Abstract
Vaccination is the most effective intervention for the primary prevention of COVID-19. Several studies have been conducted in sub-Saharan African countries on the acceptance and associated factors of COVID-19 vaccine. This review and meta-analysis aimed to recapitulate the pooled magnitude of vaccine acceptance and its favoring factors in sub-Saharan African countries. PUBMED, MEDLINE, Science Direct, Web of Science, and SCOPUS were the main databases searched from 15 March to 5 June 2022; and all the articles written in the English language were included. Also, some articles were retrieved from biomedical peer-reviewed journal sites and Google scholar. The quality of thirty-five selected articles was evaluated using an adapted scale for evaluating cross-sectional studies based on the Newcastle-Ottawa Scale. The result of the review and meta-analysis revealed that COVID-19 vaccine acceptance rate varied across studies. In a pooled analysis, factors such as; higher-level perception of infection risk (OR (95% CI (2.7 (2.1, 3.4))), perceived vaccine safety (13.9 (9.2, 20.9)), virus-related good knowledge (2.7 (2.3, 3.2)) and appropriate attitude (5.9 (4.4, 7.8)), adherence to safety precautions (5.5 (4.8, 6.2)), and infection experience (4.4 (2.8, 6.9)) were positively affected the COVID-19 vaccine acceptance. Also, vaccine acceptance was found to be high among males and chronically ill individuals. Thus, understanding factors that enhance vaccine acceptance would support planners to augment vaccine uptake in the region.
Keywords: COVID-19, Vaccine, Acceptance, Sub-Saharan, Africa, meta-Analysis
1. Introduction
The novel coronavirus disease 2019 (COVID-19) was first identified in one of China’s cities, Wuhan, in December 2019 [1]. Thereafter, the infection spread to more than 200 countries around the world and the World Health Organization finally declared the disease a pandemic [2]. COVID-19 has had extraordinary influences on the health and well-being of people all over the world and on the economy of many countries [3,4]. The lasting solution to COVID-19 would be, most likely, a safe and globally implemented vaccination program [5]. Mass vaccination is a crucial step in decreasing the spread of infectious diseases by inducing herd immunity [6,7]. Similarly, the spread of COVID-19 could be reduced or controlled by effective vaccines and widespread vaccination. However, trust is a significant factor in either acceptance or hesitance of vaccines [8,9] in general and the COVID-19 vaccine in particular.
Globally, COVID-19 vaccine acceptance varies widely. It was reported as greater than 90% in Malaysia [10,11], 74% in South-East Asia, 52% in Eastern Mediterranean [12], and as low as 43% in Greece [11], and about 20% in Lebanon [10]. In sub-Saharan African countries, the level of acceptance ranged from 28% [13] in the Democratic Republic of Congo (DRC) to 92% [14,15] in Ethiopia. The acceptance rate was found to be below 50% in most studies that surveyed healthcare workers [13,[16], [17], [18]] and university and school communities [19–22].
Studies identified that acceptance, intention, and willingness to get vaccinated significantly varied between the sex of respondents [13,15,17,19,20,[23], [24], [25], [26], [27]]. Also, some studies identified that a higher perceived level of COVID-19 risk positively contributed to the acceptance [23,[28], [29], [30], [31], [32]]. Moreover, good knowledge [13,26,30,[33], [34], [35]], appropriate attitude [13,28,33], and a higher level of adherence to safety precautions [34,36,37] were found to positively influence vaccine acceptance. Some studies pointed out that participants who had been infected by COVID-19 [21,29,38] have a better acceptance of the vaccine.
Despite the fatality of disease and free-of-charge distribution of vaccines in developing countries, the level of acceptance of vaccines in most cases remained low. This systematic review and meta-analysis aimed to assess the pooled prevalence of acceptance and to identify collective predictors of acceptance of the COVID-19 vaccines. The findings could provide insight for policymakers to focus intervention on significant factors that increase vaccine acceptance and hence increase vaccine uptake and vaccination levels and combat the spread of infection.
2. Materials and methods
2.1. Review protocol
A review protocol was not done for this review and meta-analysis and it was not registered. However, we checked frequently whether it was registered so far or not and confirmed that there is no registered protocol with the same title in sub-Saharan African studies in PROSPERO (https://www.crd.york.ac.uk/prospero/#searchadvanced).
2.2. Search strategy
In this systematic review and meta-analysis, research articles were searched irrespective of the study setting. We searched articles from the 15th of March to the 5th of June 2022. PUBMED, MEDLINE, Science Direct, Web of Science, and SCOPUS were the main databases used to search the articles. In addition, some articles were further identified from biomedical peer-reviewed journal sites and Google scholar. We used a search term (((((((((((COVID-19) OR Coronavirus) OR Corona) AND Vaccine) OR Immunization) AND Acceptance) OR Willingness) OR intention) OR Intent) AND Associated factors) AND Africa) OR sub-Sharan Africa and identified about 61,169 articles published up to May 31, 2022.
2.3. Evidence evaluation
All identified articles were first evaluated based on their titles and abstracts by both authors. Then, the selected 191 studies were fully evaluated for eligibility again by both authors. Finally, the quality of eligible articles was evaluated by using an adapted scale for evaluating cross-sectional studies based on the Newcastle-Ottawa Scale (NOS) [[39], [40], [41]]. NOS is one of the most common scales to assess quality and risk of bias in observational studies [42,43]. Each article was evaluated for clearly stated objectives, subject selection, comparability, and outcome. The scores are categorized as very good (9–10 points), good (7–8 points), satisfactory (5–6 points), and unsatisfactory (0–4 points). Then, both authors evaluated each article individually and in case of disagreement, the final evaluation result was made based on a common consensus.
2.4. Inclusion criteria
We included articles for review and meta-analysis based on the following criteria.
-
-
Publication year: all published articles up to the 31st of May 2022 were included.
-
-
Place of study: all studies conducted in sub-Saharan African countries
-
-
Language: articles reported/published in the English language were used (articles written in a non-English language were not found).
