Abstract
An outbreak of coronavirus disease 2019 (COVID-19) has spread globally, with over 500 million cases and 6 million deaths to date. COVID-19 is associated with a systemic inflammatory response and abnormalities of the extracellular matrix (ECM), which is also involved in inflammatory storms. Upon viral infection, ECM proteins are involved in the recruitment of inflammatory cells and interference with target organ metabolism, including in the lungs. Additionally, serum biomarkers of ECM turnover are associated with the severity of COVID-19 and may serve as potential targets. Consequently, understanding the expression and function of ECM, particularly of the lung, during severe acute respiratory syndrome of the coronavirus 2 infection would provide valuable insights into the mechanisms of COVID-19 progression. In this review, we summarize the current findings on ECM, such as hyaluronic acid, matrix metalloproteinases, and collagen, which are linked to the severity and inflammation of COVID-19. Some drugs targeting the extracellular surface have been effective. In the future, these ECM findings could provide novel perspectives on the pathogenesis and treatment of COVID-19.
Keywords: COVID-19, SARS-CoV-2, Extracellular Matrix, Inflammation
Core Tip: Coronavirus disease 2019 (COVID-19) shows abnormal expression of the extracellular matrix (ECM). The ECM is a type of macromolecular network secreted by cells into the extracellular stroma, which is also the major component of connective tissue. It can trigger various activities biologically essential for tissue homeostasis and normal organ development. Upon severe acute respiratory syndrome coronavirus 2 infection, ECM proteins are involved in the recruitment of inflammatory cells and interference with target organ metabolism, including that of the lung. The manuscript addresses the current findings of ECM, which are linked to the severity and inflammation of COVID-19, and the roles of ECM in COVID-19.
INTRODUCTION
In December 2019, an outbreak of novel coronavirus-infected pneumonia, called coronavirus disease 2019 (COVID-19), led to a global pandemic. It has a high incidence rate with a rapid rate of transmission and has spread worldwide[1]. Up to now, World Health Organization statistics indicate that more than 600 million people have been infected with COVID-19 (Source: https://covid19.who.int. Last accessed date October 10, 2022). With further evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the Omicron variant has emerged as the dominant strain[2]. Therefore, the development of effective countermeasures is important to combat COVID-19.
COVID-19 shows a systemic inflammatory response and abnormal expression of the extracellular matrix (ECM), which regulates homeostasis and injury repair responses[3,4], has a crucial structure with a dynamic and complicated organization, and can trigger various activities biologically essential for tissue homeostasis and normal organ development[5]. For instance, the tissue architecture of the lung forms during embryonic development by epithelial branching and is determined by the pulmonary ECM, which changes in composition and distribution over time and provides mechanical stability and elasticity to the tissue. The ECM comprises the interstitial connective tissue matrix and basement membrane separating the epithelium from the surrounding matrix. Hynes et al[6] reported the most integrated list of proteins, constituting approximately 300 proteins, which form the core matrisome and include 36 proteoglycans, 43 collagen subunits, and 200 complex glycoproteins. The interstitial connective tissue matrix contains collagen type I, fibronectin, proteoglycans, glycosaminoglycans, tenascin C, and elastin and provides a structural scaffold for the tissue. Among the components of the basement membrane are laminins, collagen type IV, heparan sulphate proteoglycans (HSPGs), entactin, nidogen and glycoproteins, such as integrins and hemidesmosomes, that bind to ECM proteins[7]. Among these, proteoglycans, glycosaminoglycans, collagens, and elastin are the main molecules of the ECM. During normal development, elements of the ECM interact with epithelial cells constantly via ligands as cell receptors, such as integrins and hemidesmosomes. Thus, ECM can deliver signals that regulate adhesion, migration, proliferation, differentiation, survival, and apoptosis[8]. It can also seal and release local growth factors, such as transforming growth factor (TGF)-β, epidermal growth factor, fibroblast growth factor, and other signalling molecules. However, its components change dynamically. Cleavage of ECM components regulates ECM abundance, composition, and structure, thereby influencing cell behaviour[9]. ECM can be cleaved by different families of proteases, such as matrix metalloproteinases (MMPs), adamalysins (ADAMs and ADAMTS), meprins, MMP inhibitors, and other enzymes (e.g., Ser proteases). Cells constantly reconstruct and remodel the ECM through synthesis, degradation, reassembly, and chemical modifications, which are complex and firmly regulated to maintain tissue homeostasis. ECM is also involved in inflammatory storms. ECM proteins help recruit inflammatory cells. However, their characteristics and functional mechanisms in COVID-19 remain obscure. Here, we review the roles of the ECM in COVID-19 with underlying regulatory mechanisms and application prospects and challenges.
ALTERATIONS IN ECM MOLECULES IN COVID-19
Hyaluronan (HA) is a key ECM compound in every vital organ system that plays a crucial role in pulmonary biological functions. Respiratory abnormalities can be triggered by HA when production and degradation are imbalanced[10]. HA is produced by three HA synthases (HAS1, HAS2, and HAS3); in particular, HAS2 generates HAs of a molecular mass greater than that of HAS1 and HAS3[11]. HA functions as a scaffold in ECM and contributes to the thickness of the endothelial glycocalyx under normal conditions. Furthermore, HA, particularly high-molecular-weight HA (HMW-HA), promotes the self-renewal survival activity of alveolar macrophages and type II alveolar epithelial cells. It is responsible for permeability selectivity and is involved in the mechanosensory effect of the endothelial glycocalyx in blood vessels upon blood folding[12].
In deceased COVID-19 patients, lungs at autopsy confirmed that HA obstructs alveoli with a presence in the exudate and plugs, as well as in thickened perialveolar interstitium compared with staining in normal lung tissue[13]. The pathophysiology of HA elevation in COVID-19 should be understood. Infection with SARS-CoV-2 causes a cytokine storm and releases abundant proinflammatory cytokines, such as interleukin-1β and tumour necrosis factor-α, which leads to HAS2 overexpression[14]. Therefore, HMW-HA production increases remarkably and can absorb abundant water molecules owing to their hygroscopic properties. Hellman et al[13] reported that fatal cases of COVID-19 were associated with the accumulation of hyaluronic acid in the alveolar spaces of the lungs and occurrences of hypoxemia and respiratory failure. A mouse model has also been shown to develop lesions in the lungs due to HA[15]. In a prospective study, the authors found that the COVID-19 cytokine milieu stimulates aberrant synthesis and degradation of HA, and HA fragments present at elevated levels in COVID-19 patient plasma directly induce endothelial barrier dysfunction[16]. This finding was corroborated by other studies[17,18]. Therefore, HA storms are likely to occur during the progression of COVID-19. Hällgren et al[19] demonstrated that HA levels in both bronchoalveolar lavage fluid and serum increased in adults with severe acute respiratory distress syndrome (ARDS). SARS-CoV-2 infection leads to the accumulation of HA, similar to severe influenza[20]. In severe lung inflammation, HA is destroyed by the reactive oxygen species produced from neutrophils that can breakdown HMW-HA into small fragments, including low-molecular-weight HA and oligo-HA[21]. This could further boost the release of cytokines and be a feedback link of the HA storm. Thus, HA storms increase the severity and lead to a poor prognosis of COVID-19.
