Abstract
Autoimmune pancreatitis (AIP) is a rare disease clinically characterized by obstructive jaundice, unintentional weight loss, acute pancreatitis, focal pancreatic mass, and diabetes. AIP is classified into two subtypes - type 1 and type 2 - according to pathological findings, clinical features, and serology test results, but some cases may be defined as type not otherwise in the absence of pathological findings and inflammatory bowel disease. To address the differences in diagnostic criteria by country, standard diagnostic criteria for AIP were proposed in 2011 by an international consensus of expert opinions. Differential diagnosis of AIP from pancreatic ductal adenocarcinoma is important but remains challenging for clinicians. Fortunately, all subtypes of AIP show dramatic response to steroid treatment. This review discusses the current perspectives on the diagnosis and management of AIP in clinical practice.
Keywords: Autoimmune pancreatitis, Pancreatic cancer, International consensus diagnostic criteria, Steroid
Core Tip: Autoimmune pancreatitis (AIP) is a rare disease characterized by obstructive jaundice, acute pancreatitis, and focal pancreatic mass. Lymphoplasmacytic sclerosing pancreatitis (type 1) and idiopathic duct centric pancreatitis (type 2) are histopathologically distinct. Typical imaging features and pathologic findings are crucial in the diagnosis of AIP to distinguish it from pancreatic ductal adenocarcinoma. Responses to steroid treatment are dramatic, but relapses are common. A careful approach to maintenance treatment is thus required.
INTRODUCTION
Autoimmune pancreatitis (AIP) is a rare, unique, and clinically distinct form of pancreatitis, characterized by histological features of a lymphoplasmacytic infiltrate and fibrosis, frequent elevations of serum immunoglobulin G4 (IgG4), and a dramatic response to steroid treatment[1]. In 1961, Sarles et al[2] first reported a case of chronic inflammatory sclerosis of the pancreas with hypergammaglobulinemia, and in 1995, Yoshida et al[3] first used the term AIP to describe a pancreatic mass responding to steroid treatment. In 2001, Hamano et al[4] reported that an increase in serum IgG4 in patients with AIP could be used as a diagnostic marker to differentiate AIP from similar diseases. AIP should be distinguished from pancreatic ductal adenocarcinoma and extrahepatic cholangiocarcinoma to avoid unnecessary surgery or delayed management. However, clinicians may find it difficult to differentiate them because of overlapping clinical and imaging findings, such as stenosis of the bile duct, focal mass of the pancreas, diffuse pancreatic hypertrophy, and obstructive jaundice.
EPIDEMIOLOGY
The incidence and prevalence of AIP are not well established owing to a lack of epidemiological data. AIP accounts for 5%-6% of chronic pancreatitis cases[5]. Type 1 AIP is reported more frequently in Asia, whereas type 2 AIP is more common in Europe and the Americas. According to a 2016 nationwide survey in Japan, the overall prevalence rate of AIP was 100.6 per 100000 persons, and the annual incidence rate was 31.4 per 100000 persons, which was twice as high as in the 2011 survey[6,7]. The male-to-female ratio was 2.94:1, and the mean age was 68.1 years[6]. In a multicenter study in Korea, the mean age of patients with AIP was 56 years, the male-to-female ratio was 2.5:1, and less than 10% of the patients had type 2 AIP[8]. An Italian study reported that the male-to-female ratio for type 2 AIP was 1:1.1, and the average age at onset was 34.4 years[9]. In a study reported in Germany in 2017, the incidence of AIP was less than 1 per 100000 persons[10].
CLASSIFICATION AND HISTOPATHOLOGICAL FEATURES
AIP is divided into two subtypes (type 1 or type 2), according to histopathological findings and clinical features. According to the International Consensus Diagnostic Criteria (ICDC)[1], AIP diagnosis requires a combination of the following five cardinal features: (1) Imaging features (pancreatic parenchyma and pancreatic duct); (2) Serology (IgG4, IgG, and antinuclear antibody); (3) Extrapancreatic organ involvement; (4) Histopathology of the pancreas; and (5) Response to steroid therapy. The imaging features and the steroid response are known to be the same in both type 1 and type 2 AIP, but the serologic findings, extrapancreatic organ involvement, and pancreatic histopathology are distinct between them. “Definitive type 1 AIP” can be diagnosed as a surrogate criterion for AIP without considering histopathology; however, "Definitive type 2 AIP" requires histopathological confirmation.
The histopathological features of lymphoplasmacytic sclerosing pancreatitis (LPSP) in type 1 AIP include: (1) Dense infiltration of plasma cells and lymphocytes, particularly periductal ones; (2) Storiform fibrosis; (3) Venulitis with lymphocytes and plasma cells often leading to obliteration of the affected veins; and (4) Abundant [> 10 cells per high-power field (HPF)] IgG4-positive plasma cells. The European guidelines recommend that the number of IgG4-positive plasma cells should exceed 50 cells/HPF in surgical specimens and 10 cells/HPF in biopsy specimens [average of three hotspots (400×)] for diagnosing type I AIP, and the IgG4/IgG ratio should be at least 40%[11]. However, an increased number of IgG4-positive plasma cells alone is insufficient for diagnosing type 1 AIP[12,13].
The primary histopathological feature of type 2 AIP is idiopathic duct-centric pancreatitis (IDCP). IDCP and LPSP share characteristics of periductal lymphoplasmacytic infiltrate and storiform fibrosis, whereas the characteristics of granulocyte epithelial lesions (GELs) are observed in IDCP alone. GELs are intraluminal and intraepithelial neutrophils in medium- and small-sized ducts, as well as in acini, often leading to the destruction and obliteration of the duct lumen[14,15]. Type 2 AIP typically has absented or very few (< 10 cells/HPF) IgG4-positive plasma cells.
According to the ICDC, AIP type not otherwise specified (NOS) is defined as the absence of histological criteria and inflammatory bowel disease (IBD). Type NOS AIP is rarely diagnosed, and some studies have reported that this subtype has clinically similar characteristics to type 2 AIP and can therefore be converted to type 2 AIP[16,17].
CLINICAL MANIFESTATIONS
Clinical manifestations of AIP mainly include obstructive jaundice, weight loss, acute pancreatitis, focal pancreatic mass, abdominal pain, diabetes mellitus (DM), and inflammatory bowel disease. The most common clinical symptom of type 1 AIP is obstructive jaundice, whereas that of type 2 AIP is acute pancreatitis[9,18,19]. Type 1 AIP is more commonly diagnosed in men, with the fifth to seventh decade of life being the predominant onset age[7,18]. In contrast, the incidence of type 2 AIP is similar in men and women, with the third to fourth decade of life being the predominant onset age[9,19].
Type 1 AIP belongs to the spectrum of IgG4-related diseases involving the bile duct (sclerosing cholangitis), salivary gland (sialadenitis), lacrimal gland (dacryoadenitis), kidney (tubulointerstitial nephritis), retroperitoneum (retroperitoneal fibrosis), lungs, thyroid gland, lymph nodes, pituitary gland, and aorta (Figure 1). Development of extrapancreatic involvement may occur antecedent to, concurrently with, or metachronously with the diagnosis of type 1 AIP[20]. Conversely, type 2 AIP does not typically involve other organs but is accompanied by IBD in 15%-45% of cases[18,19]. A multicenter international study comparing 204 patients with type 1 AIP vs 64 patients with type 2 AIP found that patients with type 2 AIP were more likely to develop concurrent IBD, particularly ulcerative colitis (16% vs 1%, respectively)[18]. According to a systematic review, the pooled estimate for the prevalence of DM at the time of diagnosis of type 1 AIP was 44%, which was 11% higher than that of type 2 AIP[21]. In addition, the pooled estimated prevalence of exocrine insufficiency at the time of AIP diagnosis was 45%. Thus, the presence of DM and pancreatic exocrine insufficiency in AIP indicates that more than one-third of patients with AIP develop significant impairment of their pancreatic function. The clinicopathological characteristics of type 1 and type 2 AIP are summarized in Table 1.
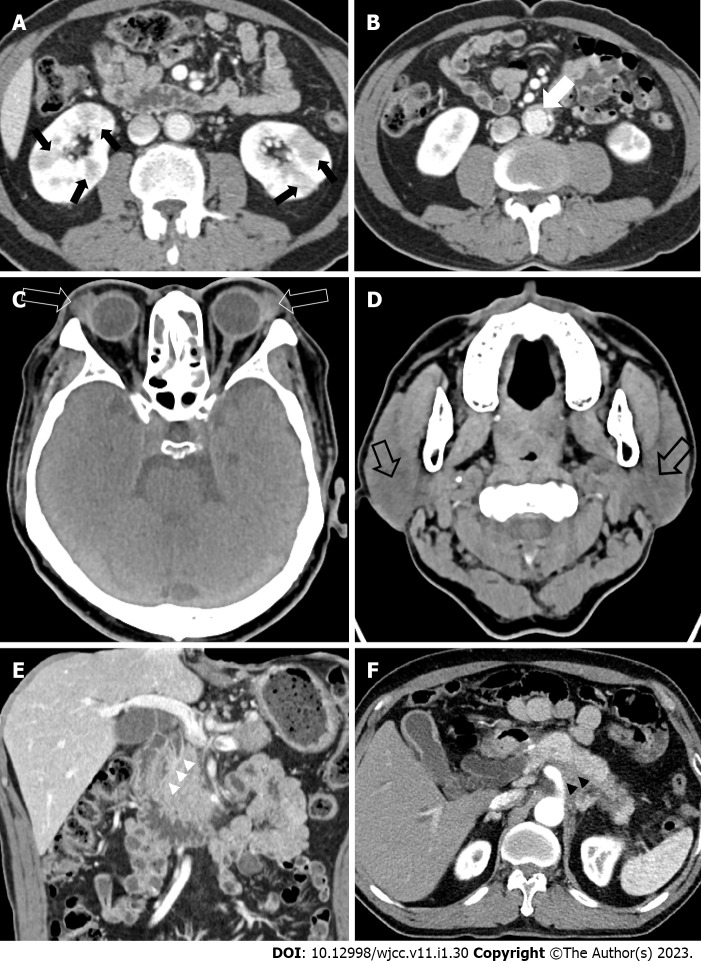
Figure 1.
