Abstract
Cirrhosis is an emerging major cause of the development of hepatocellular carcinoma (HCC), but in non-alcoholic fatty liver disease (NAFLD), up to 50% of patients with HCC had no clinical or histological evidence of cirrhosis. It is currently challenging to propose general recommendations for screening patients with NAFLD without cirrhosis, and each patient should be evaluated on a case-by-case basis based on the profile of specific risk factors identified. For HCC screening in NAFLD, a valid precision-based screening is needed. Currently, when evaluating this population of patients, the use of non-invasive methods can guide the selection of those who should undergo a screening and surveillance program. Hence, the objective of the present study is to review the epidemiology, the pathophysiology, the histopathological aspects, the current recommendations, and novel perspectives in the surveillance of non-cirrhotic NAFLD-related HCC.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Hepatocellular carcinoma, Genetic variants, Microbiota, Obesity
Core Tip: Cirrhosis is an emerging major cause of the development of hepatocellular carcinoma (HCC), but in non-alcoholic fatty liver disease (NAFLD), up to 50% of patients with HCC had no clinical or histological evidence of cirrhosis. In the present study, we evaluated data regarding the epidemiology, the pathophysiology, the histopathological aspects, the current recommendations, and novel perspectives in the surveillance of non-cirrhotic NAFLD-related HCC. We believe that using non-invasive methods can guide the selection of patients who need to undergo screening and a surveillance program.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) has been traditionally defined by the evidence of hepatic steatosis by imaging or histology and by the lack of secondary causes of hepatic fat accumulation such as significant alcohol consumption, long-term use of steatogenic medications, hereditary disorders and other causes of chronic liver diseases[1]. Recently, there has been a proposal to rename NAFLD to metabolic associated fatty liver disease (MAFLD), thus eliminating the need to exclude other causes of liver diseases and adopting inclusive criteria according to coexistence with other liver diseases[2]. The diagnosis of MAFLD is based on histological, imaging or blood biomarker evidence of fat accumulation in the liver (steatosis) in addition to one of the following criteria, namely overweight/obesity, type 2 diabetes mellitus (DM) or evidence of metabolic dysregulation[2].
NAFLD is a well-known cause of chronic liver disease, compromising more than 25% of the global population, and up to 25% may have nonalcoholic steatohepatitis (NASH) with or without fibrosis. NASH with fibrosis is the most active form of disease which is associated with significant morbidity and mortality due to complications of liver cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC)[3].
Cirrhosis is an emerging major cause of the development of HCC, but in NAFLD, up to 50% of patients with HCC had no clinical or histological evidence of cirrhosis[4-6].
NAFLD and the components of metabolic syndrome, especially obesity and DM, are independently associated with HCC development and contribute to the risk of HCC in a non-cirrhotic liver[7,8]. Non-cirrhotic NAFLD patients have a 2.5-fold higher risk of developing HCC than other etiologies of chronic liver disease without cirrhosis[9].
The objective of the present study is to review the epidemiology, the pathophysiology, the histopathological aspects, the current recommendations and novel perspectives in the surveillance of non-cirrhotic NAFLD-related HCC.
EPIDEMIOLOGY OF NAFLD-RELATED HCC
Liver cancer, most of which corresponds to HCC[10,11], ranks sixth among the most common malignancies and second among the leading causes of cancer-related death worldwide. HCC affected 11.6/100000 individuals in 2020, leading to a mortality rate of 10.7/100000[12]. Remarkably, despite major advances in the treatment of viral hepatitis, it is estimated that the incidence rate of liver cancer will keep increasing until 2030, which can be partly explained by a striking increase in the incidence of NAFLD-related HCC[13].
NAFLD affects approximately one-fourth of individuals in the world[14], reinforcing its importance in the etiology of HCC[15]. In 2019, 36300 new cases of HCC and 34700 HCC-related deaths were attributed to NAFLD[16]. The increasing burden of NAFLD will probably lead to a growth in the age-standardized incidence rate of NAFLD-related liver cancer, with an estimated average percentage change of 2.12 between 2018 and 2030[13]. The growing importance of NAFLD as a cause of HCC becomes apparent when two cohorts from South America, a continent with a high prevalence of NAFLD, are compared. While from 2005 to 2015, 9% of HCC cases were attributed to NAFLD[17], 34% of cases were associated with NAFLD from 2019 to 2020[18].
Most cases of HCC develop in cirrhotic livers. Nevertheless, it is noteworthy that HCC may also occur in NAFLD without cirrhosis[15,19-21]. Aside from cirrhosis, diabetes and other metabolic traits, older age, male sex, alcohol consumption and tobacco smoking also seem to be risk factors for developing HCC in patients with NAFLD[10,22-24]. In cirrhosis associated with NAFLD, the annual incidence of HCC is reported as 0.5% and 2.6%[25,26].
In a large retrospective cohort study of European primary care databases, including 136703 patients with NAFLD and matched controls, the incidence rate of HCC was 0.3 per 1000 person-years among individuals with NAFLD, which was significantly higher than among controls, with a hazard ratio of 3.51. The risk of developing HCC was higher according to the Fibrosis-4 (FIB-4) score, which might reflect the odds of having cirrhosis[22].
In another retrospective cohort study performed using a large American administrative database, including 296707 individuals with NAFLD and an equal number of matched controls, HCC was diagnosed in 490 patients with NAFLD and 55 controls. This translated into an annual incidence rate of HCC of 0.21 cases per 1000 person-years among individuals with NAFLD, which was significantly higher than among controls (0.02 cases per 1000 person-years). In a subgroup analysis, the annual incidence rate of HCC was 10.6 per 1000 person-years among individuals with cirrhosis, 0.08 per 1000 person-years among those with NAFLD without cirrhosis and 0.02 per 1000 person-years among controls[27]. Nonetheless, the study had substantial methodological limitations, especially regarding misclassification risks and lack of database granularity. Therefore, its results should be interpreted with caution.
Regarding non-cirrhotic HCC, a meta-analysis has demonstrated that around 38% of NAFLD-related HCCs are diagnosed in individuals without cirrhosis[9]. However, it should be emphasized that the risk of liver cancer is substantially higher in patients with NAFLD and cirrhosis when compared to those without cirrhosis. A recent meta-analysis found an incidence of 3.78 vs 0.03/100 person-years in patients with non-cirrhotic NAFLD[28].
Table 1 shows the studies that evaluated the incidence/prevalence of HCC and risk factors in patients with NAFLD without cirrhosis.
Table 1.