-
-
Study design: all studies were conducted using a cross-sectional design and all eligible articles are included in the review and meta-analysis.
-
-
Outcome: in the included articles, concepts such as “acceptance”, “intention”, and “willingness” were used interchangeably to measure a similar idea “whether participants were willing to get vaccinated when vaccines are available” and we considered both terms as acceptance.
2.5. Data analysis
The main findings of each study were described in the table and narrated in texts. In addition, the data from each identified article were extracted on the Microsoft Excel spreadsheet and exported to STATA 14 software for meta-analysis.
The analysis to determine the pooled prevalence of the COVID-19 vaccine acceptance was done on 35 articles. Then, the pooled odds ratio of factors associated with the acceptance was done on different explanatory variables and the statistical significance was determined at the p-value of <0.05 of the estimates and >0.05 of the I2.
2.6. Heterogeneity and publication bias
Heterogeneity among the included studies was checked by its corresponding I2 test statistics. For significant variability between studies as of I2 > 75% and its corresponding p-value of <0.05, subgroup analysis was done to minimize variability. The presence of publication bias was checked by Egger’s statistics, and a p-value of >0.05 was used to determine the absence of bias.
3. Results
3.1. Description of the studies
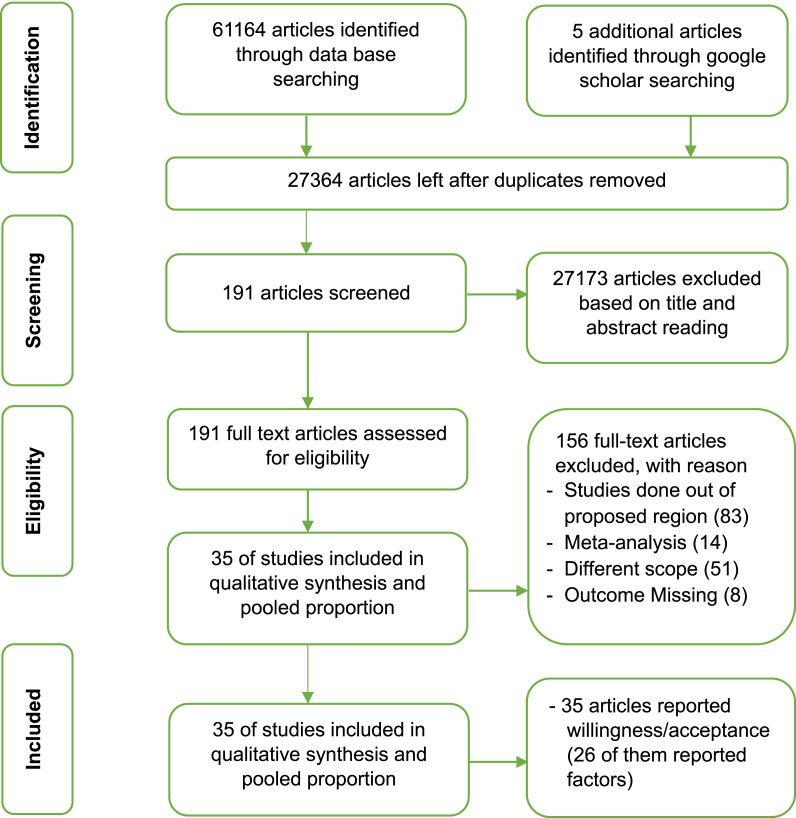
A total of 61,164 articles were identified through database searching and 5 more articles were identified through google scholar search. After removing 33,805 duplicates and 27,173 unrelated papers, 191 articles were identified for further screening. Then, the identified 191 related articles were fully evaluated for eligibility and 156 of them were excluded with reasons. Finally, 35 articles that fulfilled the selection criterion were included in the review, and 26 of them were further screened for meta-analysis of factors associated with acceptance of COVID-19 vaccine [Fig. 1]. Out of 35 studies, 13 were online surveys. Only six studies were conducted at the community level and the rest 16 were done in the facility settings. Details of the description of selected articles were presented in the table [Table 1].
Fig. 1.
Flow diagram of the studies selection process.
Table 1.
COVID-19 vaccine acceptance rate in sub-Saharan African countries, results from cross-sectional studies conducted between 2020 and 2021.