MMP
MMPs are a family of zinc-dependent enzymes that can degrade most extracellular matrix proteins[5]. They can be derived from several cell types, including epithelial cells, fibroblasts, and endothelial cells in the lungs, and they play important roles in several vital mechanisms and pathways, such as tumour infiltration and metastasis, regulation of inflammatory and immune responses, ECM degradation, normal tissue repair and remodelling, and proliferation and signalling pathways. To date, 23 MMPs have been identified in humans[22], all of which are released as inactive zymogens (preproenzymes) secreted from cells into the extracellular space. Subsequently, MMPs are processed proteolytically or by modifying the thiol group by oxidation for activation[7]. The development and progression of many acute and chronic lung disorders are associated with excessive MMP synthesis and degradation, which can result in cell injury and pulmonary fibrosis.
This paper summarizes the involvement of MMPs in regulating SARS-CoV-2 (Table 1). MMPs are differentially expressed in patients with COVID-19 in association with the occurrence and development of the disease. Syed et al[23] found that MMP-1 and vascular endothelial growth factor (VEGF)-A are excessively elevated in COVID-19 and associated with the severity of COVID-19. MMP-1 is an interstitial collagenase capable of degrading collagen types I, II, and III and plays a crucial role in vascular remodelling and diseases[24]. It is necessary for tissue development and maintenance, but its overexpression promotes hyperactivation of MMP-1/PAR1 signalling, which can increase the expression of VEGF receptor 2, decrease vascular endothelial cell function and lead to excessive recruitment and activation of inflammatory cells[25]. Furthermore, in idiopathic pulmonary fibrosis (IPF), it is described as a prospective peripheral blood biomarker[26]. The 6-mo follow-up after hospital discharge of patients with COVID-19 revealed that approximately half of the patients with moderate or severe COVID-19 developed impaired pulmonary diffusion and early fibrotic changes in association with elevated MMP-1[27]. According to a comparative study of COVID-19 and influenza A patients, elevated MMP-1 and MMP-3 Levels were found only among COVID-19 patients[28]. Thus, MMP-1 may play a role in tissue damage associated with SARS-CoV-2 infection, and MMP-1 Levels may be a prognostic biomarker of COVID-19.
Table 1.
Dysregulation of the extracellular matrix in coronavirus disease 2019
|
ECM
|
Alterations in COVID-19
|
Suggested ECM role in disease
|
In other inflammatory and fibrotic lung disorders
|
| MMP-1 | Increased MMP-1 levels have been detected in the serum of patients, which correlated with the severity of COVID-19 and early fibrosis with this condition[23] | Drive endothelial cell destruction, such as by over-activating the mmp-1/PAR1 pathway, increasing VEGF-2 receptor expression, and causing endothelial damage. Involved in collagen deposition in IPF | Emphysema, Asthma: MMP-1 levels raised |
| MMP-2 | Increased MMP-2 levels have been detected in the CSF samples of COVID-19 patients with neurological syndrome[30]. Downregulated MMP-2 levels have been detected in the plasma samples with this condition, which correlated with the mortality rate of COVID-19[31] | Involved in disruption of the blood-brain barrier. Being Involved in the regulation of angiogenesis may lead to vascular remodeling. Play an anti-inflammatory effect in the endothelial dysfunction | Asthma, Idiopathic Pneumonia: MMP-2 levels raised. pulmonary fibrosis (IPF): Disproportionate extracellular matrix degradation |
| MMP-3 | Increased MMP-3 levels have been detected in serum of individuals with this condition[37,40] | Associated with activation of MMP-9 and enhanced synthesis of procollagen | Asthma: MMP-3 levels raised |
| MMP-7 | Increased MMP-7 levels have been detected in the serum of obese-diabetic patients with novel coronavirus pneumonia-infected[36]. Elevated in COVID-19 patients with early fibrotic changes[27] | Contribute to airway epithelial damage and inflammation as well as Play a profibrotic effect | Asthma, Cystic fibrosis (CF), ARDS: MMP-7 levels raised |
| MMP-8 | / | / | COPD, Emphysema, Asthma: MMP-8 levels raised |
| MMP-9 | Increased MMP-9 levels have been detected in the circulation of COVID-19 patients with respiratory failure and with obese-diabetic[36] | Associated with respiratory failure. Linked to inflammation-induced tissue remodeling. Contributes to the disruption of alveolar epithelial basement membrane | COPD, Emphysema, Asthma, IPF, UIP, ALI, ARDS: MMP-9 levels raised |
| MMP-10 | The CSF levels of MMP-10 correlated with the degree of neurologic dysfunction exhibited[41] | Associated with neurodegeneration | / |
| MMP-12 | / | / | COPD, Emphysema: MMP-12 levels raised |
| MMP-14 | Increased MMP-14 levels in lung tissue have been detected COVID-19 patients[32] | Implicated in COVID-19 pathogenesis | COPD: MMP-14 levels raised |
| HA | Increased HA levels have been detected in lungs of deceased COVID-19 patients[13]. HA fragments present at elevated levels in COVID-19 patient plasma[16] | Induce endothelial barrier dysfunction | ARDS: HA levels raised |
| Proteogly-cans | / | / | COPD, IPF, Asthma, Bronchiolitis obliterans syndrome: Versican levels raised |
| Collagen | 16 collagens are downregulated or diminished in COVID-19[52]. Collagen deposits on Lung Samples of deceased COVID-19 patients[51] | Damage of mechanical characteristics of lungs | Asthma, COPD, IPF: Display differing collagen deposition |
| ADAMs | Increased ADAM12 and ADAM17 levels have been detected in serum of COVID-19 patients[56-58] | ADAM17 may be associated with viral entry. ADAM12 plays a role in inflammation and endothelial cell permeability | / |
| ADAMTS | ADAMTS13 decreased significantly with increasing COVID-19 severity[59,60] | Associated with vascular microthrombotic disease | / |
COVID-19: Coronavirus disease 2019; ALI: Acute lung injury; ARDS: Adult respiratory distress syndrome; COPD: Chronic obstructive pulmonary disease; ECM: Extracellular matrix; HA: Hyaluronan; MMP: Matrix metalloproteinase; IPF: Idiopathic pulmonary fibrosis; UIP: Usual interstitial pueumonia; VEGF-2: Vascular endothelial growth factor 2.