Studies that included the incidence/prevalence and risk factors for hepatocellular carcinoma in non-alcoholic fatty liver disease without cirrhosis
|
Ref.
|
Study design
|
Aim
|
Number of patients
|
Results and conclusion
|
| Mohamad et al[5], 2016 | Retrospective | To characterize patients with NAFLD and HCC comparing cirrhotic vs non-cirrhotic patients | All patients with NAFLD and HCC between 2003-2012 (n = 83) | 36 (43.4%) NAFLD HCC non-cirrhotic vs 47 (56.6%) NAFLD HCC cirrhotic patients. HCC patients without cirrhosis are more likely to present at an older age with larger tumor and higher rates of tumor recurrence |
| Piscaglia et al[6], 2016 | Multicenter observational prospective | To assess the clinical features of patients with NAFLD-related HCC and to compare to those with HCV related HCC | N = 756 (145 NAFLD vs 611 HCV) | Cirrhosis was present in about 50% of NAFLD-HCC patients, in contrast to the near totality of HCV-HCC. Survival was significantly shorter in patients with NAFLD-HCC than in those with HCV-HCC (25.5 mo vs 33.7 mo) |
| Stine et al[9], 2018 | Systematic review with meta-analysis | To compare the prevalence of NAFLD-related HCC to other chronic liver diseases | 19 studies (n = 168571) | The prevalence of NAFLD-related HCC in patients with NASH without cirrhosis is approximately 38% compared with 14% for other liver diseases |
| Tobari et al[24], 2020 | Prospective | To evaluate the characteristics of HCC in non-cirrhotic NAFLD | 48 non-cirrhotic HCC vs 71 cirrhotic HCC patients | In patients with non-cirrhotic NAFLD, important risk factors for HCC were male gender, alcohol consumption, and the FIB-4 index. HCC recurrence and survival were only influenced by the tumor stage |
| Kanwal et al[27], 2018 | Retrospective | To estimate the risk of incident HCC among patients with NAFLD | 296707 NAFLD vs 296707 matched controls | NAFLD individuals with cirrhosis had the highest annual incidence of HCC. 20% of NAFLD patients with HCC had no evidence of cirrhosis. The absolute risk of HCC in patients without cirrhosis is too low to recommend HCC surveillance |
| Orci et al[28], 2022 | Systematic review with meta-analysis | Evaluate the pooled HCC incidence in patients with NAFLD at distinct severity stages | 18 studies (470404 individuals) | Evidence documenting the risk in patients with NASH or simple steatosis is limited, but the incidence of HCC in these populations may lie below thresholds used to recommend a screening (0.03 per 100 person-years) |
| Donati et al[34], 2017 | Sectional | To evaluate whether the MBOAT7 rs641738 risk T allele predisposes to HCC in NAFLD patients stratified by the presence of severe fibrosis | 765 Italian NAFLD patients | The MBOAT7 rs641738 T allele is associated with reduced MBOAT7 expression and may predispose to HCC in patients without cirrhosis |
| Demirtaş et al[71], 2021 | Retrospective | To investigate the characteristics and survival course of non-cirrhotic individuals with HCC | N = 384 HCC; 43 (11.2%) without cirrhosis; 10 (23%) with NAFLD | HCC in non-cirrhotic liver is diagnosed at more advanced stage and with larger tumor size. The overall survival is shorter in HCC without cirrhosis, due to late recognition |
NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; HCC: Hepatocellular carcinoma; HCV: Hepatitis C virus; MBOAT7: Membrane-bound o-acyltransferase domain-containing 7.
PATHOPHYSIOLOGY OF NAFLD-RELATED HCC
The pathophysiology and etiology of NASH progression to HCC are not entirely known, and many mechanisms have been proposed. Neoplastic transformation of NAFLD is driven by metabolic imbalance, lipotoxicity consequent to hepatocyte lipid overload, oxidative stress and immunological aspects, whereas many other factors such as genetic markers, gut dysbiosis and alcohol or tobacco abuse may interact as risk modifiers[29].
Genetic factors
Three main single-nucleotide polymorphisms (SNPs) have been described as associated with a higher risk of steatosis, fibrosis and even HCC, Patatin-like phospholipase domain-containing 3 (PNPLA3), membrane-bound o-acyltransferase domain-containing 7 (MBOAT7), and transmembrane 6 superfamily member 2 (TM6SF2) genes[30].
The variant in the PNPLA3 gene is the strongest genetic variant predisposing from fatty liver to HCC, and its frequency ranges from 17% to 49% according to ethnicity and the geographic distribution of NAFLD[31]. This variant codifies adiponutrin, a protein responsible for the export of lipids from the liver. The substitution of a single nucleotide (from isoleucine to methionine – I148M) modifies the function of adiponutrin, leading to the accumulation of triglycerides, retinyl esters in lipid droplets in both hepatocytes and hepatic stellate cells, leading to fibrogenesis and tumorigenesis. Patients with at least one G allele, primarily those with GG homozygosis, have a higher risk of developing steatosis, fibrosis and HCC[32]. A subgroup analysis from a systematic review involving 9915 patients showed an association between the PNPLA3 rs738409 SNP and HCC among patients with NASH or alcohol-related cirrhosis with an odds ratio of 1.67 and a 95% confidence interval of 1.27-2.21, but not among patients with cirrhosis of other etiologies[33].
Studies investigating MBOAT7 association with HCC are scarce. In a cohort of 765 Italian patients with NAFLD, especially those without advanced fibrosis, the MBOAT7 rs641738 variant was strongly associated with HCC. On the other hand, it showed no association with HCC in a validation cohort of 358 patients with NAFLD without cirrhosis in the United Kingdom[34].
TM6SF2 polymorphism is also associated with increased liver fat content in NASH, advanced hepatic fibrosis and cirrhosis. TM6SF2 variants have a moderate to significant effect on the risk of NAFLD. Additionally, the E167K allele has an allelic odds ratio of 1.82 for steatosis[30]. Whether or not the variant is associated with an increased risk of NAFLD-related fibrosis and HCC remains to be determined.
Recently, the odd-skipped related transcription factor 1 (Osr1) has been reported as a novel tumor suppressor gene, as well as a potential prognostic biomarker in gastric cancer. Some authors suggest that Osr1 plays an essential role in regulating cell survival, cell inflammation, and macrophage migration in the liver. Accordingly, Osr1 was identified as a novel repressor gene in the progression of NAFLD/NASH[35]. So far, the role of Osr1 in the progression of NAFLD towards HCC development is not established.
Human telomerase reverse transcriptase (hTERT) mutations are associated with familial liver diseases. Telomere length and germline hTERT mutations were evaluated to determine their association with NAFLD-HCC. The authors observed an association between shorter peripheral blood telomeres and NAFLD-HCC development and found that rare germline mutations in hTERT predispose NAFLD progression to HCC, potentially assisting the identification of high-risk individuals[36].
Metabolic imbalance
Insulin resistance (IR) is the key pathogenic event associated with the development of hepatic steatosis and is also related to the development of HCC[37].