| Study | Country | Year of survey | Outcome | Type of survey | Study population | Quality score (10) | Total population | Acceptance | Acceptance rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Emmanuel Lamptey et al. [23] | Ghana | Oct.–Dec./2020 | Acceptance | Online | GP3 | 8 | 1000 | 541 | 54.1 |
| Tlale LB et al. [36] | Botswana | Feb./2021 | Acceptance | Community | GP3 | 9 | 5300 | 3689 | 69.6 |
| Mesele M. [24] | Ethiopia | Apr./2021 | Acceptance | Community | GP3 | 8 | 415 | 189 | 45.5 |
| Ahmed M.A.M. et al. [44] | Somalia | Dec.–Jan./20211 | Acceptance | Online | GP3 | 7 | 4543 | 3488 | 76.8 |
| Dula J. et al. [45] | Mozambique | Mar./2021 | Acceptance | Online | GP3 | 7 | 1878 | 1340 | 71.4 |
| Ditekemena J.D. et al. [46] | DRC2 | Aug.–Sep./2020 | Acceptance | Online | GP3 | 8 | 4131 | 2310 | 55.9 |
| Eze U A et al. [25] | Nigeria | Nov.–Jan./20211 | Acceptance | Community | GP3 | 7 | 358 | 237 | 66.2 |
| Acheampong T. et al. [47] | Ghana | Feb./2021 | Willingness | Online | GP3 | 7 | 2345 | 1197 | 51.0 |
| McAbee L. et al. [48] | Zimbabwe | May/2021 | Intention | Community | GP3 | 8 | 551 | 307 | 55.7 |
| Adedeji-Adenola H. et al. [38] | Nigeria | Jun./2021 | Willingness | Online | GP3 | 7 | 1051 | 856 | 81.4 |
| Echoru et al. [49] | Uganda | Jul.–Sep./2020 | Acceptance | Online | GP3 | 7 | 1067 | 572 | 53.6 |
| Amo-Adjei et al. [50] | Ghana | Apr.–May/2021 | Willingness | Community | GP3 | 7 | 415 | 291 | 70.1 |
| Abebe et al. [30] | Ethiopia | Mar./2021 | Acceptance | Community | GP3 | 7 | 492 | 308 | 62.6 |
| Belsti et al. [27] | Ethiopia | Feb.–Mar./2021 | Willingness | Online | GP3 | 7 | 1184 | 372 | 31.4 |
| Oyekale A.S. et al. [14] | Ethiopia | Feb./2021 | Willingness | Online | GP3 | 7 | 2178 | 2011 | 92.3 |
| Kabamba Nzaji et al. [13] | DRC2 | Mar.–Apr./2020 | Acceptance | Facility | HCW4 | 7 | 613 | 170 | 27.7 |
| Adeniyi O.V. et al. [51] | South Africa | Nov.–Dec./2020 | Acceptance | Facility | HCW4 | 9 | 1308 | 1179 | 90.1 |
| Alle Y.F. et al. [16] | Ethiopia | Feb.–Mar./2021 | Acceptance | Facility | HCW4 | 8 | 327 | 135 | 41.3 |
| Alhassan et al. [17] | Ghana | Sep. – Oc./2020 | Willingness | Facility | HCW4 | 8 | 1142 | 520 | 45.5 |
| Oduwole E.O. et al. [52] | South Africa | Feb.–Mar./2021 | Willingness | Online | HCW4 | 7 | 1015 | 781 | 76.9 |
| F.A. Gbeasor-Komlanvi et al. [18] | Togo | Feb.–Mar./2021 | Acceptance | Online | HCW4 | 7 | 1115 | 492 | 44.1 |
| Berihun et al. [33] | Ethiopia | May/2021 | Acceptance | Facility | CD5 | 8 | 416 | 247 | 59.4 |
| Mesfin et al. [26] | Ethiopia | Mar.–Apr./2021 | Intention | Facility | CD5 | 7 | 398 | 134 | 33.7 |
| C. Kassa Mekonnen et al. [35] | Ethiopia | Feb.–Mar./2021 | Intention | Facility | CD5 | 7 | 423 | 270 | 63.8 |
| Tadele Admasu [29] | Ethiopia | May–Aug./2021 | Willingness | Facility | CD5 | 8 | 422 | 230 | 54.5 |
| Kanyike et al. [19] | Uganda | Mar./2021 | Acceptance | Facility | UC6 | 7 | 600 | 224 | 37.3 |
| Sahile et al. [20] | Ethiopia | May–Jul./2021 | Acceptance | Facility | UC6 | 8 | 407 | 162 | 39.8 |
| Handebo S et al. [53] | Ethiopia | Dec.–Jan./20211 | Intention | Facility | UC6 | 8 | 301 | 165 | 54.8 |
| Mustapha M. et al. [21] | Nigeria | Mar.–Jun./2021 | Acceptance | Online | UC6 | 7 | 440 | 176 | 40.0 |