Gelatinases, including gelatinases A (MMP-2) and B (MMP-9), whose expression increases during inflammation, cleave several types of collagens, such as types IV and V[29]. MMP-2 and MMP-9 are significantly elevated in asthma, acute lung injury, ARDS, and IPF. MMP2, a constitutively expressed MMP in the brain, is involved in central nervous system pathology, and MMP9 is a major inducible MMP released in neuroinflammatory responses. MMP-2 and MMP-9 levels are significantly elevated in the cerebrospinal fluid of patients with COVID-19 who have neurological syndrome compared to those without neurological syndrome[30]. In addition, patients with respiratory failure due to COVID-19 have elevated MMP-9 in the circulation. D Avila-Mesquita et al[31] reported compared to patients with mild COVID-19, patients with severe COVID-19 had lowered plasma MMP-2 levels and highly elevated plasma MMP-9 levels. Furthermore, compared to COVID-19 survivors, COVID-19 nonsurvivors had higher MMP-2 and MMP-8 levels in the lung[32]. Pedro V suggested that overexpression of MMP-2 and MMP-8 caused lipid peroxidation, which resulted in intensive destruction of lung tissue in severe COVID-19 cases[32]. Regulation of MMP expression and activity is complex. Mmp2−/− mice display more pronounced interstitial and perivascular fibrosis in response to angiotensin II[33]. SARS-CoV-2 binds to angiotensin converting enzyme 2 for intracellular invasion, thereby increasing vasoconstriction and inflammation. An MMP-2 deficiency leads to inflammation, and low levels are just as harmful to the cardiovascular system as high levels[34]. Taken together, when MMP-2 activity falls below baseline, the bioavailability of proinflammatory cytokines could be normally cleaved and inactivated by MMP-2 elevation, leading to the production of cytokines in the circulation, which stimulates systemic inflammation. MMP-9 is secreted by many cells, such as neutrophils and macrophages, and is correlated with inflammation. When activated by cytokines and lipopolysaccharides, macrophages can produce MMPs at sites of inflammation. To determine whether MMP concentrations are increased in COVID-19, two studies measured plasma concentrations in patients with COVID-19[35]. Studies have shown that patients with obesity, diabetes, and COVID-19 have elevated alveolar M2 macrophages and produce more MMP-7 and MMP-9, which promote fibrogenesis and lead to lung stiffening[35,36]. Therefore, MMP-9 may be an early indicator of respiratory failure in COVID-19, consistent with the results of Gelzo et al[37]. MMP-7, or fibrotic genes, is overexpressed in the lung tissue of patients with IPF and ARDS compared to normal lung tissue[38]. Recently, an observational and prospective study showed a significant increase in the detection of MMP-7 and MMP-14 in lung tissue from COVID-19 patients compared to that from non-COVID-19 subjects[32]. Another MMP in BALF that is upregulated during the initial phases of ARDS is MMP-28, which is associated with increased alveolar neutrophils[39].
MMP-3 plays a role in the pathogenesis of acute inflammation-induced lung injury. Shi et al[40] suggested that MMP-3, which activates other MMPs in the family, may be a valuable biomarker of COVID-19. It is elevated in COVID-19 in the initial phase of lung inflammation, which is possibly related to the activation of MMP-9 and augmented synthesis of procollagen I[37]. Nevertheless, after one week of hospitalization, an increase in MMP-3 serum levels in patients with COVID-19 was not observed, indicating that MMP-3 activity contributes most in the early stages of lung inflammation caused by COVID-19[37]. The level of MMP-10 in cerebrospinal fluid of COVID-19-positive patients and healthy controls was related to their degree of neurologic dysfunction[41].
Many other MMPs are altered in respiratory diseases[42] but have not yet been discovered or reported in COVID-19. In addition to the aforementioned enzymes, MMP-12, MMP-15, MMP-11, and MMP-13 cause acute lung injury and ARDS[43].
PROTEOGLYCANS
Proteoglycans are the major component of the basement membrane, intracellular granules, and ECM. They influence multiple cellular events structurally and functionally, including proliferation, differentiation, and gene expression. Proteoglycans, such as decorin, versican, perlecan, and aggrecan, are a family of widely differing protein molecules whose structure is characterized by a core protein molecule with one or more attached glycosaminoglycan side chains interspersed among collagen fibrils[19].
Decorin is a proteoglycan molecule rich in leucine that plays a critical role in ECM assembly and regulates cell growth, proliferation, adhesion, inflammation, and fibrogenesis. Decorin and TGF-β have a strong relationship. Therefore, as decorin is an inhibitor of TGF-β, it reduces tissue fibrosis in the kidney and lung in various diseases[44]. Moreover, it strengthens the immune system and improves conditions mediated by oxidative stress. Therefore, it has anti-inflammatory and anti-fibrillogenic effects that make it a potential drug for COVID-19-related complications, particularly in cases of lung fibrosis, although without direct evidence currently[44,45].
Perlecan, or HSPG2, is a structurally conserved heparan sulphate-bearing proteoglycan. It is fully secreted into the extracellular space, where it is incorporated into the basement membrane and intertwined with collagen type IV, facilitating epithelial and endothelial cell attachments and regulating cellular behaviour[46]. Versican is a versatile molecule that plays roles in cell adhesion, migration, proliferation, and inflammatory responses, and its protein levels are increased in cytokine- and growth factor-stimulated lung fibroblasts[47]. It is involved in remodelling in inflammatory lung disorders, such as asthma, chronic obstructive pulmonary disease, IPF, and bronchiolitis obliterans syndrome[47]. It is a potential target for modulating the inflammatory response in COVID-19[48].
