Abstract
Objectives
In Egypt, an integrated surveillance for acute respiratory infections (ARIs) was established in 2016 to identify the causes of ARIs. The surveillance system includes 19 governmental hospitals. In response to the coronavirus disease 2019 (COVID-19) pandemic, the World Health Organisation (WHO) requested surveillance adaptation to address the emerging challenges. This study aims to describe the experience in Egypt of adapting ARI surveillance to the COVID-19 pandemic.
Methods
WHO case definitions were used to identify patients with ARIs. Nasopharyngeal/oropharyngeal swabs were collected for SARS-CoV-2 and influenza testing. Demographic and clinical information were obtained by interviewing patients at the hospitals. During the COVID-19 pandemic, the first two outpatients daily and every fifth admitted patient were enrolled in the study. To determine the status of ARIs in Egypt during the pandemic, patient demographic, clinical and laboratory data from 2020 to 2022 were obtained and descriptive analyses were performed.
Results
Overall, 18,160 patients were enrolled in the study, including 7923 (43.6%) seen at outpatient clinics and 10,237 (56.4%) inpatients. Of the study participants, 6453 (35.5%) tested positive for ARIs, including 5620 (87.1%) for SARS-CoV-2, 781 (12.1%) for influenza and 52 (0.8%) for SARS-CoV-2/influenza coinfection. SARS-CoV-2 was the cause for 95.3% of admitted patients and 65.4% of outpatients. Influenza subtypes included A/H3 (55.7%), Influenza-B (29.1%) and H1/pdm09 (14.2%). Compared with influenza, SARS-CoV-2 tended to infect the elderly, in warm weather and in urban governorates, and resulted in more hospitalisations, longer hospital stays and higher case fatalities (16.3% vs 6.6%, p < 0.001).
Conclusions
ARI surveillance in Egypt was successfully adapted to the COVID-19 pandemic and effectively described the clinical characteristics and severity of circulating viruses. Surveillance reported the re-emergence of influenza with a severe course and high fatality. Surveillance is essential for monitoring the activity of respiratory viruses with the aim of guiding clinical management, including preventative and control measures.
Keywords: Acute respiratory diseases, COVID-19, SARS-CoV-2, Influenza viruses, Sentinel surveillance, Egypt
1. Introduction
Coronavirus disease 2019 (COVID-19) has highlighted the need for multicomponent surveillance systems to monitor the epidemiology, virology and health consequences of respiratory viruses. Surveillance provides timely information on the pandemic situation and disease trends, and also assesses the burden that respiratory viruses place on healthcare systems [1].
Experts suggest that shifting from influenza to COVID-19 surveillance could make it difficult to avert future influenza pandemics. An integrating influenza and SARS CoV-2 surveillance was recommended to support the response to the COVID-19 pandemic, monitor long-term epidemiological trends, characterise virological features and study the evolution of the two viruses to inform public health interventions [2,3].
To establish comprehensive COVID-19 surveillance, the WHO recommended surveillance adaptation, strengthening existing surveillance systems, including COVID-19 as a notifiable disease, enhancing case identification and immediate reporting, contact tracing, and strengthening laboratory capacities [1].
Egypt started monitoring influenza activity in 1999 by establishing influenza-like illness (ILI) surveillance at eight outpatient clinics. In 2007, severe acute respiratory infection (SARI) surveillance was introduced at eight hospitals [5]. With the addition of pneumonia in 2016, the system was integrated to become acute respiratory infection (ARI) surveillance and expanded to include 19 hospitals, three of which participated during the pandemic, to cover all regions within the country.
As soon as the COVID-19 pandemic emerged, Egypt started adapting its pandemic preparedness plan to cope with the imminent pandemic. Adapting surveillance to help early detection of cases for effective case management, monitor disease trends, provide information for risk assessments and guide response measures was the most important pillar of the plan [3].
To prepare for future pandemics, it is necessary to describe how ARI surveillance systems adapted to the COVID-19 pandemic in terms of adapting case definitions, case enrolment, as well as laboratory capacity for the new disease. The WHO called for sharing experiences and lessons learned from challenges and successes in adapting influenza surveillance during the COVID-19 pandemic [3]. This study aims to describe the process of adapting the integrated ARI surveillance system in Egypt to the COVID-19 pandemic situation. In addition, this study used surveillance data to investigate the epidemiology and clinical features of ARIs during the pandemic.
2. Methods
Sentinel surveillance is one of five systems used for monitoring ARIs in Egypt, along with the National Electronic Diseases surveillance (NEDSS), the syndromic surveillance, the mortality surveillance and the event-based surveillance systems. Sentinel sites were selected to represent the whole population in all geographic areas, age groups and socioeconomic categories. At each site, a surveillance team (consisting of surveillance officer, laboratory focal point, nurses and data entry technicians) was assigned to implement surveillance activities.
WHO definitions of ILI, SARI and pneumonia were used to enrol patients in the study [6,7]. All patients with SARI and pneumonia admitted to sentinel sites were invited to participate in the surveillance, while the first two cases with ILI symptoms attending outpatient clinics every day were enrolled.
Participants provided nasopharyngeal and oropharyngeal swabs (NP/OP) that were kept in nitrogen tanks and sent weekly for real-time polymerase chain reaction (RT-PCR) testing for seasonal influenza viral types A and B at the Central Public Health Laboratory (CPHL). Positive Influenza A specimens were further tested to determine the subtype as H1pdm09, H3 or mixed Influenza infection using the US Centers for Disease Control (CDC) protocol [8].
