Abstract
Objective:
We sought to evaluate and describe the maternal and obstetric morbidity associated with Listeria infection in pregnancy.
Methods:
Retrospective cohort of pregnant women using the 2007–2018 National Inpatient Sample. Pregnant women with discharge diagnosis codes consistent with Listeria infection were identified. Outcomes of deliveries complicated by Listeria infection were compared to those of delivery without this infection. The primary outcome was a composite of severe maternal morbidity. Secondary outcomes included components of the composite, maternal length of stay, mode of delivery, stillbirth, and preterm delivery.
Results:
We identified 134 maternity associated hospitalizations for Listeria (weighted national estimate 666), of which 72 (weighted national estimate of 358) were delivery admissions. Delivery admissions complicated by Listeria resulted in higher rates of severe maternal morbidity than those without, (30.9% vs. 1.6%, p<0.001). In adjusted analyses, women with Listeria had 21.2-fold higher risk of severe maternal morbidity (95% CI: 14.0, 31.9) when compared to those without Listeria.
Specifically, Listeria delivery admissions had higher rates of acute respiratory distress syndrome (2.8% vs. 0.1%, p<0.001), mechanical ventilation (1.4% vs. 0.0%, p<0.001), sepsis (28.1% vs. 0.1%, p<0.001), and shock (1.4% vs. 0.0%, p<.001). Listeria delivery admissions also had higher rates of preterm birth (61.3% vs. 7.7%, p<0.001) and stillbirth (13.5% vs. 0.7%, p<0.001). Women hospitalized or delivered with Listeria infection were also more likely to have a cesarean delivery (57.9% vs. 32.9, p<.001) and the average length of stay for women with Listeria was also longer (4.0 days vs. 2.3 days, p<0.001).
Conclusions:
Women with Listeria infection in pregnancy have higher rates of severe maternal morbidity, specifically increased risk of sepsis, septic shock, and acute respiratory distress syndrome. Among delivery hospitalizations, these women also have higher rates of preterm birth and stillbirth.
Keywords: Listeria, Listeriosis, maternal morbidity, Preterm delivery, sepsis
INTRODUCTION
Listeria infection is acquired by consuming foods infected with the facultative anaerobic bacterium, Listeria monocytogenes. Maternal listeriosis, when symptomatic, most commonly presents as a mild febrile illness, with myalgias and possible gastrointestinal symptoms.1, 2 In severe cases, Listeriosis in pregnancy results in miscarriage, intrauterine fetal death, or neonatal meningitis and sepsis. Because fetal and neonatal outcomes are well described, evaluation and treatment algorithms for Listeriosis in pregnancy have been investigated and are detailed in both obstetrics and pediatrics literature.3–5
Most reported cases of Listeriosis are in the immunocompromised, the elderly, as well as neonates. However, pregnant women are also considered a susceptible population for Listeria monocytogenes infection, with one in seven cases of reported Listeriosis illness occurring in pregnant women.6 The estimated incidence of pregnancy related Listeriosis is 3–4 cases per 100,000 births.7 Maternal infection within the first trimester of pregnancy has an associated risk of miscarriage estimated at 65%, while infection in the 2nd or 3rd trimester has an associated risk of fetal demise estimated at 26%.8 While fetal loss is more common than neonatal death, fetal infection in surviving newborns confers a high risk of long term morbidity from neurologic deficits and physical disability, as well as a fatality rate as high as 20%.9–11 In contrast to fetal and neonatal mortality, maternal mortality associated with Listeria infection is rare and most women recover postpartum. 12
Some studies have suggested a higher degree of severity of disease for pregnant women, yet specific morbidities have not yet been detailed.13 Despite these findings, and well described fetal and neonatal morbidities, the attributable risk of Listeria infection in pregnancy to overall maternal morbidity has been incompletely described in the literature. We sought to evaluate and describe the maternal and obstetric outcomes associated with Listeria infection in pregnancy.
MATERIALS AND METHODS
We conducted a retrospective cohort study of pregnant women using the 2007–2018 National Inpatient Sample (NIS), a product of the Healthcare Cost and Utilization Project of the United States Agency for Healthcare Research and Quality. The NIS is an all-payor database including short-stay, non-Federal inpatient hospitalizations from participating states (40 in 2007, increased to 48 in 2018), and include an approximately 20% sample of all such hospitalizations.14 Collected data include patient demographic information such as age, primary payor, race, as well as the International Classification of Diseases Ninth or Tenth Revision (ICD-9/10) diagnosis and procedure codes, discharge disposition, and length of stay. Neonatal data was not available for this dataset. The NIS datasets are provided with weighting information to allow calculation of nationally representative estimates (i.e., to use the 20% sample, which is not a simple random sample, to provide estimates of all short-stay hospitalizations in the United States).