COLLAGEN
Collagen is a type of glycoprotein composed of distinct subunits that are the main structural proteins of the ECM and divided into fibrillar (collagens I–III, V, and XI) and nonfibrillar forms[19]. Its molecules polymerize to form fibrils by self-assembly, and nonfibronectin, such as type IV collagen and fibril-associated collagens, is associated with the formation of other forms of ECM. Collagen types I, II, and III are more frequently encountered and comprise approximately 80%-90% of all types. Collagen plays a clear structural role in mechanical support and dimensional stability, which provides tensile strength to the ECM, limiting the distensibility of tissues. Fibrosis is a dynamic process, and commonly, the continuous deposition and resorption of connective tissues and collagens are pivotal. During wound healing, collagen synthesis increases and ceases once it is deposited to sufficient levels in tissues. In contrast, during fibrosis, collagen synthesis is faster than absorption, and uncontrolled collagen deposition causes tissues to become stiff, resulting in scarring. Collagen types I, III, and VI predominate in the interstitium of the alveolar wall in both normal and fibrotic lungs[49]. Asthma, chronic obstructive pulmonary disease (COPD), and IPF display differing collagen deposition. Particularly, in active fibrosis in IPF, compared to healthy lung tissues, lung procollagen pro-peptides types I and III are increased[50]. Furthermore, messenger RNA of type I collagen is increased and colocalised with its precursor protein (pro-collagen) in highly activated fibroblasts in IPF, reflecting active synthesis in different lung regions[49]. In COPD, type I and III procollagen expression profiles differ with disease severity. Postmortem studies have shown collagen deposits on lung samples of COVID-19 patients[51]. In contrast, the expression of many core ECM proteins, including 12 proteoglycans, 16 collagens and 56 glycoproteins, is diminished in COVID-19 using a proteomic approach[52]. In particular, COVID-19 patients lose the collagen that is predominant in lung tissue, resulting in fundamental mechanical damage to the lungs. the collagens that predominate lung tissue are lost in the lungs of patients with COVID-19, leading to fundamental damage to the mechanical characteristics of lungs with COVID-19. There is an imbalance in the ECM in the lung tissues caused by COVID-19, which characterizes the major symptoms of severely ill patients with COVID-19 at the molecular level[52].
ADAMALYSINS
Adamalysins have two subfamilies: Disintegrin and MMPs (ADAMs) and ADAMs with a thrombospondin domain (ADAMTS)[53]. ADAMs are disintegrins and MMPs that cleave transmembrane protein ectodomains, resulting in their shedding. Their key function is shedding of growth factors, cytokines, and adhesion molecules that identify these cell surface enzymes as key mediators of various pathophysiological processes. ADAMTS can cleave ECM proteins and procollagens I, II, and III to mature forms extracellularly, playing vital roles in tissue remodelling[54]. It has been shown that several adamalysin proteins play roles in COVID-19, particularly ADAM17, which plays an essential role in its pathogenesis[55,56]. ADAM17 cleavage results in biologically active soluble angiotensin converting enzyme-2 (ACE2), which blocks or promotes viral entry into SARS-CoV-2 by binding to it[56,57]. The ADAM12 receptor and ephrin-A1, one of the ADAM12 substrates, play a role in inflammation and endothelial cell permeability. Among COVID-19 patients, some had significantly elevated concentrations of ADAM12 and ephrin-A1 in their blood serum, with critical patients having the highest levels[58]. Therefore, the inflammatory response mediated by Ephrin-A1 may be one of the major factors in the morbidity and mortality related to COVID-19. Levels of ADAMTS13, an antithrombotic metalloprotease that generates optimal-sized vWF proteins by cleaving large multimeric vWF precursor proteins, decreased significantly with increasing COVID-19 severity; the lower the activity of ADAMTS13, the higher the risk of mortality, which showed the best discriminatory ability to predict long-term mortality[17,59,60].
APPLICATION PROSPECTS OF ECM IN DIAGNOSIS AND TREATMENT
The COVID-19 pandemic is still underway, and most drugs available for COVID-19 are not specifically designed. The most effective way to combat pathogen infection is to design an effective pathogen-specific vaccine or antiviral agent. However, It is common for viruses to make errors during replication, and their proteins are constantly mutated. Each time a new pathogen emerges, the design of pathogen-specific therapies must be restarted. To date, therapeutic options are limited for COVID-19, and the treatment mitigates and repairs damage caused by pathogens, such as using corticosteroids, interleukin-6 receptor blockers, Janus kinase inhibitors, baricitinib, and sotrovimab. COVID-19 leads to lung damage and multiorgan failure that directly causes endothelial cell apoptosis, affects gas exchange and is the most refractory feature of respiratory diseases[61]. The composition and function of the pulmonary ECM is significantly disturbed in pathological tissue remodelling. Following SARS-CoV-2 infection, therapeutic approaches targeting ECM mediators are of interest, possibly preventing serious complications. Removing excess HA in patients with COVID-19 could reduce the severity of clinical morbidity[6]. Using 4-methylumbelliferone (4-MU), a competitive substrate inhibitor of UDP-glucosyltransferase, can reduce HA synthesis[62]. Hymecromone, a commercial drug containing 4-MU, has been shown through animal and clinical trials to improve lymphopenia and lung lesions in SARS-CoV-2-infected patients[15]. Moreover, decorin can treat lung fibrosis by direct application[63]. Therefore, it might be effective against pulmonary fibrosis associated with COVID-19. MMPs are imbalanced in lung diseases, such as asthma, COPD, pulmonary fibrosis, and ARDS, and inflammatory processes associated with COVID-19. Consequently, tissue inhibitors of MMPs (TIMPs) are secreted to maintain matrix equilibrium and regulate cytokine shedding. These inhibitors target MMPs to modulate their effects. TIMPs in a mouse model of lung injury reduce the inflammatory status and improve injury[64]. In particular, Timp3−/− mice have spontaneous emphysema-like alveolar damage, suggesting a role for TIMP3 in the maintenance of lung homeostasis and remodelling[65]. Increasing the concentration and bioavailability of TIMP may be therapeutic. Hardy et al[43] suggested the possibility of using new cocktails of low-dose MMP inhibitors and AMP-activated protein kinase activators to increase host tolerance to pathologies through cooperative effects. Interestingly, in a mouse model of COVID-19, the administration of the ADAM17/MMP inhibitors apratastat and TMI-1 significantly improved lung histology and prevented leukocyte infiltration[66]. Similarly, in severe cases of COVID-19, the pan-MMP inhibitor doxycycline has been preliminarily investigated[67]. Versican is a potential target for modulating the inflammatory response of COVID-19[48]. No therapeutic agents are available in clinical settings that target versican. This could also be a new direction for future research.