Computed tomography features of autoimmune pancreatitis. A: Kidneys (black arrows); B: Aorta (white arrow); C: Lacrimal glands (open white arrow); D: Parotid glands (open black arrow); E: Bile duct (white arrow heads); F: Retroperitoneum (black arrow heads).
Table 1.
Differences in the clinicopathological features of type 1 and type 2 autoimmune pancreatitis
|
|
Type 1 AIP
|
Type 2 AIP
|
| Histopathological pattern | LPSP | IDCP |
| Most common symptom | Obstructive jaundice | Acute pancreatitis |
| Overall prevalence of DM | 44% | 11% |
| IgG4-related disease | Yes | No |
| Epidemiology | Asia > USA, Europe | Europe, USA > Asia |
| Sex | M > F | M = F |
| Age predominance | > 50 | 30-40 |
| Serum IgG4 level | Elevated, esp. twice the upper limit of the standard (> 280 mg/dL) | Mostly normal |
| Tissue IgG4 stain | > 10 cells/HPF | < 10 cells/HPF |
| Histological features | Periductal lymphoplasmacytic infiltrate, Storiform fibrosis, Obliterative phlebitis | Granulocytic epithelial lesions |
| Other organ involvement | Yes | No |
| Associated with IBD | Rare | Common |
| Steroid response | Excellent | Excellent |
| Recurrence | Common > 30 % | Rare < 10 % |
AIP: Autoimmune pancreatitis; USA: United States of America; LPSP: Lymphoplasmacytic sclerosing pancreatitis; IDCP: Idiopathic duct-centric pancreatitis; DM: Diabetes mellitus; IgG4: Immunoglobulin G4; HPF: High-power field; M: Male; F: Female; IBD: Inflammatory bowel disease.
DIAGNOSIS
Imaging methods
The imaging findings of AIP can be divided into parenchymal images using computed tomography (CT) or magnetic resonance imaging (MRI) and pancreatic duct imaging using endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP). The imaging findings of type 1 AIP and type 2 AIP are similar[22].
CT and MRCP
Imaging of the pancreatic parenchyma: The typical radiologic findings of AIP include diffuse enlargement of the pancreas, a capsule-like rim, and homogeneous delayed enhancement[18,23]. Diffuse or focal pancreatic edema by marked lymphocyte and plasma cell infiltration with fibrosis is observed as a “sausage-like appearance,” with a straightened edge of the pancreas[24]. The pancreatic parenchymal speckled/dotted enhancement on MRI is useful for differentiating AIP from pancreatic ductal adenocarcinoma (PDAC)[25]. A “capsule-like rim” reflecting dense fibrosis refers to a hypotonic band around the pancreas on CT and MRI scans and is another important finding to distinguish AIP from PDAC[26,27] (Figure 2). However, this characteristic is less sensitive for small lesions, and caution should therefore be taken[28]. AIP usually exhibits specific and homogenous delay enhancement. Conversely, heterogeneous delay enhancement is observed in PDAC owing to tumor necrosis, hemorrhage, or degeneration[27,28].
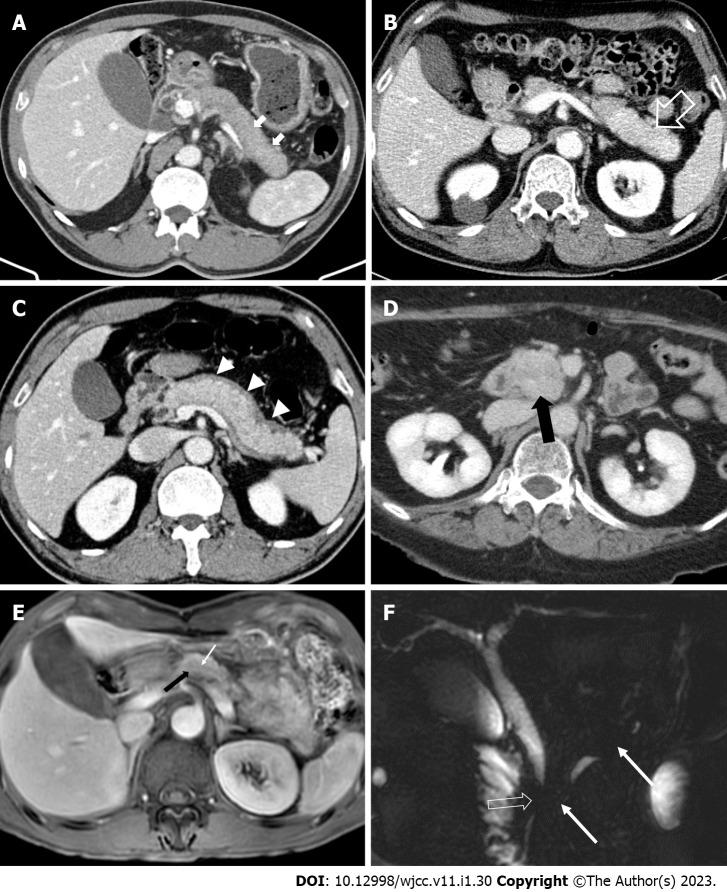
Figure 2.
Computed tomography and magnetic resonance imaging findings of autoimmune pancreatitis. A: Axial computed tomography (CT) image shows “sausage-like appearance” of diffuse enlargement of the pancreas with absence of pancreatic clefts (white arrows); B: Delayed enhancement (open white arrow) with swelling of the pancreatic tail; C: Hypotonic band (white arrow heads), called the “capsule-like rim”, around the pancreas; D: Axial CT image demonstrates a homogenous enhanced mass (black arrow) with proven autoimmune pancreatitis in the pancreas head mimicking pancreatic ductal adenocarcinoma; E: A pancreatic-phase dynamic contrast-enhanced magnetic resonance imaging (MRI) shows a mild arterial enhanced mass-like lesion (black arrow) with a penetrating duct sign (white arrow); F: An MRI cholangiopancreatography image shows irregular narrowing of the main pancreatic duct (white arrows) without upstream dilatation and short segment stricture of the common bile duct (open white arrow).
Imaging of the pancreatic duct: A duct penetrating sign of skipped narrowing of the main pancreatic duct (MPD) is a useful finding differentiating AIP from PDAC[24,26,27,29-31]. Stenosis of the pancreatic duct caused by periductal fibrosis is commonly observed in AIP; it resembles the tapering of the tip of an icicle and is called an "icicle sign"[32,33]. Linear enhancement along the MPD is often observed in AIP. This reflects inflammation around the MPD[24,34]. Unlike AIP, PDAC exhibits upstream pancreatic duct dilatation and an abrupt ductal cut of the pancreatic duct[27].
ERCP
ERCP is a useful modality for evaluating MPD. The characteristic ERCP findings of AIP are greater than one-third (5 cm) narrowing of the diffuse or segmental main pancreatic duct, multiple skipped narrowing with no upstream dilatation of the MPD above the narrowing (< 5 mm), and side branches arising from the narrowed segments[35,36]. PDAC presents as a focal narrowing of the MPD and a short single MPD stricture with upstream dilatation[36-40]. ERCP is more useful than MRCP for differentiating focal AIP from PDAC[41,42], but differentiation is difficult when the narrowing of the pancreatic duct is short (within approximately 3 mm)[35].
ERCP has the advantage of evaluating the pancreatic duct in addition to the performance of drainage of the pancreatic duct/bile duct and biopsy with cytology. However, ERCP is more invasive than MRCP, and procedure-related complications may occur as a result. ERCP may limit the evaluation of the upstream area of the MPD in case of MPD obstruction[36]. Thus, ERCP is being replaced by MRCP for the observation of the pancreatic duct owing to the improved quality and lesser invasiveness of MRCP.
Endoscopic ultrasonography
Endoscopic ultrasonography (EUS) can be used to evaluate the pancreatic parenchyma and pancreatic ducts to aid in the differentiation of AIP from PDAC (Figure 3). In EUS, diffuse hypoechoic areas, diffuse enlargement, extrahepatic bile duct wall thickening, and peripancreatic hypoechoic margins are more characteristic in AIP than in PDAC but are also observed in other forms of pancreatitis[43,44]. EUS alone is limited in the diagnosis of AIP. To overcome this limitation, trials have been performed to help diagnose AIP using contrast-enhanced EUS (CE-EUS) or elastography[45-48]. The most characteristic finding in the differentiation between focal type AIP and PDAC using CE-EUS was hyper- to iso-enhancement without irregular internal vessels (specificity, 94%)[49]. In addition, one report has indicated that measuring the strain ratio through elastography together with CE-EUS helps to differentiate it from PDAC[50]. However, CE-EUS and elastography are limited, and the most important role of EUS in diagnosing AIP is for tissue acquisition for histopathological confirmation.
