Abstract
Background: Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection during pregnancy is related with adverse maternal, fetal, and neonatal outcomes. Placental SARS-CoV-2 involvement may include various degrees of inflammation and malperfusion leading to diverse pregnancy complications. Methods: Placental, fetal and umbilical cord samples of three fetal demise cases that occurred in the context of maternal SARS-CoV-2 infections were analyzed. Cases were notified to the Colombian SARS-CoV-2 National Surveillance System. RT-PCR and immunohistochemistry (IHC) analysis were employed to identify potential tissue viral involvement. Results: RT-PCR and IHC confirmed the presence of viral genomes and antigens in placental and umbilical cord tissues. Histopathological analysis revealed findings consistent with placental malperfusion and inflammation. Conclusions: SARS-CoV-2 infection during pregnancy can lead to placental dysfunction and damage compromising fetal survival. Many questions regarding SARS-CoV-2 dynamics during pregnancy including placental physiopathology and in utero transmission are still pending definitive answers.
Keywords: SARS-CoV-2, Pregnancy, Placenta, Fetus, Pathology
Background
In March 2020, the World Health Organization (WHO) confirmed the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) as the causative agent of the coronavirus disease 2019 (COVID-19) and declared the beginning of a global pandemic (Zhu et al., 2020). It is currently known that the respiratory route mainly transmits the SARS-CoV-2 virus, by inhaling respiratory droplets from infected individuals (Setti et al., 2020). Disease manifestations often involve the respiratory system, but other systems have shown to be widely compromised such as the central nervous system, cardiovascular system, renal system among others (Devaux et al., 2020).
As the COVID-19 pandemic advanced, a considerable amount of information related to the disease became available, allowing a broad comprehension of this entity. In spite of these advances, SARS-CoV-2 disease manifestations in some population groups such as pregnant women and their newborn infants are currently under research. Recent publications with large-sized samples describe the complications of SARS-CoV-2 infection during pregnancy, where a higher risk of preeclampsia, severe COVID-19, and maternal mortality has been documented (Villar et al., 2021, Wong et al., 2004, Zaigham and Andersson, 2020). Also, vertical SARS-CoV-2 transmission has been reported between 3 and 13% within RT-PCR positive mothers (Villar et al., 2021), although the clinical definition of this condition varies across reports making it difficult to truly assess its risk. When using the case definition proposed by Shah et al. (2020), confirmed congenital SARS-CoV-2 infection was found in 5.7% within 172 neonatal infections (Raschetti et al., 2020).
Although SARS-CoV-2 congenital infection is low, some reports found severe compromise in the fetus leading to fetal demise or preterm labor and early neonatal death (Reagan-Steiner et al., 2022, Richtmann et al., 2020). SARS-CoV-2 has been identified in various tissues and fluid samples collected from infected pregnant women and their fetuses, including placenta, umbilical cord, amniotic fluid, heart, liver, fetal pharyngeal swabs, among others (Marinho et al., 2021). In this context, we describe the histopathological and immunohistochemical findings in three cases of fetal deaths that occurred in pregnancies of women with confirmed SARS-CoV-2 infection prior to the beginning of SARS-CoV-2 vaccination of pregnant women in Colombia, and compare our findings with other reported cases in the literature.
Methods
The Colombian National Institute of Health (INS, Spanish for Instituto Nacional de Salud), leads the national Public Health Surveillance System (Sivigila), which captures information related to SARS-CoV-2 and other diseases. Sivigila captures information through individual data collection forms from nationwide SARS-CoV-2 notifications, performing weekly reports. SARS-CoV-2 cases are classified as laboratory confirmed when viral RNA is detected in respiratory or other samples via real time polymerase chain reaction (RT-PCR) or antigen testing.
Between July 2020 to June 2021, the INS received notification of 3 cases of pregnant women with confirmed SARS-CoV-2 infection, who had an adverse pregnancy outcome (fetal demise) and had tissue samples available for analysis. The INS received placental, umbilical cord and fetal tissue samples for molecular and histopathological analysis. Clinical data was obtained from hospital records, including fetal autopsy findings and placental macroscopic descriptions.