| Zewude and Habtegiorgis [31] | Ethiopia | Mar./2021 | Willingness | Facility | UC6 | 7 | 319 | 147 | 46.1 |
| Uzochukwu IC et al. [22] | Nigeria | Jan.–Feb./2021 | Willingness | Online | UC6 | 7 | 349 | 121 | 34.7 |
| Taye E.B. et al. [28] | Ethiopia | Aug.–Sep./2021 | Acceptance | Facility | PL7 | 8 | 519 | 322 | 60.0 |
| Mose A. et al. [34] | Ethiopia | Jan./2021 | Acceptance | Facility | PL7 | 8 | 396 | 280 | 70.7 |
| Mose [37] | Ethiopia | Feb.–Mar./2021 | Willingness | Facility | PL7 | 7 | 630 | 384 | 60.9 |
| Hailemariam S. et al. [32] | Ethiopia | Feb.–Mar./2021 | Intention | Facility | PL7 | 7 | 412 | 129 | 31.3 |
Study was started in 2020 end ended in 2021;
Democratic Republic of Congo;
General Population;
Health Care Workers;
Chronic Disease sick patients,
University/College/School Community;
Pregnant/Lactating Mothers
3.2. Acceptance of COVID-19 vaccine
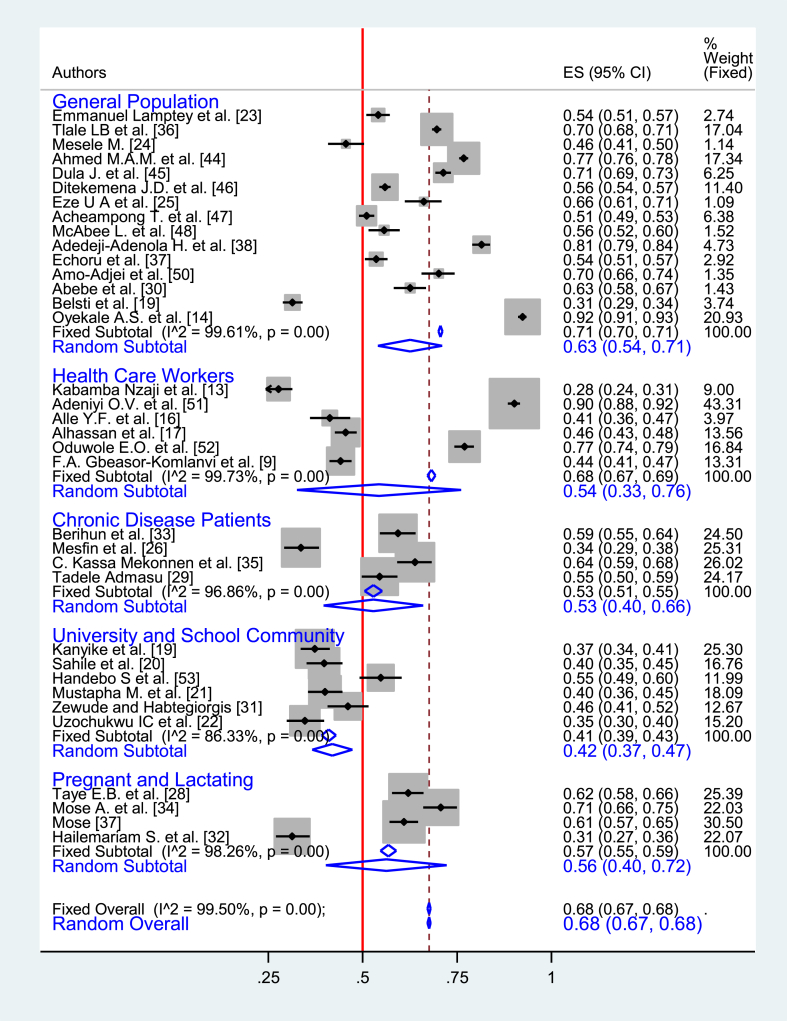
Among 35 studies included in this review, all of them were cross-sectional quantitative studies. In these studies, the COVID-19 vaccine acceptance rate ranged from 28% (95% CI: 24%–31%) in DRC [13] to 92% (95% CI: 91%–93%) in Ethiopia [14]. In about 65% of the studies, the COVID-19 vaccine acceptance rate was more than 50%. Among the total population of 38,460 of 35 studies, 26,908 were general population and 5520 were health care workers. The pooled prevalence of the vaccine acceptance that was derived from Random Effect Analysis (REA) was 68% (95% CI: 67%–68%). In the pooled prevalence, there was considerable heterogeneity between studies (I2 = 99.5%, p < 0.001) that couldn’t be resolved by a sub-group analysis. However, in a sub-group analysis, the pooled prevalence of vaccine acceptance from REA among healthcare workers was 54% (95% CI: 33%–76%), while it was 63% (95% CI: 54%–71%) among the general population [Fig. 2].
Fig. 2.
Subgroup analysis of the prevalence of COVID-19 vaccine acceptance in sub-Saharan African countries, 2020–2021.
3.3. Factors associated with COVID-9 vaccine acceptance
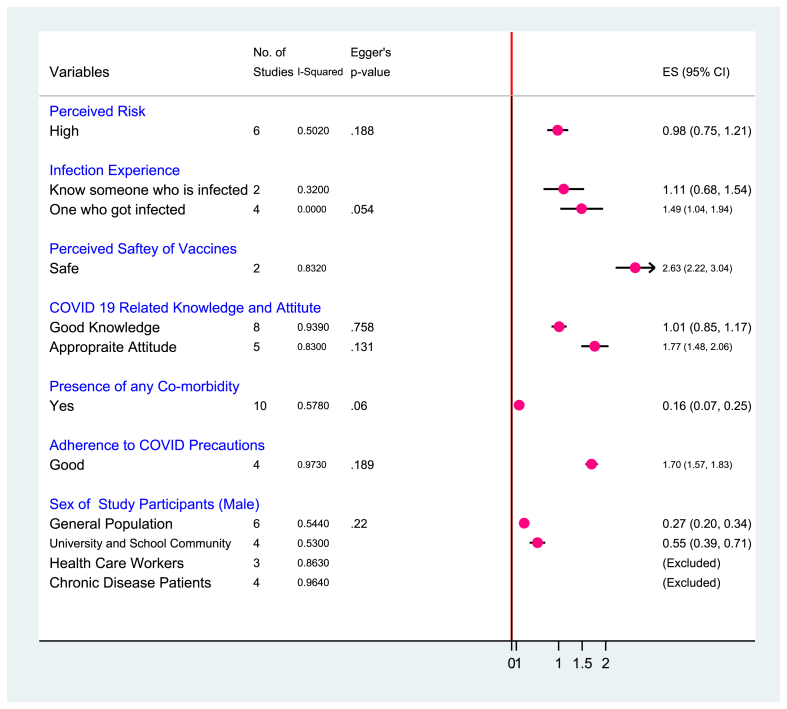
A forest plot of six studies [28–33] was done to assess the pooled effect of the perceived level of COVID-19 risk on vaccine acceptance. The studies showed no significant evidence of small study bias (Egger’s p = 0.188). The REA displayed that moderate heterogeneity was observed between studies (I2 = 50.2%, p = 0.074). As a result, the pooled effect of higher-level perception of disease risk was found to increase vaccine acceptance ((coff. (0.98 (0.75, 1.21))) [OR: 2.7, 95% CI: 2.1, 3.4]) [Fig. 3].
Fig. 3.
A meta-analysis of factors associated with the COVID-19 vaccine acceptance in sub-Saharan African countries, 2020–2021.
In two studies [24,33] (I2 = 32.0%, p = 0.225), knowing someone who was infected with COVID-19 increased vaccine acceptance ((coff. (1.11 (0.68, 1.54))) [OR: 3.0, 95% CI: 1.9, 4.7]). Similarly, in four studies [21,24,29,38] (I2 = 0.0%, p = 0.430, Egger’s p = 0.054), self-experience of COVID-19 infection increased vaccine acceptance ((coff. (1.49 (1.03, 1.94))) [OR: 4.4, 95% CI: 2.8, 6.9]). Also, in two studies [48,51] having substantial heterogeneity (I2 = 83.2%, p = 0.015), a high level of perception of vaccine safety was found to increase vaccine acceptance ((coff. (2.63 (2.22, 3.04))) [OR: 13.9, 95% CI: 9.2, 20.9]) [Fig. 3].