Participant demographic, clinical, laboratory and disease outcome data were collected using standardised forms. The completed forms were entered using a web-based application at hospitals. Data are maintained at MoHP/Department of Epidemiology and Surveillance (DES) and analysed regularly. Biweekly and quarterly reports are shared with decision-makers and stakeholders.
During the pandemic, Egypt adapted ARI surveillance to include SARS-CoV-2 in addition to influenza [3]. Data collection tools and data screens for COVID-19 were developed and incorporated into the web-based application. Case definitions for COVID-19 were developed using WHO guidelines and distributed to all hospitals. As the pandemic progressed, the WHO definition was revised several times based on clinical and epidemiological data updates to improve sensitivity in case detection. All health facilities in Egypt were requested to report suspected COVID-19 cases daily to the MoHP [6].
Staff and resources were diverted to support the pandemic response during the early stages of the COVID-19 pandemic [9]. Every fifth hospitalised patient with ARI and the first two outpatients with ILI were enrolled in the study every day to compensate for the lack of surveillance teams and laboratory capacity.
Two NP/OP swabs were collected from each patient during the pandemic, one of which was immediately tested for SARS-CoV-2 in the hospital, while the other was kept in a nitrogen tank until transported weekly to a regional or the CPHL in Cairo for SARS-CoV-2 confirmation and influenza virus testing. CPHL serves as the National Influenza Centre (NIC); it is recognised by the WHO as a component of the Global Influenza Surveillance and Response System [3]. PCR capacity was increased during the pandemic to test more than 4000 samples/day, and a network of 55 regional and governorate level laboratories was established across the country to increase testing capacity for SARS-CoV-2. Electronic connections were established between the regional laboratories and the CPHL and MoHP, and new staff and specialists were hired from other departments within the CPHL. For early identification of evolving variants, and timely sharing of samples with WHO collaborating centers, laboratory equipment were upgraded and reagents were provided for 10 mutants.
Newly hired staff were trained on biosecurity, specimen collection, handling and transfer, as well as testing for the SARS-CoV-2 pandemic strain and influenza viruses in accordance with protocols and procedures developed in collaboration with the WHO. Employees were provided with specimen collection kits, packaging materials, reagents, supplies and laboratory protocols. Policies for vaccination of all staff working with potential pandemic viruses against SARS-CoV-2 and seasonal influenza were developed.
In order to detect the antigenic and genetic changes, antiviral susceptibility and pathogenicity of the pandemic strain, adequate laboratory virological surveillance was maintained.
Before the first case of COVID-19 was identified in Egypt, all suspected influenza cases, possible causative Coronaviruses (cov 229E, NL63, OC43 and HKU1), as well as respiratory pathogens were tested. Egypt CPHL received primers and probes specific to testing the first 100 specimens for SARS-CoV-2 from the WHO on 1 February 2020, allowing the first case of COVID-19 to be confirmed. CPHL specialists attended training on coronavirus testing conducted by the African CDC in Senegal on 3–6 February 2020 and received another type of b nCoV kits (African CDC-Modular Dx Kit -Wuhan CoV E & Rdrp gene) to manage the surge of patients.
A surge plan was activated to manage the increased number of ARI samples, laboratory testing capacity was upgraded with additional laboratory equipment supplies and well-trained staff. An efficient specimen transport system from sentinel sites to CPHL was established, taking in to account the possible disruption of routine transport systems during the pandemic. A decentralisation plan was implemented for filling nitrogen tanks at regional laboratories. Protocols to pack and transport specimens and viruses in accordance with local regulations were developed and implemented. Additionally, international shipping procedures that comply with WHO guidelines and international transport regulations were followed.
Testing for SARS-CoV-2 was performed on NP swabs by extraction of nucleic acid using the chemagic 360 instrument (PerkinElmer Inc), while SARS-CoV-2 RNA (ORF1ab) was detected using a VIASURE SARS-CoV-2 Real-Time PCR Detection Kit (Certest Biotec SL). The RT-PCR runs were performed in triplicate and according to the manufacturer's recommendations, and the samples were confirmed as positive SARS-CoV-2 using a cobas 6800 system (Roche Holding AG).
SARS-CoV-2 sequences were performed by the CPHL sequencing laboratory, which is equipped with a NGS machine and virus-specific antibodies are tested by chemiluminescence technique. Reagents for ten mutant strains were distributed to laboratories in tourist areas for early detection of new variants. Bids were opened through the Unified Procurement Authority to supply one million kits. In collaboration with the WHO, CPHL staff visited governorate laboratories to supply reagents and equipment.
To maximise the usefulness the surveillance system, data were analysed timely to provide a regular update on the influenza and SARS-CoV-2 epidemic situation in Egypt. Data were regularly shared with the MoHP to help update case management guidelines, regular risk and severity assessments, and future predictions. Daily reports were developed and shared with relevant stakeholders to inform national and global preparedness [10].
The International Health Regulations unit of the MoHP maintained regular contact with the WHO Egypt country office and the Eastern Mediterranean Regional (EMR) office to share information, and update recommendations and report on the situation.