Hyperinsulinemia can promote the synthesis and activity of insulin-like growth factor-1, inhibiting cell proliferation and apoptosis[38], which increases the risk of hepatocellular carcinogenesis. Hyperglycemia provides a substrate for energy metabolism in tumor cells and leads to a glycosylation reaction activating the inflammatory signaling cascades and generating reactive oxygen species (ROS) to induce HCC development[39]. IR may also directly accelerate hepatocarcinogenesis by stimulating hepatic neovascularization[40].
These events affect cell growth by inducing the transcription of the protooncogenes, so fibrosis and carcinogenesis are promoted in the liver. Additionally, hyperinsulinemia increases hepatic lipid accumulation and leads to oxidative stress due to the increased beta-oxidation of free fatty acids and the formation of ROS. There is positive feedback between oxidative stress in mitochondria and endoplasmic reticulum (ER) through ER stress, further contributing to cell injury and carcinogenesis in NASH. In contrast to insulin-mediated apoptosis inhibition, hepatic lipotoxicity activates proapoptotic cell signals. Another recently discovered mechanism involves the association between lipolysis and autophagy, with conflicting evidence due to its double-natured, divergent role in NASH-associated HCC[41].
Lipotoxicity
Lipotoxicity is the dysregulation of intracellular lipid components resulting in the accumulation of harmful lipids, which are associated with cellular damage and death[42]. Lipotoxicity causes cellular damage as lipids alter the biology and function of intracellular organelles, such as the ER and mitochondria. Also, a direct modification of intracellular signaling pathways may occur, deregulating the metabolic and inflammatory pathways[43].
The ER is an intracellular organelle that engages in many critical cellular processes, including folding membranes and secreted proteins, synthesizing lipids and sterols, and storing free calcium. Disturbance of any of these processes results in stress on the ER and interrupts the protein folding process. When ER stress cannot be restored, the apoptotic pathway is stimulated, leading to cell death to eliminate the stressed cells[44].
ER stress is linked to the development and progression of liver inflammation. Because it is a crucial mediator of liver inflammation, the immunoglobulin protein promotes the inflammatory response associated with NASH[45]. ER stress has been identified as a mediator of NAFLD-promoted HCC in vitro. Also, enhanced ER stress increases tumor necrosis factor production by macrophages, leading to tumor formation[46].
Oxidative stress
Oxidative stress results from an imbalance between the excessive formation of prooxidants (ROS and/or reactive nitrogen species) and limited antioxidant defenses, leading to cell death and tissue damage[47].
In NAFLD, there are some mechanisms for producing mitochondrial ROS. Thus, mitochondrial dysfunction and ROS production are exacerbated. In this context, some hepatocytes may develop adaptive cell survival and proliferation mechanisms that promote precancerous transformation and/or tumor growth[48].
Immunological aspects
During the progression of NAFLD from steatosis to NASH and more advanced stages of NASH with liver fibrosis, the immune system plays an important role. There are inflammation triggers within hepatic (lipid overload, lipotoxicity, oxidative stress) and extra-hepatic systems (gut-liver axis, adipose tissue, skeletal muscle), resulting in unique immune-mediated pathomechanisms in NAFLD[49].
Immune cells play a role in hepatocarcinogenesis through processes that are independent of fibrosis. Hepatocyte damage promotes neutrophil infiltration in the liver, resulting in DNA damage to other hepatocytes and promoting HCC development without fibrosis. Furthermore, lymphoid aggregates are often present in the setting of chronic inflammation. Additionally, the selective loss of CD4+ T lymphocytes occurs, which was shown to be critical for the progression of HCC[41].
Although immunological response can promote HCC, the immune system also plays an important role in suppressing tumor growth through immunosurveillance. Furthermore, HCC actively promotes tumor tolerance by inducing immunosuppression, and the fibrotic microenvironment leads to the overproduction of transforming growth factor beta, a potent immunosuppressant, thereby promoting disease progression[41].
Microbiota
Increased gut permeability and altered microbiome composition are associated with NAFLD and its disease severity, contributing to hepatocarcinogenesis[50].
The gut microbiota has been described as a cofactor in liver disease progression and in the development of HCC through the interaction with immune compartments via the gut–liver axis. Dysbiosis characterizes the microbiota of patients with NAFLD-cirrhosis, with compositional and functional shifts occurring with HCC development. It has been suggested that the gut microbiota in NAFLD-HCC is characterized by a distinctive microbiome/metabolomic profile and can modulate the peripheral immune response[50]. Human metagenomic data support an emerging core microbiome signature that characterizes NAFLD-cirrhosis, with increased Ruminococcus gnavus, Clostridium bolteae, Streptococcus parasanguinis, and Klebsiella pneumoniae, and a reduced number of beneficial species, including Faecalibacterium prausnitzii, Alistipes putredinis, and Eubacterium eligens. Furthermore, Veillonella parvula and Bacteroides caecimuris are also identified to distinguish NAFLD-HCC from NAFLD-cirrhosis. In agreement with these findings, rRNA analyses of patients with NAFLD-HCC have detected enrichment in Bacteroides and Ruminococcaceae, which correlated with several systemic inflammatory and immune markers[51]. Ren et al[52] also observed a decrease in butyrate-producing bacterial families, namely Ruminococcus, Oscillibacter, Faecalibacterium, Clostridium IV, and Coprococcus in patients with HCC.
Increased intestinal permeability, intestinal bacterial overgrowth and elevated serum endotoxin have been reported in NAFLD and NAFLD-HCC[53]. Endotoxemia-induced toll-like receptor 2 induction leads to cyclooxygenase-2 (COX2) mediated prostaglandin E (PGE) production, which suppresses antitumor immunity by inhibiting antitumor cytokine production from liver immune cells leading to HCC progression in a mouse model. In human non-cirrhotic NAFLD-related HCC, COX2 overexpression and excess PGE production are detected. Although these findings suggest that hepatocellular inflammation may be secondary to altered intestinal permeability and translocation of either intact bacteria or microbial cell components into the circulation, the causal link between them is not entirely clarified[53,54].
Other factors
Many factors have been associated with the potential to increase the risk of HCC in NAFLD, such as male gender, older age, ethnicity, presence of type 2 DM, obesity, any degree of alcohol consumption and smoking[27,55].
Among these, risk factors for NAFLD-related HCC, which have long been recognized, are male sex, older age and Latino ethnicity[56]. Kanwal et al[27] described in a large cohort study involving 296707 patients with NAFLD that age above 65 years was an independent risk factor for HCC. It was more often identified in men and was higher in Hispanic individuals compared to white (0.21 per 1000 patient-years) and African American individuals (0.12 per 1000 patient-years)[27].