We identified maternity-associated hospitalizations using a diagnosis code-based methodology developed by the Healthcare Cost and Utilization Project.15 We then adopted previously-published algorithms to identify postpartum and delivery hospitalizations, updating the published criteria for ICD-10 coding for discharges after October 1, 2015 when the United States adopted ICD-10 for coding (See Supplement).16,17 Listeria-related hospitalizations were assessed based on the presence of ICD-9 diagnosis code 027.0 and ICD-10 diagnosis codes A32.0-A32.9 (all Listeria-specific diagnosis codes). For identified patients, a previously validated obstetric comorbidity index was applied to identify comorbid conditions, again updated for ICD-10 by the authors.18
We performed comparisons between delivery hospitalizations affected by Listeria and delivery hospitalizations unaffected by Listeria. The rationale for this choice is that because the NIS is an inpatient-only database, there is no natural comparison group for the antepartum and postpartum readmission populations with Listeriosis included in the sample, since in the absence of a complication patients typically are not hospitalized during these time periods. In contrast, most American patients give birth in hospitals. The primary outcome for our study was a composite of severe maternal morbidity (SMM). As defined by the Centers for Disease Control and Prevention (CDC), SMM includes myocardial infarction, aneurysm, acute renal failure, respiratory distress syndrome, amniotic fluid embolism, cardiac arrest, disseminated intravascular coagulopathy, eclampsia, heart failure, puerperal cerebrovascular disorders, pulmonary edema, severe anesthesia complications, sepsis, shock, sickle cell disease with crisis, venous thrombotic embolism, blood product transfusion, hysterectomy, tracheostomy, and mechanical ventilation. Secondary outcomes assessed included several individual components of the SMM composite (including shock, acute respiratory distress syndrome, mechanical ventilation, sepsis, and transfusion), maternal length of stay, mode of delivery, and preterm delivery.
Statistical methods were used which could accommodate the complex nature of the NIS dataset as a weighted sample designed to generate national estimates. Categorical variables were analyzed using weighted Chi-squared tests. Continuous variables were compared using weighted linear regression. Logistic regression models developed to control for confounders, adjusting for age, primary payor, race, and comorbid conditions and weighted to reflect the design of the NIS dataset. Weighted logistic regression was used for binary outcomes, and Poisson and gamma/log-link models for length of stay and inpatient costs, respectively. Because the odds ratios generated by logistic regression can be difficult to interpret, they were converted to adjusted relative risks.19 There were minimal missing data for most variables, including primary payer, zip code household income (<1%); an exception was race which was missing in 10.7% of observations. These variables had the most common value imputed for observations with missing values. A two-sided alpha value of 0.05 was pre-specified to be statistically significant. Data were analyzed in Stata Statistical Software, Version 16.1 (Statacorp, College Station, TX). As the NIS is a Limited Data Set as defined by the Health Insurance Portability and Accountability Act, this study was determined to be exempt from review by the Duke Health Institutional Review Board. This study was supported by the National Center for Advancing Translational Sciences (TL1-TR002555) [JJF]. Data acquisition was also supported by funding from the Foundation for Women and Girls with Blood Disorders to JJF. The funding sources had no role in the design, conduct, or publication of this study.
RESULTS
A total of 134 pregnancy-related hospitalizations related to Listeria were found in the NIS sample among, which corresponds to a national estimate of 666 hospitalizations over the 2007–2018 study period. Henceforth, we will report results based on the national estimates. Most hospitalizations with Listeria infection included a delivery (53.8%). (Figure 1) When compared to delivery hospitalizations without Listeria infection, no statistically significant differences between cohorts based on race, household income, or primary expected payer were seen (Table 1). Most women in both cohorts were either White or Hispanic (Table 1). Overall maternal medical comorbidities were similar between the two cohorts, but women admitted with Listeria infection had higher rates of preexisting HIV, pulmonary hypertension, gestational diabetes, and preeclampsia with severe features or eclampsia.