Histopathological and immunohistochemical studies
Maternal (placenta and umbilical cord) and fetal tissues (if available) were fixed in 40% neutral buffered formaldehyde and were histologically processed. The SARS-CoV-2 nucleocapsid protein was detected in tissues by 1) immunohistochemistry using the MACH 4 Universal AP Polymer kit detection system (Biocare Medical LLC, Concord, CA, USA), according to the manufacturer's instructions; and 2) immunofluorescence (IF). For IF detection, background fluorescence was reduced using sodium borohydride (1 mg/ml). Sections were blocked (5% BSA) and incubated overnight at 4 °C with primary antibodies. Sections were rinsed and incubated with Alexa Fluor 488 and Alexa Fluor 555-conjugated secondary antibodies. Images were captured using an LSM 900 confocal microscope (Carl Zeiss, Thornwood, NY) and analysis was performed using the ZEN blue ver. 3.4 imaging software. For the negative controls, tissues were incubated with normal rabbit serum. As a positive control, a cell block of SARS-CoV-2 Vero 6 infected cells was used.
RNA extraction and RT-PCR
FFPE tissue sections from maternal (placenta and umbilical cord) and fetal tissues (if available) were deparaffinized with Xylol and incubated in lysis buffer (10 mM Tris-HCl, 2 mM EDTA, 1% SDS, 2 mg/ml proteinase K) and at 56 °C overnight. Total RNA was extracted using the automated TANBead® Nucleic Acid Extraction Kit following the manufacturer’s recommendations and analyzed for the presence of viral RNA by using a quantitative reverse transcription PCR assay targeting the E gene (Corman et al., 2020). Positive and negative samples were included at each run.
Ethical considerations
The information used for this analysis comes from secondary data sources that were previously reviewed and anonymized, and they do not represent any risk to the community. Since the data is extracted from Sivigila, the approval of the Institutional Review Board or the use of informed consent was not necessary. In Colombia, in the context of public health emergencies or in those in which scientific research for public health purposes so requires, a clinical autopsy may be performed without the informed consent of patients or legal guardians (“Decreto 786 de 1990, 1990”). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Case description
Case 1
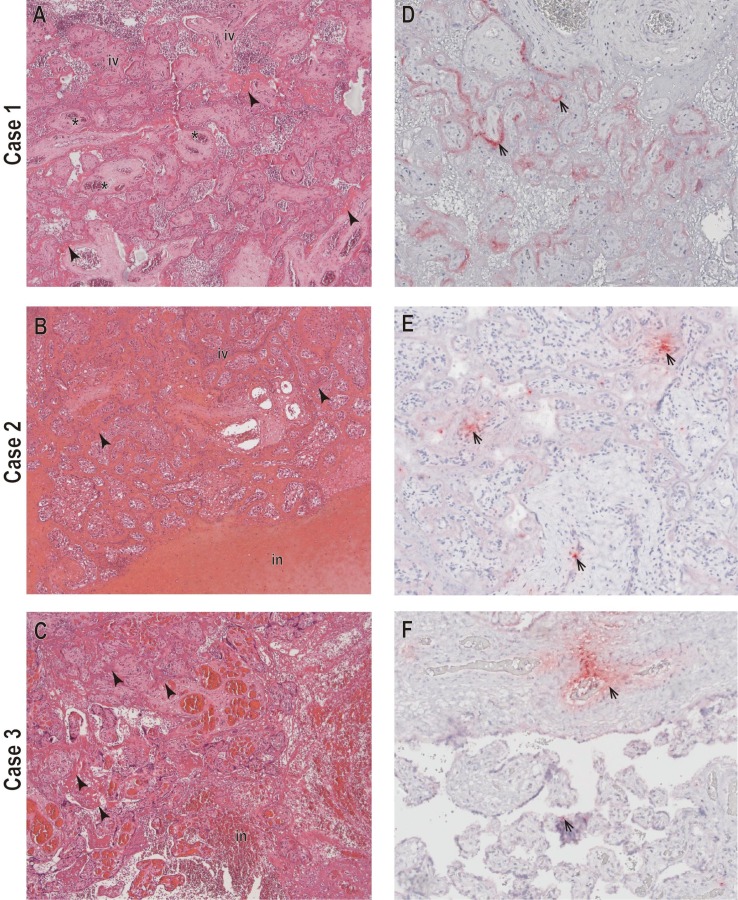
A 34-year-old pregnant woman (gravida 2, parity 2), without pregnancy complications, no history of prior SARS-CoV-2 infection and negative screening tests for STORCH agents (syphilis, toxoplasma, rubella, cytomegalovirus, and herpes). At 36 weeks of gestation the patient presented flu-like symptoms including headache, cough, and non-quantified fever. Two days after the beginning of the symptoms, she consulted the emergency department with lower abdominal pain, scant vaginal bleeding, and persistence of flu-like symptoms and was admitted for inpatient care. A RT-PCR for SARS-CoV-2 was performed in a respiratory sample, obtaining a positive result. No other respiratory agents were tested in this sample. An obstetric ultrasound confirmed fetal death and pregnancy was terminated via cesarean