The preventive sector of MoHP was given access to surveillance data for follow-up of patients and contacts through phone calls or visits. Symptomatic contacts were further investigated for early case detection [11]. Contact tracing and management procedures were developed and distributed at all levels of the healthcare system and an online application was developed within the national reporting system for contact tracing.
In order to describe the epidemiology of ARIs during the COVID-19 pandemic, surveillance data from January 2020 to April 2022 were collected. Descriptive data analyses were performed using Epi info7. Patients admitted to hospitals with SARI were compared to those seen in outpatient clinics with ILI to identify differences in their demographic characteristics and causative agents. Additionally, SARS-CoV-2 patients were compared with influenza patients to identify differences in epidemiology, clinical severity and outcome. The Chi-squared test was used to compare categorical variables, and the t-test and analysis of variance (ANOVA) were used for continuous variables, with a significance level of 0.05.
3. Results
Overall, 18,160 patients with ARIs were identified between January 2020 and April 2022, including 7923 (43.6%) patients seen at ILI sites and 10,237 (56.4%) at SARI sites. Of the study participants, 6453 (35.5%) had positive ARI test results, including 5620 (87.1%) for SARS-CoV-2, 781 (12.1%) for influenza viruses and 52 (0.8%) for SARS-CoV-2/influenza coinfection. A higher rate of positivity was identified among SARI than ILI patients (45.7% vs 22.4%, p < 0.001). The majority of SARI cases (95.3%) were caused by SARS-CoV-2, compared to 65.4% of ILI cases; influenza viruses were the cause of more ILI than SARI cases (32.8% vs 4.2%, p < 0.001) [see Table 1].
Table 1.
Characteristics of patients seen at influenza-like illness (ILI) outpatient clinics and severe acute respiratory infections (SARI) admitted to surveillance hospitals, the integrated acute respiratory infections surveillance system, Egypt 2010–2022.
| Characteristic | Total ARI (n = 18,160) |
ILI (n = 7923) |
SARI (n = 10,237) |
RR | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||||
| Mean age in years ± SD | 40.1 ± 23 | 26.8 ± 19 | 50.3 ± 21 | NA | NA | <0.001 | |||
| Male gender | 8640 | 47.6 | 3858 | 48.7 | 4782 | 46.7 | 1.1 | 1.0–1.01 | 0.01 |
| Age group in years | |||||||||
| 0-4 | 1926 | 10.6 | 1251 | 15.8 | 675 | 6.6 | 2.7 | 2.4–2.9 | <0.001 |
| 5-17 | 1882 | 10.4 | 1623 | 20.5 | 259 | 2.5 | 9.9 | 8.7–11.4 | <0.001 |
| 18-49 | 7121 | 39.2 | 3964 | 50.0 | 3157 | 30.8 | 2.2 | 2.1–2.4 | <0.001 |
| 50-64 | 4205 | 23.2 | 890 | 11.2 | 3315 | 32.4 | 0.3 | 0.2–0.3 | <0.001 |
| ≥65 | 3026 | 16.7 | 195 | 2.5 | 2831 | 27.7 | 0.07 | 0.06–0.08 | <0.001 |
| Region | |||||||||
| Urban governorates | 7182 | 39.5 | 2782 | 35.1 | 4400 | 43.0 | 0.7 | 0.6–0.8 | <0.001 |
| Upper Egypt | 5830 | 32.1 | 3235 | 40.8 | 2595 | 25.3 | 2.0 | 1.9–2.2 | <0.001 |
| Lower Egypt | 5017 | 27.6 | 1811 | 22.9 | 3206 | 31.3 | 0.6 | 0.6–0.7 | <0.001 |
| Frontier governorates | 131 | 0.7 | 95 | 1.2 | 36 | 0.4 | 3.4 | 2.3–5.1 | <0.001 |
| Season | |||||||||
| Sept–Nov | 4537 | 25.0 | 2088 | 26.4 | 2449 | 23.9 | 1.1 | 1.1–1.2 | <0.001 |
| Dec–Feb | 5719 | 31.5 | 2836 | 35.8 | 2883 | 28.2 | 1.4 | 1.3–1.5 | <0.001 |
| Mar–May | 4278 | 23.6 | 1654 | 20.9 | 2624 | 25.6 | 0.8 | 0.7–0.8 | <0.001 |
| Jun–Aug | 3626 | 20.0 | 1345 | 17.0 | 2281 | 22.3 | 0.7 | 0.7–0.8 | <0.001 |
| Positive for tested viruses | 6453 | 35.5 | 1778 | 22.4 | 4675 | 45.7 | 2.9 | 2.7–3.1 | <0.001 |
| Positive for SARS-CoV2 | 5620 | 30.9 | 1163 | 14.7 | 4457 | 43.5 | 10.8 | 9.1–12.8 | <0.001 |
| Positive for both SARS-CoV-2 and influenza | 52 | 0.3 | 32 | 0.4 | 20 | 0.2 | 0.23 | 0.13–0.41 | <0.001 |
| Positive for influenza viruses: | 781 | 4.3 | 583 | 7.4 | 198 | 1.9 | 0.09 | 0.08–0.10 | <0.001 |
| - Influenza A/H1pdm09 | 111 | 14.2 | 74 | 12.7 | 37 | 18.7 | 1.6 | 1.02–2.4 | 0.020 |
| - Influenza A/H3 | 435 | 55.7 | 347 | 59.5 | 88 | 44.4 | 0.54 | 0.39–0.75 | <0.001 |
| - Influenza B | 227 | 29.1 | 159 | 27.3 | 68 | 34.3 | 1.4 | 1.0–2.0 | 0.031 |
| - > one influenza viral infection | 8 | 1.0 | 3 | 0.5 | 5 | 2.5 | 5.0 | 1.2–21.2 | 0.016 |
CI, confidence interval; SD, standard deviation.