Figure 1:
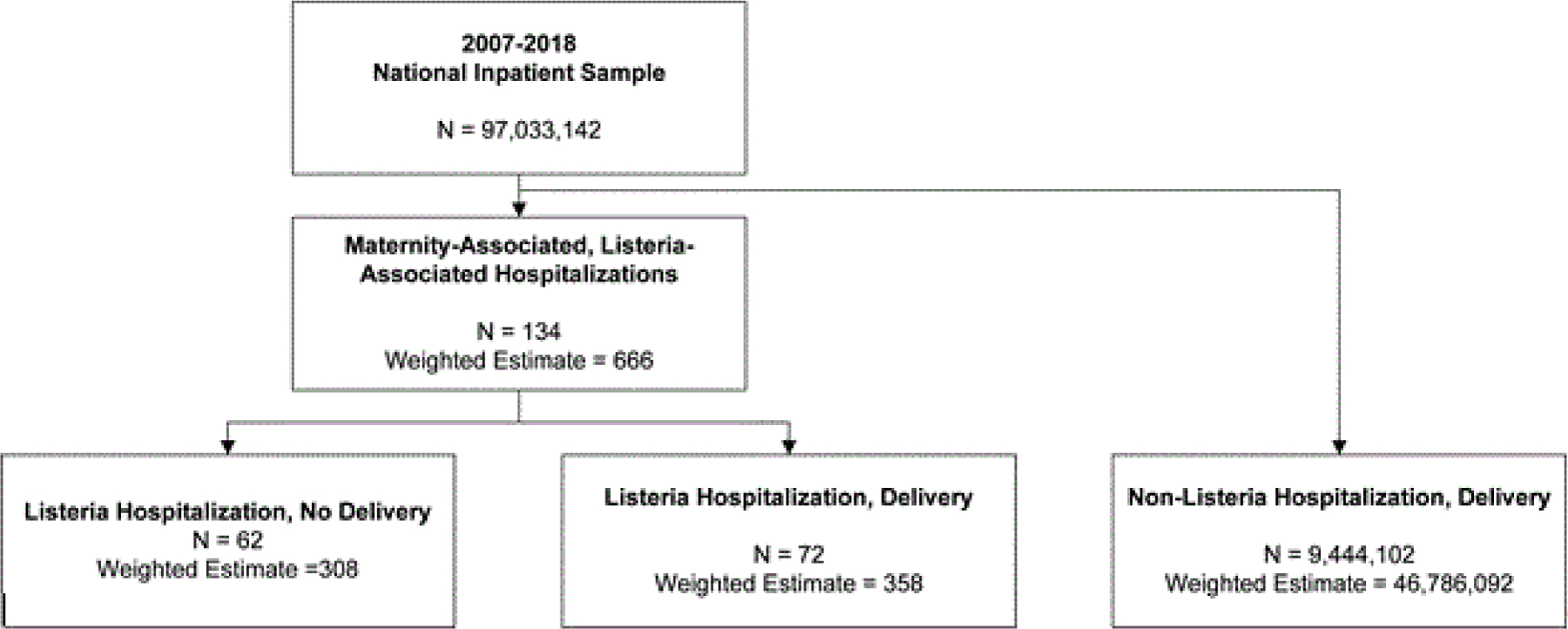
Derivation of study cohort from 2007 – 2018 National Inpatient Sample.
Table 1.
Maternal demographics and obstetrical outcomes for all deliveries, 2007 – 2018, from National Inpatient Sample. P-values by weighted linear regression for continuous variables and weighted chi2 test for binary/categorical variables.
