Sudden cardiac death, predominantly due to ventricular tachycardia and fibrillation (VT/VF), has a staggering death toll, by some estimates up to 10 million deaths/year worldwide(Rubart & Zipes, 2005). Underlying heart disease increases the risk of VT/VF and arrhythmic death.(Rubart & Zipes, 2005) Physiological (uniform/orderly) impulse propagation in the myocardium is critically dependent on proper organization of the myocardial bundles and cell-cell communication.(Kleber & Rudy, 2004) Myocardial fibrosis is a major structural alteration of the myocardium as a result of various disease processes. Cardiac injury (e.g. infarction, focal inflammation) results in the formation of a fibrotic scar.(Rutherford et al., 2012) Other cardiac diseases such as hypertension that are associated with cardiac hypertrophyare also associated with myocardial fibrosis. Fibrosis is a central problem in arrhythmia biology, by distorting myocardial architecture and interrupting electrical propagation pathways it setsthe stage for non-uniform impulse propagation. These altered ‘zig zag’ conduction pathways form reentrant circuits that sustain VT/VF.(Nguyen et al., 2014) The pattern and location of fibrosis also profoundly influences the formation of arrhythmogenic circuits in the heart.(Nguyen et al., 2014) Fibrotic scars secondary to myocardial infarction have been the subject of intense study over several decades. These scars (usually encompassing a perfusion bed of a coronary artery) contain strands of viable myocardial bundles interspersed with fibrotic tissue that can create reentrant circuits(Janse & Wit, 1989). The translational potential of understanding myocardial scars by the experimental mapping of reentry in experimental models has resulted in substantial clinical benefit.(Ajijola et al., 2013) Clinical translation of these experimental findings resulted in mapping of VT circuits in humans. The development of catheter ablation of VT as a clinical therapy followed and is an excellent example of the direct beneficial consequence of these scientific advances.(Shivkumar, 2019)
In contrast to fibrosis/scars secondary to infarction, patchy fibrosis seen with diseases such as hypertrophy can result in complex circuits and set the stage for additional electrophysiological effects such as triggered activity (Qu et al., 2022) secondary to myofibroblast-myocyte interactions and source sink effects.(Nguyen et al., 2012) Complex fibrosis (as seen in cardiac hypertrophy) has been the subject of intense experimental investigation. In this issue of the Journal (ref to be inserted by Journal), Khwaounjoo et al report the risk of VT/VF in the spontaneous hypertensive rat (SHR), a well-established model, characterizing the three-dimensional aspects of fibrosis and associated electrophysiological changes with mapping. They demonstrate proarrhythmic changes with increasing age and hypertensive heart disease in great detail, placing the structural and electrophysiological variables in context. Their study strongly supports the concept that arrhythmic risk is predicted by several left ventricular measures, but most importantly fibrosis. The strength of the study is extremely detailed quantification of structure, fibrosis and epicardial action potential duration dispersion. The study exclusively uses programmed electrical stimulation induced VT/VF risk as a surrogate for spontaneous VT/VF risk. Therefore, their results are relevant only to SHR producing a vulnerable substrate via fibrosis but does not explore whether SHR also promotes the other contributors to arrhythmogenesis, such as the emergence of triggers. Studies in SHR model (also cited by the authors) show the emergence of oxidative-stress induced early afterdepolarizations as a result of the source-sink mismatches caused by fibrosis, directly inducing spontaneous VT/VF.(Nguyen et al., 2016) Thus taken together the data in the present study provides a comprehensive picture of the well characterized SHR model. The emerging paradigm would be that aging and hypertrophy cause both fibrosis and dispersion of repolarization. Changes secondary to fibrosis such as conduction block, combined with source-sink mismatch and enhanced formation of triggers can act in concert to provide both the trigger and the substrate for lethal arrhythmias (Figure).