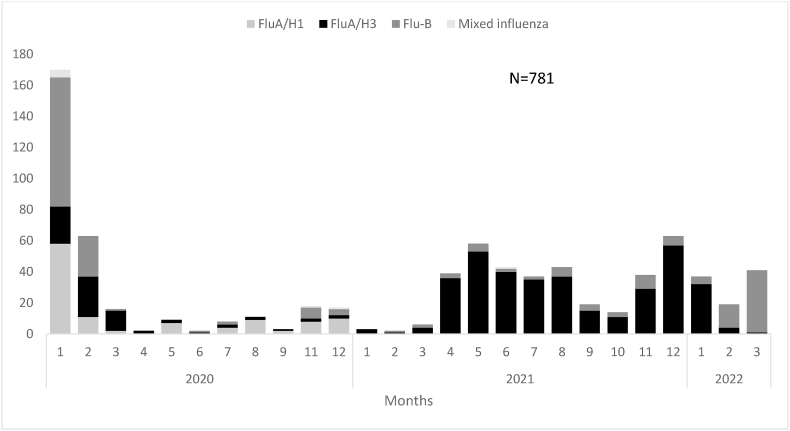
Of 781 influenza viral infections, the A/H3 subtype was the cause of more than half (435 [55.7%]), followed by Influenza subtype B (Influenza B) (227 [29.1%]) and influenza A/H1pdm09 (111 [14.2%]). Influenza A/H3 and B were the main subtypes isolated from SARI patients, representing 44.4% and 34.3%, respectively. Among ILI patients, A/H3 caused 59.5% of influenza infections, followed by Influenza B (27.3%) and H1pdm09 (12.7%). SARI patients had a higher percentage of influenza A/H1pdm09 than ILI patients (18.7% vs 14.2%), while ILI patients had a higher percentage of influenza A/H3 than SARI patients (59.5% vs 44.4%) (Table 1).
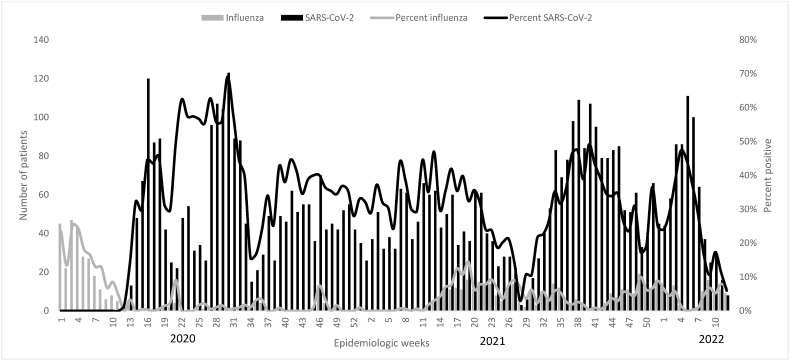
Two distinct waves of SARS-CoV-2 occurred during the summers of 2020 and 2021, and two influenza waves corresponded to the 2020 and 2021 influenza seasons. While influenza occurred sporadically in 2020 and re-emerged in spring 2021 with a peak of A/H3 subtype, it then peaked in the 2021–2022 influenza season with A/H3 and Influenza B subtypes (see Fig. 1, Fig. 2).
Fig. 1.
Distribution of SARS-CoV-2 and influenza by time, the integrated acute respiratory infections surveillance system, Egypt 2010–2022.
Fig. 2.
Influenza virus subtypes isolated from patients seen at the integrated acute respiratory infections surveillance system, Egypt, 2020–2022.
The demographic, epidemiological and clinical characteristics of ARI patients differed by viral cause; SARS-CoV-2 tended to infect older females throughout the year in urban governorates and Lower Egypt, while influenza viruses mainly infected younger males during the winter to spring months in Upper Egypt (see Table 2). Compared with influenza patients, SARS-CoV-2 patients took more time before seeking medical advice (3.9 vs 2.8 days, p < 0.001) and needed hospital admission more frequently (82.4% vs 5.9%, p < 0.001).
Table 2.
Comparison between characteristics of patients with SARS-CoV-2 and influenza, the integrated acute respiratory infections surveillance system, Egypt 2010–2022.