| Listeria Admissions | ||||
|---|---|---|---|---|
| Overall (N=134) (Weighted N = 666) |
Delivery Admissions (N=72) (Weighted N = 358) |
Non-Listeria Deliveries (N=9,444,102) (Weighted N = 46,786,092) |
P (Listeria delivered vs. non-Listeria Delivered) | |
| Mean (Standard Deviation) or % | ||||
| Demographics | ||||
| Age in years at admission | 30.1 (0.5) | 30.6 (6.5) | 28.7 (5.8) | 0.09 |
| Primary expected payer | 0.97 | |||
| Medicare | * | 0 (0) | 328,724 (0.7) | |
| Medicaid | 256 (38.4) | 137 (38.4) | 20,119,751 (43.1) | |
| Private | 355 (53.3) | 205 (57.1) | 23,561,861 (50.4) | |
| Self-pay | 50 (7.5) | 16 (4.5) | 1,332,830 (2.9) | |
| No Charge | * | 0 (0.0) | 65,659 (0.1) | |
| Other | * | 0 (0.0) | 1,304,151 (2.8) | |
| Zip Code Median Household Income ($1000s) | 0.21 | |||
| Quartile 1 (Lowest) | 139 (21.0) | 59 (16.8) | 12,771,473 (27.7) | |
| Quartile 2 | 163 (24.7) | 108 (30.5) | 11,635,583 (25.3) | |
| Quartile 3 | 192 (29.1) | 88 (25.0) | 11,342,644 (24.6) | |
| Quartile 4 (Highest) | 166 (25.2) | 98 (27.7) | 10,289,758 (22.3) | |
| Race (uniform) | 0.19 | |||
| White | 249 (41.8) | 149 (46.2) | 21,950,785 (52.6) | |
| Black | 64 (10.8) | 24 (7.5) | 6,035,439 | |
| Hispanic | 177 (29.8) | 109 (33.7) | 9,080,988 (5.6) | |
| Asian or Pacific Islander | 50 (8.4) | 21 (6.4) | 2,341,216 (5.6) | |
| Other | 55 (9.2) | 0 (0.0) | 337,029 (0.8) | |
| Admission Type | ||||
| Antepartum | 177 (26.5) | |||
| Delivery | 358 (53.8) | |||
| Postpartum | 50 (7.6) | |||
| Early/abnormal | 81 (12.1) | |||
| Selected Maternal Comorbid Conditions | ||||
| Gestational Diabetes Mellitus | 60 (9.0) | 50 (14.0) | 3,048,897 (6.5) | 0.01 |
| HIV/AIDS | 11 (1.6) | * | 49,711 (0.1) | <0.001 |
| Preexisting Diabetes Mellitus | * | * | 3,048,897 (6.5) | 0.61 |
| Prior cesarean birth | 54 (8.2) | 44 (12.4) | 7,941,948 (17.0) | 0.28 |
| Pulmonary Hypertension | * | * | 9,997 (0.0) | <0.001 |
| Multiples gestation | 20 (3.0) | * | 857,947 (1.8) | 0.57 |
| Asthma | 30 (4.5) | 15 (4.2) | 1,803,397 (3.9) | 0.89 |
| BMI greater or equal to 40 | 35 (5.2) | 25 (6.9) | 2,933,517 (6.3) | 0.83 |
| Preexisting cardiac disease | 0 (0.0) | 0 (0.0) | 125,509 (0.3) | 0.67 |
| Chronic hypertension | 15 (2.2) | * | 792,228 (1.7) | 0.83 |
| Chronic renal disease | 0 (0.0) | 0 (0.0) | 105,857 (0.2) | 0.69 |
| Connective tissue or autoimmune disease | 14 (2.1) | 0 (0.0) | 55,612 (0.1) | 0.77 |
| Placenta previa | * | * | 256,891 (0.5) | 0.33 |
| Preeclampsia with severe features | 30 (4.5) | 30 (8.3) | 829,895 (1.8) | <0.001 |
| Gestational hypertension / preeclampsia without severe features | * | * | 3,202,524 (6.8) | 0.27 |
| Tobacco use disorder | 24 (3.6) | * | 2,496,308 (5.3) | 0.37 |
| Alcohol abuse | 0 (0.0) | 0 (0.0) | 55,175 (0.1) | 0.78 |
| Drug abuse | * | * | 842,122 (1.8) | 0.61 |
| Congenital heart disease | 0 (0.0) | 0 (0.0) | 43,587 (0.1) | 0.80 |
| Sickle cell disease | 0 (0.0) | 0 (0.0) | 68,188 (0.1) | 0.75 |
| Placental abruption | * | * | 131,076 (1.1) | 0.02 |
| Placenta accreta spectrum | 0 (0.0) | 0 (0.0) | 14,066 (0.1) | 0.84 |
| Obstetrical Outcomes | ||||
| Any Severe Maternal Morbidity | 220 (33.1) | 111 (30.9) | 747m873 (1.6) | <0.001 |
| Acute myocardial infarction | 0 (0.0) | 0 (0.0) | 1,171 (0.0) | 0.97 |
| Aneurysm | 0 (0.0) | 0 (0.0) | 1,136 (0.0) | 0.97 |