section. A macerated female fetus with a thrombosed nuchal cord was obtained. Fetal autopsy findings are described in Table 1 . No additional infectious agent testing was performed on tissue samples. The placental examination performed at INS revealed multiple intraparenchymal hematomas that compromised more than 30% or the placental disc. Mild acute intervillositis with accumulation of polymorphonuclear neutrophils and an area of acute infarction was observed (Fig. 1 A). The presence of SARS-CoV-2 RNA in the placenta was confirmed by RT-PCR, and viral antigens were observed inside the trophoblasts cytoplasm of chorionic villi via immunohistochemical analysis (Fig. 1D). Other histologic findings of the placenta are described in Table 2 .
Table 1.
Clinical and pathology findings of the three fetal demise cases in pregnancies with confirmed SARS-CoV-2 infection.
| Case number and notification date (DD/MM/YYYY) | Fetal sex and GA at pregnancy termination (weeks, days) | Fetal anthropometry* (Weight, g); (Height, cm); (HC, cm) | Macroscopic findings | Microscopic findings | Case conclusion |
|---|---|---|---|---|---|
|
1 (01/08/2020) |
Female; 36 2/7 | 2205 (z-score: 0.17); 47 (z-score: −1.26); 32.5 (z-score: 0.18) | -Macerated body, with sloughing of more than 50% of the total body surface -Overlapping head sutures due to softening of the encephalic structures. -Generalized visceral congestion with serohematic fluid in pleural, pericardial, and peritoneal cavities. -External petechiae and congestive appearance in both lungs. -Hepatic subcapsular hematoma. |
-Pulmonary lytic changes, interstitial edema, and alveolar hemorrhage. -Presence of interstitial hemorrhagic foci in the heart. |
Intrauterine death greater than 48 h with signs of hypoxic-ischemic injury. |
|
2 (14/06/2021) |
Female; 25 2/7 | 700 (z-score: −0.10); NA; NA | -Positional deformity of upper and lower limbs with bilateral dorsiflexion of the feet and hands, and external rotation of the hip. –Thoracic, pericardial, and peritoneal cavities with abundant hemorrhagic effusion. | -Heart: no inflammatory process. -Lung: tubular development phase, consistent with the second trimester, without inflammatory process or morphological alteration in development. | Symmetric intrauterine growth restriction and possible fetal pulmonary hypertension. |
|
3 (30/06/2021) |
Female 37 1/7 | 2630 (z-score: −0.53); 47 (z-score: −0.24) | Loose nuchal cord. Discrete skin sphacelation. | NA | NA |
Abbreviations: GA: gestational age, g: grams, cm: centimeters, NA: Not available.
*Fetal anthropometric features were calculated via Intergrwoth-21st newborn growth standards, accounting for sex and gestational age.
Fig. 1.
Histopathological and immunohistochemical findings in placental tissues from mothers infected with SARS-CoV-2. A-C) Prominent perivillous fibrin deposition (arrowheads), placental infarction (in), partial obliteration of fetal vessels (asterisks), and intervillositis with polymorphonuclear cell accumulations (in) (×100). D-F) SARS-CoV-2 nucleocapsid staining, mainly observed at the periphery of the chorionic villi (arrows), suggesting a trophoblast infection (×200).
Table 2.