Figure:
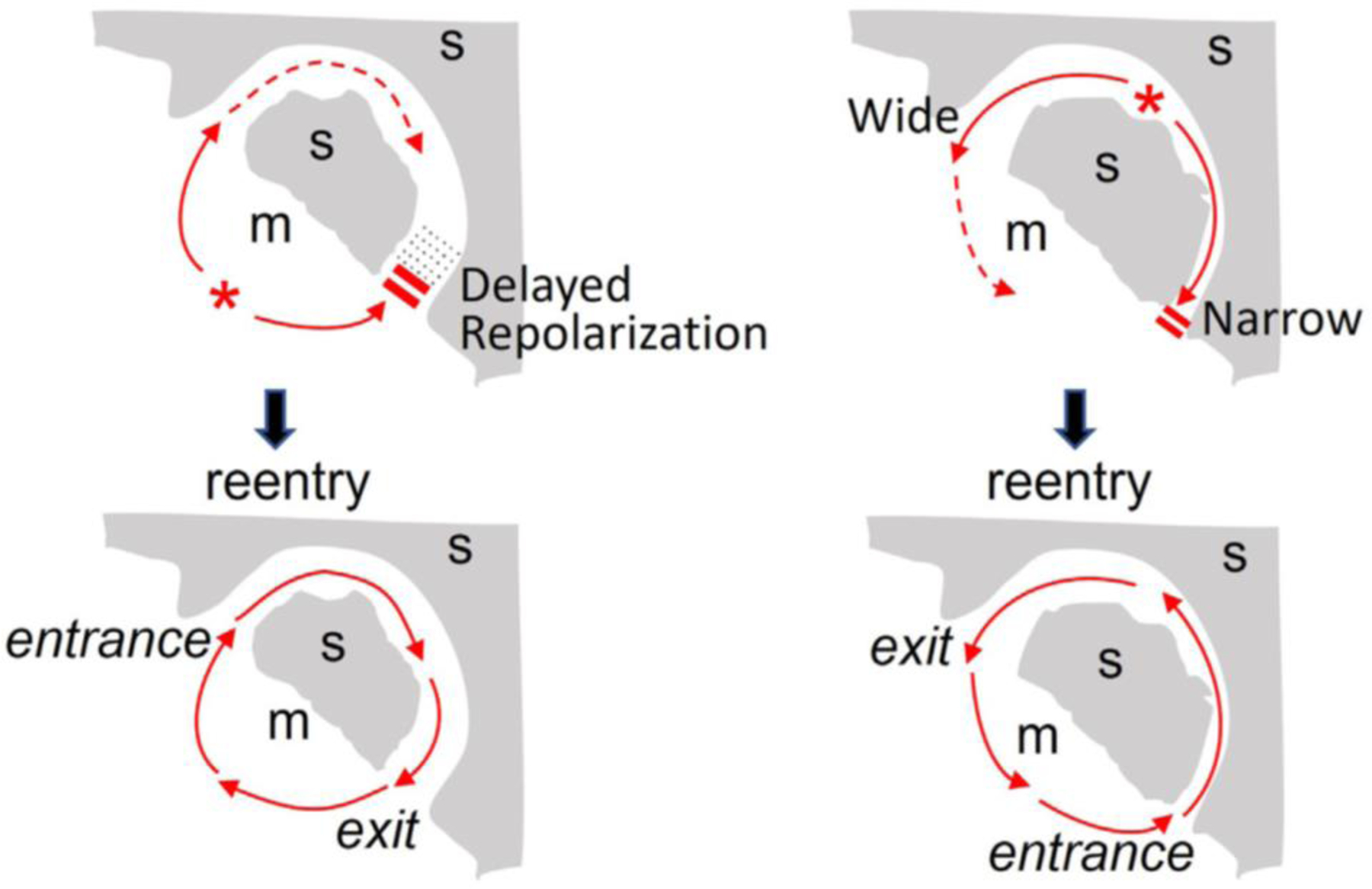
Reentry initiation via trigger (premature ventricular complex [PVC]) and substrate interactions. Schematic diagrams showing a PVC (*) inducing anatomic reentry using a myocardial channel (m) embedded in a scar (s) (gray shading) via two mechanisms: delayed repolarization mediated unidirectional conduction block (left) and narrow exit (source-sink mismatch) mediated unidirectional conduction block (right). Image modified from ref(Qu et al., 2022).