COVID-19-related level of knowledge and attitude was found to determine vaccine acceptance. Eight studies [13,20,26,30,[33], [34], [35],37] (I2 = 93.9%, p = 0.000, Egger’s p = 0.758), revealed that good level of COVID-19 related knowledge predicted vaccine acceptance ((coff. (1.01 (0.85, 1.17))) [OR: 2.7, 95% CI: 2.3, 3.2]). At the time, five studies that evaluated the attitude [13,28,33,34,37] (I2 = 83.0%, p = 0.000, Egger’s p = 0.131) showed that appropriate level of attitude towards COVID-19 increased vaccine acceptance ((coff. (1.77 (1.48, 2.06))) [OR: 5.9, 95% CI: 4.4, 7.8]). Likewise, a good level of adherence to COVID-19 precautions in four studies [13,34,36,37] (I2 = 97.3%, p = 0.000, Egger’s p = 0.189), found to increase vaccine acceptance ((coff. (1.70 (1.57, 1.83))) [OR: 5.5, 95% CI: 4.8, 6.2]) [Fig. 3].
Ten studies [21,26,30,34,36,38,[44], [45], [46],51] (I2 = 57.8%, p = 0.011, Egger’s p = 0.06), revealed that the presence of any comorbidity increases vaccine acceptance ((coff. (0.16 (0.07, 0.25))) [OR: 1.2, 95% CI: 1.1, 1.3]). On the other hand, six [[23], [24], [25],27,36,38] (I2 = 54.4%, p = 0.052, Egger’s p = 0.22) and four [[19], [20], [21],31] (I2 = 53.0%, p = 0.095, Egger’s p = 0.22) studies showed that being male sex increased vaccine acceptance among general population and University/School community respectively [Fig. 3].
4. Discussion
According to scientific estimates, depending on the vaccine efficacy and population heterogeneity, about 60–90% protected or immune population would be needed to break the alarming level of spread of the COVID-19 virus [7,54,55]. Thus, estimating the COVID-19 vaccine acceptance and identifying its determinants can be helpful for administrators and policymakers to plan intervention measures required to increase community awareness and ensure vaccine coverage, which in turn would help to control virus spread and reduce the negative impact of the disease [56,57].
In this review and meta-analysis, the COVID-19 vaccine acceptance rate in sub-Saharan African countries was considerably heterogeneous. In the region, a pooled prevalence of vaccine acceptance was 68% in aggregate and 63% among the general population, and 54% among healthcare workers. Though acceptance rates largely vary, irrespective of the surveyed population, the pooled vaccine acceptance rate of the eligible studies done early in 2020 (before midyear) and those included in this review was 78%. The acceptance rate has fallen to 54% in studies conducted in late 2020 (after midyear). Similarly, the pooled vaccine acceptance rate from studies conducted early in 2021 raised to 69% and dropped to 59% in studies done in late 2021. From this pattern, one can understand that early 2020 was the time World Health Organization declared COVID-19 a pandemic [2], and its devastating impact forced individuals to accept survival measures. People’s cultural beliefs, trust in government, and actual observation of disease progression, as well as reported findings [58,59] of slow progress, might have altered an individual’s attitude towards vaccination.
A pooled analysis of determinants of the COVID-19 vaccine acceptance identified that knowledge about the virus, its severity, and being infected or having relatives who were infected, were positively increased vaccine acceptance. It is acceptable that people who knew about the disease or had an infection experience would value their health. Those who believe nothing worth more than health; could accept interventions that improve their health [45,60]. Also, people’s vaccine acceptance behavior could be affected by healthcare workers' knowledge and perception [61]. Healthcare workers' knowledge and confidence were found to be related to their level of education [[61], [62], [63]]. Which in turn is associated with vaccine confidence, and may contribute to higher vaccine acceptance, trust, and uptake [64,65].
An appropriate attitude about COVID-19 and a good level of compliance with its safety measures appears to be associated with vaccine acceptance. Attitude and compliance are mainly related to knowledge as evidenced by other findings [66]. Knowledge, predominantly of healthcare providers has a paramount impact in modeling the behavior of the general population [61]. Good adherence and attitude could also be linked with trust in institutions and government [67].
Besides, vaccine acceptance in this review was found affected by the sex of respondents. Males are more willing to accept the vaccine than females as a finding from similar studies [12,68,69]. Studies pointed out that males have relatively good health-seeing behavior [70,71], a lower belief in rumors, and a higher risk perception for the disease [72,73]; while females were reported to have adopted more negative views about vaccination [74]. In addition, health-related knowledge and treatment awareness coupled with socio-cultural factors such as beliefs and traditions are factors affecting African women’s health seeing behavior in general [75].
Due to few articles reporting vaccine hesitance and uptake, this review and meta-analysis mainly focused on the acceptance of COVID-19 vaccine in sub-Saharan African countries. Some socio-demographic factors such as age, education, and income were not analyzed in this review for the reason of mismatching categories used to measure them among studies.
5. Conclusion
COVID-19 vaccine acceptance rate varied among studies in sub-Saharan African countries. The analysis revealed that modifiable and actionable factors played a significant role in determining vaccine acceptance.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e13037.
Contributor Information
Temesgen Worku Gudayu, Email: teme.worku@gmail.com.
Hibist Tilahun Mengistie, Email: hibisttilahun21@gmail.com.