Histopathology and immunohistochemistry findings in placental and fetal tissues of the three cases with confirmed SARS-CoV-2 infection during pregnancy.
| Case | Placental macroscopic fetaures | Placental microscopic features | SARS-CoV-2 RT-PCR ** | SARS-CoV-2 IHC |
|---|---|---|---|---|
| 1 | Weight*: 550 g (75th percentile). Placental disc measurements: 18.5 × 15 cm. Presence of multiple intraparenchymal hematomas (hemorrhagic areas) compromising 30% of the placental disc. | -Chorioamniotic membranes with predominantly neutrophilic inflammation. Chorioamniotic junction band with lamellar necrosis and transfer of neutrophils to the amniotic epithelium. -Villous sclerosis foci, hemorrhagic infarcts with excess in perivillous fibrin deposits, obliteration, and vascular occlusion of fetal vessels. -Mild acute intervillitis with accumulation of polymorphonuclear neutrophils and area of acute infarction. -The maternal side shows hematic dissection and endovascular thrombi. |
Positive in placental tissue | Positive in trophoblast cells of the chorionic villus. |
| 2 | NA | -Placenta with a maturation pattern consistent with the second trimester with excess perivillous fibrin deposits and Hofbauer cell hyperplasia. -Acute placental infarction with a 30% extension, presence of focal ischemic changes. -Moderate (grade II) chronic lymphoplasmacytic villitis. | Positive in placental tissue and umbilical cord Negative in fetal heart and lung tissue |
Positive in mesenchymal and endothelial cells of the chorionic villi. Positive staining in some mesenchymal cells of the umbilical cord. |
| 3 | Weight*: 432 g (25th percentile) | -Placenta with a maturation pattern consistent with the third trimester, excess perivillous fibrin deposits and Hofbauer cell hyperplasia. -Acute placental infarction with extension of approximately 30%. - Hyperplasia of syncytial cell knots. | Positive in placental tissue | Positive in mesenchymal and endothelial cells of the chorionic villi. Positive staining in some syncytial cells. |
NA: Not available. RT-PCR: reverse transcription polymerase chain reaction. IHC: immunohistochemistry.
*Placental weight percentiles calculated according to Raymond et al (2018). **Viral genome sequencing could not be performed on any of the FFPE samples due to fixation and subsequent nucleic acid degradation, resulting in fragmented RNA transcripts that were inadequate to obtain appropriate sequencing results.
Case 2
A 20-year-old primigravida with low maternal weight negative results for STORCH agents and no history of prior SARS-CoV-2 infection presented to the emergency department at 24 5/7 weeks of gestation, with general malaise, non-quantified fever, dry cough, anosmia, and hypogeusia. A positive SARS-CoV-2 antigen test from a respiratory was obtained. No other respiratory agents were tested in this sample. She had a normal fetal evaluation and was sent home with symptom management but no antiviral medications. Four days later, the patient consulted the emergency department presenting with lower abdominal pain, and upon admission, no fetal heart rate was detected. An obstetric ultrasound confirmed fetal death. A vaginal delivery was performed obtaining a female fetus. Fetal autopsy findings are described in Table 1. No additional infectious agent testing was performed on tissue samples. Placental, umbilical cord, lung and heart tissues were examined at the INS; placental findings included excess perivillous fibrin deposits and Hofbauer cell hyperplasia. An acute placental infarction with extension of approximately 30% of the placental disc was described, as well as moderate chronic lymphoplasmacytic villitis (Fig. 1B). A positive SARS-CoV-2 RT-PCR test from placental tissue was obtained. Umbilical cord examination revealed normal structures without inflammation, but IHC found positive staining in umbilical cord mesenchymal cells.
