the supplementary methods.
Measurement of n-3 PUFA biomarkers
Biomarker levels of n-3 PUFAs (ALA, EPA, DPA, and DHA) were measured by each cohort in plasma phospholipids, erythrocyte phospholipids, total plasma, total serum, cholesterol esters, or several lipid compartments using gas chromatography (supplementary methods). Individual fatty acid levels were expressed as the proportion of total fatty acids.
Outcome measurement
All outcomes were determined using estimated glomerular filtration rate (eGFR) at baseline and follow-up visits, with the same definitions in all the 19 participating cohorts. The primary outcome was incident CKD using the same definition as used by the Chronic Kidney Disease Genetics (CKDGen) Consortium.33 Briefly, incident CKD was defined as an eGFR that decreased to <60 mL/min/1.73 m2 during follow-up among participants with baseline eGFR≥60 mL/min/1.73 m2. eGFR was estimated using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation with calibrated serum or plasma creatinine.34 If creatinine levels were not calibrated and were obtained with a Jaffé assay before 2009, then data were corrected by multiplying creatinine levels by 0.95 before eGFR calculation, as described previously.35 A more stringent definition of incident CKD was used in a sensitivity analysis: both eGFR<60 mL/min/1.73 m2 and <75% of baseline eGFR during follow-up among participants with baseline eGFR≥60 mL/min/1.73 m2.36
We also assessed two secondary outcomes: annual absolute change in eGFR from baseline to the last follow-up as a continuous variable; and ≥40% decrease in eGFR from baseline to the last follow-up.37 Unlike the primary outcomes, analysis of the secondary outcomes comprised all participants, including those with baseline eGFR<60 mL/min/1.73 m2.
Cohort analyses
A prespecified and harmonised protocol with the same participant inclusion and exclusion criteria, exposures, outcomes, covariates, and analytical methods were sent to all cohorts to perform de novo analyses of individual data. For the outcomes of incident CKD and ≥40% decrease in eGFR, Cox proportional hazards models were used to estimate hazard ratios, considered to approximate relative risk, for cohorts with eGFR available in two or more follow-up visits. In cohorts with data available for only one follow-up eGFR, Poisson regression models were used to estimate relative risks. For the outcome of annual absolute change in eGFR from baseline to the last follow-up, multivariable linear regression was used. All analyses estimated robust standard errors.
Because n-3 PUFA biomarker levels were measured in different lipid compartments in the participating cohorts, they were evaluated as continuous variables using study specific interquintile range (difference between midpoint of lowest fifth (10th centile) and highest fifth (90th centile)).29 30 31 32 To assess potential nonlinear associations, n-3 PUFA levels were also evaluated as variables (in fifths) using study specific cut-off points. In the primary multivariable model (model 1), analyses were adjusted for age, sex, race, clinical centre or field site, education, occupation, body mass index, smoking, alcohol intake, physical activity, prevalent coronary heart disease, and use of lipid lowering drugs at baseline, when applicable. In model 2, additional covariates that could confound or mediate the association of n-3 PUFAs with incident CKD were considered, including prevalent diabetes mellitus, urine albumin-creatinine ratio, systolic blood pressure, and use of antihypertensive drugs at baseline (see supplementary methods for definitions of all covariates). Participants with missing data on n-3 PUFAs were excluded from the analysis. Missing data on covariates were handled according to the usual practice of each cohort and study investigators (see supplementary methods for cohort specific details).
In each study, a statistical interaction was evaluated by fitting the n-3 PUFA biomarker (continuous), each potential effect modifier, and their cross product. The prespecified effect modifiers included age (≥60 v <60 years), race, hypertension, diabetes, coronary heart disease, and eGFR (60-89 v ≥90 mL/min/1.73 m2) at baseline. These modifiers were selected by considering their potential biological relevance.
Data pooling and meta-analysis
Effect estimates from each participating cohort were provided to the lead author in standardised electronic forms and pooled using inverse variance weighted meta-analysis. Results from participating cohorts were pooled overall and within each specific type of lipid compartment. For studies with measures in multiple compartments, we prioritised the compartments in the overall pooled analysis on the basis of which best reflect long term intake, as prespecified in the following order: erythrocyte phospholipids, plasma phospholipids, cholesterol esters, and total plasma or serum.22 In sensitivity analyses, the overall pooled analyses were performed after excluding data from one cohort at a time or using alternative biomarker compartments. To assess potential nonlinear relations, exploratory analysis was conducted to model the association within each compartment using meta-regression with restricted cubic splines constructed from variables (in fifths) using study specific cut-off points.28 29 30 31 32 38 Interaction terms were pooled using inverse variance weighted meta-regression with Bonferroni correction for multiple testing, and such analysis was considered exploratory. All meta-analyses were performed using Stata (version 16.0) or R (version 4.1.0), with two tailed P<0.05 considered statistically significant.
Patient and public involvement
Patients and members of the public were not directly involved in this research owing to lack of funding, staff, and infrastructure to facilitate their involvement in this pooled analysis of data from 19 participating cohorts.
Results
Study cohorts
The primary outcome analysis included 25 570 participants without prevalent CKD from 19 cohorts in a total of 12 countries (table 1). Among the 19 cohorts, the mean age of participants ranged from 49 to 77 years and the mean body mass index from 23.2 to 28.3. The weighted median follow-up duration was 11.3 years. Sixteen cohorts recruited men and women, and most (n=15) recruited predominantly white participants (supplementary table S2). The mean baseline eGFR ranged from 76.1 to 99.8 mL/min/1.73 m2 (supplementary table S3), and the mean annual change in eGFR ranged from −6.2 to 0.3 mL/min/1.73 m2/year. In total, 4944 participants (19.3%) developed the primary outcome of incident CKD during follow-up (supplementary table S4).
Table 1.