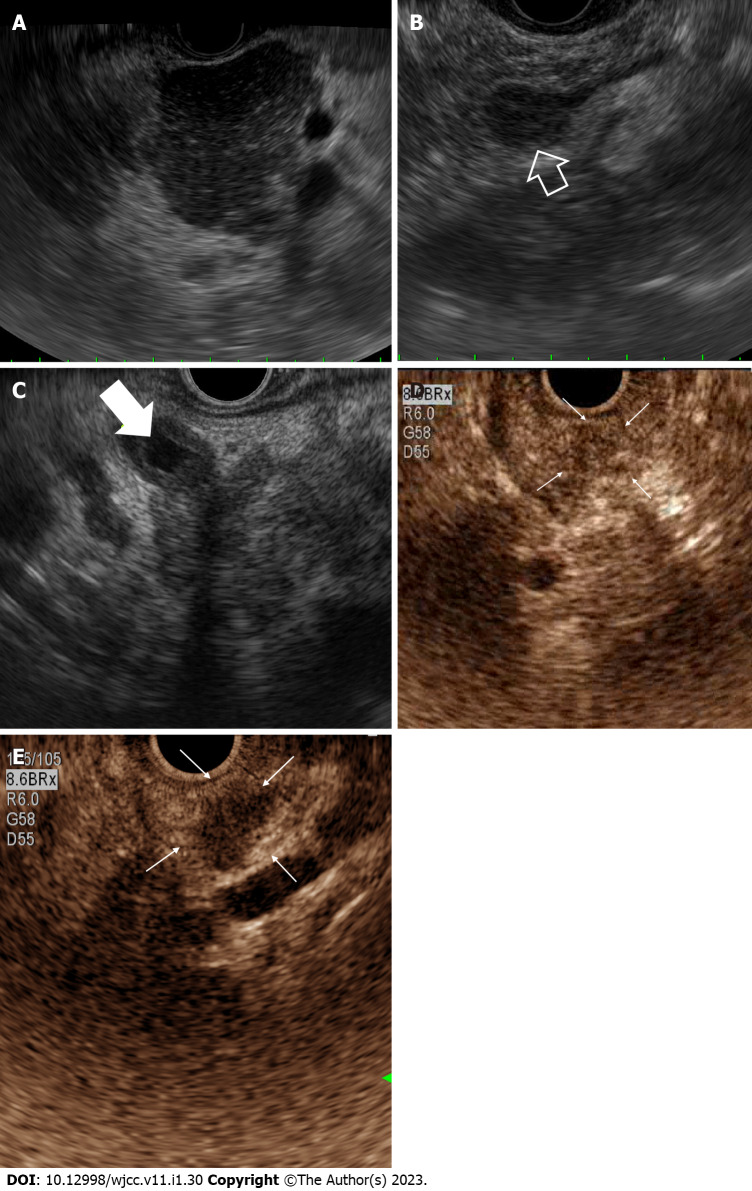
Figure 3.
Endoscopic ultrasound appearance of autoimmune pancreatitis. A: Hypoechoic diffuse pancreatic enlargement with patchy and coarse heterogeneous parenchyma; B: Mass-like lesion (open white arrow); C: Common bile duct wall thickening (white arrow) with heterogeneous pancreatic parenchyma; D and E: Contrast-enhanced endoscopic ultrasound features of mass forming autoimmune pancreatitis showing (D) homogenous hyper-enhancement mass (white arrows) without irregular internal vessel in the arterial phase and (E) wash-out (white arrows) in the venous phase.
Endoscopic ultrasound-guided fine needle aspiration and fine needle biopsy: EUS-guided tissue acquisition from patients with AIP is important not only for the differential diagnosis from PDAC but also for the differentiation between type 1 and type 2 AIP. No significant difference was observed between fine needle aspiration (FNA) and FN biopsy (FNB) in the diagnosis of PDAC, and both can be used to differentiate PDAC in patients with AIP[51-55]. However, histopathological diagnosis of AIP requires a larger amount of tissue than diagnosis of PDAC, so AIP diagnosis with FNA is uncertain[53,56]. The needles used for FNB have been developed to allow the acquisition of intact histologic core specimens with preserved tissue architecture[57]. According to a recent meta-analysis of nine FNA studies and seven FNB studies, the diagnostic yields for AIP with level 1 or 2 histology criteria were 55.8% in FNA and 87.2% in FNB, indicating the superiority of FNB[58]. The size of the needle affects the amount of tissue that can be obtained, so a 19-gauge needle has a greater diagnostic yield than a 22-gauge needle (82%-89% vs 61%-69%)[58,59]. However, caution is required because large gauge needles are difficult to handle and carry a higher risk of complications[59,60]. Diagnosis of AIP is not always simple, and in some cases distinguishing it from pancreatic cancer is difficult; while the occurrence of concomitant pancreatic tumors (benign and malignant) in patients with AIP has been documented in up to 7% of cases[61]. Therefore, surgery may be considered for patients in whom suspicion of malignant/premalignant lesions cannot be excluded even after detailed diagnostic workup such as biopsy through EUS or ERCP[62].
Serology: Elevation of serum IgG or IgG4 is often observed in AIP, and the ICDC recommends serum IgG4 as a level 1 serological marker for type 1 AIP[1]. An increase in IgG4 is usually accompanied by type 1 AIP (seropositive), whereas type 2 AIP is seronegative. Serum IgG4 is reported to range from 135 to 140 mg/dL as the upper limit of the standard[63]. Elevated serum IgG4 Level (> 140 mg/dL) is used as a diagnostic criterion for AIP, with a sensitivity of 86% and a specificity of 90%-96%[35,64,65]. Serum IgG4 elevations greater than twice the upper limit of the standard (> 280 mg/dL) are highly specific for AIP (specificity increased to 96%); however, the positive predictive value is less than 50%[4,65-69]. This is due to the presence of elevated IgG4 Levels in approximately 10% of patients with PDAC or other diseases (e.g., parasitic diseases, chronic pancreatitis, primary biliary cirrhosis, primary sclerosing cholangitis, or Sjogren’s syndrome)[4,35,65,70-73]. In addition, although LPSP with typical IgG4+ plasma cell abundance, a histological finding of type 1 AIP, was observed, the level of IgG4 in serum was sometimes lower than the cutoff value, so the level of IgG4 did not rise in all patients with type 1 AIP[74].Patients with increased IgG4 concentrations have high disease activity, a high incidence of jaundice at onset, and a number of extrapancreatic manifestations[74,75]. However, IgG4 Levels are not sufficiently correlated with the onset of complications or recurrence[11,74,76,77]; therefore, serological markers such as autotaxin are being studied for their relevance to relapse[78].
The c-antineutrophil cytoplasmic antibodies tends to be increased in some patients with type 2 AIP, which can help to distinguish type 1 from type 2[79]. However, despite research on various biomarkers (e.g., antibodies against the following: Carboanhydrase II, plasminogen-binding protein, lactoferrin, Alpha 2A amylase, cationic trypsinogen gene 1 (PRSS1) and PRSS2, and pancreatic secretory trypsin inhibitor/serine protease inhibitor, Kazal type 1 for the diagnosis of AIP and its differentiation from PDAC, verification of their specificity and sensitivity for commercialization is still insufficient[75,80].
Elevation of carbohydrate antigen (CA) 19-9 Levels are commonly observed in pancreatic cancer (sensitivity of 79%-95%, specificity of 82%-91%); however, up to 38% of patients with AIP also have values > 100 U/mL[81-83]. Therefore, the measurement of CA19-9 or IgG4 alone is insufficient to differentiate between AIP and PDAC. However, one study reported that the combination of IgG4 > 100 mg/dL and CA19-9 < 74 U/mL was more likely to indicate AIP than PDAC (sensitivity of 94%, specificity of 100%)[83]. In addition, serum eosinophilia, raised total serum IgE levels, and serum microRNAs may be used to differentiate between AIP and PDAC[11,75,84,85].
Diagnosis of AIP is still challenging even with a combination of imaging, histological, and serological tests. Therefore, follow up with a thorough clinical history and physical examination is required. In the case of type 1 AIP, additional imaging tests may be considered to determine other organ involvement; in contrast, in the case of type 2 AIP, the presence or absence of IBD can be confirmed through colonoscopy. The improvement of the lesion can be confirmed after an empirical short-term (2 wk) steroid treatment if AIP is suspected but a definitive diagnosis is not possible despite the performance of several tests, and no malignancy is found in histopathological examinations[86]. If the lesion worsens on follow-up imaging after 2 wk, biopsy may be performed again, and surgical treatment may be considered if necessary. However, a steroid trial with patients in whom differentiation from malignancy is an issue could result in delayed pancreatic cancer surgery and subsequent cancer progression, so sufficient information should be provided to the patient. In addition, novel biomarkers that have recently attracted attention may help distinguish PDAC from AIP in the future.
TREATMENT
The standard treatment for AIP is steroid therapy, which can successfully induce remission for both type 1 and type 2 AIP[87]. Therefore, the response to steroid treatment is sometimes used as a diagnostic criterion[1]. After induction of remission, the recurrence rate of type 1 AIP was higher than that of type 2 AIP (31% vs 9%), and the need for treatment maintenance was suggested owing to the high recurrence of type 1 AIP[77,87-89].
Indications
Treatment of AIP is required for symptomatic patients with pancreatic involvement (e.g., obstructive jaundice, abdominal pain, and back pain) or other organ involvement (e.g., jaundice due to bile duct stricture). In the case of asymptomatic patients, indications for treatment include persistent pancreatic mass on imaging tests or persistent abnormalities in liver function tests in patients with IgG4-related sclerosing cholangitis[87]. Some patients with AIP (approximately 10%-25%) improve spontaneously without intervention or steroid treatment, so "watchful waiting" may be considered in asymptomatic patients[76,87,88,90,91].