| Characteristic | SARS-CoV-2 (n = 5620) |
Influenza (n = 781) |
p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Mean age years ±SD | |||||
| Age group (years) | |||||
| 0-4 | 103 | 1.8 | 154 | 19.7 | <0.001 |
| 5-17 | 201 | 3.6 | 211 | 27.0 | |
| 18-49 | 296 | 37.3 | 311 | 39.8 | |
| 50-64 | 1815 | 32.3 | 62 | 7.9 | |
| ≥65 | 1405 | 25.0 | 43 | 5.5 | |
| Male gender | 2475 | 44.0 | 412 | 52.8 | <0.001 |
| Seasonality | |||||
| Sept–Nov | 1690 | 30.1 | 92 | 11.8 | <0.001 |
| Dec–Feb | 1368 | 24.3 | 374 | 47.9 | |
| Mar–May | 1304 | 23.2 | 171 | 21.9 | |
| Jun–Aug | 1258 | 22.4 | 144 | 18.4 | |
| Region | |||||
| Urban | 2213 | 39.4 | 210 | 26.9 | <0.001 |
| Lower Egypt | 1920 | 34.2 | 99 | 12.7 | |
| Upper Egypt | 1467 | 26.1 | 451 | 57.7 | |
| Frontier governorates | 20 | 0.4 | 21 | 2.7 | |
| Mean duration from onset to first seen at HCF (days) | 3.9 ± 3.7 | 2.8 ± 2.2 | <0.001 | ||
| Home treated | 1163 | 20.7 | 583 | 74.6 | <0.001 |
| Hospitalised | 4457 | 79.3 | 198 | 25.4 | |
| Mean age of hospitalised patients (years) | 55.3 ± 16.0 | 37.7 ± 25.3 | <0.001 | ||
| Male gender | 1953 | 43.8 | 103 | 52.0 | 0.01 |
| With chronic disease | 1463 | 32.8 | 48 | 24.2 | <0.01 |
| Severity among hospitalised patients | |||||
| Pneumonia | 3298 | 74.0 | 108 | 54.5 | <0.001 |
| Bilateral | 2054 | 62.3 | 57 | 52.8 | 0.02 |
| Unilateral | 1244 | 37.7 | 51 | 47.2 | |
| ICU admitted | 712 | 16.0 | 28 | 14.1 | 0.250 |
| Mechanical ventilator | 193 | 4.3 | 7 | 3.5 | 0.294 |
| Mean length of hospital stay (days) | 7.2 ± 6.7 | 5.4 ± 5.0 | <0.001 | ||
| Died in hospital | 727 | 16.3 | 13 | 6.6 | <0.001 |
HCF, healthcare facility; ICU, intensive care unit; SD, standard deviation.
Among the 10,237 patients hospitalised with SARI, 4457 (43.5%) had SARS-CoV-2, 198 (1.9%) had influenza and 20 (0.2%) had SARS-CoV-2 and influenza viral coinfection; 5562 (54.3%) tested negative for both viruses (Table 1, Table 2). SARS-CoV-2 hospitalised patients were significantly older, female and had comorbidities, while influenza hospitalised patients were younger, males and without comorbidities (55.5 ± 16.0 vs 37.7 ± 25.3 years, 56.2% vs 48.0%, 32.8% vs 24.2%, respectively). Patients with SARS-CoV-2 in hospital had a more severe course of disease in terms of pneumonia, bilateral pneumonia, length of hospital stay and died more frequently than those with influenza (74.0% vs 54.5%, 62.3% vs 52.8%, 7.2 ± 6.7 vs 5.4 ± 5.0 days, and 16.3% vs 6.6%, respectively). There was no significant difference in intensive care unit (ICU) admissions or mechanical ventilation between SARS-CoV-2 and influenza (16.0% vs 14.1% and 4.3% vs 3.5%, respectively) [see Table 2].
4. Discussion
This is the first study to describe how the integrated ARI surveillance system in Egypt was adapted to the COVID-19 pandemic situation using the WHO key strategy. This study also reports the surveillance findings.
During the COVID-19 pandemic, Egypt experienced disruptions to influenza sentinel surveillance systems that resulted from repurposing personnel, supplies, facilities and laboratories. During the course of this study, the practical and standardised approach for maintaining, strengthening and adapting the influenza sentinel virological surveillance system based on the WHO strategy to meet the evolving public health needs arising from the COVID-19 pandemic was discussed [12].
During the pandemic, other countries used different models to address the challenges. Spanish surveillance efforts focused on people aged >65 years, the immunocompromised and those with underlying diseases, given the number of natural infections and the fact that most people are protected against severe COVID-19 [13]. According to WHO, it is better to have a few sites with good data than many sites with poor data. To provide quality clinical, laboratory and epidemiological data, Egypt adopted the WHO strategy of influenza sentinel surveillance. In addition, COVID-19 was added to the reportable diseases list of the NEDSS so that an overview of the COVID-19 situation could be provided daily.
It is important to note that several limitations exist in the surveillance system in Egypt. For example, surveillance cannot accurately estimate the burden of COVID-19 in Egypt. MoHP assesses influenza burden using sentinel surveillance data based on WHO methods [14]. Surveillance data in Egypt can be used to estimate the burden of SARS-CoV-2 in the same way. Additionally, there is no mechanism for the early detection of ARI outbreaks and thus providing early warnings. Continuous baseline and threshold calculation is recommended for the early detection of ARI outbreaks and to contribute to the country's pandemic preparedness [15].
The study showed that incorporation of SARS-CoV-2 into the influenza surveillance system was useful to describe the epidemiological and virus trends, detect co-circulation of influenza and SARS-CoV-2, and illustrate how these two diseases affect health systems during the COVID-19 pandemic.
Surveillance indicated higher rates of viral infections among ARI patients, both in the outpatient clinics and inpatient wards, compared with the rates reported from Egypt influenza surveillance during the inter-pandemic phase [4,16,17]. This finding reflects the surge of SARS-CoV-2 when first introduced into a naïve population and could indicate high sensitivity of the surveillance. Additionally, the results emphasise the importance of monitoring viral infection rates over time in order to enable early detection of epidemics and to describe disease trends. Other explanations for these results could include the change in healthcare-seeking behaviour during the COVID-19 pandemic as well as the triage protocol used to prioritise the moderate-to-severe patients for case management [18].