Baseline characteristics of 19 prospective cohorts that participated in analysis of association of n-3 PUFA biomarkers with incident chronic kidney disease (as part of FORCE Consortium)
| Study | Country | Study design | Sample size (n) | Median follow-up (years) | Age, years (mean±SD) | Sex (% female) | BMI (mean±SD) | Biomarker measurement | |
|---|---|---|---|---|---|---|---|---|---|
| Lipid compartment | Year of sampling | ||||||||
| AOC | Netherlands | Cohort of patients with myocardial infarction | 2026 | 3.4 | 68.9±5.4 | 20.6 | 27.7±3.6 | Cholesterol esters | 2002-2006 |
| ARIC | USA | Population based cohort of middle aged adults | 3526 | 25.8 | 53.9±5.6 | 47.7 | 27.0±4.6 | Plasma phospholipids | 1987-1989 |
| CCCC | Taiwan | Population based cohort | 1074 | 1.0 | 56.8±9.7 | 42.1 | 23.9±3.2 | Total serum | 1990-1991 |
| CHS | USA | Population based cohort of older adults | 1608 | 5.6 | 73.3±4.2 | 63.0 | 26.7±4.5 | Plasma phospholipids | 1992-1993 |
| EPIC-Norfolk | United Kingdom | Population based cohort | 926 | 13.8 | 59.2±7.5 | 50.2 | 26.0±3.5 | Plasma phospholipids | 1993–1997 |
| EPIC-Potsdam | Germany | Population based cohort | 253 | 19.6 | 49.0±7.9 | 56.1 | 25.3±3.4 | Erythrocyte phospholipids | 2008 |
| FDPS | Finland | Randomised trial cohort of patients with impaired glucose tolerance at baseline | 355 | 4.0 | 55.1±7.2 | 67.5 | 31.0±4.7 | Total serum | 1993-1998 |
| FHS | USA | Population based cohort | 1895 | 5.8 | 54.1±8.1 | 50.3 | 28.3±5.4 | Erythrocyte phospholipids | 2008 |
| Hisayama | Japan | Population based cohort | 2713 | 10.0 | 60.2±11.4 | 58.4 | 23.2±3.4 | Total serum | 2002-2003 |
| InCHIANTI | Italy | Population based cohort | 830 | 9.1 | 64.7±15.3 | 52.2 | 27.1±0.3 | Total plasma | 1998-2000 |
| KIHD | Finland | Population based cohort of men | 1082 | 20.0 | 52.0±5.2 | 0.0 | 26.7±3.2 | Total serum | 1991 |
| MAS | Australia | Population based cohort of older adults without dementia at baseline | 395 | 6.0 | 77.0±4.3 | 51.9 | 27.1±4.2 | Total plasma | 2005-2006 |
| MESA | USA | Population based cohort | 1682 | 9.5 | 58.7±9.1 | 53.3 | 27.8±5.4 | Plasma phospholipids | 2000-2002 |
| METSIM | Finland | Population based cohort of men | 1152 | 4.8 | 55.0±5.6 | 0.0 | 26.3±3.4 | Erythrocyte phospholipids, plasma phospholipids, cholesterol esters | 2006-2010 |
| NHAPC | China | Population based cohort | 2046 | 6.0 | 58.0±5.9 | 57.6 | 24.5±3.6 | Erythrocyte phospholipids | 2005 |
| PIVUS | Sweden | Population based cohort of older adults | 893 | 9.9 | 70.2±0.2 | 47.6 | 26.9±4.1 | Plasma phospholipids | 2001 |
| ULSAM | Sweden | Population based cohort of men | 1055 | 21.3 | 49.6±0.6 | 0.0 | 25.0±4.4 | Cholesterol esters | 1970-1973 |
| WHIMS | USA | Randomised trial cohort of older postmenopausal women | 1686 | 15.3 | 69.2±3.4 | 100.0 | 28.3±5.5 | Erythrocyte phospholipids | 1995 |
| 3C | France | Population based cohort | 373 | 4.2 | 76.1±4.6 | 62.7 | 26.1±4.0 | Erythrocyte phospholipids, total plasma* | 1999-2001 |
AOC=Alpha Omega Cohort; ARIC=Atherosclerosis Risk in Communities Study; BMI=body mass index; CCCC=Chin-Shan Community Cardiovascular Cohort Study; CHS=Cardiovascular Health Study; EPIC-Norfolk=European Prospective Investigation into Cancer and Nutrition (Norfolk); EPIC-Potsdam=European Prospective Investigation into Cancer and Nutrition (Potsdam); FDPS=Finnish Diabetes Prevention Study; FHS=Framingham Heart Study; FORCE=Fatty Acids and Outcomes Research Consortium; InCHIANTI=Invecchiare in Chianti Study; KIHD=Kuopio Ischaemic Heart Disease Risk Factor Study; MAS=Memory and Ageing Study; MESA=Multi-Ethnic Study of Atherosclerosis; METSIM=Metabolic Syndrome in Men; n-3 PUFA=omega 3 polyunsaturated fatty acid; NHAPC=Nutrition and Health of Ageing Populations in China; PIVUS=Prospective Investigation of the Vasculature in Uppsala Seniors; SD=standard deviation; ULSAM=Uppsala Longitudinal Study of Adult Men; WHIMS=Women’s Health Initiative Memory Study; 3C=Three City Study.
Data shown are for 373 participants with n-3 PUFA measurement in erythrocyte phospholipids, which were used in overall pooled analysis. Total plasma n-3 PUFA biomarkers were also measured in larger sample of 1019 participants.
Most of the participating cohorts measured fatty acid levels in erythrocyte or plasma phospholipids (n=11), followed by total plasma or serum (n=7), and cholesterol esters (n=3; table 1). The proportion of total fatty acids for each n-3 PUFA varied between different lipid compartments (supplementary table S5; supplementary fig S1).
Seafood n-3 PUFAs and incident CKD
Higher levels of total seafood derived n-3 PUFAs were associated with 8% lower risk of incident CKD per interquintile range with low heterogeneity in the observed associations between cohorts (relative risk 0.92, 95% confidence interval 0.86 to 0.98; P=0.009, I2=9.9%; table 2 and fig 1). Findings were not appreciably altered by further adjustment for covariates that could be potential confounders or mediators (0.91, 0.85 to 0.97; P=0.006, I2=17.2%). Similar results were obtained when the n-3 PUFAs were assessed categorically—participants with total seafood n-3 PUFA level in the highest fifth had 13% lower risk of incident CKD than those in the lowest fifth (0.87, 0.80 to 0.96; P=0.005, I2=0.0%; table 2 and supplementary fig S2). Protective associations were found for the individual seafood n-3 PUFAs (table 2), and associations appeared consistent across lipid compartments (fig 1; supplementary table S6). Little evidence could be found of nonlinear associations between total seafood n-3 PUFAs and incident CKD in any of the biomarker compartments (supplementary fig S3).
Table 2.
Association of seafood n-3 PUFA biomarkers with primary outcome of incident CKD
| n-3 PUFA biomarker (No of studies; participants with incident CKD)* | Model† | Per interquintile range | Highest fifth v lowest fifth | |||
|---|---|---|---|---|---|---|
| Relative risk (95% CI) | I2 (%) | Relative risk (95% CI) | I2 (%) | |||
| EPA (19; 4940) | 1 | 0.94 (0.89 to 1.00) | 0.0 | 0.92 (0.84 to 1.00) | 0.0 | |
| 2 | 0.94 (0.88 to 0.99) | 0.0 | 0.91 (0.83 to 0.99) | 7.0 | ||
| DPA (16; 4350) | 1 | 0.94 (0.88 to 1.00) | 0.0 | 0.89 (0.81 to 0.98) | 0.0 | |
| 2 | 0.94 (0.80 to 1.01) | 0.0 | 0.90 (0.82 to 0.99) | 0.0 | ||
| DHA (19; 4944) | 1 | 0.93 (0.87 to 1.00) | 27.0 | 0.89 (0.81 to 0.97) | 0.0 | |
| 2 | 0.93 (0.87 to 0.99) | 30.1 | 0.89 (0.81 to 0.97) | 8.4 | ||
| EPA+DPA+DHA‡ (19; 4939) | 1 | 0.92 (0.86 to 0.98) | 9.9 | 0.87 (0.80 to 0.96) | 0.0 | |
| 2 | 0.91 (0.85 to 0.97) | 17.2 | 0.88 (0.80 to 0.96) | 0.0 | ||
| ALA (19; 4940) | 1 | 1.00 (0.94 to 1.06) | 5.8 | 0.98 (0.89 to 1.07) | 0.0 | |
| 2 | 0.99 (0.93 to 1.05) | 0.0 | 0.97 (0.88 to 1.06) | 0.0 | ||
Effect estimates were pooled using inverse variance weighted meta-analysis.
ALA=α linolenic acid; CKD=chronic kidney disease; DHA=docosahexaenoic acid; DPA=docosapentaenoic acid; EPA=eicosapentaenoic acid; n-3 PUFA=omega 3 polyunsaturated fatty acid.