Clinical variables such as diagnosis of type 2 DM and obesity are also significant risk factors among patients with NAFLD. They can act independently or jointly with NAFLD to increase the risk of HCC development[56]. Type 2 DM doubled the risk of developing this outcome[19]. DM is a recognized risk factor for HCC regardless of the etiology of liver disease, and some authors suggest that DM has the strongest association with HCC[57], being related to the duration of DM and adequate glycemic control[58]. On the other hand, it is unclear if the correlation between DM and HCC in patients without cirrhosis applies, as a recent study evaluating the differences between cirrhotic and non-cirrhotic HCC in NAFLD found an inverse association between DM and HCC in the non-cirrhotic group, emphasizing that non-cirrhotic HCC tended to occur in older patients and those with a lower body mass index[59].
Obese patients with cirrhosis were 47 times more likely to have HCC than persons without liver disease, and there is strong evidence that obesity impacts HCC development and promotes an increase in mortality, especially in those with early age onset and the presence of visceral fat[58].
Obesity is a well-known risk factor for many cancers but is significantly linked to liver cancer[60]. A study from the Mayo Clinic has shown that the diagnosis of type 2 DM increased the risk of HCC by fourfold. Therefore, it is recommended that type 2 DM in every individual with NAFLD should be investigated due to its association with more advanced disease and increased risk of HCC[61].
Alcohol consumption is independently associated with a higher risk of HCC in individuals with NAFLD[62]. Some studies suggest that the increased risk would apply only to those with heavy alcohol use (e.g., > 50 g/d or ≥ 3 drinks/d or ≥ 7 drinks/d), better supporting the recent definition of MAFLD instead of NAFLD. The additive effect of alcohol in those with NAFLD might explain the increase of HCC in this specific group[63].
The study by Ascha et al[64] suggested that any degree of alcohol consumption may increase the risk of HCC occurrence in patients who, by the classic definition, do not have a significant intake. The deleterious effects of continuous and excessive ethanol intake on the liver are well established; however, there is uncertainty regarding the impact of mild to moderate ethanol consumption[65].
In the same way, elevated alanine aminotransferase has been proposed as an independent factor associated with an increased HCC risk[65].
Environmental factors such as tobacco smoking are associated with insulin resistance, the development of NAFLD and liver cancer. Current and former smoking is associated with a 70% and 40% increased risk of liver cancer, respectively[66]. Similarly, in a meta-analysis of 81 studies, the pooled odds ratios for HCC development were 1.55 in current smokers vs 1.39 in former smokers[67]. Currently, there is no specific data on the risk of smoking in NAFLD-related HCC.
Many studies[22,27,54,57,64,65,68] have assessed the risk of HCC or other liver complications in patients with non-cirrhotic NAFLD, but they have many limitations. Most of them were retrospective and heterogeneous in terms of the inclusion criteria; did not have data on liver fibrosis stages; or had a short follow-up to assess complex outcomes such as HCC or complications of cirrhosis. In addition, most of them had relatively few cases of HCC diagnosed.
HISTOPATHOLOGICAL ASPECTS OF NAFLD-RELATED HCC
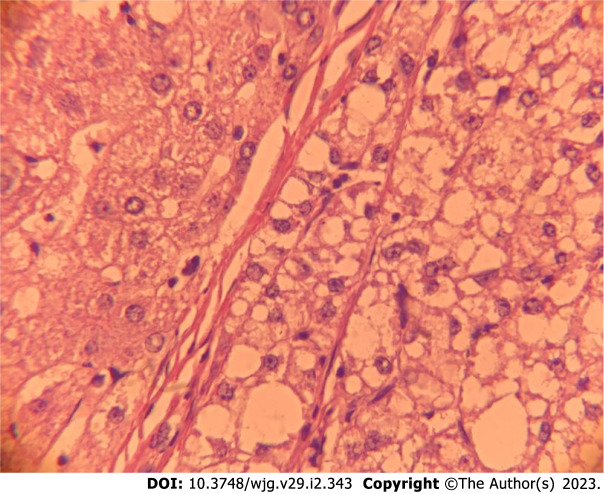
Patients with NAFLD and HCC without cirrhosis have larger tumors, but more often, they have well-differentiated tumors and a single nodule compared to those with cirrhosis[59,69,70]. On the other hand, due to late diagnosis, some cases have a higher rate of vascular invasion and extra-hepatic metastasis[71]. Frequently, the nontumor liver has significant steatosis and histological findings of steatohepatitis[72] (Figure 1).
Figure 1.
Histopathological aspect of a non-alcoholic fatty liver disease-related hepatocellular carcinoma in a non-cirrhotic liver. On the left side: Non-cirrhotic liver with steatosis. On the right side: Hepatocellular carcinoma, steatohepatitis variant with steatosis, hepatocellular ballooning and macro-trabecular arrangement.
Paradis et al[69], studying patients with HCC and metabolic risk factors, demonstrated that the neoplasia in 5 of 31 patients with NASH without cirrhosis developed on a preexisting liver cell adenoma.
Approximately 90% of HCCs are the conventional subtype, but patients with NAFLD with or without cirrhosis or patients with metabolic risks can present a histological subtype of HCC identified as a steatohepatitis-related variant[73]. Macroscopically, the nodule is golden-yellow in color and slightly firm because of steatosis and fibrosis[74].
The histologic features of this subtype are macrovesicular steatosis, ballooning malignant hepatocytes, lymphocytic inflammation, Mallory Denk bodies and pericellular fibrosis, often with a “chicken-wire pattern”[75]. More frequently, neoplasia has a trabecular arrangement and minimal mitotic activity[74,75].
Regarding immunohistochemistry, a study evaluating 62 cases demonstrated a similarity between steatohepatitis HCC in non-cirrhotic livers and inflammatory liver cell adenomas, demonstrating a higher expression of C-reactive protein and serum amyloid A[70].
These tumors also have distinct molecular features: They frequently showed IL-6/JAK/STAT activation and less often Wnt/β catenin/CTNNB1 and P53 pathway alterations[76].
CURRENT RECOMMENDATIONS ON SURVEILLANCE FOR HCC IN NAFLD
HCC in the setting of NASH is known to occur even in the absence of liver cirrhosis, an event previously mainly related to hepatitis B virus infection. Thus, knowing whom to screen for HCC and which patient population deserves surveillance is essential.
The objective of screening and surveillance in patients with cirrhosis is to reduce mortality, as this patient population will develop HCC. Cost-effectiveness studies suggest that an incidence of 1.5% per year or more would warrant HCC surveillance in cirrhotic patients, regardless of etiology[23,77]. Recent analysis has confirmed the importance of surveillance in patients with cirrhosis, resulting in longer survival[78]. In Brazil, when we performed screening in a population of more than 500 patients with cirrhosis, the prevalence of HCC was around 5%[79]. Likewise, when we followed a cohort of 450 patients with cirrhosis, the estimated cumulative incidence of HCC was 2.6% in the first year, 15.4% in the fifth year and 28.8% in the tenth year, demonstrating the relevance of carrying out a surveillance program[80].