| Acute renal failure | * | 0 (0.0) | 35,725 (0.1) | 0.82 |
| Adult respiratory distress syndrome | 15 (2.3) | * | 35,003 (0.1) | <0.001 |
| Amniotic fluid embolism | 0 (0.0) | 0 (0.0) | 2,193 (0.0) | 0.95 |
| Cardiac arrest/ventricular fibrillation | 0 (0.0) | 0 (0.0) | 3,756 (0.0) | 0.94 |
| Conversion of cardiac rhythm | 0 (0.0) | 0 (0.0) | 3,527 (0.0) | 0.94 |
| Disseminated intravascular coagulation | * | 0 (0.0) | 116,450 (0.2) | 0.68 |
| Eclampsia | 0 (0.0) | 0 (0.0) | 35,565 (0.1) | 0.82 |
| Heart failure/arrest during surgery or procedure | 0 (0.0) | 0 (0.0) | 3,979 (0.0) | 0.94 |
| Puerperal cerebrovascular disorders | 0 (0.0) | 0 (0.0) | 13,564 (0.0) | 0.89 |
| Pulmonary edema / Acute heart failure | * | * | 14,967 (0.0) | 0.88 |
| Severe anesthesia complications | 0 (0.0) | 0 (0.0) | 5,693 (0.0) | 0.93 |
| Sepsis | 189 (28.4) | 101 (28.1) | 27,404 (0.1) | <0.001 |
| Shock | * | * | 22,299 (0.0) | <0.001 |
| Sickle cell disease with crisis | * | 0 (0.0) | 4,887 (0.0) | 0.93 |
| Air and thrombotic embolism | 0 (0.0) | 0 (0.0) | 10,915 (0.0) | 0.90 |
| Blood products transfusion | 41 (6.1) | 15 (4.2) | 511,425 (1.1) | 0.01 |
| Hysterectomy | 0 (0.0) | 0 (0.0) | 45,778 (0.1) | 0.79 |
| Temporary tracheostomy | 0 (0.0) | 0 (0.0) | 1,135 (0.0) | 0.97 |
| Mechanical ventilation | * | * | 6,157(0.1) | <0.001 |
| Gestational age (weeks) | 27.8 (0.9) | 33.8 (5.2) | 38.3 (3.2) | <0.001 |
| Preterm birth | 43 (59.9) | 42 (61.3) | 1,222,538 (10.1) | <0.001 |
| Stillbirth | 13 (8.6) | * | 91,053 (0.8) | <0.001 |
| Delivery type | <0.001 | |||
| SVD | 149 (42.1) | 1,222,538 (10.1) | ||
| OVD | 0 (0.0) | 2,218,172 (4.8) | ||
| C/S | 205 (57.9) | 15,350,839 (32.9) | ||
| Disposition of patient | 0.72 | |||
| Home | 118 (77.0) | 319 (89.0) | 45,825,136 (98.0) | |
| Transfer | 0 (0.0) | 49,080 (0.1) | ||
| SNF/LTC | * | 0 (0.0) | 21,214 (0.0) | |
| Home Health | 32 (21.0) | 40 (11.0) | 837,590 (1.8) | |
| AMA | * | 0 (0.0) | 36,093 (0.1) | |
| Died | 0 (0.0) | 2,784 (0.0) | ||
| Unknown | 0 (0.0) | 977 (0.0) | ||
| Length of stay | 6.1 (0.5) | 5.4 (4.0) | 2.6 (2.3) | <0.001 |
indicates value ≤ 10; exact value suppressed due to privacy limitations from data vendor.
Deliveries following admission with Listeria infection resulted in higher rates of severe maternal morbidity, with nearly one third of the women with Listeria infection experiencing severe morbidity, (30.9% vs. 1.6%, p<0.001; Figure 2). In adjusted analyses, women admitted with Listeria infection had 21.2-fold higher adjusted relative risk of severe maternal morbidity (95% CI: 14.0, 31.9) when compared to those without Listeria infection. Deliveries following admission with Listeria infection had higher rates of acute respiratory distress syndrome (ARDS) (2.8% vs. 0.1%, p<0.001), mechanical ventilation (1.4% vs. 0.0%, p<0.001), sepsis (28.1% vs. 0.1%, p<0.001), shock (1.4% vs. 0.0%, p<0.001), and blood product transfusion (4.2% vs. 1.1%, p<0.01). In adjusted analyses, women hospitalized for Listeria infection in pregnancy had 522.8-fold higher risk of sepsis (95% CI: 345.3, 791.6), 25.5-fold higher risk of ARDS (95% CI: 6.37, 1010.92), 102.8-fold higher risk of mechanical ventilation (95% CI: 14.7, 719.7), 22.6-fold higher risk of shock (95% CI: 3.2, 159.6).