Small difference in number of participants with incident CKD was due to missing measurement for specific n-3 PUFA fatty acids in some cohorts.
Model 1 adjusted for age, sex, race, clinical centre or field site, education, occupation, body mass index, smoking, alcohol intake, physical activity, prevalent coronary heart disease, and use of lipid lowering drugs, when applicable. Model 2 adjusted for all covariates in model 1 and also adjusted for prevalent diabetes mellitus, urine albumin-creatinine ratio, systolic blood pressure, use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and use of other antihypertensive drugs.
DPA was not available in three of the cohorts (CCCC—Chin-Shan Community Cardiovascular Cohort, InCHIANTI—Invecchiare in Chianti Study, and ULSAM—Uppsala Longitudinal Study of Adult Men), therefore the sum in these cohorts was calculated as EPA+DHA.
Fig 1.
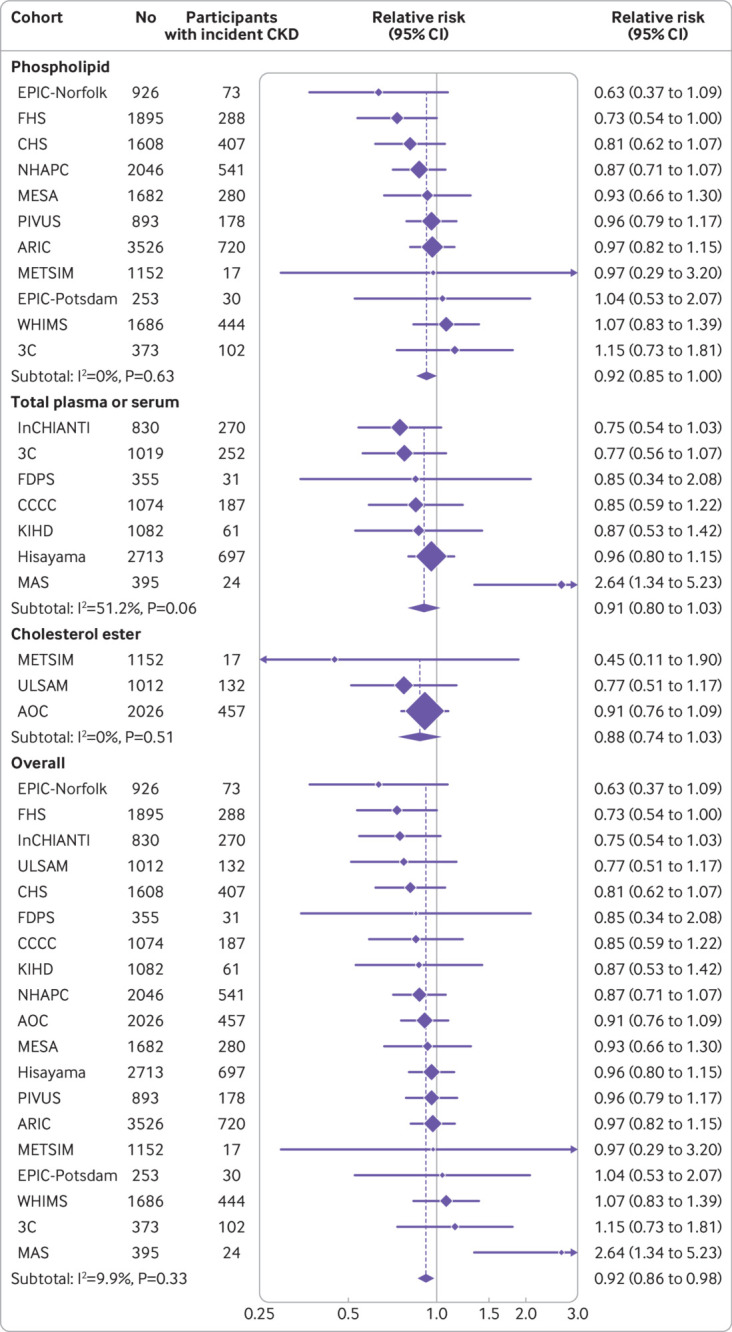
Association of total seafood n-3 PUFAs (eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid) with incident CKD. Incident CKD was defined as an eGFR<60 mL/min/1.73 m2 during follow-up among participants with baseline eGFR≥60 mL/min/1.73 m2. Analyses were adjusted for age, sex, race, field centre if applicable, education, occupation, body mass index, smoking, physical activity, alcohol intake, prevalent coronary heart disease, and use of lipid lowering drugs. Study specific estimates per interquintile range (difference between midpoint of lowest fifth and highest fifth) of total n-3 PUFAs were pooled separately for different lipid compartments and overall. AOC=Alpha Omega Cohort; ARIC=Atherosclerosis Risk in Communities Study; CCCC=Chin-Shan Community Cardiovascular Cohort Study; CHS=Cardiovascular Health Study; CKD=chronic kidney disease; eGFR=estimated glomerular filtration rate; EPIC-Norfolk=European Prospective Investigation into Cancer and Nutrition (Norfolk); EPIC-Potsdam=European Prospective Investigation into Cancer and Nutrition (Potsdam); FDPS=Finnish Diabetes Prevention Study; FHS=Framingham Heart Study; InCHIANTI=Invecchiare in Chianti Study; KIHD=Kuopio Ischaemic Heart Disease Risk Factor Study; MAS=Memory and Ageing Study; MESA=Multi-Ethnic Study of Atherosclerosis; METSIM=Metabolic Syndrome in Men; n-3 PUFA=omega 3 polyunsaturated fatty acid; NHAPC=Nutrition and Health of Ageing Populations in China; PIVUS=Prospective Investigation of the Vasculature in Uppsala Seniors; WHIMS=Women’s Health Initiative Memory Study; ULSAM=Uppsala Longitudinal Study of Adult Men; 3C=Three City Study
When incident CKD was defined using a more stringent definition (new onset of eGFR<60 mL/min/1.73 m2 and <75% of baseline eGFR) in a sensitivity analysis, the results were largely consistent with the primary outcome analysis. Higher levels of total seafood n-3 PUFAs were associated with a lower risk of incident CKD (0.91, 0.84 to 0.99 per interquintile range; P=0.009, I2=27.3%; supplementary table S7). In additional sensitivity analyses, the association of total seafood n-3 PUFAs with incident CKD remained significant after excluding data from one cohort at a time, or using alternative biomarker compartments in the overall pooled results (supplementary table S8).
Seafood n-3 PUFAs and secondary outcomes
Total seafood n-3 PUFA level was not associated with the outcome of ≥40% decrease in eGFR in continuous analyses, but was associated with a lower risk (about 15%) for those in the highest fifth versus those in the lowest fifth (relative risk 0.85, 95% confidence interval 0.74 to 0.98; P=0.03, I2=44.1%; table 3).
Table 3.