Initial treatment
Steroid therapy is recommended as the first-line treatment unless there is a contraindication for the use of steroids for induction of remission[87-89,92,93]. Assessment of the response to treatment using imaging and serological tests is recommended within 1-2 wk after initiation of steroid therapy[86]. To induce remission, an initial dose of 0.6-1.0 mg/kg/day of prednisone, which is maintained for 2-4 wk and then gradually reduced is recommended[87]. In general, the dose is reduced to 5-10 mg/day every 1-2 wk until a daily dose of 20 mg is reached, after which the dose is reduced by 5 mg every 2 wk. Another acceptable regimen is to take 40 mg/day for 4 wk and then taper by 5 mg/week until ceasing treatment[94]. The remission treatment period should be maintained for at least 12 wk. Administration of high-dose corticosteroids (approximately 30-40 mg/day) leads to a faster induction of remission than conservative management does[76]. Since low-dose prednisolone (less than 20 mg/day) has a limited remission rate, a minimum of 20 mg/day is generally required to induce remission[95,96]. Maintenance treatment to prevent AIP recurrence is not recommended for type 2 AIP or for type 1 AIP with low pre-treatment disease activity (i.e., involvement in the pancreas alone with segmental/focal lesions without any other organ involvement or complete radiological remission with normalized IgG/IgG4 in rapid response to steroid treatment). However, maintenance treatment with low-dose glucocorticoids or steroid-sparing agents may be helpful in some patients with type 1 AIP, such as those with remarkably high serum IgG4 Levels before treatment (e.g., more than four times the normal upper limits), diffuse enlargement of the pancreas, delayed radiological remission, persistently high serum IgG4 after treatment (more than two times the normal upper limits), more than two other organs involved, or association with proximal IgG4-related sclerosing cholangitis before treatment[88,94,97-110]. Low-dose (2.5-7.5 mg/day) glucocorticoid maintenance therapy is recommended to lower the recurrence rate, and a maintenance period of 1-3 years is recommended, but this remains controversial[111,112]. According to a recent meta-analysis, the glucocorticoid treatment group indicated calculated pooled relapse rates of 46.6% for less than 6 mo, 44.3% for 6-12 mo, 34.1% for 12-36 mo, and 27.0% for more than 36 mo[111].
Concerns have been raised about the side effects of long-term steroid treatment, and according to one study, most complications occurred after 3 years or after the accumulated amount of steroid exceeded 10000 mg[112,113]. Complications included poor glycemic control, osteoporosis, steroid myopathy, fungal infections, bacterial infections, cerebral infarctions, and atherosclerosis. Several studies have shown that people with AIP often have DM at diagnosis (33%-78%)[114-117]. DM improves in some patients when standard corticosteroid therapy is administered[114,116-118]. Therefore, steroid treatment may be considered even in patients with diabetes, and steroid treatment is particularly recommended in patients with AIP who have glucose intolerance[117]. Some patients do not relapse after cessation of steroids after achieving remission; however, some patients relapse during steroid reduction or require a relatively high-dose maintenance therapy. Therefore, the maintenance treatment period should be determined in consideration of each patient's age, comorbidities, recurrence risk, and individual preference for treatment[111].
There are rare cases of steroid non-response in patients with AIP[88,92,119]. Rituximab is recommended as an alternative treatment in the setting of steroid-refractory AIP[120-122]. Immunomodulatory agents, such as thiopurines (azathioprine and 6-mercaptopurine), mycophenolate mofetil, and methotrexate, are relatively inexpensive and can be administered to reduce the cumulative steroid dose, but immunomodulator monotherapy is ineffective[87,121]. Therefore, the use of immunomodulators in combination with low-dose steroids is recommended[121].
In patients with AIP with obstructive jaundice, biliary stenting is usually performed along with biopsy through ERCP[77,123]. Some studies have reported that obstructive jaundice should be treated with steroid treatment without biliary stents because AIP responds rapidly to steroid treatment[124,125]. However, as AIP is sometimes difficult to differentiate from pancreatic cancer or concomitant cholangiocarcinoma, empirical steroid treatment can be challenging without insertion of a biliary stent and biopsy. In cases of biliary stent insertion, early biliary stent removal after diagnosis of AIP according to the guidelines should be considered owing to stent related complications such as migration or stent dysfunction.
Treatment of relapse
In patients with recurrent AIP, steroids and steroid-sparing agents may be used. Steroid re-administration or dose increase is recommended in patients who relapse after successful induction of remission with initial steroid therapy[87]. In most patients with relapsed AIP, re-remission can be achieved with administration of prednisolone at the same dose as the initial treatment, although a more gradual reduction is required. Immune-modulators or rituximab are generally used as steroid-sparing agents[91,121,126-130]. Rituximab is used as a single agent for induction of remission, but immune-modulators are not as effective as single agents. Thiopurines and mycophenolate require 6-8 wk of overlapping treatment with steroids[102,121,126]. Patients with jaundice may require 4-6 wk of steroid treatment owing to the late onset of action of rituximab[121,127].
PROGNOSIS
In patients with AIP, the prevalence of endocrine and exocrine insufficiency at the time of AIP diagnosis is high. In a recent meta-analysis, the pooled estimate rate for the overall prevalence of DM in patients at the time of AIP (combined type 1 and type 2 AIP) diagnosis was 37%[21]. At the time of AIP diagnosis, the prevalence of DM was higher in Asian countries than in Western countries, and the pooled estimate of the prevalence of DM was 44% in type 1 AIP and 11% in type 2 AIP. The pooled estimated prevalence of diabetes at follow up in patients with AIP with or without steroid treatment was 42% and 44%, respectively[21]. In studies conducted in Asian and Western countries, respectively, the pooled estimate rate of DM at follow up was 49% and 34%. In some studies, glycemic control was improved by steroid treatment when AIP was accompanied by DM[116-118]; however, in a previous meta-analysis, the prevalence of DM increased during long-term follow up[21,131]. The pooled prevalence of exocrine pancreatic insufficiency in patients with AIP treated with steroids was 36%, which was improved from the time of diagnosis (45%). However, validation is warranted as other studies report an additional risk of exocrine pancreatic insufficiency during follow up in patients with AIP[131]. Histological analysis, changes in pancreatic volume and structure, obstruction of the main pancreatic duct, and decreased stimulation of the exocrine function suggest that islet cells in patients with AIP are fibrotic, and lymphoplasmatic cell infiltration is associated with endocrine and exocrine insufficiency of the pancreas[132-134]. Improvements in histological findings in patients with AIP following steroid treatment can sometimes lead to improvements in endocrine and exocrine function, but further studies and long-term follow up are still required to verify this. Furthermore, one study showed that chronic pancreatitis progressed in 22% of patients with AIP, and approximately 40% of the patients developed pancreatic stones[135,136]; as such, the occurrence of chronic pancreatitis-related complications is also expected to increase.
Patients with AIP may be associated with an increased risk of developing malignant disease than the general population[137-139]. A considerable number of patients with AIP are diagnosed with cancer at the time of AIP diagnosis or within 1 year, with cancer lesions occurring more often in other organs, such as the stomach, lung and prostate, than the pancreas[137,140,141]. These results suggest that AIP may be related to cancer more in other organs than in the pancreas itself. Based on this phenomenon, some studies have argued that because cancer and AIP occur simultaneously, AIP can sometimes occur in coexistence with cancer as a paraneoplastic syndrome[137,140]. Studies on the incidence of PDAC in patients with AIP have reported conflicting results[140,142,143]. However, additional research is required, as one case report indicated that PDAC and cholangiocarcinoma developed during follow up after AIP treatment[144,145]. Studies on patients with IgG4-related diseases have also shown that various malignant diseases (e.g., lung cancer, colorectal cancer, pancreatic cancer, bladder cancer, lymphoma, and leukemia) may also occur with AIP[141,146-148],which suggests that IgG4-related diseases are associated with an increased risk of malignant disease. Although whether AIP is a risk factor for malignancy remains controversial careful attention should be paid to the occurrence of malignancy at the time of AIP diagnosis and during follow up.
CONCLUSION
AIP is a rare disease that can be difficult to diagnose and treat unless the clinician has a high index of suspicion for AIP. Obstructive jaundice, narrowing of the bile ducts, and focal masses in the pancreas can occur in AIP and may result in a misdiagnosis of pancreaticobiliary cancer; therefore, performing a cytology test or biopsy along with imaging and serology testing is required. Although most patients respond well to steroid treatment, relapse is frequent. Therefore, standardization of the duration and the dose of maintenance treatment is necessary. Patients who are resistant to steroid therapy may be treated with rituximab or immunomodulatory medications, although more research to support this regimen is required. Finally, long-term follow up is required in patients with AIP because endocrine and exocrine insufficiency of the pancreas may persist or be induced and are associated with the development of malignant tumors.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 12, 2022
First decision: October 19, 2022
Article in press: December 19, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Caba O, Spain; Dugic A, Sweden; Kitagawa K, Japan; Vujasinovic M, Sweden S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
Contributor Information
Seong-Hun Kim, Division of Gastroenterology, Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju 54907, South Korea.
Yun Chae Lee, Division of Gastroenterology, Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju 54907, South Korea.
Hyung Ku Chon, Department of Internal Medicine, Institution of Wonkwang Medical Science, Wonkwang University School of Medicine and Hospital, Iksan 54538, South Korea. gipb2592@wku.ac.kr.