Abbreviations
- DRC
Democratic Republic of Congo
- REA
Random Effect Analysis
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Ge H., Wang X., Yuan X., Xiao G., Wang C., Deng T., et al. The epidemiology and clinical information about COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(6):1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed.: Atenei Parmensis. 2020;91(1):157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucero-Prisno D.E., Adebisi Y.A., Lin X. Current efforts and challenges facing responses to 2019-nCoV in Africa. Global Health Res. Policy. 2020;5(1):1–3. doi: 10.1186/s41256-020-00148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogunkola I.O., Adebisi Y.A., Imo U.F., Odey G.O., Esu E., Lucero‐Prisno D.E., III Rural communities in Africa should not be forgotten in responses to COVID‐19. Int. J. Health Plann. Manag. 2020;35(6):1302–1305. doi: 10.1002/hpm.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeRoo S.S., Pudalov N.J., Fu L.Y. Planning for a COVID-19 vaccination program. JAMA. 2020;323(24):2458–2459. doi: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 6.Anderson R.M., May R.M. Oxford University Press; 1992. Infectious Diseases of Humans: Dynamics and Control. [Google Scholar]
- 7.Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latkin C.A., Dayton L., Yi G., Konstantopoulos A., Boodram B. Trust in a COVID-19 vaccine in the US: a social-ecological perspective. Soc. Sci. Med.(1982) 2021;270 doi: 10.1016/j.socscimed.2021.113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson H., Leask J., Aggett S., Sevdalis N., Thomson A. A multidisciplinary research agenda for understanding vaccine-related decisions. Vaccines. 2013;1(3):293–304. doi: 10.3390/vaccines1030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakeel C.S., Mujeeb A.A., Mirza M.S., Chaudhry B., Khan S.J. Global COVID-19 vaccine acceptance: a systematic review of associated social and behavioral factors. Vaccines. 2022;10(1):110. doi: 10.3390/vaccines10010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q., Yang L., Jin H., Lin L. Vaccination against COVID-19: a systematic review and meta-analysis of acceptability and its predictors. Prev. Med. 2021;150 doi: 10.1016/j.ypmed.2021.106694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norhayati M.N., Yusof R.C., Azman Y.M. Systematic review and meta-analysis of COVID-19 vaccination acceptance. Front. Med. 2021;8 doi: 10.3389/fmed.2021.783982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nzaji M.K., Ngombe L.K., Mwamba G.N., Ndala D.B.B., Miema J.M., Lungoyo C.L., et al. Acceptability of vaccination against COVID-19 among healthcare workers in the Democratic Republic of the Congo. Pragmatic Observational Res. 2020;11:103. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyekale A.S. Willingness to take COVID-19 vaccines in Ethiopia: an instrumental variable probit approach. Int. J. Environ. Res. Publ. Health. 2021;18(17):8892. doi: 10.3390/ijerph18178892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanyanda S., Markhof Y., Wollburg P., Zezza A. Acceptance of COVID-19 vaccines in sub-Saharan Africa: evidence from six national phone surveys. BMJ Open. 2021;11(12) doi: 10.1136/bmjopen-2021-055159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alle Y.F., Oumer K.E. Attitude and associated factors of COVID-19 vaccine acceptance among health professionals in Debre Tabor Comprehensive Specialized Hospital, North Central Ethiopia; 2021: cross-sectional study. Virusdisease. 2021;32(2):272–278. doi: 10.1007/s13337-021-00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhassan R.K., Owusu-Agyei S., Ansah E.K., Gyapong M. COVID-19 vaccine uptake among health care workers in Ghana: a case for targeted vaccine deployment campaigns in the global south. Hum. Resour. Health. 2021;19(1):1–12. doi: 10.1186/s12960-021-00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gbeasor-Komlanvi F., Afanvi K., Konu Y., Agbobli Y., Sadio A., Tchankoni M., et al. Prevalence and factors associated with COVID-19 vaccine hesitancy in health professionals in Togo, 2021. Public Health Pract. 2021;2 doi: 10.1016/j.puhip.2021.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanyike A.M., Olum R., Kajjimu J., Ojilong D., Akech G.M., Nassozi D.R., et al. Acceptance of the coronavirus disease-2019 vaccine among medical students in Uganda. Trop. Med. Health. 2021;49(1):1–11. doi: 10.1186/s41182-021-00331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahile A.T., Mulugeta B., Hadush S., Fikre E.M. COVID-19 vaccine acceptance and its predictors among college students in Addis Ababa, Ethiopia, 2021: a cross-sectional Survey. Patient Prefer. Adher. 2022;16:255. doi: 10.2147/PPA.S348132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustapha M., Lawal B.K., Sha’aban A., Jatau A.I., Wada A.S., Bala A.A., et al. Factors associated with acceptance of COVID-19 vaccine among University health sciences students in Northwest Nigeria. PLoS One. 2021;16(11) doi: 10.1371/journal.pone.0260672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzochukwu I.C., Eleje G.U., Nwankwo C.H., Chukwuma G.O., Uzuke C.A., Uzochukwu C.E., et al. COVID-19 vaccine hesitancy among staff and students in a Nigerian tertiary educational institution. Ther. Adv. Infect. Dis. 2021;8 doi: 10.1177/20499361211054923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamptey E., Serwaa D., Appiah A.B. A nationwide survey of the potential acceptance and determinants of COVID-19 vaccines in Ghana. Clin. Exp. Vaccine Res. 2021;10(2):183. doi: 10.7774/cevr.2021.10.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mesele M. COVID-19 vaccination acceptance and its associated factors in Sodo Town, Wolaita Zone, Southern Ethiopia: cross-sectional study. Infect. Drug Resist. 2021;14:2361. doi: 10.2147/IDR.S320771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eze U.A., Ndoh K.I.N., Ibisola B.A., Onwuliri C.D., Osiyemi A., Ude N., et al. 2021. Determinants for Acceptance of COVID-19 Vaccine Among Nigerians. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesfin Y., Argaw M., Geze S., Zewdu B.T. Factors associated with intention to receive COVID-19 vaccine among HIV positive patients attending ART clinic in Southwest Ethiopia. Patient Prefer. Adherence. 