Association of seafood n-3 PUFA biomarkers with secondary outcome of ≥40% decrease in eGFR from baseline
| n-3 PUFA biomarker (No of studies; participants with ≥40% decrease in eGFR)* | Model† | Per interquintile range | Highest fifth v lowest fifth | ||||
|---|---|---|---|---|---|---|---|
| Relative risk (95% CI) | I2 (%) | Relative risk (95% CI) | I2 (%) | ||||
| EPA (16; 2554) | 1 | 0.99 (0.91 to 1.07) | 0.0 | 0.85 (0.74 to 0.98) | 19.5 | ||
| 2 | 0.98 (0.91 to 1.06) | 7.6 | 0.84 (0.73 to 0.97) | 26.1 | |||
| DPA (13; 2110)) | 1 | 0.92 (0.82 to 1.02) | 42.5 | 0.85 (0.73 to 0.98) | 21.8 | ||
| 2 | 0.92 (0.82 to 1.03) | 42.1 | 0.86 (0.74 to 1.00) | 33.7 | |||
| DHA (16; 2554) | 1 | 0.93 (0.84 to 1.03) | 52.2 | 0.88 (0.76 to 1.01) | 46.2 | ||
| 2 | 0.93 (0.83 to 1.03) | 54.3 | 0.88 (0.76 to 1.02) | 47.1 | |||
| EPA+DPA+DHA‡ (16; 2552) | 1 | 0.95 (0.86 to 1.04) | 45.2 | 0.85 (0.74 to 0.98) | 44.1 | ||
| 2 | 0.94 (0.85 to 1.03) | 46.4 | 0.85 (0.74 to 0.98) | 45.3 | |||
| ALA (16; 2553) | 1 | 0.98 (0.89 to 1.07) | 35.8 | 0.97 (0.85 to 1.12) | 7.1 | ||
| 2 | 0.94 (0.86 to 1.04) | 18.9 | 0.95 (0.82 to 1.09) | 0.0 | |||
Effect estimates were pooled using inverse variance weighted meta-analysis.
ALA=α linolenic acid; CKD=chronic kidney disease; DHA=docosahexaenoic acid; DPA=docosapentaenoic acid; eGFR=estimated glomerular filtration rate; EPA=eicosapentaenoic acid; n-3 PUFA=omega 3 polyunsaturated fatty acid.
Number of cohorts contributing to this analysis was lower than the primary outcome because three cohorts were excluded due to low number of participants with ≥40% decrease in eGFR (FDPS—Finnish Diabetes Prevention Study, MAS—Memory and Ageing Study, and METSIM—Metabolic Syndrome in Men). The small difference in number of participants with ≥40% decrease in eGFR was due to missing measurement for specific n-3 PUFA fatty acids in some cohorts.
Model 1 adjusted for age, sex, race, clinical centre or field site, education, occupation, body mass index, smoking, alcohol intake, physical activity, prevalent coronary heart disease, and use of lipid lowering drugs, when applicable. Model 2 adjusted for all covariates in model 1 and also adjusted for prevalent diabetes mellitus, urine albumin-creatinine ratio, systolic blood pressure, use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and use of other antihypertensive drugs.
DPA was not available in three of the cohorts (CCCC—Chin-Shan Community Cardiovascular Cohort, InCHIANTI—Invecchiare in Chianti Study, and ULSAM—Uppsala Longitudinal Study of Adult Men), therefore the sum in these cohorts was calculated as EPA+DHA.
Higher levels of total seafood n-3 PUFAs, especially DHA, were associated with a slower annual decline in eGFR (table 4). For instance, the annual decline in eGFR was 0.07 mL/min/1.73 m2 lower (95% confidence interval 0.02 to 0.13; P=0.007, I2=42.2%) for people with total seafood n-3 PUFA level in the highest fifth than those in the lowest fifth.
Table 4.
Association of seafood n-3 PUFA biomarkers with secondary outcome of annual change in eGFR
| n-3 PUFA biomarker (No of studies; total participants)* | Model† | Per interquintile range | Highest fifth v lowest fifth | |||
|---|---|---|---|---|---|---|
| Mean difference (95% CI) | I2 (%) | Mean difference (95% CI) | I2 (%) | |||
| EPA (19; 28 804) | 1 | 0.02 (−0.01 to 0.05) | 26.6 | 0.03 (−0.02 to 0.09) | 37.2 | |
| 2 | 0.02 (−0.01 to 0.05) | 28.3 | 0.04 (−0.01 to 0.09) | 37.0 | ||
| DPA (16; 25 102) | 1 | 0.03 (−0.02 to 0.07) | 46.4 | 0.03 (−0.03 to 0.09) | 7.8 | |
| 2 | 0.02 (−0.02 to 0.06) | 38.8 | 0.02 (−0.04 to 0.08) | 2.6 | ||
| DHA (19; 28 837) | 1 | 0.05 (0.01 to 0.09) | 36.6 | 0.08 (0.02 to 0.13) | 32.9 | |
| 2 | 0.05 (0.01 to 0.08) | 33.0 | 0.07 (0.01 to 0.12) | 27.9 | ||
| EPA+DPA+DHA‡ (19; 28 798) | 1 | 0.04 (0.01 to 0.08) | 32.0 | 0.07 (0.02 to 0.13) | 42.2 | |
| 2 | 0.04 (0.00 to 0.07) | 29.8 | 0.07 (0.01 to 0.12) | 36.2 | ||
| ALA (19; 28 826) | 1 | −0.03 (−0.06 to 0.00) | 24.1 | −0.05 (−0.10 to 0.01) | 15.1 | |
| 2 | −0.03 (−0.06 to 0.01) | 20.3 | −0.05 (−0.10 to 0.01) | 3.4 | ||
Data shown are adjusted mean difference (95% CI) in the change of eGFR (mL/min/1.73 m2) per year. Effect estimates were pooled using inverse variance weighted meta-analysis.
ALA=α linolenic acid; DHA=docosahexaenoic acid; DPA=docosapentaenoic acid; eGFR=estimated glomerular filtration rate; EPA=eicosapentaenoic acid; n-3 PUFA=omega 3 polyunsaturated fatty acid.
The small difference in number of participants with data on annual change in eGFR was due to missing measurement for specific n-3 PUFAs in some cohorts.
Model 1 adjusted for age, sex, race, clinical centre or field site, education, occupation, body mass index, smoking, alcohol intake, physical activity, prevalent coronary heart disease, and use of lipid lowering drugs, when applicable. Model 2 adjusted for all covariates in model 1 and also adjusted for prevalent diabetes mellitus, urine albumin-creatinine ratio, systolic blood pressure, use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and use of other antihypertensive drugs.
DPA was not available in three of the cohorts (CCCC—Chin-Shan Community Cardiovascular Cohort, InCHIANTI—Invecchiare in Chianti Study, and ULSAM—Uppsala Longitudinal Study of Adult Men), therefore the sum in these cohorts was calculated as EPA+DHA.
ALA and incident CKD
ALA was not associated with incident CKD, with low heterogeneity in the observed associations between cohorts (relative risk 1.00 per interquintile range, 95% confidence interval 0.94 to 1.06; P=0.94, I2=5.8%; table 2 and supplementary fig S4). No significant association was observed in analyses using a more stringent definition of incident CKD (supplementary table S7). No significant association was observed in the secondary outcomes of ≥40% decrease in eGFR (0.98, 0.89 to 1.07; P=0.08, I2=35.8%; table 3) and annual change in eGFR (mean difference −0.03 mL/min/1.73 m2 per interquintile range, 95% confidence interval −0.06 to 0.00; P=0.16, I2=24.1%; table 4).
Effect modification analyses
The association of n-3 PUFAs with the primary outcome of incident CKD was not modified by key demographic and clinical characteristics at baseline, including age, race, hypertension, diabetes, coronary heart disease, and eGFR (P for pooled interaction terms >0.05). No difference in association was observed based on study location, follow-up duration, and sample size (supplementary tables S9-11).