NAFLD, with or without NASH, is a hepatic manifestation of metabolic syndrome and predisposes to HCC in cirrhotic and non-cirrhotic patients. Despite the high prevalence of NAFLD in the general population, as previously mentioned, it is believed that the incidence of HCC in these patients with non-advanced disease is not sufficiently high for a universal surveillance program to be proposed. In a systematic review, considering only studies that include patients with or without cirrhosis, the incidence of HCC in NAFLD patients with cirrhosis was 15% at 10 years, while the incidence in NAFLD patients without cirrhosis was 2.7% and 23 per 100000 person-years[81]. Given the lowest risk of HCC in non-cirrhotic livers (approximately 0.1 to 0.8 per 1000 patient-years), the development of cost-effective HCC surveillance strategies to identify high-risk NAFLD patients without cirrhosis are needed[58].
Although type 2 DM and obesity have been implicated as independent risk factors for HCC, studies establishing a clear link with HCC in non-cirrhotic livers are scarce[82]. Therefore, it becomes essential to assess the benefits of predictive models based on clinical data to identify patients with HCC in the population of NAFLD patients without cirrhosis.
Some authors use different tools to stratify patients according to the risk of developing HCC. Thus, FIB-4 was evaluated in European databases, including more than 18 million individuals. When the NAFLD group was classified according to the FIB-4 score, it was possible to identify which patients were at greater risk. When compared to individuals with a FIB-4 score < 1.30, those with a score between 1.30 and 2.67 had a risk ratio for HCC of 3.74, and those with a score > 2.67 had a risk ratio for HCC of 25.2[22]. Although not accepted by all[27], it is possible that the FIB-4 score can be used in selected patients for surveillance.
In a European longitudinal study, Younes et al[83] applied various scores (NAFLD fibrosis score - NFS, FIB-4, BARD, APRI) and the Hepamet fibrosis score to predict HCC in 1173 patients with NAFLD (75% non-cirrhotic). These patients were followed for a mean period of 81 mo, with 17 patients (1.5%) developing HCC. The NFS performed significantly better than the other non-invasive scores (C-index: 0.901 ± 0.0302; AUROC = 0.889 ± 0.048)[83].
The latest European Association for the Study of the Liver guideline recommends surveillance in patients with metabolic syndrome or NASH in the presence of significant fibrosis on histology or elastography. However, it is noted that the role of surveillance for NAFLD patients without cirrhosis is unclear[84].
Recently, at a meeting of experts, an evidence-based review was performed addressing the risk of HCC in patients with NAFLD. This review concluded that NAFLD patients with evidence of advanced fibrosis, even when suggested by non-invasive markers, should be considered for HCC screening. Thus, the need for surveillance would be indicated when there is an agreement between two non-invasive tests with different methodologies (FIB-4 and elastography, for example). These results were endorsed by the American Gastroenterological Association[85].
NOVEL PERSPECTIVES IN SURVEILLANCE FOR HCC IN NAFLD
The most validated predictive factor for HCC development in NAFLD is the presence of advanced fibrosis. However, many other factors may be considered to identify those at high risk for liver cancer, even though we still do not have enough evidence to change HCC surveillance strategies in NAFLD[86].
In addition to surveillance based on imaging and serological methods, mainly ultrasound and alpha-fetoprotein, there are no scores or predictive models with enough strength to use in the daily surveillance of NAFLD-related HCC. The development of novel tools might help risk stratification and accurately identify high-risk patients, even those without cirrhosis, leading to individualized surveillance strategies.
In future studies, some of these clinical scores should be combined with genetic risk factors for risk stratification of patients with NAFLD, since the genetic markers currently available still have limitations. As noted, different genetic polymorphisms have varying effects on HCC risks; the 17-β hydroxysteroid dehydrogenase 13-HSD17B13, for example, has protective effects, while others such as the PNPLA3 (variant I148M) increase HCC risk[58].
The combination of genetic polymorphisms to determine a genetic risk score has shown a low accuracy with a sensitivity of 43% and specificity of 79% in the prediction of HCC with an AUROC of only 0.65[85]. Moreover, the genetic polymorphisms are not ready to be used in clinical routine due to high cost and low availability. Another large study by Bianco et al[87] investigated the polygenic risk score (PRS) in a German and an Italian cohort with NAFLD compared to the general population regarding the development of HCC. The polygenic risk score (PRS) was composed of TM6SF2-GCKR-MBOAT7 combined in hepatic fat PRS (PRSHFC), further adjusted for HSD17B13 (PRS-5). This study showed a strong association between hepatic fat and HCC. The PRS improved the accuracy of HCC detection and may help stratify HCC risk in individuals with dysmetabolism, including those without severe liver fibrosis[87].
Also, multiple new panels, including biomarkers such as multiprotein-based and circulating tumor-derived DNA-based (“liquid biopsy”) panels[88], as well as abbreviated magnetic resonance imaging protocols and other imaging-based protocols, are currently under investigation as potential screening tests. Studies investigating the accuracy of liquid biopsies are ongoing. Liquid biopsy strategies for sampling tumor products in the bloodstream include substances such as circulating tumor cells (CTCs), circulating tumor DNA (ct-DNA) and extracellular vesicles (EVs)[88]. CTCs include cells released from primary or metastatic tumor sites, CT-DNA consists of DNA from cellular necrosis or apoptosis, and EVs are cell membrane-derived particles such as apoptotic bodies, micro-vesicles and exosomes, containing molecular cargoes specific to the origin cell with an essential role in cell-to-cell communication[88]. Data from a systematic review with 67 studies evaluated liquid biopsy techniques for early-stage HCC detection, including studies evaluating CTCs, ct-DNA and EVs. They have shown good accuracy for HCC detection, with higher accuracy than alpha-fetoprotein (AFP) for distinguishing patients with HCC from controls and the capacity to identify AFP-negative HCC patients. In this study, combinations with AFP were superior to AFP alone[62]. When included in a panel, a liquid biopsy was also associated with poorer survival (EVs and ct-DNA)[89] and with tumor progression.
Some blood-based biomarkers, such as lectin-bound AFP (AFP-L3) and des-gamma carboxyprothrombin (DCP), have been proposed for detecting HCC in some regions like Japan and are under investigation in other countries. Moreover, there is an increased interest in early detection panels using multiple combined biomarkers. The best example is GALAD, which combines demographic and clinical variables with blood-based biomarkers such as gender, age, AFP, AFP-L3, and DCP[90]. In a multinational case-control study, its sensitivity was 60%–80% for detecting early-stage HCC[90]. The GALAD panel was recently evaluated in a case-control study of 125 patients with NAFLD. It showed a similar diagnostic performance at a cut-off of -0.63, with a sensitivity and specificity of 68% and 95%, respectively, for early-stage HCC[91]. Interestingly, in the prospective study arm, the GALAD score identified patients who developed HCC as early as 1.5 years before their diagnosis[91]. However, although it is a promising tool, it is not yet available for clinical use since it still needs to be validated in phase III and IV studies.