References
- 1.Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 2.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 4.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 5.Nishimori I, Tamakoshi A, Otsuki M Research Committee on Intractable Diseases of the Pancreas, Ministry of Health, Labour, and Welfare of Japan. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol. 2007;42 Suppl 18:6–8. doi: 10.1007/s00535-007-2043-y. [DOI] [PubMed] [Google Scholar]
- 6.Masamune A, Kikuta K, Hamada S, Tsuji I, Takeyama Y, Shimosegawa T, Okazaki K Collaborators. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol. 2020;55:462–470. doi: 10.1007/s00535-019-01658-7. [DOI] [PubMed] [Google Scholar]
- 7.Kanno A, Masamune A, Okazaki K, Kamisawa T, Kawa S, Nishimori I, Tsuji I, Shimosegawa T Research Committee of Intractable Diseases of the Pancreas. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2011. Pancreas. 2015;44:535–539. doi: 10.1097/MPA.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 8.Ryu JK, Chung JB, Park SW, Lee JK, Lee KT, Lee WJ, Moon JH, Cho KB, Kang DW, Hwang JH, Yoo KS, Yoo BM, Lee DH, Kim HK, Moon YS, Lee J, Lee HS, Choi HS, Lee SK, Kim YT, Kim CD, Kim SJ, Hahm JS, Yoon YB. Review of 67 patients with autoimmune pancreatitis in Korea: a multicenter nationwide study. Pancreas. 2008;37:377–385. doi: 10.1097/MPA.0b013e31817a0914. [DOI] [PubMed] [Google Scholar]
- 9.Ikeura T, Manfredi R, Zamboni G, Negrelli R, Capelli P, Amodio A, Caliò A, Colletta G, Gabbrielli A, Benini L, Okazaki K, Vantini I, Frulloni L. Application of international consensus diagnostic criteria to an Italian series of autoimmune pancreatitis. United European Gastroenterol J. 2013;1:276–284. doi: 10.1177/2050640613495196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider A, Michaely H, Weiss C, Hirth M, Rückert F, Wilhelm TJ, Schönberg S, Marx A, Singer MV, Löhr JM, Ebert MP, Pfützer RH. Prevalence and Incidence of Autoimmune Pancreatitis in the Population Living in the Southwest of Germany. Digestion. 2017;96:187–198. doi: 10.1159/000479316. [DOI] [PubMed] [Google Scholar]
- 11.Löhr JM, Beuers U, Vujasinovic M, Alvaro D, Frøkjær JB, Buttgereit F, Capurso G, Culver EL, de-Madaria E, Della-Torre E, Detlefsen S, Dominguez-Muñoz E, Czubkowski P, Ewald N, Frulloni L, Gubergrits N, Duman DG, Hackert T, Iglesias-Garcia J, Kartalis N, Laghi A, Lammert F, Lindgren F, Okhlobystin A, Oracz G, Parniczky A, Mucelli RMP, Rebours V, Rosendahl J, Schleinitz N, Schneider A, van Bommel EF, Verbeke CS, Vullierme MP, Witt H UEG guideline working group. European Guideline on IgG4-related digestive disease - UEG and SGF evidence-based recommendations. United European Gastroenterol J. 2020;8:637–666. doi: 10.1177/2050640620934911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011;64:237–243. doi: 10.1136/jcp.2010.085613. [DOI] [PubMed] [Google Scholar]
- 13.Detlefsen S, Mohr Drewes A, Vyberg M, Klöppel G. Diagnosis of autoimmune pancreatitis by core needle biopsy: application of six microscopic criteria. Virchows Arch. 2009;454:531–539. doi: 10.1007/s00428-009-0747-5. [DOI] [PubMed] [Google Scholar]
- 14.Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119–1127. doi: 10.1097/00000478-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 16.de Pretis N, Vieceli F, Brandolese A, Brozzi L, Amodio A, Frulloni L. Autoimmune pancreatitis not otherwise specified (NOS): Clinical features and outcomes of the forgotten type. Hepatobiliary Pancreat Dis Int. 2019;18:576–579. doi: 10.1016/j.hbpd.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 17.de Pretis N, Frulloni L. Autoimmune pancreatitis type 2. Curr Opin Gastroenterol. 2020;36:417–420. doi: 10.1097/MOG.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 18.Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J, Pickartz T, Lohr M, Schneider A, Frulloni L, Webster GJ, Reddy DN, Liao WC, Wang HP, Okazaki K, Shimosegawa T, Kloeppel G, Go VL. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809–814. doi: 10.1097/MPA.0b013e3182258a15. [DOI] [PubMed] [Google Scholar]
- 19.Hart PA, Levy MJ, Smyrk TC, Takahashi N, Abu Dayyeh BK, Clain JE, Gleeson FC, Pearson RK, Petersen BT, Topazian MD, Vege SS, Zhang L, Chari ST. Clinical profiles and outcomes in idiopathic duct-centric chronic pancreatitis (type 2 autoimmune pancreatitis): the Mayo Clinic experience. Gut. 2016;65:1702–1709. doi: 10.1136/gutjnl-2015-309275. [DOI] [PubMed] [Google Scholar]
- 20.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 21.Lanzillotta M, Tacelli M, Falconi M, Arcidiacono PG, Capurso G, Della-Torre E. Incidence of endocrine and exocrine insufficiency in patients with autoimmune pancreatitis at diagnosis and after treatment: a systematic review and meta-analysis. Eur J Intern Med. 2022;100:83–93. doi: 10.1016/j.ejim.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Negrelli R, Boninsegna E, Avesani G, Zamboni GA, Brozzi L, Frulloni L, Manfredi R, Pozzi Mucelli R. Type 1 and Type 2 Autoimmune Pancreatitis: Distinctive Clinical and Pathological Features, But Are There Any Differences at Magnetic Resonance? Pancreas. 2018;47:1115–1122. doi: 10.1097/MPA.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 23.Kamisawa T, Kim MH, Liao WC, Liu Q, Balakrishnan V, Okazaki K, Shimosegawa T, Chung JB, Lee KT, Wang HP, Lee TC, Choudhuri G. Clinical characteristics of 327 Asian patients with autoimmune pancreatitis based on Asian diagnostic criteria. Pancreas. 2011;40:200–205. doi: 10.1097/mpa.0b013e3181fab696. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, Fujinaga Y, Notohara K, Koyama T, Inoue D, Irie H, Gabata T, Kadoya M, Kawa S, Okazaki K Working Group Members of The Research Program on Intractable Diseases from the Ministry of Labor, Welfare of Japan. Diagnostic imaging guide for autoimmune pancreatitis. Jpn J Radiol. 2020;38:591–612. doi: 10.1007/s11604-020-00971-z. [DOI] [PubMed] [Google Scholar]
- 25.Kwon JH, Kim JH, Kim SY, Byun JH, Kim HJ, Lee MG, Lee SS. Differentiating focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma: contrast-enhanced MRI with special emphasis on the arterial phase. Eur Radiol. 2019;29:5763–5771. doi: 10.1007/s00330-019-06200-0. [DOI] [PubMed] [Google Scholar]
- 26.Hur BY, Lee JM, Lee JE, Park JY, Kim SJ, Joo I, Shin CI, Baek JH, Kim JH, Han JK, Choi BI. Magnetic resonance imaging findings of the mass-forming type of autoimmune pancreatitis: comparison with pancreatic adenocarcinoma. J Magn Reson Imaging. 2012;36:188–197. doi: 10.1002/jmri.23609. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kim JH, Kim SY, Byun JH, Kim HJ, Kim MH, Lee MG, Lee SS. Comparison of diagnostic performance between CT and MRI in differentiating non-diffuse-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Eur Radiol. 2018;28:5267–5274. doi: 10.1007/s00330-018-5565-1. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama Y, Fujinaga Y, Kadoya M, Ueda K, Kurozumi M, Hamano H, Kawa S. Characteristic magnetic resonance features of focal autoimmune pancreatitis useful for differentiation from pancreatic cancer. Jpn J Radiol. 2012;30:296–309. doi: 10.1007/s11604-011-0047-2. [DOI] [PubMed] [Google Scholar]
- 29.Muhi A, Ichikawa T, Motosugi U, Sou H, Sano K, Tsukamoto T, Fatima Z, Araki T. Mass-forming autoimmune pancreatitis and pancreatic carcinoma: differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging. 2012;35:827–836. doi: 10.1002/jmri.22881. [DOI] [PubMed] [Google Scholar]
- 30.Furuhashi N, Suzuki K, Sakurai Y, Ikeda M, Kawai Y, Naganawa S. Differentiation of focal-type autoimmune pancreatitis from pancreatic carcinoma: assessment by multiphase contrast-enhanced CT. Eur Radiol. 2015;25:1366–1374. doi: 10.1007/s00330-014-3512-3. [DOI] [PubMed] [Google Scholar]
- 31.Choi SY, Kim SH, Kang TW, Song KD, Park HJ, Choi YH. Differentiating Mass-Forming Autoimmune Pancreatitis From Pancreatic Ductal Adenocarcinoma on the Basis of Contrast-Enhanced MRI and DWI Findings. AJR Am J Roentgenol. 2016;206:291–300. doi: 10.2214/AJR.15.14974. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Kim YK, Jeong WK, Lee WJ, Choi D. Pancreatic duct "Icicle sign" on MRI for distinguishing autoimmune pancreatitis from pancreatic ductal adenocarcinoma in the proximal pancreas. Eur Radiol. 2015;25:1551–1560. doi: 10.1007/s00330-014-3548-4. [DOI] [PubMed] [Google Scholar]
- 33.Shankar A, Srinivas S, Kalyanasundaram S. Icicle sign: autoimmune pancreatitis. Abdom Radiol (NY) 2020;45:245–246. doi: 10.1007/s00261-019-02323-6. [DOI] [PubMed] [Google Scholar]