Discussion
Principal findings
This de novo pooled analysis of 19 cohort studies gathered data from more than 25 000 patients as part of the FORCE consortium. We found that higher levels of seafood n-3 PUFA biomarkers were associated with a modestly lower risk of incident CKD and slower decline in renal function, whereas these associations were not found with higher levels of plant n-3 PUFAs (ALA). The results were consistent across a range of sensitivity analyses, highlighting the robustness of the findings. Similar trends were observed using secondary outcomes of >40% decrease in eGFR and annual changes in eGFR. These findings support a favourable role for increased intake of seafood n-3 PUFAs for the primary prevention of CKD.
Comparison with previous studies
Randomised controlled trials found increased intake of seafood n-3 PUFAs reduced blood pressure, a key risk factor for CKD development.9 Conversely, ALA generally exhibits weaker to no effect on metabolic risk factors such as lipid, glucose, and inflammatory profile in randomised controlled trials compared with EPA and DHA.39 Furthermore, findings from previous experimental animal studies provide strong biological plausibility similar to our results of the beneficial effects of seafood n-3 PUFAs on CKD.40 41 42 For example, in a mouse model of nephrectomy induced chronic renal failure, supplementation with EPA and DHA for 12 weeks mitigated tubulointerstitial injury through reductions in fibrosis, inflammation, and oxidative stress.40 Increased endogenous production of DHA by a transgenic technique also protected against the development of kidney fibrosis and inflammation in mice with unilateral ureter obstruction induced nephropathy.41 Renoprotective mechanisms might be mediated through the endogenous conversion of n-3 PUFAs to specialised inflammation resolving mediators such as resolvins.43 44 Most previous experiments focused on EPA and DHA, but less is known about DPA, although limited evidence also suggests DPA has favourable properties on cardiometabolic profile.45 46
Although our findings do not prove a causal relation between seafood n-3 PUFAs and CKD risk, they are supportive and consistent with current clinical guidelines that recommend adequate intake of seafood as part of healthy dietary patterns, especially when seafood replaces the intake of less healthy foods.47 48 49 Although ALA could be enzymatically transformed to the longer chain n-3 PUFAs like EPA and DHA, such endogenous conversion only occurs at a low rate27 and our findings suggest intake of this plant derived n-3 PUFA alone might not maintain renal health. However, although ALA is an essential fatty acid, circulating ALA generally has a weaker correlation with ALA intake compared with the seafood derived n-3 PUFAs,22 50 51 potentially explained by a higher oxidation rate of ALA compared with other fatty acids.52 Therefore, if ALA intake were associated with incident CKD, we might not be able to detect such an association using an ALA biomarker. In our study, the levels of EPA and DHA probably mostly reflected variations in dietary intake from seafood, rather than n-3 PUFA supplements, because relatively small proportions of the general population used n-3 PUFA supplements, especially for studies that were conducted before the early 2000s when fish oil supplements were infrequently used (most of the included cohorts).53 Current dietary guidelines generally recommend at least two servings per week of oily fish to provide around 250 mg/day of long chain n-3 PUFAs for the general population.27 49
Given the renoprotective effects of seafood n-3 PUFAs observed in experimental animal studies, human trials have also assessed the use of n-3 PUFA supplementation to prevent decline in kidney function among patients with existing CKD or kidney failure. A recent systematic review of randomised controlled trials found n-3 PUFA supplementation could improve lipid profile and reduce oxidative stress, but not blood pressure in patients with CKD.54 Another recent systematic review of randomised controlled trials found n-3 PUFA supplementation could prevent the progression to kidney failure in patients with CKD who were not receiving renal replacement therapy, although the certainty of evidence is low.55 Therefore, current clinical guidelines do not recommend the use of n-3 PUFA supplementation to prevent further decline in renal function for those with existing CKD, although using n-3 PUFA supplementation to treat hypertriglyceridaemia is recommended.56 Our findings highlight the need for large randomised controlled trials to assess increased intake of seafood n-3 PUFAs for the primary prevention of CKD and decline in renal function. One such randomised controlled trial of 1312 patients with type 2 diabetes found that fish oil supplementation (daily dose of 1 g, containing 465 mg EPA and 375 mg DHA) did not affect the change in eGFR over five years.57 Several other randomised controlled trials have also reported null or small beneficial effects of n-3 PUFA supplementation on kidney function, but these were secondary outcomes or post hoc analyses and were not powered to detect incident CKD as a primary outcome.58 59 Further, randomised controlled trials of n-3 PUFA supplementation often included participants with sufficient seafood intake, in which additional n-3 PUFA supplementation might not confer additional cardiometabolic benefits.60 Therefore, future randomised controlled trials could target increased seafood intake, instead of n-3 PUFA supplementation, because consumption of seafood can replace the intake of less healthy foods. Finally, all these trials tested n-3 PUFA supplementation mainly in high risk populations, limiting the applicability of the study findings to the general population.
Strengths and limitations of this study
Our study has several strengths. Our literature search only identified two high quality prospective observational analyses of the association of n-3 PUFA biomarkers with incident CKD or decline in kidney. These two studies were included in our pooled analysis with updated patient numbers and harmonised analytical protocols.61 62 Our findings expand upon these previous individual cohort based analyses with a sevenfold larger sample size and enhanced statistical power to more precisely quantify the association of n-3 PUFAs with CKD. The large sample size also enabled a detailed evaluation of potential effect modifiers of the association between n-3 PUFAs and CKD. By collaborating with participating cohorts using a standardised approach to define exposures, covariates, and outcome variables, we reduced potential heterogeneity that is inherent in a publication based meta-analysis. Our collaborative approach enabled the inclusion of most of the cohorts with available exposure and outcome data, which reduced the likelihood of publication bias.
The use of n-3 PUFA biomarkers allowed us to distinguish between individual n-3 PUFAs that could have differing biological effects,26 and avoid measurement errors related to self-reported dietary intake. Conducting analyses across cohorts from diverse demographic, dietary, and medical backgrounds with different incident CKD rates enhanced the generalisability of our findings, and the use of several related secondary outcomes and different sensitivity analyses enhanced the robustness of the findings. However, more studies are needed to further understand the generalisability of our findings, especially in countries experiencing high incidence of CKD such as China and India.
Our study also has some limitations. The n-3 PUFA biomarkers and covariates were only measured once at baseline and this might lead to increasing random misclassification over time, which would tend to bias the associations towards the null. However, n-3 PUFA biomarkers have good long term reproducibility as determined in a previous study with repeated within person measurements across 13 years.63 While we standardised our methods across cohorts as much as possible, fatty acid assays, determination of outcomes, and covariate measurements varied by cohort and might contribute to the heterogeneity in our findings. Among different prespecified covariates, many participating cohorts did not have data for urine albumin-creatinine ratio. However, in cohorts that measured urine albumin-creatinine ratio, association of n-3 PUFA levels with incident CKD was similar whether it was adjusted for or not (results not shown). Although, the possibility of residual confounding by other unmeasured or imprecisely measured covariates could not be excluded, the differential association of plant derived ALA and seafood n-3 PUFAs with incident CKD suggests residual confounding was unlikely to entirely account for our findings. In this analysis, data were not adjusted for n-6 PUFA biomarker levels because experimental evidence on their relation with kidney function was limited. Because n-6 PUFA levels are associated with a lower CVD risk,30 future studies assessing their association with incident CKD are warranted, pending more experimental work assessing their possible impact on CKD.