After basic serological tests, elastographic techniques are the cornerstone for NAFLD's non-invasive staging of liver fibrosis. Vibration-controlled transient elastography (VCTE) can also assess steatosis through the controlled attenuation parameter and is considered the point of care method among elastography-related techniques[40]. 2D-Shear wave elastography and point-shear wave elastography have the additional capacity to evaluate the macroscopic aspect of the liver and identify nodular lesions as patients with NAFLD-related cirrhosis should have an ultrasound every six months to screen for HCC. Thus, the elastography evaluation and the evaluation of liver lesions have been studied as additional methods for HCC surveillance[92]. A recent study in type 2 DM individuals with NAFLD who had VCTE at baseline and were followed for 50 mo has shown that those with liver stiffness > 13 kPa had a higher incidence of liver decompensation and HCC[93].
Boursier et al[94] evaluated the prognostic significance of liver stiffness in NAFLD. They proposed defining a new fibrosis classification stage based on liver stiffness by VCTE categorized in seven different classes of liver fibrosis: LSM1 (2.0 to 4.6 kPa), LSM2 (4.6 to 6.1 kPa), LSM3 (6.1 to 8.8 kPa), LSM4 (8.8 to 12.0 kPa), LSM5 (12.0 to 18.0 kPa), LSM6 (18.0 to 38.6 kPa) and LSM7 (greater than 38.6 kPa to 75 kPa). In this study, overall survival decreased as liver stiffness increased. For instance, overall survival for LSM1 in ten years was close to 100%, whereas, for LSM7, it was near 30%. The authors evaluated liver-related deaths in this study, not specifically HCC[94]. As a reflection, based on the data presented, we could suggest performing elastography in patients with NAFLD, and, when a greater liver stiffness is evidenced, they would be selected to join a screening and surveillance program.
CONCLUSION
It is currently challenging to propose general recommendations for screening patients with NAFLD without cirrhosis, and each patient should be evaluated on a case-by-case basis based on the profile of specific risk factors identified. For HCC screening in NAFLD, a valid precision-based screening is needed.
Currently, when evaluating this population of patients, we believe that the use of non-invasive methods can guide the selection of patients who will undergo a screening and surveillance program. So far, ultrasound with or without AFP is still the screening method of choice and should be used for all NAFLD patients with advanced fibrosis. In the future, it is possible that new technologies and liquid biopsy methods might add precision in screening these large populations, including those without cirrhosis.
Footnotes
Conflict-of-interest statement: There is no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 12, 2022
First decision: October 4, 2022
Article in press: November 18, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Geng TY, China; Lin J, China; Salvadori M, Italy S-Editor: Wang JL L-Editor: Webster JR P-Editor: Wang JL
Contributor Information
Cristiane Valle Tovo, Department of Internal Medicine, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre 90050170, RS, Brazil. cris.tovo@terra.com.br.
Angelo Zambam de Mattos, Department of Internal Medicine, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre 90050170, RS, Brazil.
Gabriela Perdomo Coral, Department of Internal Medicine, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre 90050170, RS, Brazil.
Giovana D P Sartori, Department of Internal Medicine, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre 90050170, RS, Brazil.
Livia Villela Nogueira, Department of Internal Medicine, Fundação Técnico Educacional Souza Marques, RJ 21491-630, RJ, Brazil.
Gustavo Tovo Both, Department of Internal Medicine, Universidade Luterana do Brasil, Canoas 92425-350, RS, Brazil.
Cristiane A Villela-Nogueira, Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro 21491-630, RJ, Brazil.
Angelo A de Mattos, Department of Internal Medicine, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre 90050170, RS, Brazil.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Long MT, Noureddin M, Lim JK. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology. 2022;163:764–774.e1. doi: 10.1053/j.gastro.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung C, Yeoh SW, Patrick D, Ket S, Marion K, Gow P, Angus PW. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol. 2015;21:1189–1196. doi: 10.3748/wjg.v21.i4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, Hanouneh IA, Alhaddad R, Alkhouri N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10:632–639. doi: 10.1007/s12072-015-9679-0. [DOI] [PubMed] [Google Scholar]
- 6.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 7.Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234–1245. doi: 10.1007/s10620-016-4085-6. [DOI] [PubMed] [Google Scholar]
- 8.Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR. Diabetes Mellitus Heightens the Risk of Hepatocellular Carcinoma Except in Patients With Hepatitis C Cirrhosis. Am J Gastroenterol. 2016;111:1573–1580. doi: 10.1038/ajg.2016.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, Argo CK. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696–703. doi: 10.1111/apt.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Global Cancer Observatory-GLOBOCAN 2020. Available from: https://gco.iarc.fr/
- 13.Liu Z, Xu K, Jiang Y, Cai N, Fan J, Mao X, Suo C, Jin L, Zhang T, Chen X. Global trend of aetiology-based primary liver cancer incidence from 1990 to 2030: a modelling study. Int J Epidemiol. 2021;50:128–142. doi: 10.1093/ije/dyaa196. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 15.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 16.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debes JD, Chan AJ, Balderramo D, Kikuchi L, Gonzalez Ballerga E, Prieto JE, Tapias M, Idrovo V, Davalos MB, Cairo F, Barreyro FJ, Paredes S, Hernandez N, Avendaño K, Diaz Ferrer J, Yang JD, Carrera E, Garcia JA, Mattos AZ, Hirsch BS, Gonçalves PT, Carrilho FJ, Roberts LR. Hepatocellular carcinoma in South America: Evaluation of risk factors, demographics and therapy. Liver Int. 2018;38:136–143. doi: 10.1111/liv.13502. [DOI] [PubMed] [Google Scholar]
- 18.Farah M, Diaz Ferrer J, Baca EL, Mattos A, Arrese M, Prieto Ortiz JE, Balderramo D, Carrera E, Boonstra A, Debes JD. Changing epidemiology of hepatocellular carcinoma in South America: a report from the ESCALON Network. Hepatology. 2021;74 Suppl 1:681A. doi: 10.1016/j.aohep.2022.100876. [DOI] [PubMed] [Google Scholar]
- 19.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Geh D, Manas DM, Reeves HL. Hepatocellular carcinoma in non-alcoholic fatty liver disease-a review of an emerging challenge facing clinicians. Hepatobiliary Surg Nutr. 2021;10:59–75. doi: 10.21037/hbsn.2019.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 22.Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Waterworth DM, Kendrick S, Sattar N, Alazawi W. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Strazzabosco M, Giannini EG. Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective? Cancers (Basel) 2020;12 doi: 10.3390/cancers12061422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobari M, Hashimoto E, Taniai M, Kodama K, Kogiso T, Tokushige K, Yamamoto M, Takayoshi N, Satoshi K, Tatsuo A. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J Gastroenterol Hepatol. 2020;35:862–869. doi: 10.1111/jgh.14867. [DOI] [PubMed] [Google Scholar]