It is also worth highlighting that additional pathophysiological mechanisms are likely to be operational in the genesis of arrhythmias relating to fibrosis. Even in the presence of a discrete region of scar tissue in the heart creating the substrate for VT, it is known that reentrant arrhythmias require areas of functional block/conduction changes that allow impulse propagation in preferential directions.(Tung et al., 2012; Ajijola et al., 2013; Tung et al., 2013) Thus, clinical occurrence of VT reflects the balance between macro structure and functional control in real life. Neural remodeling is a key contributor to functional control of circuits and arrhythmogenesis in the setting of scars.(Fukuda et al., 2015; Zhu et al., 2022)
To conclude, the current publication provides much needed data on the complex changes to the myocardial architecture in the setting of cardiac hypertrophy. Future studies should build on this framework to further elucidate the arrhythmic implications of fibrosis. Experiments that can address functional changes relating to cardiac innervation (and functional control) as it has been done in post-infract scars(Zhu et al., 2022) will add an important dimension to our understanding of this disease. The authors are to be congratulated for this elegant study.
Supplementary Material
Footnotes
Conflicts of Interest: None
REFERENCES
- Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A & Shivkumar K. (2013). Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol 305, H1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kanazawa H, Aizawa Y, Ardell JL & Shivkumar K. (2015). Cardiac innervation and sudden cardiac death. Circ Res 116, 2005–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse MJ & Wit AL. (1989). Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev 69, 1049–1169. [DOI] [PubMed] [Google Scholar]
- Kleber AG & Rudy Y. (2004). Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84, 431–488. [DOI] [PubMed] [Google Scholar]
- Nguyen TP, Qu Z & Weiss JN. (2014). Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol 70, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TP, Sovari AA, Pezhouman A, Iyer S, Cao H, Ko CY, Bapat A, Vahdani N, Ghanim M, Fishbein MC & Karagueuzian HS. (2016). Increased susceptibility of spontaneously hypertensive rats to ventricular tachyarrhythmias in early hypertension. J Physiol 594, 1689–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TP, Xie Y, Garfinkel A, Qu Z & Weiss JN. (2012). Arrhythmogenic consequences of myofibroblast-myocyte coupling. Cardiovasc Res 93, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Liu MB, Olcese R, Karagueuzian H, Garfinkel A, Chen PS & Weiss JN. (2022). R-on-T and the initiation of reentry revisited: Integrating old and new concepts. Heart Rhythm 19, 1369–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubart M & Zipes DP. (2005). Mechanisms of sudden cardiac death. J Clin Invest 115, 2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford SL, Trew ML, Sands GB, LeGrice IJ & Smaill BH. (2012). High-resolution 3-dimensional reconstruction of the infarct border zone: impact of structural remodeling on electrical activation. Circ Res 111, 301–311. [DOI] [PubMed] [Google Scholar]
- Shivkumar K. (2019). Catheter Ablation of Ventricular Arrhythmias. N Engl J Med 380, 1555–1564. [DOI] [PubMed] [Google Scholar]
- Tung R, Mathuria N, Michowitz Y, Yu R, Buch E, Bradfield J, Mandapati R, Wiener I, Boyle N & Shivkumar K. (2012). Functional Pace-Mapping Responses for Identification of Targets for Catheter Ablation of Scar-Mediated Ventricular Tachycardia. Circulation Arrhythmia and electrophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung R, Mathuria NS, Nagel R, Mandapati R, Buch EF, Bradfield JS, Vaseghi M, Boyle NG & Shivkumar K. (2013). Impact of local ablation on interconnected channels within ventricular scar: mechanistic implications for substrate modification. Circulation Arrhythmia and electrophysiology 6, 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Rajendran PS, Hanna P, Efimov IR, Salama G, Fowlkes CC & Shivkumar K. (2022). High-resolution structure-function mapping of intact hearts reveals altered sympathetic control of infarct border zones. JCI Insight 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