CONCLUSION
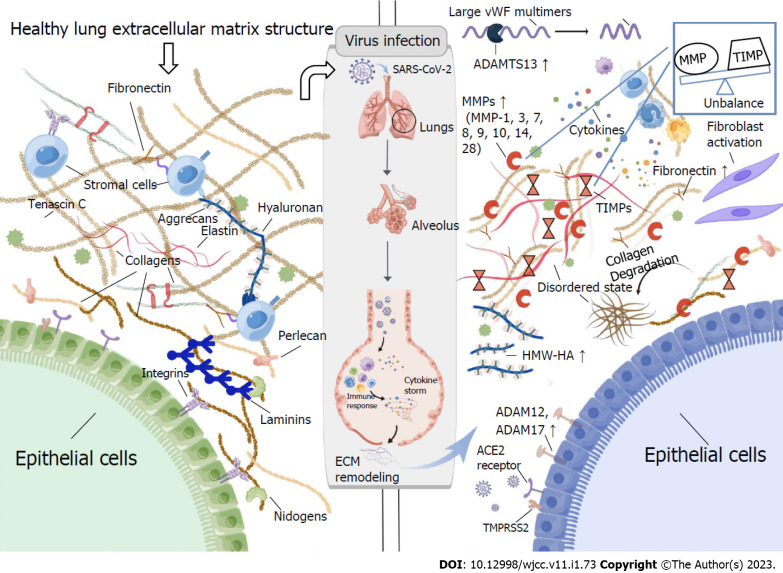
Extracellular matrix remodelling is broadly involved in several physiological, pathological, and homeostatic states. Its mediators can alter the fate of organs as they are present in and associated with cellular and circulating patterns. Furthermore, SARS-CoV-2 infection in the human body changes the matrix balance (Figure 1). For example, in patients with COVID-19, MMP-1[23,27], MMP-3[37,40], MMP-7[35,36], MMP-8[32], MMP-9[35-37], MMP-10[41], MMP-14[32], and MMP-28[39] secretion increases at sites of inflammation, which leads to pathological remodelling of the lung ECM. Among them, MMP-1 and MMP-9 have been implicated in pulmonary fibrosis[27,35]. It is possible, therefore, that COVID-19 results in endothelial cell damage through the upregulation of MMPs, which results in further inflammation and the spread of cytokines. ADAM17 mediates active ectodomain shedding of ACE2, which blocks or promotes viral entry into SARS-CoV-2 by binding to it[57]. On the other hand, increased ADAM17 expression in the majority of patients with severe COVID-19 demonstrated that ADAM17 activity itself may facilitate viral entry. ADAMTS13’s main role is cleaving vWF[60]. Reduced activity of ADAMTS13 in COVID-19 patients results in aggregation of vWF platelets, vessel occlusion, and microvascular thrombosis[59].
Figure 1.
The extracellular matrix of a healthy lung and its remodelling after infection with severe acute respiratory syndrome coronavirus 2. Tenascin C, collagen, elastin, integrins, laminins, perlecan, hyaluronan, aggrecan, and fibronectin are crucial components of the healthy lung matrix structure. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry into the cell via the effects of angiotensin converting enzyme-2 (ACE2) and transmembrane serine protease 2 (TMPRSS2). As a result of identifying the virus, immune cells, specifically macrophages, release proinflammatory mediators that activate more immune cells, creating an uncontrolled cycle of the inflammatory response “cytokine storm”. Extracellular matrix (ECM) remodelling occurs in response to lung injuries, including matrix metalloproteinase (MMP) overexpression and an imbalance between MMPs and tissue inhibitors of MMPs (TIMPs), increased deposition of ECM fragments and collagen, massive formation and deposition of fibronectin, and accumulation of hyaluronan (HA). Moreover, ADAM12, ADAM17, and ADAMTS13 expressions are elevated during SARS-CoV-2 infection. ACE2 angiotensin-converting enzyme 2, TMPRSS2 transmembrane protease serine 2, ADAM17 ADAM metalloprotease domain 17, ADAM12 ADAM metalloprotease domain 12, ADAMTS13 ADAMs with thrombospondin domain 12, HMW-HA high-molecular-weight hyaluronan, MMPs matrix metalloproteinases, TIMPs tissue inhibitors of matrix metalloproteinases. ACE2: Angiotensin converting enzyme-2; ECM: Extracellular matrix; HA: Hyaluronan; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; MMP: Matrix metalloproteinase; TIMP: Tissue inhibitors of MMP; TMPRSS2: Transmembrane serine protease 2; HMW-HA: High-molecular-weight HA.
As previously mentioned, the ECM is regulated by SARS-CoV-2 infection and is involved in the pathogenesis of infection. An overactive ECM can aggravate a disease, and regulation of overactive ECM can alleviate it. As the specific mechanisms targeting this aspect are unclear, the therapeutic course for ECM should be explored as possible targets for long-term therapy, as cured patients still develop sequelae. Studies have shown that multiple organ systems are affected to varying degrees by COVID-19 sequelae[68]. Patients with mild-to-moderate COVID-19 usually develop mild-to-moderate pulmonary fibrosis[69]. Severe or critical COVID-19 shows variable degrees of fibrosis, ranging from early interstitial to severe obliteration of alveolar structure[70]. Indicators of ECM could be used as tools for identification during the acute phase of disease in COVID-19 survivors who are risk of developing permanent pulmonary damage and fibrosis.
In conclusion, further work is required to better understand the underlying mechanisms of the extracellular matrix in COVID-19 and how the dysregulation of the different ECM components leads to disease manifestation.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 23, 2022
First decision: November 25, 2022
Article in press: December 23, 2022
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beyoglu MA, Turkey; Crocé LS, Italy; Mukhopadhyay A, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Jia-Jia Huang, School of Medicine, Ningbo University, Ningbo 315000, Zhejiang Province, China.
Chu-Wen Wang, School of Medicine, Ningbo University, Ningbo 315000, Zhejiang Province, China.
Ying Liu, School of Medicine, Ningbo University, Ningbo 315000, Zhejiang Province, China.
Ying-Ying Zhang, School of Medicine, Ningbo University, Ningbo 315000, Zhejiang Province, China.
Nai-Bin Yang, Department of Infectious Diseases, Ningbo First Hospital, Ningbo 315000, Zhejiang Province, China.
Yu-Chun Yu, Department of Endocrinology, Ningbo Ninth Hospital, Ningbo 315000, Zhejiang Province, China.