- 34.Kawai Y, Suzuki K, Itoh S, Takada A, Mori Y, Naganawa S. Autoimmune pancreatitis: assessment of the enhanced duct sign on multiphase contrast-enhanced computed tomography. Eur J Radiol. 2012;81:3055–3060. doi: 10.1016/j.ejrad.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Kawa S, Kamisawa T, Notohara K, Fujinaga Y, Inoue D, Koyama T, Okazaki K. Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2011. Pancreas. 2020;49:e13–e14. doi: 10.1097/MPA.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naitoh I, Nakazawa T. Endoscopic retrograde cholangiopancreatography and intraductal ultrasonography in the diagnosis of autoimmune pancreatitis and IgG4-related sclerosing cholangitis. J Med Ultrason (2001) 2021;48:573–580. doi: 10.1007/s10396-021-01114-1. [DOI] [PubMed] [Google Scholar]
- 37.Sugumar A, Levy MJ, Kamisawa T, Webster GJ, Kim MH, Enders F, Amin Z, Baron TH, Chapman MH, Church NI, Clain JE, Egawa N, Johnson GJ, Okazaki K, Pearson RK, Pereira SP, Petersen BT, Read S, Sah RP, Sandanayake NS, Takahashi N, Topazian MD, Uchida K, Vege SS, Chari ST. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: an international multicentre study. Gut. 2011;60:666–670. doi: 10.1136/gut.2010.207951. [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Kim MH, Byun JH, Lee SS, Lee SJ, Park SH, Lee SK, Park DH, Lee MG, Moon SH. Diagnostic Strategy for Differentiating Autoimmune Pancreatitis From Pancreatic Cancer: Is an Endoscopic Retrograde Pancreatography Essential? Pancreas. 2012;41:639–647. doi: 10.1097/MPA.0b013e31823a509b. [DOI] [PubMed] [Google Scholar]
- 39.Naitoh I, Nakazawa T, Hayashi K, Okumura F, Miyabe K, Shimizu S, Kondo H, Yoshida M, Yamashita H, Ohara H, Joh T. Clinical differences between mass-forming autoimmune pancreatitis and pancreatic cancer. Scand J Gastroenterol. 2012;47:607–613. doi: 10.3109/00365521.2012.667147. [DOI] [PubMed] [Google Scholar]
- 40.Jung YJ, Moon SH, Kim MH. Role of Endoscopic Procedures in the Diagnosis of IgG4-Related Pancreatobiliary Disease. Chonnam Med J. 2021;57:44–50. doi: 10.4068/cmj.2021.57.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kodama M, Kamata N. Can MRCP replace ERCP for the diagnosis of autoimmune pancreatitis? Abdom Imaging. 2009;34:381–384. doi: 10.1007/s00261-008-9401-y. [DOI] [PubMed] [Google Scholar]
- 42.Lee LK, Sahani DV. Autoimmune pancreatitis in the context of IgG4-related disease: review of imaging findings. World J Gastroenterol. 2014;20:15177–15189. doi: 10.3748/wjg.v20.i41.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoki N, Mizuno N, Sawaki A, Tajika M, Takayama R, Shimizu Y, Bhatia V, Yamao K. Diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. J Gastroenterol. 2009;44:154–159. doi: 10.1007/s00535-008-2294-2. [DOI] [PubMed] [Google Scholar]
- 44.Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927–936. doi: 10.1016/s0016-5107(04)02230-8. [DOI] [PubMed] [Google Scholar]
- 45.Faccioli N, Crippa S, Bassi C, D'Onofrio M. Contrast-enhanced ultrasonography of the pancreas. Pancreatology. 2009;9:560–566. doi: 10.1159/000225960. [DOI] [PubMed] [Google Scholar]
- 46.Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–720. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 47.Hocke M, Ignee A, Dietrich CF. Contrast-enhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy. 2011;43:163–165. doi: 10.1055/s-0030-1256022. [DOI] [PubMed] [Google Scholar]
- 48.Kanno A, Ikeda E, Ando K, Nagai H, Miwata T, Kawasaki Y, Tada Y, Yokoyama K, Numao N, Ushio J, Tamada K, Lefor AK, Yamamoto H. The Diagnosis of Autoimmune Pancreatitis Using Endoscopic Ultrasonography. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10121005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho MK, Moon SH, Song TJ, Kim RE, Oh DW, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Contrast-Enhanced Endoscopic Ultrasound for Differentially Diagnosing Autoimmune Pancreatitis and Pancreatic Cancer. Gut Liver. 2018;12:591–596. doi: 10.5009/gnl17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Abdulkader-Nallib I, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses: contrast-enhanced harmonic (CEH-EUS), quantitative-elastography (QE-EUS), or both? United European Gastroenterol J. 2017;5:236–246. doi: 10.1177/2050640616640635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M, Sato Y, Hikichi T, Ohira H. Endoscopic Ultrasonography-Guided Fine Needle Aspiration Can Be Used to Rule Out Malignancy in Autoimmune Pancreatitis Patients. J Ultrasound Med. 2017;36:2237–2244. doi: 10.1002/jum.14265. [DOI] [PubMed] [Google Scholar]
- 52.Khalid A, Dewitt J, Ohori NP, Chen JH, Fasanella KE, Sanders M, McGrath KM, Nikiforova M. EUS-FNA mutational analysis in differentiating autoimmune pancreatitis and pancreatic cancer. Pancreatology. 2011;11:482–486. doi: 10.1159/000331505. [DOI] [PubMed] [Google Scholar]
- 53.Imai K, Matsubayashi H, Fukutomi A, Uesaka K, Sasaki K, Ono H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis. 2011;43:869–874. doi: 10.1016/j.dld.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–349. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 55.Facciorusso A, Wani S, Triantafyllou K, Tziatzios G, Cannizzaro R, Muscatiello N, Singh S. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc. 2019;90:893–903.e7. doi: 10.1016/j.gie.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Estrada P, Pfau P. Diagnosing autoimmune pancreatitis: choosing your weapon. Gastrointest Endosc. 2020;91:382–384. doi: 10.1016/j.gie.2019.11.037. [DOI] [PubMed] [Google Scholar]
- 57.de Pretis N, Crinò SF, Frulloni L. The Role of EUS-Guided FNA and FNB in Autoimmune Pancreatitis. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11091653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon SB, Moon SH, Song TJ, Kim JH, Kim MH. Endoscopic ultrasound-guided fine needle aspiration vs biopsy for diagnosis of autoimmune pancreatitis: Systematic review and comparative meta-analysis. Dig Endosc. 2021;33:1024–1033. doi: 10.1111/den.13866. [DOI] [PubMed] [Google Scholar]
- 59.Facciorusso A, Barresi L, Cannizzaro R, Antonini F, Triantafyllou K, Tziatzios G, Muscatiello N, Hart PA, Wani S. Diagnostic yield of endoscopic ultrasound-guided tissue acquisition in autoimmune pancreatitis: a systematic review and meta-analysis. Endosc Int Open. 2021;9:E66–E75. doi: 10.1055/a-1293-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K, Notohara K, Shimosegawa T. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594–602. doi: 10.1016/j.gie.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 61.Xiang P, Zhang X, Wang C, Lang Y, Xu L, Huang L, Shen J, Feng ST. Pancreatic tumor in type 1 autoimmune pancreatitis: a diagnostic challenge. BMC Cancer. 2019;19:814. doi: 10.1186/s12885-019-6027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikolic S, Ghorbani P, Pozzi Mucelli R, Ghazi S, Baldaque-Silva F, Del Chiaro M, Sparrelid E, Verbeke CS, Löhr JM, Vujasinovic M. Surgery in Autoimmune Pancreatitis. Dig Surg. 2022;39:32–41. doi: 10.1159/000521490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deheragoda MG, Church NI, Rodriguez-Justo M, Munson P, Sandanayake N, Seward EW, Miller K, Novelli M, Hatfield AR, Pereira SP, Webster GJ. The use of immunoglobulin g4 immunostaining in diagnosing pancreatic and extrapancreatic involvement in autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1229–1234. doi: 10.1016/j.cgh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 64.Choi EK, Kim MH, Lee TY, Kwon S, Oh HC, Hwang CY, Seo DW, Lee SS, Lee SK. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 Levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007;35:156–161. doi: 10.1097/MPA.0b013e318053eacc. [DOI] [PubMed] [Google Scholar]
- 65.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, Vege SS, Farnell MB. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 66.Raina A, Krasinskas AM, Greer JB, Lamb J, Fink E, Moser AJ, Zeh HJ 3rd, Slivka A, Whitcomb DC. Serum immunoglobulin G fraction 4 Levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Arch Pathol Lab Med. 2008;132:48–53. doi: 10.5858/2008-132-48-SIGFLI. [DOI] [PubMed] [Google Scholar]
- 67.Bojková M, Dítě P, Dvořáčková J, Novotný I, Floreánová K, Kianička B, Uvírová M, Martínek A. Immunoglobulin G4, autoimmune pancreatitis and pancreatic cancer. Dig Dis. 2015;33:86–90. doi: 10.1159/000368337. [DOI] [PubMed] [Google Scholar]
- 68.Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, Petersen BT, Topazian MA, Vege SS. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097–1103. doi: 10.1016/j.cgh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 69.Culver EL, Sadler R, Simpson D, Cargill T, Makuch M, Bateman AC, Ellis AJ, Collier J, Chapman RW, Klenerman P, Barnes E, Ferry B. Elevated Serum IgG4 Levels in Diagnosis, Treatment Response, Organ Involvement, and Relapse in a Prospective IgG4-Related Disease UK Cohort. Am J Gastroenterol. 2016;111:733–743. doi: 10.1038/ajg.2016.40. [DOI] [PubMed] [Google Scholar]