Figure 2:
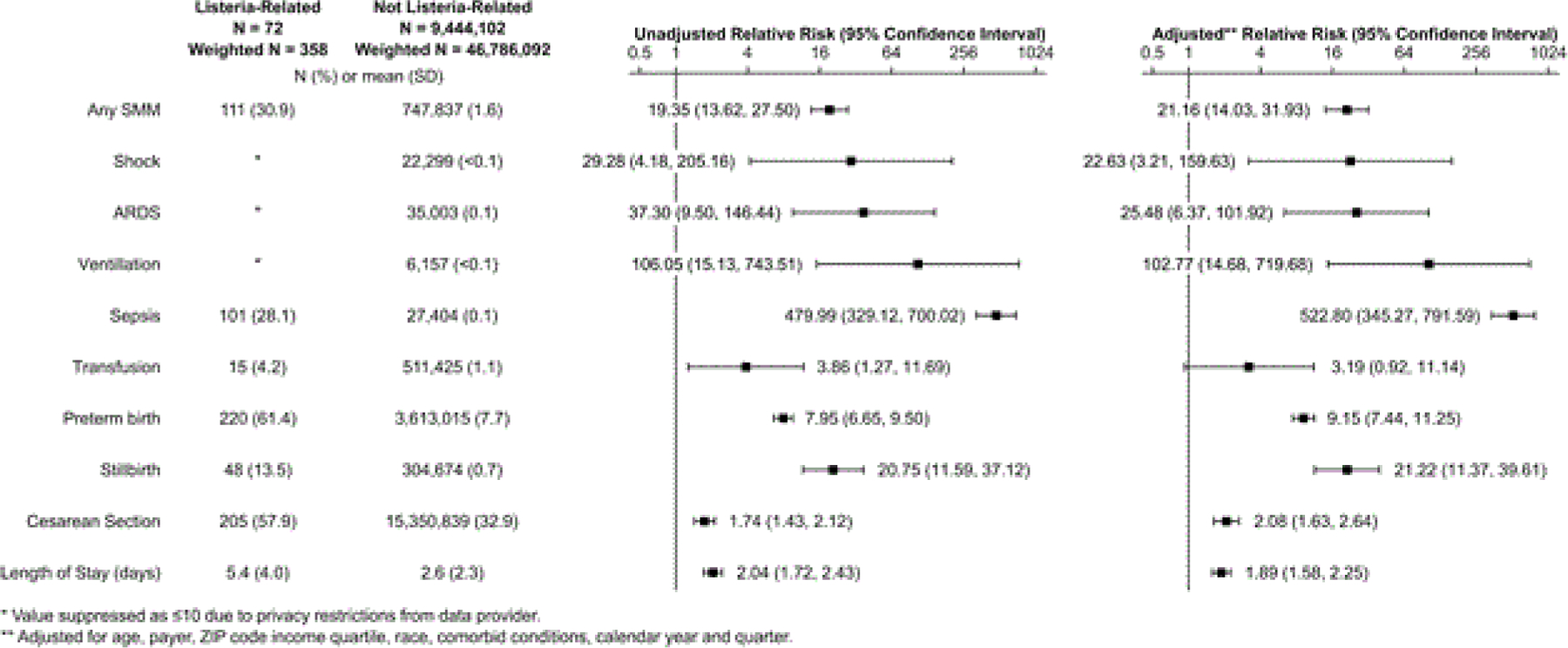
Unadjusted and covariate outcomes of delivery hospitalizations, comparing those associated with listeria to those not associated.
From the fetal/neonatal perspective, deliveries following admission with Listeria infection also had higher rates of preterm birth (61.4% vs. 7.7%, p<0.001) and stillbirth (13.5% vs. 0.7%, p<0.001) when compared to the non-Listeria cohort. In adjusted analysis, women hospitalized for Listeria infection in pregnancy had 9.2-fold higher risk of preterm birth (95% CI: 7.4, 11.3) and 21.2-fold higher risk of stillbirth (95% CI: 11.4, 39.6). Women hospitalized with Listeria infection were also more likely to have a cesarean delivery (57.9% vs. 32.9%, p<0.001; ARR 2.1, 95% CI 1.6, 2.6) and the average length of stay for these women was also longer (4.0 days vs. 2.3 days, p<0.001; ARR 1.9, 95 CI: 1.6, 2.3).
DISCUSSION
In this study, we found that women hospitalized with Listeria infection in pregnancy have much higher rates of severe maternal morbidity compared to women hospitalized without Listeria infection. Specifically, these patients experience higher rates of sepsis, septic shock, ARDS, and need for mechanical ventilation during delivery admission hospitalizations. This study also found significantly increased risk of preterm birth, stillbirth, and cesarean delivery, as well as longer lengths of stay, associated with hospitalization for Listeria infection in pregnancy.