2021;15:2731. doi: 10.2147/PPA.S342801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belsti Y., Gela Y.Y., Akalu Y., Dagnew B., Getnet M., Seid M.A., et al. Willingness of Ethiopian population to receive COVID-19 vaccine. J. Multidiscip. Healthc. 2021;14:1233. doi: 10.2147/JMDH.S312637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taye E.B., Taye Z.W., Muche H.A., Tsega N.T., Haile T.T., Tiguh A.E. COVID-19 vaccine acceptance and associated factors among women attending antenatal and postnatal cares in Central Gondar Zone public hospitals, Northwest Ethiopia. Clin. Epidemiol. Global Health. 2022;14 doi: 10.1016/j.cegh.2022.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Admasu F.T. Knowledge and proportion of COVID-19 vaccination and associated factors among cancer patients attending public hospitals of Addis Ababa, Ethiopia, 2021: a Multicenter Study. Infect. Drug Resist. 2021;14:4865. doi: 10.2147/IDR.S340324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abebe H., Shitu S., Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect. Drug Resist. 2021;14:2015. doi: 10.2147/IDR.S312116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zewude B., Habtegiorgis T. Willingness to take COVID-19 vaccine among people most at risk of exposure in Southern Ethiopia. Pragmatic Observational Res. 2021;12:37. doi: 10.2147/POR.S313991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hailemariam S., Mekonnen B., Shifera N., Endalkachew B., Asnake M., Assefa A., et al. Predictors of pregnant women’s intention to vaccinate against coronavirus disease 2019: a facility-based cross-sectional study in southwest Ethiopia. SAGE Open Med. 2021;9 doi: 10.1177/20503121211038454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berihun G., Walle Z., Berhanu L., Teshome D. Acceptance of COVID-19 vaccine and determinant factors among patients with chronic disease visiting Dessie Comprehensive Specialized Hospital, Northeastern Ethiopia. Patient Prefer. Adherence. 2021;15:1795. doi: 10.2147/PPA.S324564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mose A., Yeshaneh A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in Southwest Ethiopia: institutional-based cross-sectional study. Int. J. Gen. Med. 2021;14:2385. doi: 10.2147/IJGM.S314346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekonnen C.K., Demissie N.G., Beko Z.W., Ferede Y.M., Abate H.K. Intent to get vaccinated against COVID-19 pandemic and its associated factors among adults with a chronic medical condition. Int. J. Africa Nurs. Sci. 2022;16 doi: 10.1016/j.ijans.2022.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tlale L.B., Gabaitiri L., Totolo L.K., Smith G., Puswane-Katse O., Ramonna E., et al. Acceptance rate and risk perception towards the COVID-19 vaccine in Botswana. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0263375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mose A. Willingness to receive COVID-19 vaccine and its determinant factors among lactating mothers in Ethiopia: a cross-sectional study. Infect. Drug Resist. 2021;14:4249. doi: 10.2147/IDR.S336486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adedeji-Adenola H., Olugbake O.A., Adeosun S.A. Factors influencing COVID-19 vaccine uptake among adults in Nigeria. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0264371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson J., Welch V., Losos M., Tugwell P. Ottawa Hospital Research Institute; Ottawa: 2011. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; pp. 1–12. 2(1) [Google Scholar]
- 40.Herzog R., Álvarez-Pasquin M., Díaz C., Del Barrio J.L., Estrada J.M., Gil Á. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Publ. Health. 2013;13(1):1–17. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naafs J.C., Vendrig L.M., Limpens J., Van Der Lee H., Duijnhoven R.G., Marchal J., et al. Cognitive outcome in congenital central hypothyroidism: a systematic review with meta-analysis of individual patient data. Eur. J. Endocrinol. 2020;182(3):351–361. doi: 10.1530/EJE-19-0874. [DOI] [PubMed] [Google Scholar]
- 42.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 43.Luchini C., Stubbs B., Solmi M., Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa scale. World J. Meta-Anal. 2017;5(4):80–84. [Google Scholar]
- 44.Ahmed M.A., Colebunders R., Gele A.A., Farah A.A., Osman S., Guled I.A., et al. COVID-19 vaccine acceptability and adherence to preventive measures in Somalia: results of an online survey. Vaccines. 2021;9(6):543. doi: 10.3390/vaccines9060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dula J., Mulhanga A., Nhanombe A., Cumbi L., Júnior A., Gwatsvaira J., et al. COVID-19 vaccine acceptability and its determinants in Mozambique: an online survey. Vaccines. 2021;9(8):828. doi: 10.3390/vaccines9080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ditekemena J.D., Nkamba D.M., Mutwadi A., Mavoko H.M., Siewe Fodjo J.N., Luhata C., et al. COVID-19 vaccine acceptance in the Democratic Republic of Congo: a cross-sectional survey. Vaccines. 2021;9(2):153. doi: 10.3390/vaccines9020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acheampong T., Akorsikumah E.A., Osae-Kwapong J., Khalid M., Appiah A., Amuasi J.H. Examining vaccine hesitancy in Sub-Saharan Africa: a survey of the knowledge and attitudes among adults to receive COVID-19 vaccines in Ghana. Vaccines. 2021;9(8):814. doi: 10.3390/vaccines9080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAbee L., Tapera O., Kanyangarara M. Factors associated with COVID-19 vaccine intentions in eastern Zimbabwe: a cross-sectional study. Vaccines. 2021;9(10):1109. doi: 10.3390/vaccines9101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Echoru I., Ajambo P.D., Keirania E., Bukenya E.E. Sociodemographic factors associated with acceptance of COVID-19 vaccine and clinical trials in Uganda: a cross-sectional study in western Uganda. BMC Publ. Health. 2021;21(1):1–8. doi: 10.1186/s12889-021-11197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amo-Adjei J., Nurzhynska A., Essuman R., Lohiniva A.-L. Trust and willingness towards COVID-19 vaccine uptake: a mixed-method study in Ghana, 2021. Arch. Publ. Health. 