- 25.Banini BA, Sanyal AJ. NAFLD-related HCC. Adv Cancer Res. 2021;149:143–169. doi: 10.1016/bs.acr.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Mattos ÂZ, Debes JD, Dhanasekaran R, Benhammou JN, Arrese M, Patrício ALV, Zilio AC, Mattos AA. Hepatocellular carcinoma in nonalcoholic fatty liver disease: A growing challenge. World J Hepatol. 2021;13:1107–1121. doi: 10.4254/wjh.v13.i9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828–1837.e2. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, Bruix J, Reig M, Toso C. Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clin Gastroenterol Hepatol. 2022;20:283–292.e10. doi: 10.1016/j.cgh.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Valenti L, Pedica F, Colombo M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Dig Liver Dis. 2022;54:154–163. doi: 10.1016/j.dld.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Meroni M, Longo M, Tria G, Dongiovanni P. Genetics Is of the Essence to Face NAFLD. Biomedicines. 2021;9 doi: 10.3390/biomedicines9101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machado CM, Leite NC, França PH, Cardoso CR, Salles GF, Villela-Nogueira CA. PNPLA3 gene polymorphism in Brazilian patients with type 2 diabetes: A prognostic marker beyond liver disease? Nutr Metab Cardiovasc Dis. 2019;29:965–971. doi: 10.1016/j.numecd.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, Waljee AK. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:325–334. doi: 10.1038/ajg.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, Petta S, Maier S, Rosso C, De Luca L, Vanni E, Grimaudo S, Romagnoli R, Colli F, Ferri F, Mancina RM, Iruzubieta P, Craxi A, Fracanzani AL, Grieco A, Corradini SG, Aghemo A, Colombo M, Soardo G, Bugianesi E, Reeves H, Anstee QM, Fargion S, Valenti L. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. doi: 10.1038/s41598-017-04991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Liu Z, Lynch EC, He L, Cheng H, Liu L, Li Z, Li J, Lawless L, Zhang KK, Xie L. Osr1 regulates hepatic inflammation and cell survival in the progression of non-alcoholic fatty liver disease. Lab Invest. 2021;101:477–489. doi: 10.1038/s41374-020-00493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donati B, Pietrelli A, Pingitore P, Dongiovanni P, Caddeo A, Walker L, Baselli G, Pelusi S, Rosso C, Vanni E, Daly A, Mancina RM, Grieco A, Miele L, Grimaudo S, Craxi A, Petta S, De Luca L, Maier S, Soardo G, Bugianesi E, Colli F, Romagnoli R, Anstee QM, Reeves HL, Fracanzani AL, Fargion S, Romeo S, Valenti L. Telomerase reverse transcriptase germline mutations and hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancer Med. 2017;6:1930–1940. doi: 10.1002/cam4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Minicis S, Agostinelli L, Rychlicki C, Sorice GP, Saccomanno S, Candelaresi C, Giaccari A, Trozzi L, Pierantonelli I, Mingarelli E, Marzioni M, Muscogiuri G, Gaggini M, Benedetti A, Gastaldelli A, Guido M, Svegliati-Baroni G. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS One. 2014;9:e97136. doi: 10.1371/journal.pone.0097136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 39.Jabir NR, Ahmad S, Tabrez S. An insight on the association of glycation with hepatocellular carcinoma. Semin Cancer Biol. 2018;49:56–63. doi: 10.1016/j.semcancer.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, Yoshii J, Yanase K, Namisaki T, Yamazaki M, Moriya K, Tsujimoto T, Kawaratani H, Akahane T, Uemura M, Fukui H. Impact of insulin resistance on the progression of chronic liver diseases. Int J Mol Med. 2008;22:801–808. [PubMed] [Google Scholar]
- 41.Grgurevic I, Bozin T, Mikus M, Kukla M, O'Beirne J. Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: From Epidemiology to Diagnostic Approach. Cancers (Basel) 2021;13 doi: 10.3390/cancers13225844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wouters K, van Bilsen M, van Gorp PJ, Bieghs V, Lütjohann D, Kerksiek A, Staels B, Hofker MH, Shiri-Sverdlov R. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS Lett. 2010;584:1001–1005. doi: 10.1016/j.febslet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 43.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang K, Kaufman RJ. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods Enzymol. 2008;442:395–419. doi: 10.1016/S0076-6879(08)01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J, Koike K, Kaufman RJ, Karin M. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–343. doi: 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matés JM, Segura JA, Alonso FJ, Márquez J. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82:273–299. doi: 10.1007/s00204-008-0304-z. [DOI] [PubMed] [Google Scholar]
- 48.Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303–1307. doi: 10.1136/gut.2009.199661. [DOI] [PubMed] [Google Scholar]
- 49.Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–1160. doi: 10.1016/j.jhep.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187. doi: 10.1038/s41467-020-20422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 52.Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014–1023. doi: 10.1136/gutjnl-2017-315084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwabe RF, Greten TF. Gut microbiome in HCC - Mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230–238. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 55.Fassio E, Barreyro FJ, Pérez MS, Dávila D, Landeira G, Gualano G, Ruffillo G. Hepatocellular carcinoma in patients with metabolic dysfunction-associated fatty liver disease: Can we stratify at-risk populations? World J Hepatol. 2022;14:354–371. doi: 10.4254/wjh.v14.i2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, Asch SM, El-Serag HB. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:808–819. doi: 10.1002/hep.31014. [DOI] [PubMed] [Google Scholar]
- 58.Shah PA, Patil R, Harrison SA. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology. 2022 doi: 10.1002/hep.32542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bengtsson B, Stål P, Wahlin S, Björkström NK, Hagström H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019;39:1098–1108. doi: 10.1111/liv.14087. [DOI] [PubMed] [Google Scholar]
- 60.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, Roberts LR. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:907–916. doi: 10.1002/hep.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen VL, Xu D, Wicha MS, Lok AS, Parikh ND. Utility of Liquid Biopsy Analysis in Detection of Hepatocellular Carcinoma, Determination of Prognosis, and Disease Monitoring: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:2879–2902.e9. doi: 10.1016/j.cgh.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021;74:458–465. doi: 10.1016/j.jhep.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 64.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 65.Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141:1307–1314. doi: 10.1002/ijc.30784. [DOI] [PubMed] [Google Scholar]