Qi Jiang, Department of Digestive, Ningbo First Hospital, Ningbo 315000, Zhejiang Province, China.
Qi-Fa Song, Medical Data Center, Ningbo First Hospital, Ningbo 315000, Zhejiang Province, China.
Guo-Qing Qian, Department of Infectious Diseases, Ningbo First Hospital, Ningbo 315000, Zhejiang Province, China. bill.qian@outlook.com.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med . 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O'Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med . 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, Bitterman PB, Corley RA, Ding BS, Engler AJ, Hansen KC, Hagood JS, Kheradmand F, Lin QS, Neptune E, Niklason L, Ortiz LA, Parks WC, Tschumperlin DJ, White ES, Chapman HA, Thannickal VJ. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol . 2018;73:77–104. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarker H, Haimour A, Toor R, Fernandez-Patron C. The Emerging Role of Epigenetic Mechanisms in the Causation of Aberrant MMP Activity during Human Pathologies and the Use of Medicinal Drugs. Biomolecules . 2021;11 doi: 10.3390/biom11040578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol . 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol . 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol . 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V, Ricard-Blum S, Schmelzer CEH, Duca L, Durbeej M, Afratis NA, Troeberg L, Franchi M, Masola V, Onisto M. A guide to the composition and functions of the extracellular matrix. FEBS J . 2021;288:6850–6912. doi: 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- 9.Löffek S, Schilling O, Franzke CW. Series "matrix metalloproteinases in lung health and disease": Biological role of matrix metalloproteinases: a critical balance. Eur Respir J . 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 10.Johnson P, Arif AA, Lee-Sayer SSM, Dong Y. Hyaluronan and Its Interactions With Immune Cells in the Healthy and Inflamed Lung. Front Immunol . 2018;9:2787. doi: 10.3389/fimmu.2018.02787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem . 2007;282:36777–36781. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 12.Zöller M. CD44, Hyaluronan, the Hematopoietic Stem Cell, and Leukemia-Initiating Cells. Front Immunol . 2015;6:235. doi: 10.3389/fimmu.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellman U, Karlsson MG, Engström-Laurent A, Cajander S, Dorofte L, Ahlm C, Laurent C, Blomberg A. Presence of hyaluronan in lung alveoli in severe Covid-19: An opening for new treatment options? J Biol Chem . 2020;295:15418–15422. doi: 10.1074/jbc.AC120.015967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sur S, Steele R, Isbell TS, Ray R, Ray RB. Circulatory Exosomes from COVID-19 Patients Trigger NLRP3 Inflammasome in Endothelial Cells. mBio . 2022;13:e0095122. doi: 10.1128/mbio.00951-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Ling Y, Zhao F, Li W, Song Z, Wang L, Li Q, Liu M, Tong Y, Chen L, Ru D, Zhang T, Zhou K, Zhang B, Xu P, Yang Z, Song Y, Xu J, Zhu T, Shan F, Yu W, Lu H. Hymecromone: a clinical prescription hyaluronan inhibitor for efficiently blocking COVID-19 progression. Signal Transduct Target Ther . 2022;7:91. doi: 10.1038/s41392-022-00952-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Queisser KA, Mellema RA, Middleton EA, Portier I, Manne BK, Denorme F, Beswick EJ, Rondina MT, Campbell RA, Petrey AC. COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight . 2021;6 doi: 10.1172/jci.insight.147472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, Kühn J, Braune S, Göbel U, Thölking G, Gröschel A, Pavenstädt H, Vink H, Kümpers P. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis . 2021;24:145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ontong P, Prachayasittikul V. Unraveled roles of hyaluronan in severe COVID-19. EXCLI J . 2021;20:117–125. doi: 10.17179/excli2020-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hällgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis . 1989;139:682–687. doi: 10.1164/ajrccm/139.3.682. [DOI] [PubMed] [Google Scholar]
- 20.Bell TJ, Brand OJ, Morgan DJ, Salek-Ardakani S, Jagger C, Fujimori T, Cholewa L, Tilakaratna V, Östling J, Thomas M, Day AJ, Snelgrove RJ, Hussell T. Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis. Matrix Biol . 2019;80:14–28. doi: 10.1016/j.matbio.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol . 2015;6:150. doi: 10.3389/fimmu.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol . 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syed F, Li W, Relich RF, Russell PM, Zhang S, Zimmerman MK, Yu Q. Excessive Matrix Metalloproteinase-1 and Hyperactivation of Endothelial Cells Occurred in COVID-19 Patients and Were Associated With the Severity of COVID-19. J Infect Dis . 2021;224:60–69. doi: 10.1093/infdis/jiab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukhova GK, Schönbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation . 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 25.Mazor R, Alsaigh T, Shaked H, Altshuler AE, Pocock ES, Kistler EB, Karin M, Schmid-Schönbein GW. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Chem . 2013;288:598–607. doi: 10.1074/jbc.M112.417451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res . 2012;159:218–227. doi: 10.1016/j.trsl.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safont B, Tarraso J, Rodriguez-Borja E, Fernández-Fabrellas E, Sancho-Chust JN, Molina V, Lopez-Ramirez C, Lope-Martinez A, Cabanes L, Andreu AL, Herrera S, Lahosa C, Ros JA, Rodriguez-Hermosa JL, Soriano JB, Moret-Tatay I, Carbonell-Asins JA, Mulet A, Signes-Costa J. Lung Function, Radiological Findings and Biomarkers of Fibrogenesis in a Cohort of COVID-19 Patients Six Months After Hospital Discharge. Arch Bronconeumol . 2022;58:142–149. doi: 10.1016/j.arbres.