- 70.Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, Chari S, Lindor KD. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070–2075. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 71.Aalberse RC, Van Milligen F, Tan KY, Stapel SO. Allergen-specific IgG4 in atopic disease. Allergy. 1993;48:559–569. doi: 10.1111/j.1398-9995.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 72.Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, Futamura S, Rivitti EA, Diaz LA. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 73.Bhol K, Mohimen A, Ahmed AR. Correlation of subclasses of IgG with disease activity in pemphigus vulgaris. Dermatology. 1994;189 Suppl 1:85–89. doi: 10.1159/000246938. [DOI] [PubMed] [Google Scholar]
- 74.Paik WH, Ryu JK, Park JM, Song BJ, Park JK, Kim YT, Lee K. Clinical and pathological differences between serum immunoglobulin G4-positive and -negative type 1 autoimmune pancreatitis. World J Gastroenterol. 2013;19:4031–4038. doi: 10.3748/wjg.v19.i25.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dugic A, Verdejo Gil C, Mellenthin C, Vujasinovic M, Löhr JM, Mühldorfer S. The Clinical Utility of Soluble Serum Biomarkers in Autoimmune Pancreatitis: A Systematic Review. Biomedicines. 2022;10 doi: 10.3390/biomedicines10071511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirano K, Tada M, Isayama H, Yagioka H, Sasaki T, Kogure H, Nakai Y, Sasahira N, Tsujino T, Yoshida H, Kawabe T, Omata M. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007;56:1719–1724. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, Frulloni L, Go VL, Gress TM, Kim MH, Kawa S, Lee KT, Lerch MM, Liao WC, Löhr M, Okazaki K, Ryu JK, Schleinitz N, Shimizu K, Shimosegawa T, Soetikno R, Webster G, Yadav D, Zen Y, Chari ST. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–1776. doi: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukiage A, Fujino H, Miki D, Ishii Y, Serikawa M, Tsuge M, Imamura M, Aikata H, Hayes CN, Chayama K. Clinical Usefulness of Serum Autotaxin for Early Prediction of Relapse in Male Patients with Type 1 Autoimmune Pancreatitis. Dig Dis Sci. 2021;66:1268–1275. doi: 10.1007/s10620-020-06338-8. [DOI] [PubMed] [Google Scholar]
- 79.Detlefsen S, de Vos JD, Tanassi JT, Heegaard NHH, Fristrup C, Schaffalitzky de Muckadell OB. Value of anti-plasminogen binding peptide, anti-carbonic anhydrase II, immunoglobulin G4, and other serological markers for the differentiation of autoimmune pancreatitis and pancreatic cancer. Medicine (Baltimore) 2018;97:e11641. doi: 10.1097/MD.0000000000011641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sánchez Castañón M, Zuliani V, Amodio A, Campagnola P, Granato A, Gabbrielli A, Benini L, López Hoyos M, Frulloni L. Role of Amylase-α2A Autoantibodies in the Diagnosis of Autoimmune Pancreatitis. Pancreas. 2015;44:1078–1082. doi: 10.1097/MPA.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 81.Safi F, Roscher R, Bittner R, Schenkluhn B, Dopfer HP, Beger HG. High sensitivity and specificity of CA 19-9 for pancreatic carcinoma in comparison to chronic pancreatitis. Serological and immunohistochemical findings. Pancreas. 1987;2:398–403. doi: 10.1097/00006676-198707000-00006. [DOI] [PubMed] [Google Scholar]
- 82.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 83.van Heerde MJ, Buijs J, Hansen BE, de Waart M, van Eijck CH, Kazemier G, Pek CJ, Poley JW, Bruno MJ, Kuipers EJ, van Buuren HR. Serum level of Ca 19-9 increases ability of IgG4 test to distinguish patients with autoimmune pancreatitis from those with pancreatic carcinoma. Dig Dis Sci. 2014;59:1322–1329. doi: 10.1007/s10620-013-3004-3. [DOI] [PubMed] [Google Scholar]
- 84.Chang MC, Liang PC, Jan S, Yang CY, Tien YW, Wei SC, Wong JM, Chang YT. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 Levels. Pancreatology. 2014;14:366–372. doi: 10.1016/j.pan.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 85.Yan T, Ke Y, Chen Y, Xu C, Yu C, Li Y. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19-9, globulin, eosinophils and hemoglobin. PLoS One. 2017;12:e0174735. doi: 10.1371/journal.pone.0174735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moon SH, Kim MH, Park DH, Hwang CY, Park SJ, Lee SS, Seo DW, Lee SK. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? Gut. 2008;57:1704–1712. doi: 10.1136/gut.2008.150979. [DOI] [PubMed] [Google Scholar]
- 87.Okazaki K, Chari ST, Frulloni L, Lerch MM, Kamisawa T, Kawa S, Kim MH, Lévy P, Masamune A, Webster G, Shimosegawa T. International consensus for the treatment of autoimmune pancreatitis. Pancreatology. 2017;17:1–6. doi: 10.1016/j.pan.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T, Takatori H, Yamakita K, Kubota K, Hamano H, Okamura K, Hirano K, Ito T, Ko SB, Omata M. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504–1507. doi: 10.1136/gut.2008.172908. [DOI] [PubMed] [Google Scholar]
- 89.Maire F, Le Baleur Y, Rebours V, Vullierme MP, Couvelard A, Voitot H, Sauvanet A, Hentic O, Lévy P, Ruszniewski P, Hammel P. Outcome of patients with type 1 or 2 autoimmune pancreatitis. Am J Gastroenterol. 2011;106:151–156. doi: 10.1038/ajg.2010.314. [DOI] [PubMed] [Google Scholar]
- 90.Kubota K, Kamisawa T, Hirano K, Hirooka Y, Uchida K, Ikeura T, Shiomi H, Ohara H, Shimizu K, Arakura N, Kanno A, Sakagami J, Itoi T, Ito T, Ueki T, Nishino T, Inui K, Mizuno N, Yoshida H, Sugiyama M, Iwasaki E, Irisawa A, Okazaki K, Kawa S, Shimosegawa T, Takeyama Y, Chiba T. Clinical course of type 1 autoimmune pancreatitis patients without steroid treatment: a Japanese multicenter study of 97 patients. J Hepatobiliary Pancreat Sci. 2018;25:223–230. doi: 10.1002/jhbp.541. [DOI] [PubMed] [Google Scholar]
- 91.Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 92.Kamisawa T, Okazaki K, Kawa S, Ito T, Inui K, Irie H, Nishino T, Notohara K, Nishimori I, Tanaka S, Nishiyama T, Suda K, Shiratori K, Tanaka M, Shimosegawa T Working Committee of the Japan Pancreas Society and the Research Committee for Intractable Pancreatic Disease supported by the Ministry of Health, Labour and Welfare of Japan. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J Gastroenterol. 2014;49:961–970. doi: 10.1007/s00535-014-0945-z. [DOI] [PubMed] [Google Scholar]
- 93.Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, Chari ST, Della-Torre E, Frulloni L, Goto H, Hart PA, Kamisawa T, Kawa S, Kawano M, Kim MH, Kodama Y, Kubota K, Lerch MM, Löhr M, Masaki Y, Matsui S, Mimori T, Nakamura S, Nakazawa T, Ohara H, Okazaki K, Ryu JH, Saeki T, Schleinitz N, Shimatsu A, Shimosegawa T, Takahashi H, Takahira M, Tanaka A, Topazian M, Umehara H, Webster GJ, Witzig TE, Yamamoto M, Zhang W, Chiba T, Stone JH Second International Symposium on IgG4-Related Disease. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015;67:1688–1699. doi: 10.1002/art.39132. [DOI] [PubMed] [Google Scholar]
- 94.Park DH, Kim MH, Oh HB, Kwon OJ, Choi YJ, Lee SS, Lee TY, Seo DW, Lee SK. Substitution of aspartic acid at position 57 of the DQbeta1 affects relapse of autoimmune pancreatitis. Gastroenterology. 2008;134:440–446. doi: 10.1053/j.gastro.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 95.Buijs J, van Heerde MJ, Rauws EA, de Buy Wenniger LJ, Hansen BE, Biermann K, Verheij J, Vleggaar FP, Brink MA, Beuers UH, Kuipers EJ, Bruno MJ, van Buuren HR. Comparable efficacy of low- vs high-dose induction corticosteroid treatment in autoimmune pancreatitis. Pancreas. 2014;43:261–267. doi: 10.1097/MPA.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 96.Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, Johnson GJ, Pereira SP, Chapman RW, Webster GJM, Barnes E. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 2014;109:1675–1683. doi: 10.1038/ajg.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Sawabu N. Long-term prognosis of duct-narrowing chronic pancreatitis: strategy for steroid treatment. Pancreas. 2005;30:31–39. [PubMed] [Google Scholar]
- 98.Masamune A, Nishimori I, Kikuta K, Tsuji I, Mizuno N, Iiyama T, Kanno A, Tachibana Y, Ito T, Kamisawa T, Uchida K, Hamano H, Yasuda H, Sakagami J, Mitoro A, Taguchi M, Kihara Y, Sugimoto H, Hirooka Y, Yamamoto S, Inui K, Inatomi O, Andoh A, Nakahara K, Miyakawa H, Hamada S, Kawa S, Okazaki K, Shimosegawa T Research Committee of Intractable Pancreas Diseases in Japan. Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut. 2017;66:487–494. doi: 10.1136/gutjnl-2016-312049. [DOI] [PubMed] [Google Scholar]
- 99.Ito T, Nishimori I, Inoue N, Kawabe K, Gibo J, Arita Y, Okazaki K, Takayanagi R, Otsuki M. Treatment for autoimmune pancreatitis: consensus on the treatment for patients with autoimmune pancreatitis in Japan. J Gastroenterol. 2007;42 Suppl 18:50–58. doi: 10.1007/s00535-007-2051-y. [DOI] [PubMed] [Google Scholar]