ARI sentinel surveillance proved to be useful in the detection and monitoring of the spread of known and emerging respiratory pathogens, including seasonal influenza and any respiratory pathogen with epidemic potential in a timely manner [19]. The integrated sentinel surveillance system in Egypt was effective in describing the COVID-19 pandemic situation accurately, as evidenced by the similarity in time distribution with the official national COVID-19 data [20]. ARI surveillance provided better quality data on the circulated influenza viruses’ epidemiological and virological characteristics by type and subtype than the official system amidst the pandemic. ARI surveillance should be maintained, and early outbreak detection should be strengthened through timely data collection, analysis and reporting.
Surveillance reported an unprecedented decline of influenza viruses during the COVID-19 pandemic. Several studies have suggested that the application of epidemiological measures, such as wearing masks, social distancing and other interventions, are responsible [21,22]. However, this assumption cannot fully explain the epidemiological patterns of different respiratory viruses observed during the pandemic [22]. An additional reason could be the viral interference phenomenon in which a virus prevents or partially inhibits the infection of another virus within the same host [23,24]. Popovic and Minceva argued that Gibbs energies of binding and growth ratios may influence the outcome of the competition between the competing viruses [25]. There is also a possibility that SARS-CoV-2 predominated during the first year of the pandemic due to lack of natural immunity, but as the pandemic progressed, natural and vaccine-induced immunity to SARS-CoV-2 was established, resulting in influenza viruses re-emerging.
According to this study, during the pandemic, influenza viruses caused severe disease in younger healthy individuals, with a high fatality rate and a predominance of A/H3 subtype. This pattern is different to that reported by Fahim et al. in Egypt during the pre-pandemic phase, with higher fatality rates and severe outcomes among older people and a predominance of A/H126. Some studies suggest that the A/H3 virus has declined in its ability to efficiently infect susceptible hosts but, according to this and other studies, influenza-associated hospitalisations and deaths are highest during seasons where A/H3 dominates [26,27]. Due to reduced influenza viruses circulating in 2020, the severity of the A/H3 subtype could have changed due to a prolonged absence of natural exposure to influenza viruses. It is important to recognise that the upcoming influenza season will probably be more severe than average and will likely impact young adults particularly hard due to waning immunity levels during the surge of COVID-19. Studies are needed to better describe viral interference phenomenon and predict severity of upcoming influenza seasons to guide vaccination and prevention strategies.
Despite the significantly lower fatality rate from influenza than SARS-CoV-2 found in this study, it is still higher than the rate reported during the inter-pandemic period [26]. Furthermore, high rates of ICU admissions and mechanical ventilation due to influenza were reported in this study. It is essential to increase public awareness about influenza vaccination and educate clinicians on the proper diagnosis and treatment of influenza to reduce the burden of influenza on healthcare systems. Research is needed to determine the potential causes of high case fatality rate (CFR) during COVID-19 so that CFR from ARIs can be reduced. Prioritisation policies for hospital care during the SARS-CoV-2 pandemic should also be reviewed [28].
The US ARI surveillance that targets a wide of range of respiratory viruses has described the different patterns of respiratory viruses during COVID-19 pandemic 2020–2021. The influenza activity was low with an unusual increase in respiratory syncytical virus (RSV) and pre-pandemic levels of the common human coronaviruses and parainfluenza viruses after notably low circulation [22]. ARI surveillance in Egypt successfully described the pattern of influenza and SARS-CoV-2, where a large percentage of ARI patients tested negative for both SARS-CoV-2 and influenza. Multiple respiratory pathogen testing is therefore necessary due to the changes in the circulation of respiratory viruses as the pandemic evolves and immunity to SARS-CoV-2 and other respiratory viruses increases and decreases. It also demonstrates the need to expand the testing panel for ARI in Egypt to better characterise the circulation patterns of different respiratory viruses in different times and geographical areas.
The current study results revealed that epidemiological characteristics of the patients with SARS-CoV-2 differ significantly from those with influenza. Differentiation between patients infected with SARS-CoV-2 and influenza could help clinicians in case management. For example, glucocorticoids should be used with caution in influenza patients, while dexamethasone should be used with caution in patients who have influenza and SARS-CoV-2 coinfection [29]. Furthermore, identifying different causes of ARI would help in estimating the burden of different viral agents and in designing effective preventive strategies, including vaccination policies. Additionally, multipathogen testing for ARIs and susceptibility to therapeutics is important to guide clinical management and save time and resources [22].
This study indicated that SARS-CoV-2 occurred all year around, while the influenza season peaks in winter. Seasonality of SARS-CoV-2 is controversial, early in the pandemic, it was believed that COVID-19 spreads faster in winter than in summer like influenza and other respiratory viruses, but this proved inaccurate as many countries experienced COVID-19 peaks in summer. Studies suggested many environmental factors are probable determinants of COVID-19 transmission at the seasonal scale, including air drying capacity, ultraviolet radiation, air temperature and ground level ozone [30]. Additional studies are required to identify the environmental factors that enhance SARS-CoV-2 transmission to help tailor preventive strategies, including vaccination campaigns.