Conclusion
In this large de novo pooled analysis across 19 cohorts with more than 25 000 patients, higher seafood n-3 PUFA levels were associated with a lower incident CKD risk and a slower decline in renal function. Although the magnitude of these associations was modest, our findings suggest adequate consumption of seafood and oily fish should be part of healthy dietary patterns. Additionally, further randomised controlled trials are warranted to assess the potential beneficial role of seafood n-3 PUFAs in preventing and managing CKD.
What is already known on this topic
Animal studies suggest omega 3 polyunsaturated fatty acids (n-3 PUFAs) have beneficial effects on kidney function, but evidence from human studies is limited
Most previous studies on chronic kidney disease (CKD) assessed self-reported intake of n-3 PUFAs using dietary questionnaires, which are subject to errors related to misreporting and inaccuracy of food composition databases
What this study adds
Higher seafood n-3 PUFA levels (eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid) were associated with a lower incident CKD risk and a slower decline in renal function
Plant derived n-3 PUFA level (α linolenic acid) was not associated with incident CKD
Findings are consistent with dietary guidelines recommending seafood and oily fish consumption as part of healthy dietary patterns, and provide strong evidence for further trials analysing seafood derived n-3 PUFAs in CKD prevention
Web extra.
Extra material supplied by authors
Web appendix: Supplementary online content
Contributors: KLO and JHYW designed the study and drafted the manuscript. KLO, MM, KAR, NH, XFP, RM, AT, AK, IHdB, DSS, DM, and JHY contributed to the development of study proposal and protocol. MM, CMR, HK, LMS, ACvW, JMG, EKH, YYC, KLC, AMF, RNL, FI, NGF, NJW, AB, SJ, OK, MBS, VDdM, JT, MU, JL, NT, WSH, KY, YH, TN, TT, LF, SBa, JKV, AV, TJ, AT, AP, SBu, PSS, MKS, SSR, MYT, ACW, MLaa, MLan, XY, LS, HL, XL, CN, JA, UR, LL, MLG, CS, and CH contributed to cohort specific data (principal investigators, analysts, associates, and trainees). KLO and LH performed the pooled analysis of cohort data. KLO, MM, KAR, XFP, WSH, FQ, AT, AK, IhdB, DSS, DM, and JHYW contributed to the primary writing team. All authors contributed to the data interpretation, critically revised the manuscript and approved the final version for publication. KLO is the guarantor of this manuscript and accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: Cohort specific funding is outlined in the supplementary methods. KLO was supported by the Australian National Health and Medical Research Council Career Development Fellowship (1122854) and the University of New South Wales Safety Net Fellowship. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication in the analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication. We operated independently from the funders.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest and declare: support from Australian National Health and Medical Research Council Career Development Fellowship and the University of New South Wales Safety Net Fellowship for the submitted work; MM reports research funding from Resolve to Save Lives, World Health Organisation and North western University, and support as invited speaker in the Nordic Dairy Congress 2022. CMR and LMS report research funding from the US National Institute of Health (NIH). CMR reports participation on the Data Safety Monitoring Boards of the SUPER and ADEPT trials and leadership role as associate editor of Diabetes Care. ACvW reports research funding from Jaap Schouten Foundation. JMG reports research funding from Jaap Schouten Foundation, EU Horizon 2020, and Ministry of Health, Welfare and Sports, The Netherlands, and leadership role as the Vice President of the Dutch Health Council. EKH reports research funding from Dutch Kidney Foundation. FI and NGF receive the MRC Epidemiology Unit core support. NGF reports research fundings from the NIHR Cambridge Biomedical Research Centre Theme on Nutrition, Diet and Lifestyle. JT declares conference support from the University of Antioquia, Colombia and possession of stocks from Orion Pharma. MU declares possession of stocks from Orion Pharma. WSH declares possession of stock in OmegaQuant Analytics, LLC (a laboratory that offers blood fatty acid testing to healthcare providers, researchers and consumers). PSS reports research funding from the National Health and Medical Research Council of Australia and honoraria from Biogen Australia and Roche Australia. ACW reports research funding from the US Department of Agriculture/Agricultural Research Service, NIH, National Cattlemen’s Beef Association and Hass Avocado Board Avocado Nutrition Research Center. JA has received research honoraria for lectures from AstraZeneca and Novartis, and has participated in the advisory board for AstraZeneca and Boerhinger Ingelheim, unrelated to the present study. UR reports research funding from Swedish Research Council Forma and Swedish Diabetes Foundation. RM reports research funding from the US NIH, Gates Foundation, Nestle and Danone, and consulting fees from Development Initiatives with leadership role as chair of the Independent Expert Group, Global Nutrition Report. AT declares as co-chair of ClinGen Gout Genetic Curation Panel and reports research funding from the US National Institute of Health; AK reports research funding from the German Research Foundation. DM reports research funding from the US NIH, the Gates Foundation, The Rockefeller Foundation, Vail Innovative Global Research, and the Kaiser Permanente Fund at East Bay Community Foundation; personal fees from Acasti Pharma and Barilla; scientific advisory board, Beren Therapeutics, Brightseed, Calibrate, Elysium Health, Filtricine, HumanCo, Instacart, January Inc., Perfect Day, Tiny Organics, and (ended) Day Two, Discern Dx, and Season Health; stock ownership in Calibrate and HumanCo; and chapter royalties from UpToDate. No other relationships or activities that could appear to have influenced the submitted work were reported.
The lead author (KLO) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned in the protocol have been explained.
Dissemination to participants and related patient and public communities: We plan to share the results of this study through press releases, presentations, institutional newsletters, and multiple social media platforms, including Twitter and LinkedIn.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
All the 19 participating cohorts have ethics approval from their respective institutions with appropriate data sharing agreements that enabled the current project of pooled data analysis (Alpha Omega Cohort—AOC: Medical Ethics Committee South-West Holland at the Haga Hospital and local ethics committees of all participating hospitals (approval No L01.049); Atherosclerosis Risk in Communities Study—ARIC: Johns Hopkins Bloomberg School of Public Health Institutional Review Board (approval No 11012/CR1014); CCCC—Chin-Shan Community Cardiovascular Cohort Study: Institutional Review Board of the National Taiwan University Hospital (approval No 201003001R); CHS—Cardiovascular Health Study: University of Washington Human Subject Committee (approval No STUDY00000109); EPIC-Norfolk—European Prospective Investigation into Cancer and Nutrition (Norfolk): Norwich Local Ethics Committee (approval No 98CN01); EPIC-Potsdam—European Prospective Investigation into Cancer and Nutrition (Potsdam): Ethics Committee of the State of Brandenburg, Germany (approval No AS 29/93 and S 9/2002); FDPS—Finnish Diabetes Prevention Study: the Ethics Committee of the National Public Health Institute of Helsinki (dated 6 March 1992, approval No n/a); FHS—Framingham Heart Study, and WHIMS—Women’s Health Initiative Memory Study: University of South Dakota Institutional Review Board (approval No IRB-21-136); Hisayama: The Kyushu University Institutional Review Board for Clinical Research (approval No 2021-457); InCHIANTI—Invecchiare in Chianti Study: Internal Review Board of the Intramural Research Program of the National Institutes of Health (approval No AG001050; exemption 11796); KIHD—Kuopio Ischaemic Heart Disease Risk Factor Study: Ethics Working Committee of Kuopio University (approval No 01/12/1983), and Ethics Committee of Kuopio University and Kuopio University Hospital (approval No 143/97); MAS—Memory and Ageing Study: Human Research Ethics Committee of the University of New South Wales (approval no. HC200506); MESA—Multi-Ethnic Study of Atherosclerosis: Intuitional Review Board Human Subjects Committee at The University of Minnesota (approval No 9805M00034), Johns Hopkins Medicine Intuitional Review Board (approval No 99-11-10-06), University of California Los Angeles Intuitional Review Board (approval No 99-057-22A), North western University Intuitional Review Board (approval No 200806-3515), Wake Forest University Intuitional Review Board (approval No BG00-035), Columbia University Medical Center Intuitional Review Board (approval No AAAA7791), and Baylor College of Medicine Intuitional Review Board (approved protocol No H-49021); METSIM—Metabolic Syndrome in Men: Ethics Committee of the Kuopio University Hospital (approval No 174/2004); NHAPC—Nutrition and Health of Ageing Populations in China: Institutional Review Board of the Institute for Nutritional Sciences, Chinese Academy of Sciences (approval No E-2005-01 for baseline survey and E-2009-01 for follow-up survey); PIVUS—Prospective Investigation of the Vasculature in Uppsala Seniors, and ULSAM—Uppsala Longitudinal Study of Adult Men: Ethics Committee of Uppsala University (approval No 2011/045, 00-419, and 251/90); 3C—Three City Study: Consultative Committee for the Protection of Persons participating in Biomedical Research at Kremlin-Bicêtre University Hospital (approval No 99-28).