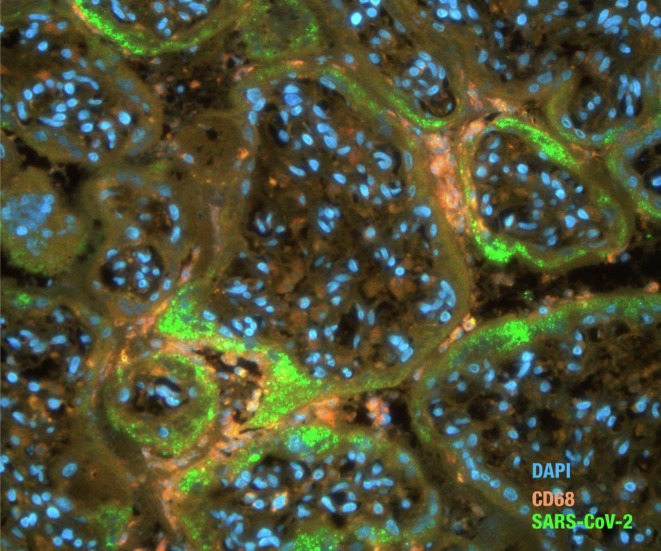
The IHC analysis identified multifocal positive staining of SARS-CoV-2 viral particles in mesenchymal and endothelial cells of the chorionic villi (Fig. 1E). In the simultaneous detection of SARS-CoV-2 viral antigens and macrophages (CD68), the viral antigens were located mainly in the peripheral regions of the chorionic villi, where numerous CD68-positive macrophages were also found (Fig. 2 ). Other placental findings are described in Table 2.
Fig. 2.
Double immunofluorescence assay in the placenta infected with SARS-CoV-2. Viral antigen detected mainly in the trophoblast layer of chorionic villi where CD68-positive cells (macrophages) were also observed. The red, green, and blue signals indicate Alexa 555, 488, and DAPI, respectively. SARS-CoV-2 IF, ×400 magnification.
Case 3
A 24-year-old, pregnant woman (gravida 2, parity 1), without any known pregnancy complications, no prior history SARS-CoV-2 infection and negative tests for STORCH agents, consulted the emergency department at 36 1/7 weeks of gestation with two days of myalgias, headache and non-quantified fever. She was found to be stable, with adequate fetal evaluation. A SARS-CoV-2 RT-PCR test was taken from a respiratory sample with a positive result. No other respiratory agents were tested in this sample. She was discharged home with symptom management and no antiviral medications. Seven days later, she presented to the emergency department with a non-fetid white vaginal discharge and uterine contractions. An obstetric ultrasound confirmed fetal death. A vaginal delivery was performed obtaining a female fetus with a loose nuchal cord. No fetal autopsy was performed and no additional infectious agent testing was performed on fetal tissue samples. Other fetal findings are described in Table 1.
Placental tissue was examined at INS finding excess perivillous fibrin deposits, Hofbauer cell and syncytial cell node hyperplasia. An acute placental infarction compromising over 30% of the placental disc was identified (Fig. 1C). SARS-CoV-2 RT-PCR testing in placental tissue was positive and IHC analysis identified multifocal positivity for viral particles in mesenchymal and endothelial cells of the chorionic villi, with a positive staining in some syncytial cells (Fig. 1F) (Table 2).
Discussion
We present three cases of fetal demise in the context of maternal SARS-CoV-2 infection, where placental viral involvement was confirmed through RT-PCR and IHC techniques. All pregnant women were previously healthy and had uncomplicated pregnancies. SARS-CoV-2 infection was confirmed by RT-PCR and/or antigen test in respiratory samples, all cases occurred in an outpatient setting, with mild and oligosymptomatic forms of COVID-19. The patients consulted the emergency department with respiratory symptoms, but no signs of acute fetal distress, cardiac, respiratory, or neurologic compromise was observed. In an average of 7 days after symptom onset a fetal demise was confirmed.
Placental and umbilical cord analysis have been crucial to understand diverse maternal and fetal interactions in the contexts of infective processes during pregnancy. The placenta acts as a natural defense barrier that actively protects the fetus from adverse maternal environments including infectious agents (Delorme-Axford et al., 2014). Despite these effective mechanisms, many microorganisms can breach the placenta and affect the fetus (Arora et al., 2017). SARS-CoV-2 has been found to affect the placenta in diverse ways, mainly related to maternal vascular malperfusion (MVM) (Horn et al., 2022), a feature that seems to be more prominent in placentas from SARS-CoV-2 infected women when compared to control groups (Sharps et al., 2020).
SARS-CoV-2 entry mechanisms to host cells involve the angiotensin- converting enzyme 2 (ACE2) and the transmembrane protease serine 2 (TMPRSS2), required for viral cell invasion (Lan et al., 2020). Cui et al. (2021) analyzed placental tissues from different trimesters and found that both ACE2 and TMPRSS2 are expressed throughout all trimesters of pregnancy and suggested the important role of these receptors in placental growth and function. These findings support the hypothesis that placental SARS-CoV-2 invasion and in utero fetal infection can occur, but also that the virus could lead to mechanisms of placental dysfunction and long-term consequences in the neonate (Shook et al., 2022, Edlow et al., 2021).