The distribution of influenza infections during the pandemic, most of which were reported in Upper Egypt, also differed from the pre-pandemic pattern [26]. The change in geographical distribution of different types and subtypes of ARIs over time could be attributed to the change in the performance of sentinel sites, seasonal variability of different ARIs and influenza subtypes, varying levels of immunity among different populations, or different approaches to prevention and control [26].
According to this study and others, SARS-CoV-2 results in high hospitalisation and fatality rates in the elderly with pre-existing conditions [31]. A lower level of natural immunity to the newly emerged SARS-CoV-2, especially in this age group, and the lack of effective treatment could explain this, in addition to the delay in seeking healthcare reported in this study [32]. Vaccination campaigns that focus on the elderly and patients with comorbidities should be considered to reduce CFRs from SARS-CoV-2. The reasons behind delays in seeking healthcare for COVID-19 require further studies.
5. Conclusions
With the help of the WHO key strategy, Egypt was able to adapt an integrated sentinel site ARI surveillance to the COVID-19 pandemic situation. In Egypt, surveillance was useful for identifying the circulating respiratory viruses, as well as providing useful data for describing the clinical characteristics and severity of respiratory viral infections. In 2021, influenza re-emerged with high severity and mortality, warning that it may return to its pre-pandemic patterns. Vaccination policies for both influenza and COVID-19 should be developed as soon as possible, with special consideration for high-risk groups.
To provide quality information about circulating viruses, it is crucial that influenza and SARS-CoV-2 virological surveillance be maintained along with a digitalised system in order to guide public health authorities to prepare for upcoming seasonal epidemics and the detection of emerging pandemic virus strains. Testing for multiple pathogens is recommended as an effective way to identify ARIs viral causes and guide clinical and public health policies. To reduce the burden of ARIs, surveillance information should be communicated to clinicians for updated case management and to policymakers to develop effective prevention and control strategies in Egypt.
Ethics approval
The study was exempted as non-research project by Ministry of Health and Population Ethics committee and institutional review board (IRB).
The study was conducted using data collected during patients' routine management procedures. All patients verbally consented to participate after they were fully informed about the aims of surveillance and risks associated. During participation, anonymity was maintained to preserve patients’ confidentiality.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy restrictions but are available from Egypt Ministry of Health and Population on reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization . December 2020. Public Health Surveillance for COVID-19: Interim Guidance.https://www.who.int/publications/i/item/who-2019-nCoV-surveillanceguidance-2020.8 Found at: [Google Scholar]
- 2.Owen J. WHO warns that averting flu pandemic may be harder as surveillance switches to covid-19. Br. Med. J. 2020;369 doi: 10.1136/bmj.m2441. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Global Influenza Surveillance and Response System (GISRS). Maintaining Surveillance of Influenza and Monitoring SARS-CoV-2 – Adapting and Sentinel Systems during the COVID-19 Pandemic: Interim Guidance. Geneva. WHO/2019-nCoV/Adapting_GISRS/2020.1). Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 4.Refaey S., Amin M., Labib M., Kandeel A. Influenza virus positivity and circulating subtypes among cases of influenza-like illness and severe acute respiratory infection, Egypt. Eng. Manag. J. 2012;22(7):527–536. 2015. [PubMed] [Google Scholar]
- 5.Kandeel A., Dawson P., Labib M., Said M., El-Refai S., El-Gohari A., et al. Morbidity, mortality, and seasonality of influenza hospitalizations in Egypt. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161301. November 2007-November 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Operational considerations for COVID-19 surveillance using GISRS Interim guidance. 26 March 2020. https://apps.who.int/iris/bitstream/handle/10665/331589/WHO-2019-nCoV-Leveraging_GISRS-2020.1-eng.pdf?sequence=1&isAllowed=y Found at:
- 7.Russell F.M., Reyburn R., Chan J., Tuivaga E., Lim R., Lai J., Van H.M.T., Choummanivong M., Sychareun V., Khanh D.K.T., de Campo M., Enarson P., Graham S., La Vincente S., Mungan T., von Mollendorf C., Mackenzie G., Mulholland K. Impact of the change in WHO's severe pneumonia case definition on hospitalized pneumonia epidemiology: case studies from six countries. Bull. World Health Organ. 2019 Jun 1;97(6):386–393. doi: 10.2471/BLT.18.223271. Epub 2019 Mar 27. PMID: 31210676; PMCID: PMC6560369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC protocol of realtime RTPCR for influenza A(H1N1) World Health Organization. 2009. https://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf Apr 29, [2021-04-22]
- 9.European Centre for Disease Prevention and Control, WHO Regional Office for Europe . interim guidance; 2020. Operational Considerations for Influenza Surveillance in the WHO European Region during COVID-19. [Google Scholar]
- 10.World Health Organization Laboratory testing strategy recommendations for COVID-19 Interim guidance. 21 March 2020. https://apps.who.int/iris/bitstream/handle/10665/331509/WHO-COVID-19-lab_testing-2020.1-eng.pdf Found at:
- 11.Abu El Sood H., Abu Kamer S.A., Kamel R., Magdy H., Osman F.S., Fahim M., Mohsen A., AbdelFatah M., Hassany M., Afifi S. Eid A the impact of implementing the Egypt pandemic preparedness plan for acute respiratory infections in combating the early stage of the COVID-19 pandemic. Viewpoint JMIR Public Health Surveill. 2021;7(5) doi: 10.2196/27412. February-July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Consultation to Adapt Influenza Sentinel Surveillance Systems to Include COVID-19 Virological Surveillance: Virtual Meeting, 6 – 8. October 2020. https://www.who.int/publications/i/item/WHO-WHE-GIH-GIP-2021.1 [Google Scholar]
- 13.Sanz-Muñoz I., Castrodeza Sanz J., María Eiros J. Surveillance of COVID-19 after the pandemic. How do we do it? Med. Clin. (Barc) 2022 Oct 28;159(8):396–400. doi: 10.1016/j.medcli.2022.05.010. English, Spanish, Epub 2022 Jul 15. PMID: 35933188; PMCID: PMC9283601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Regional Office for Europe Guidance for Sentinel Influenza Surveillance in Humans. May 2011. https://www.euro.who.int/__data/assets/pdf_file/0020/90443/E92738.pdf [Google Scholar]
- 15.AbdElGawad B., Refaey S., Abu El Sood H., El Shourbagy S., Mohsen A., Fahim M. Defining influenza baseline and threshold values using surveillance data - Egypt, season 2016-17. iproc. 2018;4(1) doi: 10.2196/10600. https://www.iproc.org/2018/1/e10600 URL: [DOI] [PubMed] [Google Scholar]
- 16.Elhakim M.M., Kandil S.K., Abd Elaziz K.M., Anwar W.A. Epidemiology of severe acute respiratory infection (SARI) cases at a sentinel site in Egypt, 2013-15. J. Public Health. 2020;42(3):525–533. doi: 10.1093/pubmed/fdz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Refaey S., Hassan M., Mansour A., Kandeel A. Incidence of influenza virus-associated severe acute respiratory infection in Damanhour district, Egypt, 2013. EMHJ. 2016;22(7):499–508. [PubMed] [Google Scholar]
- 18.Moynihan R., Sanders S., Michaleff Z.A., et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . World Health Organization; 2017. WHO Guidance for Surveillance during an Influenza Pandemic, 2017 Update.https://apps.who.int/iris/handle/10665/259886. License: CC BY-NC-SA 3.0 IGO [Google Scholar]
- 20.World meter. Coronavirus, COVID-19 pandemic, Egypt. https://www.worldometers.info/coronavirus/country/egypt/ Found at :
- 21.Oh D.Y., Buda S., Biere B., Reiche J., Schlosser F., Duwe S., Wedde M., von Kleist M., Mielke M., Wolff T., Dürrwald R. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January - september 2020: analysis of national surveillance data. Lancet Reg Health Eur. 2021 Jul;6 doi: 10.1016/j.lanepe.2021.100112. Epub 2021 Jun 7. PMID: 34124707; PMCID: PMC8183189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen Sonja J., Winn Amber K., Budd Alicia P., et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1013–1019. doi: 10.15585/mmwr.mm7029a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu A., Mihaylova V.T., Landry M.L., Foxman E.F. Interference between rhinovirus and influenza A virus: a clinical data analysis and experimental infection study. Lancet Microbe. 2020;1:e254–e262. doi: 10.1016/s2666-5247(20)30114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones N. How COVID-19 is changing the cold and flu season. Nature. 2020;588:388–390. doi: 10.1038/d41586-020-03519-3. [DOI] [PubMed] [Google Scholar]
- 25.Popovic M., Minceva M. Coinfection and interference phenomena are the results of multiple thermodynamic competitive interactions. Microorganisms. 2021;9:2060. doi: 10.3390/microorganisms9102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahim M., AbdElGawad B., Hassan H., Naguib A., Ahmed E., Afifi S., Abu ElSood H., Mohsen A. Epidemiology and outcome of influenza-associated infections among hospitalized patients with acute respiratory infections, Egypt national surveillance system, 2016-2019. Influenza Other Respir Viruses. 2021 Sep;15(5):589–598. doi: 10.1111/irv.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliot A., Fleming D. Surveillance of influenza‐like illness in England and wales during 1966‐2006. Euro Surveill. 2006;11:249–250. [PubMed] [Google Scholar]
- 28.D'Aeth J.C., Ghosal S., Grimm F., et al. Optimal national prioritization policies for hospital care during the SARS-CoV-2 pandemic. Nat Comput Sci. 2021;1:521–531. doi: 10.1038/s43588-021-00111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali R., Patel A., Chan K., et al. A case series of SARS-CoV-2 and influenza Co-infection. Cureus. August 31, 2021;13(8) doi: 10.7759/cureus.17597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi Y.W., Tuel A., Eltahir E.A.B. On the environmental determinants of COVID-19 seasonality. Geohealth. 2021 Jun 1;5(6) doi: 10.1029/2021GH000413. e2021GH000413, PMID: 34095688; PMCID: PMC8166213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cascella M., Rajnik M., Aleem A., et al. StatPearls Publishing; Treasure Island (FL): 2022 Jan. Features, Evaluation, and Treatment of Coronavirus (COVID-19)https://www.ncbi.nlm.nih.gov/books/NBK554776/ [Updated 2022 Feb 5]. In: StatPearls [Internet] Available from: [PubMed] [Google Scholar]
- 32.Martinez M.A. Lack of effectiveness of repurposed drugs for COVID-19 treatment. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.63537. Published 2021 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy restrictions but are available from Egypt Ministry of Health and Population on reasonable request.