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choreño-Parra JA, Jiménez-Álvarez LA, Cruz-Lagunas A, Rodríguez-Reyna TS, Ramírez-Martínez G, Sandoval-Vega M, Hernández-García DL, Choreño-Parra EM, Balderas-Martínez YI, Martinez-Sánchez ME, Márquez-García E, Sciutto E, Moreno-Rodríguez J, Barreto-Rodríguez JO, Vázquez-Rojas H, Centeno-Sáenz GI, Alvarado-Peña N, Salinas-Lara C, Sánchez-Garibay C, Galeana-Cadena D, Hernández G, Mendoza-Milla C, Domínguez A, Granados J, Mena-Hernández L, Pérez-Buenfil LÁ, Domínguez-Cheritt G, Cabello-Gutiérrez C, Luna-Rivero C, Salas-Hernández J, Santillán-Doherty P, Regalado J, Hernández-Martínez A, Orozco L, Ávila-Moreno F, García-Latorre EA, Hernández-Cárdenas CM, Khader SA, Zlotnik A, Zúñiga J. Clinical and Immunological Factors That Distinguish COVID-19 From Pandemic Influenza A(H1N1) Front Immunol . 2021;12:593595. doi: 10.3389/fimmu.2021.593595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res . 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadhosayni M, Sadat Mohammadi F, Ezzatifar F, Mahdavi Gorabi A, Khosrojerdi A, Aslani S, Hemmatzadeh M, Yazdani S, Arabi M, Marofi F, Jadidi-Niaragh F, Shomali N, Mohammadi H. Matrix metalloproteinases are involved in the development of neurological complications in patients with Coronavirus disease 2019. Int Immunopharmacol . 2021;100:108076. doi: 10.1016/j.intimp.2021.108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D Avila-Mesquita C, Couto AES, Campos LCB, Vasconcelos TF, Michelon-Barbosa J, Corsi CAC, Mestriner F, Petroski-Moraes BC, Garbellini-Diab MJ, Couto DMS, Jordani MC, Ferro D, Sbragia L, Joviliano EE, Evora PR, Carvalho Santana R, Martins-Filho OA, Polonis K, Menegueti MG, Ribeiro MS, Auxiliadora-Martins M, Becari C. MMP-2 and MMP-9 Levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed Pharmacother . 2021;142:112067. doi: 10.1016/j.biopha.2021.112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva-Neto PV, do Valle VB, Fuzo CA, Fernandes TM, Toro DM, Fraga-Silva TFC, Basile PA, de Carvalho JCS, Pimentel VE, Pérez MM, Oliveira CNS, Rodrigues LC, Bastos VAF, Tella SOC, Martins RB, Degiovani AM, Ostini FM, Feitosa MR, Parra RS, Vilar FC, Gaspar GG, Rocha JJRD, Feres O, Arruda E, Maruyama SR, Russo EMS, Viana AL, Santos IKFM, Bonato VLD, Cardoso CRB, Tanus-Santos JE, Donadi EA, Faccioli LH, Dias-Baruffi M, Fernandes APM, Gerlach RF, Sorgi CA On Behalf Of The Immunocovid Study Group. Matrix Metalloproteinases on Severe COVID-19 Lung Disease Pathogenesis: Cooperative Actions of MMP-8/MMP-2 Axis on Immune Response through HLA-G Shedding and Oxidative Stress. Biomolecules . 2022;12 doi: 10.3390/biom12050604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Berry E, Hernandez-Anzaldo S, Takawale A, Kassiri Z, Fernandez-Patron C. Matrix metalloproteinase-2 mediates a mechanism of metabolic cardioprotection consisting of negative regulation of the sterol regulatory element-binding protein-2/3-hydroxy-3-methylglutaryl-CoA reductase pathway in the heart. Hypertension . 2015;65:882–888. doi: 10.1161/HYPERTENSIONAHA.114.04989. [DOI] [PubMed] [Google Scholar]
- 34.Hardy E, Hardy-Sosa A, Fernandez-Patron C. MMP-2: is too low as bad as too high in the cardiovascular system? Am J Physiol Heart Circ Physiol . 2018;315:H1332–H1340. doi: 10.1152/ajpheart.00198.2018. [DOI] [PubMed] [Google Scholar]
- 35.Moin ASM, Sathyapalan T, Atkin SL, Butler AE. Pro-fibrotic M2 macrophage markers may increase the risk for COVID19 in type 2 diabetes with obesity. Metabolism . 2020;112:154374. doi: 10.1016/j.metabol.2020.154374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasr El-Din A, Ata KAE, Abdel-Gawad AR, Fahmy NF. Impact of High Serum Levels of MMP-7, MMP-9, TGF-β and PDGF Macrophage Activation Markers on Severity of COVID-19 in Obese-Diabetic Patients. Infect Drug Resist . 2021;14:4015–4025. doi: 10.2147/IDR.S329004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelzo M, Cacciapuoti S, Pinchera B, De Rosa A, Cernera G, Scialò F, Comegna M, Mormile M, Fabbrocini G, Parrella R, Corso G, Gentile I, Castaldo G. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci Rep . 2022;12:1212. doi: 10.1038/s41598-021-04677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, de Castro JPW, DeIuliis G, Ahangari F, Woolard T, Aurelien N, Arrojo E Drigo R, Gan Y, Graham M, Liu X, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med . 2018;24:39–49. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrell ED, Mikacenic C, Gong KQ, Kosamo S, Wurfel MM, Manicone AM. Alveolar MMP28 is associated with clinical outcomes and measures of lung injury in acute respiratory distress syndrome. Crit Care . 2020;24:141. doi: 10.1186/s13054-020-02847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi S, Su M, Shen G, Hu Y, Yi F, Zeng Z, Zhu P, Yang G, Zhou H, Li Q, Xie X. Matrix metalloproteinase 3 as a valuable marker for patients with COVID-19. J Med Virol . 2021;93:528–532. doi: 10.1002/jmv.26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remsik J, Wilcox JA, Babady NE, McMillen TA, Vachha BA, Halpern NA, Dhawan V, Rosenblum M, Iacobuzio-Donahue CA, Avila EK, Santomasso B, Boire A. Inflammatory Leptomeningeal Cytokines Mediate COVID-19 Neurologic Symptoms in Cancer Patients. Cancer Cell . 2021;39:276–283.e3. doi: 10.1016/j.ccell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HS, Kim WJ. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int J Mol Sci . 2022;23 doi: 10.3390/ijms231810546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardy E, Fernandez-Patron C. Targeting MMP-Regulation of Inflammation to Increase Metabolic Tolerance to COVID-19 Pathologies: A Hypothesis. Biomolecules . 2021;11 doi: 10.3390/biom11030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kehlet SN, Bager CL, Willumsen N, Dasgupta B, Brodmerkel C, Curran M, Brix S, Leeming DJ, Karsdal MA. Cathepsin-S degraded decorin are elevated in fibrotic lung disorders - development and biological validation of a new serum biomarker. BMC Pulm Med . 2017;17:110. doi: 10.1186/s12890-017-0455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allawadhi P, Singh V, Khurana I, Rawat PS, Renushe AP, Khurana A, Navik U, Allwadhi S, Kumar Karlapudi S, Banothu AK, Bharani KK. Decorin as a possible strategy for the amelioration of COVID-19. Med Hypotheses . 2021;152:110612. doi: 10.1016/j.mehy.2021.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirn-Safran C, Farach-Carson MC, Carson DD. Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell Mol Life Sci . 2009;66:3421–3434. doi: 10.1007/s00018-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersson-Sjöland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Törmänen K, Bjermer L, Malmström A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology . 2015;25:243–251. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung S, Potapov I, Chillara S, Del Sol A. Leveraging systems biology for predicting modulators of inflammation in patients with COVID-19. Sci Adv . 2021;7 doi: 10.1126/sciadv.abe5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaarteenaho-Wiik R, Lammi L, Lakari E, Kinnula VL, Risteli J, Ryhänen L, Pääkkö P. Localization of precursor proteins and mRNA of type I and III collagens in usual interstitial pneumonia and sarcoidosis. J Mol Histol . 2005;36:437–446. doi: 10.1007/s10735-006-9018-9. [DOI] [PubMed] [Google Scholar]
- 50.Garbuzenko OB, Ivanova V, Kholodovych V, Reimer DC, Reuhl KR, Yurkow E, Adler D, Minko T. Combinatorial treatment of idiopathic pulmonary fibrosis using nanoparticles with prostaglandin E and siRNA(s) Nanomedicine . 2017;13:1983–1992. doi: 10.1016/j.nano.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ball L, Barisione E, Mastracci L, Campora M, Costa D, Robba C, Battaglini D, Micali M, Costantino F, Cittadini G, Patroniti N, Pelosi P, Fiocca R, Grillo F. Extension of Collagen Deposition in COVID-19 Post Mortem Lung Samples and Computed Tomography Analysis Findings. Int J Mol Sci . 2021;22 doi: 10.3390/ijms22147498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leng L, Cao R, Ma J, Mou D, Zhu Y, Li W, Lv L, Gao D, Zhang S, Gong F, Zhao L, Qiu B, Xiang H, Hu Z, Feng Y, Dai Y, Zhao J, Wu Z, Li H, Zhong W. Pathological features of COVID-19-associated lung injury: a preliminary proteomics report based on clinical samples. Signal Transduct Target Ther . 2020;5:240. doi: 10.1038/s41392-020-00355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Goor H, Melenhorst WB, Turner AJ, Holgate ST. Adamalysins in biology and disease. J Pathol . 2009;219:277–286. doi: 10.1002/path.2594. [DOI] [PubMed] [Google Scholar]
- 54.Liu G, Philp AM, Corte T, Travis MA, Schilter H, Hansbro NG, Burns CJ, Eapen MS, Sohal SS, Burgess JK, Hansbro PM. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther . 2021;225:107839. doi: 10.1016/j.pharmthera.2021.107839. [DOI] [PubMed] [Google Scholar]
- 55.Patra T, Meyer K, Geerling L, Isbell TS, Hoft DF, Brien J, Pinto AK, Ray RB, Ray R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog . 2020;16:e1009128. doi: 10.1371/journal.ppat.1009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xavier LL, Neves PFR, Paz LV, Neves LT, Bagatini PB, Timmers LFSM, Rasia-Filho AA, Mestriner RG, Wieck A. Does Angiotensin II Peak in Response to SARS-CoV-2? Front Immunol . 2020;11:577875. doi: 10.3389/fimmu.2020.577875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernard I, Limonta D, Mahal LK, Hobman TC. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses . 2020;13 doi: 10.3390/v13010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendoza R, Saha N, Momeni A, Gabutan E, Alawad M, Dehghani A, Diks J, Lin B, Wang D, Alshal M, Fyke W, Wang B, Himanen JP, Premsrirut P, Nikolov DB. Ephrin-A1 and the sheddase ADAM12 are upregulated in COVID-19. Heliyon . 2021;7:e07200. doi: 10.1016/j.heliyon.2021.e07200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int J Lab Hematol . 2020;42:e211–e212. doi: 10.1111/ijlh.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Favaloro EJ, Henry BM, Lippi G. Increased VWF and Decreased ADAMTS-13 in COVID-19: Creating a Milieu for (Micro)Thrombosis. Semin Thromb Hemost . 2021;47:400–418. doi: 10.1055/s-0041-1727282. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Garron TM, Chang Q, Su Z, Zhou C, Qiu Y, Gong EC, Zheng J, Yin YW, Ksiazek T, Brasel T, Jin Y, Boor P, Comer JE, Gong B. Cell-Type Apoptosis in Lung during SARS-CoV-2 Infection. Pathogens . 2021;10 doi: 10.3390/pathogens10050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagy N, Kuipers HF, Frymoyer AR, Ishak HD, Bollyky JB, Wight TN, Bollyky PL. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol . 2015;6:123. doi: 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol . 1997;54:1205–1216. doi: 10.1016/s0006-2952(97)00343-2. [DOI] [PubMed] [Google Scholar]
- 64.Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet . 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 65.Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest . 2001;108:817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lartey NL, Valle-Reyes S, Vargas-Robles H, Jiménez-Camacho KE, Guerrero-Fonseca IM, Castellanos-Martínez R, Montoya-García A, García-Cordero J, Cedillo-Barrón L, Nava P, Filisola-Villaseñor JG, Roa-Velázquez D, Zavala-Vargas DI, Morales-Ríos E, Salinas-Lara C, Vadillo E, Schnoor M. ADAM17/MMP inhibition prevents neutrophilia and lung injury in a mouse model of COVID-19. J Leukoc Biol . 2022;111:1147–1158. doi: 10.1002/JLB.3COVA0421-195RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yates PA, Newman SA, Oshry LJ, Glassman RH, Leone AM, Reichel E. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther Adv Respir Dis . 2020;14:1753466620951053. doi: 10.1177/1753466620951053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hope AA, Evering TH. Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Infect Dis Clin North Am . 2022;36:379–395. doi: 10.1016/j.idc.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou JN, Sun L, Wang BR, Zou Y, Xu S, Ding YJ, Shen LJ, Huang WC, Jiang XJ, Chen SM. The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS One . 2021;16:e0248957. doi: 10.1371/journal.pone.0248957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barisione E, Grillo F, Ball L, Bianchi R, Grosso M, Morbini P, Pelosi P, Patroniti NA, De Lucia A, Orengo G, Gratarola A, Verda M, Cittadini G, Mastracci L, Fiocca R. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch . 2021;478:471–485. doi: 10.1007/s00428-020-02934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]