Placental tissue samples of all cases had findings consistent with MVM, including macroscopic findings such as multiple intraparenchymal hematomas (case 1), and histological findings such as endovascular thrombi, excess perivillous fibrin deposits, increased syncytial knots for gestational age and placental infarction zones (Khong et al., 2016). Case 1 showed specific findings consistent with fetal vascular malperfusion (FVM), including obliteration and vascular occlusion of fetal vessels due to collapse, and in this case a thrombosed nuchal cord was also identified. MVM and FVM are frequent findings in placentas of SARS-CoV-2 infected women, being reported as high as 46% and 35.3% respectively (Sharps et al., 2020).
Histopathology showed the presence of an inflammatory reaction, in which findings of mild acute intervillositis (case 1) and moderate chronic lymphoplasmacytic villitis (case 2) were observed. Inflammatory lesions in SARS-CoV-2 affected placentas seem to be an infrequent finding, being reported in 5–8.7% of a series of cases (Sharps et al., 2020). Although acute intervillositis has been found in a wide range of conditions, chronic lymphoplasmacytic villitis seems to be a less usual finding, and recently associated with fetal SARS-CoV-2 infection (Schwartz and Levitan, 2021). These findings raise the possibility of a direct damage of SARS-CoV-2 on the placenta (Baergen and Heller, 2020, Shanes et al., 2020).
Placental SARS-CoV-2 infection was confirmed in all three cases. IHC staining was positive for viral particles mostly present in mesenchymal and endothelial cells of the chorionic villi, but also in the syncytiotrophoblast. In utero viral transmission from the mother to the fetus occur mainly from endothelial microvasculature (maternal) to endovascular extravillous cytotrophoblasts (fetal); from infected macrophages (maternal blood) to placental trophoblast (fetal) (Delorme-Axford et al., 2014). Studies have observed an intense placental immune and inflammatory response in maternal SARS-CoV-2 infection cases (Argueta et al., 2021, Lu-Culligan et al., 2021). The examination of placental tissues from our cases detected SARS-CoV-2 RNA and protein. No simultaneous staining was observed using SARS-CoV-2 and the CD68+ macrophage marker, suggesting that viral antigens were infecting mainly the maternal tissues. In case 2, fetal heart samples were negative for viral particles but positive in umbilical cord. Umbilical cord compromise and its clinical implications in SARS-CoV-2 has not been thoroughly described and warrants further research.
Our findings are similar to recent reports that describe that SARS-CoV-2 infection is restricted to the syncytiotrophoblast of SARS-CoV-2 infected mothers (Alamar et al., 2020). However, some studies found SARS-CoV-2 in other cell types (Hofbauer cells, stromal cells, and maternal macrophages), suggesting that SARS-CoV-2 infection at earlier gestational stages could increase the viral spread from maternal to fetal tissues beyond the syncytiotrophoblast layer (Argueta et al., 2021).
The definition of SARS-CoV-2 congenital infection has been approached by different authors (Shah et al., 2020, Robaina-Castellanos & Riesgo-Rodriguez), and in 2021 the WHO released a scientific brief where definitions and categorizations of vertical SARS-CoV-2 transmission were outlined (WHO, 2021). Following the WHO categories, cases 1 and 3 could be defined in the “possible” category and case 3 in the “unlikely” category for SARS-CoV-2 in utero transmission.
Although placental involvement was present in all cases, fetal death cannot be completely and exclusively attributed to COVID-19, especially in case 1 where a possible case of fetal asphyxia due to nuchal cord occurred. Pregnancy loss including early abortions and stillbirths has been related to SARS-CoV-2 infection (Villar et al., 2021), where mechanisms of hypoxia, placental dysfunction, and maternal immune activation could act as contributing factors.