Prior studies have described clinical findings of fever, flu-like symptoms, leukocytosis, gastrointestinal symptoms, preterm contractions and preterm labor among women with confirmed cases of Listeria in pregnancy, 20, 21 yet general maternal morbidity has been incompletely described. Fortunately, maternal mortality associated with Listeria infection is rare.22 Despite rare maternal mortality, this study details the severe maternal morbidity associated with exposure to Listeria bacteria in pregnancy and subsequent maternal infection. Within our cohort, in addition to higher odds ratios of sepsis and septic shock, when compared to hospitalizations for non-infected pregnant women, we found a significant increased risk of ARDS, need for mechanical ventilation, need for blood transfusion, and increased risk of cesarean delivery among pregnant patients admitted for Listeria infection. Though ARDS is a rare occurrence in pregnant patients with estimated incidence of 16–70 per 100,000 pregnancies, it has an overall mortality rate of 11%.23 These morbid findings are of particular interest as nationwide rates of maternal sepsis and the incidence rate of Listeriosis continue to increase, contributing to increasing rates of maternal morbidity and mortality. 24 Additionally, newer Listeria outbreaks are being reported within the United States, most recently linked to packaged salads and fully cooked chicken with the numbers of illnesses, hospitalizations, and deaths nationwide prompting CDC investigation and food recalls as recently as December 22, 2021 and February 1, 2022. 25,26
Our study had several limitations related to the use of data from the NIS. First, our data was limited by use of diagnosis codes (administrative data). This data is thereby reliant on accurate coding and may underrepresent some conditions and comorbidities. This limitation includes the caveat that our assessed cohort of women were admitted to the hospital with Listeria infection, therefore it is not inclusive of presumably all women (hospitalized and not hospitalized) whom had Listeria infections in pregnancy. This dataset only included patients admitted for recognized symptoms, and more symptoms requiring hospitalization likely indicates higher bacterial load and bacteremia, which will ultimately lead to more severe maternal morbidity overall. Additionally, this data was obtained across the transition to ICD-10 which may limit its ability to be compared to prior studies. This dataset also does not include specific societal or behavioral characteristics which limit our ability to specifically identify patient factors that may have contributed to specific morbidities. This could include prenatal care use, socioeconomic factors, and behavioral habits which may impact risks of infection, particularly among minority populations, and thereby maternal outcomes.
Our study is strengthened by the size and diversity of a nationally represented cohort. This cohort also represented the most recent and up-to-date information available from hospital discharge data. Additionally, this is the first study of its kind to specifically investigate the maternal morbidities associated with Listeria infection in pregnancy.
In conclusion, women admitted to the hospital with Listeria infection in pregnancy are at significantly increased risk for severe maternal morbidity, specifically, increased risks of sepsis, septic shock, ARDS and mechanical ventilation. We found higher rates of preterm birth and stillbirth among delivery hospitalizations complicated by Listeria infection, as well as longer lengths of stay and increased relative risk of cesarean delivery. The increasing incidence of Listeriosis in the United States and the known fetal and neonatal consequences are independently concerning enough to support national efforts to educate patients and healthcare providers regarding the risks associated with severe Listeria infection in pregnancy. However, the added findings of this study, demonstrating significantly increased severe maternal morbidity and adverse obstetric outcomes further emphasize the importance of provider and patient education. It is important for patients to be counseled on recognition of symptoms in pregnancy that should prompt evaluation by a provider, and more importantly, how to prevent infection in order to reduce severe maternal morbidity. Specific efforts targeting all patients, with a specific emphasis on at-risk populations, should be the focus of future research and education in attempts to mitigate disparities in overall maternal morbidity from Listeriosis infection in pregnancy.
Supplementary Material
ACKNOLWEDGEMENTS
The authors appreciate the HCUP Data Partners who contribute data to the NIS. A complete list of partners can be found at (www.hcup-us.ahrq.gov/hcupdatapartners.jsp)
Work contained in this manuscript were made possible by the following grant from the National Institutes of Health: TL1-TR002555 [JJF]. Data acquisition was also supported by funding from the Foundation for Women and Girls with Blood Disorders to JJF. The funding sources had no role in the design, conduct, or publication of this study.
Footnotes
A preliminary version of this manuscript was presented at the Infectious Diseases Society for Obstetrics and Gynecology (IDSOG) 2020 Virtual Annual Meeting (August 13 - August 15, 2020).
DECLARATION OF INTERESTS
Jennifer Thompson is a co-author for “Treatment and Prevention of Listeria monocytogenes infection” on UpToDate. All other author(s) report(s) no conflict of interest. The authors alone are responsible for the content and writing of this manuscript.