2022;80(1):1–12. doi: 10.1186/s13690-022-00827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adeniyi O.V., Stead D., Singata-Madliki M., Batting J., Wright M., Jelliman E., et al. Acceptance of COVID-19 vaccine among the healthcare workers in the Eastern Cape, South Africa: a cross sectional study. Vaccines. 2021;9(6):666. doi: 10.3390/vaccines9060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oduwole E.O., Esterhuizen T.M., Mahomed H., Wiysonge C.S. Estimating vaccine confidence levels among healthcare staff and students of a tertiary institution in South Africa. Vaccines. 2021;9(11):1246. doi: 10.3390/vaccines9111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Handebo S., Wolde M., Shitu K., Kassie A. Determinant of intention to receive COVID-19 vaccine among school teachers in Gondar City, Northwest Ethiopia. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Billah M.A., Miah M.M., Khan M.N. Reproductive number of coronavirus: a systematic review and meta-analysis based on global level evidence. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0242128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Britton T., Ball F., Trapman P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science. 2020;369(6505):846–849. doi: 10.1126/science.abc6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weintraub R.L., Subramanian L., Karlage A., Ahmad I., Rosenberg J. COVID-19 Vaccine to Vaccination: why Leaders Must Invest in Delivery Strategies Now: analysis describe lessons learned from past pandemics and vaccine campaigns about the path to successful vaccine delivery for COVID-19. Health Aff. 2021;40(1):33–41. doi: 10.1377/hlthaff.2020.01523. [DOI] [PubMed] [Google Scholar]
- 57.Habersaat K.B., Betsch C., Danchin M., Sunstein C.R., Böhm R., Falk A., et al. Ten considerations for effectively managing the COVID-19 transition. Nat. Human Behav. 2020;4(7):677–687. doi: 10.1038/s41562-020-0906-x. [DOI] [PubMed] [Google Scholar]
- 58.Massinga Loembé M., Tshangela A., Salyer S.J., Varma J.K., Ouma A.E.O., Nkengasong J.N. COVID-19 in Africa: the spread and response. Nat. Med. 2020;26(7):999–1003. doi: 10.1038/s41591-020-0961-x. [DOI] [PubMed] [Google Scholar]
- 59.Umviligihozo G., Mupfumi L., Sonela N., Naicker D., Obuku E.A., Koofhethile C., et al. Sub-Saharan Africa preparedness and response to the COVID-19 pandemic: a perspective of early career African scientists. Wellcome Open Res. 2020;5 doi: 10.12688/wellcomeopenres.16070.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen M., Li Y., Chen J., Wen Z., Feng F., Zou H., et al. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum. Vaccines Immunother. 2021;17(7):2279–2288. doi: 10.1080/21645515.2020.1853449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karlsson L.C., Lewandowsky S., Antfolk J., Salo P., Lindfelt M., Oksanen T., et al. The association between vaccination confidence, vaccination behavior, and willingness to recommend vaccines among Finnish healthcare workers. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0224330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shekhar R., Sheikh A.B., Upadhyay S., Singh M., Kottewar S., Mir H., et al. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. 2021;9(2):119. doi: 10.3390/vaccines9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verger P., Dubé E. Restoring confidence in vaccines in the COVID-19 era. Expet Rev. Vaccine. 2020;19(11):991–993. doi: 10.1080/14760584.2020.1825945. [DOI] [PubMed] [Google Scholar]
- 64.Larson H.J., Clarke R.M., Jarrett C., Eckersberger E., Levine Z., Schulz W.S., et al. Measuring trust in vaccination: a systematic review. Hum. Vaccines Immunother. 2018;14(7):1599–1609. doi: 10.1080/21645515.2018.1459252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown A.L., Sperandio M., Turssi C.P., Leite R., Berton V.F., Succi R.M., et al. vol. 34. Cadernos de saude publica; 2018. Vaccine Confidence and Hesitancy in Brazil. [DOI] [PubMed] [Google Scholar]
- 66.Zhong B.-L., Luo W., Li H.-M., Zhang Q.-Q., Liu X.-G., Li W.-T., et al. Knowledge, attitudes, and practices towards COVID-19 among Chinese residents during the rapid rise period of the COVID-19 outbreak: a quick online cross-sectional survey. Int. J. Biol. Sci. 2020;16(10):1745. doi: 10.7150/ijbs.45221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021;27(2):225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moola S., Gudi N., Nambiar D., Dumka N., Ahmed T., Sonawane I.R., et al. A rapid review of evidence on the determinants of and strategies for COVID-19 vaccine acceptance in low-and middle-income countries. J. Global Health. 2021:11. doi: 10.7189/jogh.11.05027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zintel S., Flock C., Arbogast A.L., Forster A., von Wagner C., Sieverding M. Gender differences in the intention to get vaccinated against COVID-19: a systematic review and meta-analysis. J. Publ. Health. 2022:1–25. doi: 10.1007/s10389-021-01677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rapisarda V., Vella F., Ledda C., Barattucci M., Ramaci T. What prompts doctors to recommend COVID-19 vaccines: is it a question of positive emotion? Vaccines. 2021;9(6):578. doi: 10.3390/vaccines9060578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gagneux-Brunon A., Detoc M., Bruel S., Tardy B., Rozaire O., Frappe P., et al. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J. Hosp. Infect. 2021;108:168–173. doi: 10.1016/j.jhin.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sallam M., Dababseh D., Eid H., Al-Mahzoum K., Al-Haidar A., Taim D., et al. High rates of COVID-19 vaccine hesitancy and its association with conspiracy beliefs: a study in Jordan and Kuwait among other Arab countries. Vaccines. 2021;9(1):42. doi: 10.3390/vaccines9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akarsu B., Canbay Özdemir D., Ayhan Baser D., Aksoy H., Fidancı İ., Cankurtaran M. While studies on COVID‐19 vaccine is ongoing, the public’s thoughts and attitudes to the future COVID‐19 vaccine. Int. J. Clin. Pract. 2021;75(4) doi: 10.1111/ijcp.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akuoko C.P., Armah E., Sarpong T., Quansah D.Y., Amankwaa I., Boateng D. Barriers to early presentation and diagnosis of breast cancer among African women living in sub-Saharan Africa. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.