Recently, Horn et al. (2022) published two cases of sudden intrauterine death occurring in pregnancies with SARS-CoV-2 oligosymptomatic infection. Fetal testing for SARS-CoV-2 was negative, but positive in placental tissues, where histopathological findings included focal histiocytic chronic intervillositis and massive perivillous fibrin deposits with extensive necrosis of the villous trophoblast. The authors consider acute placental failure secondary to SARS-CoV-2 infection as the cause of fetal demise, where diffuse trophoblastic damage may be the main physiopathological event (Horn et al., 2022). These cases are similar to our findings, where placental compromise related to SARS-CoV-2 infection acts as a contributing factor to fetal demise.
Although SARS-CoV-2 sequencing could not be done on any of the analyzed cases, the INS performed national SARS-CoV-2 genomic surveillance since the beginning of the pandemic. In this sense, case 1 occurred during Colombiás first pandemic peak, were a high circulation of non-VOI/VOC SARS-CoV-2 lineages (B.1, B.1.111, and B.1.420) was registered. Cases 2 and 3 occurred during Colombia`s third peak where the Mu variant was the dominant strain (Instituto Nacional de Salud, 2022). Although no specific susceptibilities for pregnant women have been registered for the Mu variant, authors have found higher adverse pregnancy outcomes for infected women with the Alpha and Delta variants when compared to non-VOI/VOC strains, information that warrants further research (Vousden et al., 2022).
Conclusions
SARS-CoV-2 infection during pregnancy is currently being investigated, and many questions regarding viral dynamics, placental physiopathology, and in utero transmission are still pending definitive answers. SARS-CoV-2 has the potential to infect the placenta and cause direct damage that could lead to malperfusion, dysfunction, and placental failure with deleterious outcomes to the growing fetus. Although our cases presented some histopathological findings described in SARS-CoV-2 infected placentas, a hypoxic process leading to these findings and to the fetal demise should also be considered. Our results contribute to the understanding of this emerging disease and its consequences during gestation offering valuable information to address maternal and neonatal health.
Author’s disclaimers
The views expressed in the submitted article are their own and not an official position of the institution or funder.
Sources of support
No specific funding or grant was used to develop the work shown in the manuscript.
Ethical approval statement
The information used for this analysis comes from secondary data sources that were previously reviewed and anonymized, and they do not represent any risk to the community. Since the data is extracted from Sivigila, the approval of the Institutional Review Board or the use of informed consent was not necessary. In Colombia, in the context of public health emergencies or in those in which scientific research for public health purposes so requires, a clinical autopsy may be performed without the informed consent of patients or legal guardians (“Decreto 786 de 1990,” 1990). This analysis did not receive funding from external sources.
CRediT authorship contribution statement
Marcela Daza: Conceptualization, Writing – original draft, Writing – review & editing. Sheryll Corchuelo: Conceptualization, Writing – original draft, Writing – review & editing. Johana Osorio: Writing – original draft. Luis Alberto Gómez: Writing – review & editing. Edgar Parra: Writing – review & editing. Ángela Alarcón: Methodology. Marcela Mercado: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alamar I., Abu-Arja M.H., Heyman T., Roberts D.J., Desai N., Narula P., Dygulska B. A Possible Case of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a Newborn With Positive Placental In Situ Hybridization of SARS-CoV-2 RNA. J. Pediatric. Infect. Dis. Soc. 2020;9(5):636–639. doi: 10.1093/jpids/piaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueta, L. B., Lacko, L. A., Bram, Y., Tada, T., Carrau, L., Zhang, T., et al., 2021. SARS-CoV-2 Infects Syncytiotrophoblast and Activates Inflammatory Responses in the Placenta. 2021.2006.2001.446676. doi:10.1101/2021.06.01.446676 %J bioRxiv.
- Arora N., Sadovsky Y., Dermody T.S., Coyne C.B. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe. 2017;21(5):561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baergen R.N., Heller D.S. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020;23(3):177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.Es.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, D., Liu, Y., Jiang, X., Ding, C., Poon, L. C., Wang, H., Yang, H., 2021. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. 57(2), 248-256. doi:https://doi.org/10.1002/uog.22186. [DOI] [PMC free article] [PubMed]
- Delorme-Axford E., Sadovsky Y., Coyne C.B. The Placenta as a Barrier to Viral Infections. Annu Rev Virol. 2014;1(1):133–146. doi: 10.1146/annurev-virology-031413-085524. [DOI] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020;53(3):425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decreto 786 de 1990, (1990), por el cual se reglamenta parcialmente el Título IX de la Ley 09 de 1979, en cuanto a la práctica de autopsias clínicas y médico-legales, así como viscerotomías y se dictan otras disposiciones. Retrieved from: https://www.suin-juriscol.gov.co/viewDocument.asp?id=1165673.