REFERENCES
- 1.Madjunkov M, Chaudhry S, Ito S. Listeriosis during pregnancy. Arch Gynecol Obstet 2017;296:143–52. [DOI] [PubMed] [Google Scholar]
- 2.Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 2002;81:260–9. [DOI] [PubMed] [Google Scholar]
- 3.Committee Opinion No. 614: Management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol 2014;124:1241–44. [DOI] [PubMed] [Google Scholar]
- 4.Posfay-Barbe KM, Wald ER. Listeriosis. Pediatr Rev 2004;25:151–9. [DOI] [PubMed] [Google Scholar]
- 5.SMFM Statement Listeria Exposure in Pregnancy. Publications & Guidelines | SMFM.org - The Society for Maternal-Fetal Medicine. [online] 172. 2022
- 6.Wadhwa Desai R, Smith MA. Pregnancy-related listeriosis. Birth Defects Res 2017;109:324–35. [DOI] [PubMed] [Google Scholar]
- 7.Jackson KA, Iwamoto M, Swerdlow D. Pregnancy-associated listeriosis. Epidemiol Infect 2010;138:1503–9. [DOI] [PubMed] [Google Scholar]
- 8.Infectious disease/CDC update. Vital signs: Listeria illnesses, deaths, and outbreaks-United States, 2009–2011. Ann Emerg Med 2013;62:536–7. [DOI] [PubMed] [Google Scholar]
- 9.Awofisayo A, Amar C, Ruggles R, et al. Pregnancy-associated listeriosis in England and Wales. Epidemiol Infect 2015;143:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mclauchlin J Human listeriosis in Britain, 1967–85, a summary of 722 cases. 1. Listeriosis during pregnancy and in the newborn. Epidemiol Infect 1990;104:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease C, Prevention. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food−-10 states, 2007. MMWR Morb Mortal Wkly Rep 2008;57:366–70. [PubMed] [Google Scholar]
- 12.Charlier C, Perrodeau E, Leclercq A, et al. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis 2017;17:510–19. [DOI] [PubMed] [Google Scholar]
- 13.Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol 2013;2013:752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healthcare Cost and Utilization Project. Introduction to the National Inpatient Sample. In: United States Agency for Healthcare Research and Quality, ed., 2020. [Google Scholar]
- 15.Healthcare Cost and Utilization Project. HCUP Quality Control Procedures. In: United States Agency for Healthcare Research and Quality, ed., 2021. [Google Scholar]
- 16.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–36. [DOI] [PubMed] [Google Scholar]
- 17.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J 2008;12:469–77. [DOI] [PubMed] [Google Scholar]
- 18.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol 2013;122:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol 2010;63:2–6. [DOI] [PubMed] [Google Scholar]
- 20.Wang P, Chen Y, Wang H, Yang S, Xu Y, Li T. [A clinical analysis of 16 patients with maternal listeriosis]. Zhonghua Nei Ke Za Zhi 2015;54:763–7. [PubMed] [Google Scholar]
- 21.Fouks Y, Amit S, Many A, Haham A, Mandel D, Shinar S. Listeriosis in pregnancy: under-diagnosis despite over-treatment. J Perinatol 2018;38:26–30. [DOI] [PubMed] [Google Scholar]
- 22.Elinav H, Hershko-Klement A, Solt I, Glikman D, Nir-Paz R. Pregnancy-associated listeriosis: many beliefs, few facts. Lancet Infect Dis 2015;15:1128–30. [DOI] [PubMed] [Google Scholar]
- 23.Rush B, Martinka P, Kilb B, Mcdermid RC, Boyd JH, Celi LA. Acute Respiratory Distress Syndrome in Pregnant Women. Obstet Gynecol 2017;129:530–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohl AM, Pouillot R, Van Doren JM. Changing US Population Demographics: What Does This Mean for Listeriosis Incidence and Exposure? Foodborne Pathog Dis, 2017. 14(9): 524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC: Listeria Outbreak Linked to Packaged Salads by Fresh Express. Centers for Disease Control and Prevention. https://www.cdc.gov/listeria/outbreaks/packaged-salad-12-21-b/index.html. Published 2022. Accessed February 25, 2022. [Google Scholar]
- 26.CDC: Listeria Outbreak Linked to Packaged Salads by Fresh Express. Centers for Disease Control and Prevention. https://www.cdc.gov/listeria/outbreaks/packaged-salad-12-21-b/index.html. Published 2022. Accessed February 25, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