Data availability statement
Individual participant data are owned by individual participating cohorts and are available to researchers on consent from participating cohorts. For further queries or requests, please contact force@tufts.edu. Further details are available at the FORCE website: http://force.nutrition.tufts.edu.
References
- 1. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS One 2016;11:e0158765. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709-33. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushita K, Coresh J, Sang Y, et al. CKD Prognosis Consortium . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3:514-25. 10.1016/S2213-8587(15)00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoogeveen EK, Geleijnse JM, Giltay EJ, et al. Kidney function and specific mortality in 60-80 years old post-myocardial infarction patients: a 10-year follow-up study. PLoS One 2017;12:e0171868. 10.1371/journal.pone.0171868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. King-Wing Ma T, Kam-Tao Li P. Depression in dialysis patients. Nephrology (Carlton) 2016;21:639-46. 10.1111/nep.12742 [DOI] [PubMed] [Google Scholar]
- 6. Kraus MA, Fluck RJ, Weinhandl ED, et al. Intensive hemodialysis and health-related quality of life. Am J Kidney Dis 2016;68(5S1):S33-42. 10.1053/j.ajkd.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 7. Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol 2019;16:581-601. 10.1038/s41569-019-0206-1 [DOI] [PubMed] [Google Scholar]
- 8. Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J Nutr 2011;106:974-80. 10.1017/S0007114511002819 [DOI] [PubMed] [Google Scholar]
- 9. Zhang X, Ritonja JA, Zhou N, Chen BE, Li X. Omega-3 polyunsaturated fatty acids intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J Am Heart Assoc 2022;11:e025071. 10.1161/JAHA.121.025071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi HD, Chae SM. Comparison of efficacy and safety of combination therapy with statins and omega-3 fatty acids versus statin monotherapy in patients with dyslipidemia: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e13593. 10.1097/MD.0000000000013593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pei J, Zhao Y, Huang L, Zhang X, Wu Y. The effect of n-3 polyunsaturated fatty acids on plasma lipids and lipoproteins in patients with chronic renal failure--a meta-analysis of randomized controlled trials. J Ren Nutr 2012;22:525-32. 10.1053/j.jrn.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 12. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA 2019;322:1294-304. 10.1001/jama.2019.14745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russo G, Piscitelli P, Giandalia A, et al. Atherogenic dyslipidemia and diabetic nephropathy. J Nephrol 2020;33:1001-8. 10.1007/s40620-020-00739-8 [DOI] [PubMed] [Google Scholar]
- 14. Theodorakopoulou MP, Dipla K, Zafeiridis A, Sarafidis P. Εndothelial and microvascular function in CKD: Evaluation methods and associations with outcomes. Eur J Clin Invest 2021;51:e13557. 10.1111/eci.13557 [DOI] [PubMed] [Google Scholar]
- 15. Gopinath B, Harris DC, Flood VM, Burlutsky G, Mitchell P. Consumption of long-chain n-3 PUFA, α-linolenic acid and fish is associated with the prevalence of chronic kidney disease. Br J Nutr 2011;105:1361-8. 10.1017/S0007114510005040 [DOI] [PubMed] [Google Scholar]
- 16. Yuzbashian E, Asghari G, Mirmiran P, Hosseini F-S, Azizi F. Associations of dietary macronutrients with glomerular filtration rate and kidney dysfunction: Tehran lipid and glucose study. J Nephrol 2015;28:173-80. 10.1007/s40620-014-0095-7 [DOI] [PubMed] [Google Scholar]
- 17. dos Santos ALT, Duarte CK, Santos M, et al. Low linolenic and linoleic acid consumption are associated with chronic kidney disease in patients with type 2 diabetes. PLoS One 2018;13:e0195249. 10.1371/journal.pone.0195249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park I, Xun P, Tsinovoi CL, Klemmer P, Liu K, He K. Intakes of long-chain omega-3 polyunsaturated fatty acids and non-fried fish in relation to incidence of chronic kidney disease in young adults: a 25-year follow-up. Eur J Nutr 2020;59:399-407. 10.1007/s00394-019-02022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malhotra R, Cavanaugh KL, Blot WJ, Ikizler TA, Lipworth L, Kabagambe EK. Dietary polyunsaturated fatty acids and incidence of end-stage renal disease in the Southern Community Cohort Study. BMC Nephrol 2016;17:152. 10.1186/s12882-016-0371-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arab L. Biomarkers of fat and fatty acid intake. J Nutr 2003;133(Suppl 3):925S-32S. 10.1093/jn/133.3.925S [DOI] [PubMed] [Google Scholar]
- 21. Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol 2006;17:22-7. 10.1097/01.mol.0000199814.46720.83 [DOI] [PubMed] [Google Scholar]
- 22. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348-80. 10.1016/j.plipres.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 23. Dawczynski C, Massey KA, Ness C, et al. Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clin Nutr 2013;32:686-96. 10.1016/j.clnu.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 24. Masson S, Marchioli R, Mozaffarian D, et al. Plasma n-3 polyunsaturated fatty acids in chronic heart failure in the GISSI-Heart Failure Trial: relation with fish intake, circulating biomarkers, and mortality. Am Heart J 2013;165:208-15.e4. 10.1016/j.ahj.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 25. Pertiwi K, Küpers LK, de Goede J, Zock PL, Kromhout D, Geleijnse JM. Dietary and circulating long-chain omega-3 polyunsaturated fatty acids and mortality risk after myocardial infarction: a long-term follow-up of the Alpha Omega Cohort. J Am Heart Assoc 2021;10:e022617. 10.1161/JAHA.121.022617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr 2012;142:614S-25S. 10.3945/jn.111.149633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011;58:2047-67. 10.1016/j.jacc.2011.06.063 [DOI] [PubMed] [Google Scholar]
- 28. Del Gobbo LC, Imamura F, Aslibekyan S, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) . ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016;176:1155-66. 10.1001/jamainternmed.2016.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu JHY, Marklund M, Imamura F, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol 2017;5:965-74. 10.1016/S2213-8587(17)30307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marklund M, Wu JHY, Imamura F, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) . Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation 2019;139:2422-36. 10.1161/CIRCULATIONAHA.118.038908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qian F, Ardisson Korat AV, Imamura F, et al. Fatty Acids and Outcomes Research Consortium (FORCE) . n-3 Fatty acid biomarkers and incident type 2 diabetes: an individual participant-level pooling project of 20 prospective cohort studies. Diabetes Care 2021;44:1133-42. 10.2337/dc20-2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris WS, Tintle NL, Imamura F, et al. Fatty Acids and Outcomes Research Consortium (FORCE) . Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat Commun 2021;12:2329. 