- Edlow, A.G., Castro, V.M., Shook, L.L., Kaimal, A.J., Perlis, R.H., 2021. Neurodevelopmental outcomes at one year in offspring of mothers who test positive for SARS-CoV-2 during pregnancy. 2021.2012.2015.21267849. doi:10.1101/2021.12.15.21267849 %J. medRxiv.
- Horn L.C., Krücken I., Hiller G.G.R., Niedermair M., Perac K., Pietsch C., Höhn A.K. Placental pathology in sudden intrauterine death (SIUD) in SARS-CoV-2-positive oligosymptomatic women. Arch. Gynecol. Obstet. 2022;1–12 doi: 10.1007/s00404-022-06614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Salud, 2022. COVID-19 en Colombia. Retrieved from https://www.ins.gov.co/Noticias/Paginas/coronavirus-genoma.aspx.
- Khong T.Y., Mooney E.E., Ariel I., Balmus N.C., Boyd T.K., Brundler M.A., Gordijn S.J. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016;140(7):698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lu-Culligan A., Chavan A.R., Vijayakumar P., Irshaid L., Courchaine E.M., Milano K.M., Farhadian S.F. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y) 2021;2(5):591–610.e510. doi: 10.1016/j.medj.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho P.S., da Cunha A., Chimelli L., Avvad-Portari E., Andreiuolo F.D.M., de Oliveira-Szejnfeld P.S., Prata-Barbosa A. Case Report: SARS-CoV-2 Mother-to-Child Transmission and Fetal Death Associated With Severe Placental Thromboembolism. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.677001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020;11(1):5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Steiner S., Bhatnagar J., Martines R.B., Milligan N.S., Gisondo C., Williams F.B., Zaki S.R. Detection of SARS-CoV-2 in Neonatal Autopsy Tissues and Placenta. Emerg. Infect. Dis. 2022;28(3):510–517. doi: 10.3201/eid2803.211735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtmann R., Torloni M.R., Oyamada Otani A.R., Levi J.E., Crema Tobara M., de Almeida Silva C., Macoto Kondo M. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series. Case Rep Womens Health. 2020;27:e00243. doi: 10.1016/j.crwh.2020.e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.A., Levitan D. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infecting Pregnant Women and the Fetus, Intrauterine Transmission, and Placental Pathology During the Coronavirus Disease 2019 (COVID-19) Pandemic: It's Complicated. Arch. Pathol. Lab. Med. 2021;145(8):925–928. doi: 10.5858/arpa.2021-0164-ED. [DOI] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Miani A. Airborne Transmission Route of COVID-19: Why 2 Meters/6 Feet of Inter-Personal Distance Could Not Be Enough. Int. J. Environ. Res. Public Health. 2020;17(8) doi: 10.3390/ijerph17082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P.S., Diambomba Y., Acharya G., Morris S.K., Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. Gynecol. Scand. 2020;99(5):565–568. doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharps M.C., Hayes D.J.L., Lee S., Zou Z., Brady C.A., Almoghrabi Y., Heazell A.E.P. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook L.L., Sullivan E.L., Lo J.O., Perlis R.H., Edlow A.G. COVID-19 in pregnancy: implications for fetal brain development. Trends Mol. Med. 2022;28(4):319–330. doi: 10.1016/j.molmed.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J., Ariff S., Gunier R.B., Thiruvengadam R., Rauch S., Kholin A., Papageorghiou A.T. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N., Ramakrishnan R., Bunch K., Morris E., Simpson N.A.B., Gale C., Knight M. Severity of maternal infection and perinatal outcomes during periods of SARS-CoV-2 wildtype, alpha, and delta variant dominance in the UK: prospective cohort study. 2022;1(1):e000053. doi: 10.1136/bmjmed-2021-000053. %J BMJ Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2021. Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2. In (Sexual and Reproductive Health and Research, WHO ed., pp. 14).
- Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C., Tan P.Y. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020;99(7):823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]