- 66.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General's report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179:403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdel-Rahman O, Helbling D, Schöb O, Eltobgy M, Mohamed H, Schmidt J, Giryes A, Mehrabi A, Iype S, John H, Tekbas A, Zidan A, Oweira H. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: An updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10:245–254. doi: 10.1111/jebm.12270. [DOI] [PubMed] [Google Scholar]
- 68.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ohmoto Y, Amakawa K, Tsuji H, Kumada H. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 69.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 70.Taniai M, Hashimoto E, Tobari M, Kodama K, Tokushige K, Yamamoto M, Takayama T, Sugitani M, Sano K, Kondo F, Fukusato T. Clinicopathological investigation of steatohepatitic hepatocellular carcinoma: A multicenter study using immunohistochemical analysis of adenoma-related markers. Hepatol Res. 2018;48:947–955. doi: 10.1111/hepr.13203. [DOI] [PubMed] [Google Scholar]
- 71.Demirtaş CÖ, Tolu T, Keklikkıran Ç, Özdoğan OC, Gündüz F. Hepatocellular Carcinoma in Non-cirrhotic Liver Arises with a More Advanced Tumoral Appearance: A Single-Center Cohort Study. Turk J Gastroenterol. 2021;32:685–693. doi: 10.5152/tjg.2021.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]
- 73.Olofson AM, Gonzalo DH, Chang M, Liu X. Steatohepatitic Variant of Hepatocellular Carcinoma: A Focused Review. Gastroenterology Res. 2018;11:391–396. doi: 10.14740/gr1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeh MM, Liu Y, Torbenson M. Steatohepatitic variant of hepatocellular carcinoma in the absence of metabolic syndrome or background steatosis: a clinical, pathological, and genetic study. Hum Pathol. 2015;46:1769–1775. doi: 10.1016/j.humpath.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 75.Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43:737–746. doi: 10.1016/j.humpath.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage P, Nault JC, Zucman-Rossi J. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Díaz-González Á, Forner A. Surveillance for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2016;30:1001–1010. doi: 10.1016/j.bpg.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 78.Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, Yang JD, Reig M, Cabibbo G, Nahon P, Parikh ND, Marrero JA. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;77:128–139. doi: 10.1016/j.jhep.2022.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.John JA, de Mattos AA, da Silva Miozzo SA, Comerlato PH, Porto M, Contiero P, da Silva RR. Survival and risk factors related to death in outpatients with cirrhosis treated in a clinic in Southern Brazil. Eur J Gastroenterol Hepatol. 2015;27:1372–1377. doi: 10.1097/MEG.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 80.Appel-da-Silva MC, Miozzo SA, Dossin IA, Tovo CV, Branco F, de Mattos AA. Incidence of hepatocellular carcinoma in outpatients with cirrhosis in Brazil: A 10-year retrospective cohort study. World J Gastroenterol. 2016;22:10219–10225. doi: 10.3748/wjg.v22.i46.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reig M, Gambato M, Man NK, Roberts JP, Victor D, Orci LA, Toso C. Should Patients With NAFLD/NASH Be Surveyed for HCC? Transplantation. 2019;103:39–44. doi: 10.1097/TP.0000000000002361. [DOI] [PubMed] [Google Scholar]
- 82.Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11:1–18. doi: 10.4254/wjh.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Younes R, Caviglia GP, Govaere O, Rosso C, Armandi A, Sanavia T, Pennisi G, Liguori A, Francione P, Gallego-Durán R, Ampuero J, Garcia Blanco MJ, Aller R, Tiniakos D, Burt A, David E, Vecchio FM, Maggioni M, Cabibi D, Pareja MJ, Zaki MYW, Grieco A, Fracanzani AL, Valenti L, Miele L, Fariselli P, Petta S, Romero-Gomez M, Anstee QM, Bugianesi E. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. 2021;75:786–794. doi: 10.1016/j.jhep.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 84.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 85.Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020;158:1822–1830. doi: 10.1053/j.gastro.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75:1476–1484. doi: 10.1016/j.jhep.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 87.Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, Santoro L, Maier S, Liguori A, Meroni M, Borroni V, D'Ambrosio R, Spagnuolo R, Alisi A, Federico A, Bugianesi E, Petta S, Miele L, Vespasiani-Gentilucci U, Anstee QM, Stickel F, Hampe J, Fischer J, Berg T, Fracanzani AL, Soardo G, Reeves H, Prati D, Romeo S, Valenti L. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74:775–782. doi: 10.1016/j.jhep.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arvind A, Singal AG. Emerging liquid biopsy techniques for early detection of hepatocellular carcinoma, prognostication, and disease monitoring. Clin Liver Dis (Hoboken) 2022;20:18–20. doi: 10.1002/cld.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, Yi S, Shi W, Quan Q, Li K, Zheng L, Zhang H, Caughey BA, Zhao Q, Hou J, Zhang R, Xu Y, Cai H, Li G, Hou R, Zhong Z, Lin D, Fu X, Zhu J, Duan Y, Yu M, Ying B, Zhang W, Wang J, Zhang E, Zhang C, Li O, Guo R, Carter H, Zhu JK, Hao X, Zhang K. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16:1155–1161. doi: 10.1038/nmat4997. [DOI] [PubMed] [Google Scholar]
- 90.Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, Schweitzer N, Vogel A, Manns MP, Benckert J, Berg T, Ebker M, Best J, Dechêne A, Gerken G, Schlaak JF, Weinmann A, Wörns MA, Galle P, Yeo W, Mo F, Chan SL, Reeves H, Cox T, Johnson P. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol. 2016;14:875–886.e6. doi: 10.1016/j.cgh.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 91.Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, Bedreli S, Schotten C, Geier A, Berg T, Fischer J, Vogel A, Bantel H, Weinmann A, Schattenberg JM, Huber Y, Wege H, von Felden J, Schulze K, Bettinger D, Thimme R, Sinner F, Schütte K, Weiss KH, Toyoda H, Yasuda S, Kumada T, Berhane S, Wichert M, Heider D, Gerken G, Johnson P, Canbay A. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:728–735.e4. doi: 10.1016/j.cgh.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 92.Lupsor-Platon M, Serban T, Silion AI, Tirpe A, Florea M. Hepatocellular Carcinoma and Non-Alcoholic Fatty Liver Disease: A Step Forward for Better Evaluation Using Ultrasound Elastography. Cancers (Basel) 2020;12 doi: 10.3390/cancers12102778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson AL, Hayward KL, Patel P, Horsfall LU, Cheah AEZ, Irvine KM, Russell AW, Stuart KA, Williams S, Hartel G, Valery PC, Powell EE. Predicting Liver-Related Outcomes in People With Nonalcoholic Fatty Liver Disease: The Prognostic Value of Noninvasive Fibrosis Tests. Hepatol Commun. 2022;6:728–739. doi: 10.1002/hep4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, Chermak F, Bertrais S, Foucher J, Oberti F, Charbonnier M, Fouchard-Hubert I, Rousselet MC, Calès P, de Lédinghen V. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570–578. doi: 10.1016/j.jhep.2016.04.023. [DOI] [PubMed] [Google Scholar]