10.1038/s41467-021-22370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hellwege JN, Velez Edwards DR, Giri A, et al. Mapping eGFR loci to the renal transcriptome and phenome in the VA Million Veteran Program. Nat Commun 2019;10:3842. 10.1038/s41467-019-11704-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wuttke M, Li Y, Li M, et al. Lifelines Cohort Study. V. A. Million Veteran Program . A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 2019;51:957-72. 10.1038/s41588-019-0407-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhavsar NA, Köttgen A, Coresh J, Astor BC. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2012;60:233-40. 10.1053/j.ajkd.2012.02.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014;64:821-35. 10.1053/j.ajkd.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 38. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66-73. 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen H, Deng G, Zhou Q, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid versus α-linolenic acid supplementation on cardiometabolic risk factors: a meta-analysis of randomized controlled trials. Food Funct 2020;11:1919-32. 10.1039/C9FO03052B [DOI] [PubMed] [Google Scholar]
- 40. An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol 2009;297:F895-903. 10.1152/ajprenal.00217.2009 [DOI] [PubMed] [Google Scholar]
- 41. Zeng Z, Yang H, Wang Y, Ren J, Dai Y, Dai C. Omega-3 polyunsaturated fatty acids attenuate fibroblast activation and kidney fibrosis involving MTORC2 signaling suppression. Sci Rep 2017;7:46146. 10.1038/srep46146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Henao Agudelo JS, Baia LC, Ormanji MS, et al. Fish oil supplementation reduces inflammation but does not restore renal function and klotho expression in an adenine-induced CKD model. Nutrients 2018;10:1283. 10.3390/nu10091283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duffield JS, Hong S, Vaidya VS, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol 2006;177:5902-11. 10.4049/jimmunol.177.9.5902 [DOI] [PubMed] [Google Scholar]
- 44. Halade GV, Kain V, Serhan CN. Immune responsive resolvin D1 programs myocardial infarction-induced cardiorenal syndrome in heart failure. FASEB J 2018;32:3717-29. 10.1096/fj.201701173RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen J, Jiang Y, Liang Y, et al. DPA n-3, DPA n-6 and DHA improve lipoprotein profiles and aortic function in hamsters fed a high cholesterol diet. Atherosclerosis 2012;221:397-404. 10.1016/j.atherosclerosis.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 46. Guo XF, Sinclair AJ, Kaur G, Li D. Differential effects of EPA, DPA and DHA on cardio-metabolic risk factors in high-fat diet fed mice. Prostaglandins Leukot Essent Fatty Acids 2018;136:47-55. 10.1016/j.plefa.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 47.US Department of Health and Human Services and US Department of Agriculture. 2015-2020 Dietary Guidelines for Americans. 8th ed. Washington, DC: US Government Printing Office; 2015.
- 48.US Department of Agriculture and US Department of Health and Human Services. Scientific Report of the 2015 US Dietary Guidelines Advisory Committee. Washington, DC: US Government Printing Office; 2015.
- 49. Rimm EB, Appel LJ, Chiuve SE, et al. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation 2018;138:e35-47. 10.1161/CIR.0000000000000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iggman D, Ärnlöv J, Cederholm T, Risérus U. Association of adipose tissue fatty acids with cardiovascular and all-cause mortality in elderly men. JAMA Cardiol 2016;1:745-53. 10.1001/jamacardio.2016.2259 [DOI] [PubMed] [Google Scholar]
- 51. Fridén M, Rosqvist F, Kullberg J, Ahlström H, Lind L, Risérus U. Associations between fatty acid composition in serum cholesteryl esters and liver fat, basal fat oxidation, and resting energy expenditure: a population-based study. Am J Clin Nutr 2021;114:1743-51. 10.1093/ajcn/nqab221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr 2000;72:905-11. 10.1093/ajcn/72.4.905 [DOI] [PubMed] [Google Scholar]
- 53. Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association. Nutrition Committee . Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747-57. 10.1161/01.CIR.0000038493.65177.94 [DOI] [PubMed] [Google Scholar]
- 54. Fazelian S, Moradi F, Agah S, et al. Effect of omega-3 fatty acids supplementation on cardio-metabolic and oxidative stress parameters in patients with chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol 2021;22:160. 10.1186/s12882-021-02351-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saglimbene VM, Wong G, van Zwieten A, et al. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: systematic review and meta-analysis of randomized controlled trials. Clin Nutr 2020;39:358-68. 10.1016/j.clnu.2019.02.041 [DOI] [PubMed] [Google Scholar]
- 56. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis 2020;76(Suppl 1):S1-107. 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 57. de Boer IH, Zelnick LR, Ruzinski J, et al. Effect of Vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA 2019;322:1899-909. 10.1001/jama.2019.17380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoogeveen EK, Geleijnse JM, Kromhout D, et al. Effect of omega-3 fatty acids on kidney function after myocardial infarction: the Alpha Omega Trial. Clin J Am Soc Nephrol 2014;9:1676-83. 10.2215/CJN.10441013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Majithia A, Bhatt DL, Friedman AN, et al. Benefits of icosapent ethyl across the range of kidney function in patients with established cardiovascular disease or diabetes: REDUCE-IT RENAL. Circulation 2021;144:1750-9. 10.1161/CIRCULATIONAHA.121.055560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schulze MB, Minihane AM, Saleh RNM, Risérus U. Intake and metabolism of omega-3 and omega-6 polyunsaturated fatty acids: nutritional implications for cardiometabolic diseases. Lancet Diabetes Endocrinol 2020;8:915-30. 10.1016/S2213-8587(20)30148-0 [DOI] [PubMed] [Google Scholar]
- 61. Lauretani F, Semba RD, Bandinelli S, et al. Plasma polyunsaturated fatty acids and the decline of renal function. Clin Chem 2008;54:475-81. 10.1373/clinchem.2007.095521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Westing AC, Eckl MR, Küpers LK, Pertiwi K, Hoogeveen EK, Geleijnse JM. Plasma fatty acids and kidney function decline in post-myocardial infarction patients of the Alpha Omega Cohort. Nutr Metab Cardiovasc Dis 2021;31:1467-76. 10.1016/j.numecd.2021.01.012 [DOI] [PubMed] [Google Scholar]
- 63. Lai HT, de Oliveira Otto MC, Lemaitre RN, et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ 2018;363:k4067. 10.1136/bmj.k4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary online content
Data Availability Statement
Individual participant data are owned by individual participating cohorts and are available to researchers on consent from participating cohorts. For further queries or requests, please contact force@tufts.edu. Further details are available at the FORCE website: http://force.nutrition.tufts.edu.