- 100.You MW, Kim JH, Byun JH, Kim HJ, Lee SS, Kim MH, Lee MG. Relapse of IgG4-related sclerosing cholangitis after steroid therapy: image findings and risk factors. Eur Radiol. 2014;24:1039–1048. doi: 10.1007/s00330-014-3127-8. [DOI] [PubMed] [Google Scholar]
- 101.Boonstra K, Culver EL, de Buy Wenniger LM, van Heerde MJ, van Erpecum KJ, Poen AC, van Nieuwkerk KM, Spanier BW, Witteman BJ, Tuynman HA, van Geloven N, van Buuren H, Chapman RW, Barnes E, Beuers U, Ponsioen CY. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology. 2014;59:1954–1963. doi: 10.1002/hep.26977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawa S, Hamano H, Ozaki Y, Ito T, Kodama R, Chou Y, Takayama M, Arakura N. Long-term follow-up of autoimmune pancreatitis: characteristics of chronic disease and recurrence. Clin Gastroenterol Hepatol. 2009;7:S18–S22. doi: 10.1016/j.cgh.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 103.Matsubayashi H, Sawai H, Kimura H, Yamaguchi Y, Tanaka M, Kakushima N, Takizawa K, Kadooka M, Takao T, Hebbar S, Ono H. Characteristics of autoimmune pancreatitis based on serum IgG4 Level. Dig Liver Dis. 2011;43:731–735. doi: 10.1016/j.dld.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Soliman H, Vullierme MP, Maire F, Hentic O, Ruszniewski P, Lévy P, Rebours V. Risk factors and treatment of relapses in autoimmune pancreatitis: Rituximab is safe and effective. United European Gastroenterol J. 2019;7:1073–1083. doi: 10.1177/2050640619862459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kubota K, Watanabe S, Uchiyama T, Kato S, Sekino Y, Suzuki K, Mawatari H, Iida H, Endo H, Fujita K, Yoneda M, Takahashi H, Kirikoshi H, Kobayashi N, Saito S, Sugimori K, Hisatomi K, Matsuhashi N, Sato H, Tanida E, Sakaguchi T, Fujisawa N, Nakajima A. Factors predictive of relapse and spontaneous remission of autoimmune pancreatitis patients treated/not treated with corticosteroids. J Gastroenterol. 2011;46:834–842. doi: 10.1007/s00535-011-0393-y. [DOI] [PubMed] [Google Scholar]
- 106.Zhu L, Xue HD, Zhang W, Wang Q, Tan B, Lai YM, Zheng WY, Asbach P, Hamm B, Denecke T, Jin ZY. Pancreaticobiliary involvement in treated type 1 autoimmune pancreatitis: Imaging pattern and risk factors for disease relapse. Eur J Radiol. 2019;120:108673. doi: 10.1016/j.ejrad.2019.108673. [DOI] [PubMed] [Google Scholar]
- 107.Shimizu K, Tahara J, Takayama Y, Akao J, Ajihara T, Nagao K, Shiratori K, Tokushige K. Assessment of the Rate of Decrease in Serum IgG4 Level of Autoimmune Pancreatitis Patients in Response to Initial Steroid Therapy as a Predictor of Subsequent Relapse. Pancreas. 2016;45:1341–1346. doi: 10.1097/MPA.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 108.Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD, Takahashi N, Farnell MB, Vege SS. Differences in clinical profile and relapse rate of type 1 vs type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140–8; quiz e12. doi: 10.1053/j.gastro.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 109.Lee HW, Moon SH, Kim MH, Cho DH, Jun JH, Nam K, Song TJ, Park DH, Lee SS, Seo DW, Lee SK. Relapse rate and predictors of relapse in a large single center cohort of type 1 autoimmune pancreatitis: long-term follow-up results after steroid therapy with short-duration maintenance treatment. J Gastroenterol. 2018;53:967–977. doi: 10.1007/s00535-018-1434-6. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura A, Ozawa M, Watanabe T, Ito T, Muraki T, Hamano H, Koinuma M, Kawa S. Predictive Factors for Autoimmune Pancreatitis Relapse After 3 Years of Maintenance Therapy. Pancreas. 2018;47:1337–1343. doi: 10.1097/MPA.0000000000001173. [DOI] [PubMed] [Google Scholar]
- 111.Yoon SB, Moon SH, Kim JH, Park JW, Kim SE, Kim MH. Determination of the duration of glucocorticoid therapy in type 1 autoimmune pancreatitis: A systematic review and meta-analysis. Pancreatology. 2021 doi: 10.1016/j.pan.2021.05.303. [DOI] [PubMed] [Google Scholar]
- 112.Kubota K, Kamisawa T, Okazaki K, Kawa S, Hirano K, Hirooka Y, Uchida K, Shiomi H, Ohara H, Shimizu K, Arakura N, Kanno A, Sakagami J, Itoi T, Ito T, Ueki T, Nishino T, Inui K, Mizuno N, Yoshida H, Sugiyama M, Iwasaki E, Irisawa A, Shimosegawa T, Takeyama Y, Chiba T. Low-dose maintenance steroid treatment could reduce the relapse rate in patients with type 1 autoimmune pancreatitis: a long-term Japanese multicenter analysis of 510 patients. J Gastroenterol. 2017;52:955–964. doi: 10.1007/s00535-016-1302-1. [DOI] [PubMed] [Google Scholar]
- 113.Shimizu S, Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Kondo H, Nishi Y, Yoshida M, Umemura S, Hori Y, Kato A, Okumura F, Sano H, Hirata Y, Takada H, Ohara H, Joh T. Correlation between long-term outcome and steroid therapy in type 1 autoimmune pancreatitis: relapse, malignancy and side effect of steroid. Scand J Gastroenterol. 2015;50:1411–1418. doi: 10.3109/00365521.2015.1054424. [DOI] [PubMed] [Google Scholar]
- 114.Hirano K, Isogawa A, Tada M, Isayama H, Takahara N, Miyabayashi K, Mizuno S, Mohri D, Kawakubo K, Sasaki T, Kogure H, Yamamoto N, Sasahira N, Toda N, Nagano R, Yagioka H, Yashima Y, Hamada T, Ito Y, Koike K. Long-term prognosis of autoimmune pancreatitis in terms of glucose tolerance. Pancreas. 2012;41:691–695. doi: 10.1097/MPA.0b013e31823bcdee. [DOI] [PubMed] [Google Scholar]
- 115.Masuda A, Shiomi H, Matsuda T, Takenaka M, Arisaka Y, Azuma T, Kutsumi H. The relationship between pancreatic atrophy after steroid therapy and diabetes mellitus in patients with autoimmune pancreatitis. Pancreatology. 2014;14:361–365. doi: 10.1016/j.pan.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 116.Miyamoto Y, Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, Inaba Y, Kuwata G, Fujiwara T, Egashira H, Koizumi K, Sekiya R, Fujiwara J, Arakawa T, Momma K, Asano T. Short and long-term outcomes of diabetes mellitus in patients with autoimmune pancreatitis after steroid therapy. Gut Liver. 2012;6:501–504. doi: 10.5009/gnl.2012.6.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Noguchi K, Nakai Y, Mizuno S, Isayama H, Hirano K, Kanai S, Nakamura T, Uchino R, Takahara N, Kogure H, Tada M, Koike K. Insulin secretion improvement during steroid therapy for autoimmune pancreatitis according to the onset of diabetes mellitus. J Gastroenterol. 2020;55:198–204. doi: 10.1007/s00535-019-01615-4. [DOI] [PubMed] [Google Scholar]
- 118.Nishimori I, Tamakoshi A, Kawa S, Tanaka S, Takeuchi K, Kamisawa T, Saisho H, Hirano K, Okamura K, Yanagawa N, Otsuki M Research Committee on Intractable Pancreatic Diseases, the Ministry of Health and Welfare of Japan. Influence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis: findings from a nationwide survey in Japan. Pancreas. 2006;32:244–248. doi: 10.1097/01.mpa.0000202950.02988.07. [DOI] [PubMed] [Google Scholar]
- 119.Matsubayashi H, Yoneyama M, Nanri K, Sugimoto S, Shinjo K, Kakushima N, Tanaka M, Ito S, Takao M, Ono H. Determination of steroid response by abdominal ultrasound in cases with autoimmune pancreatitis. Dig Liver Dis. 2013;45:1034–1040. doi: 10.1016/j.dld.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 120.Tacelli M, Celsa C, Magro B, Barresi L, Guastella S, Capurso G, Frulloni L, Cabibbo G, Cammà C. Risk Factors for Rate of Relapse and Effects of Steroid Maintenance Therapy in Patients With Autoimmune Pancreatitis: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1061–1072.e8. doi: 10.1016/j.cgh.2018.09.051. [DOI] [PubMed] [Google Scholar]
- 121.Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, Klebig RR, Levy MJ, Pearson RK, Petersen BT, Smyrk TC, Sugumar A, Takahashi N, Vege SS, Chari ST. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 2013;62:1607–1615. doi: 10.1136/gutjnl-2012-302886. [DOI] [PubMed] [Google Scholar]
- 122.Akiyama M, Takeuchi T. IgG4-Related Disease: Beyond Glucocorticoids. Drugs Aging. 2018;35:275–287. doi: 10.1007/s40266-018-0534-6. [DOI] [PubMed] [Google Scholar]
- 123.Kamisawa T, Okazaki K, Kawa S, Shimosegawa T, Tanaka M Research Committee for Intractable Pancreatic Disease and Japan Pancreas Society. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45:471–477. doi: 10.1007/s00535-010-0221-9. [DOI] [PubMed] [Google Scholar]
- 124.Bi Y, Hart PA, Law R, Clain JE, Farnell MB, Gleeson FC, Kendrick ML, Levy MJ, Pearson RK, Petersen BT, Pisney LD, Smyrk TC, Takahashi N, Topazian MD, Vege SS, Chari ST. Obstructive jaundice in autoimmune pancreatitis can be safely treated with corticosteroids alone without biliary stenting. Pancreatology. 2016;16:391–396. doi: 10.1016/j.pan.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 125.Meng QQ, Gao Y, Hu LH, Qian W, Lin H, Zhang YR, Pan J, Li ZS, Xin L, Wang LW. Limited Role of Endoscopic Biliary Drainage Before Steroid Treatment for Autoimmune Pancreatitis With Significant Obstructive Jaundice. Pancreas. 2021;50:e65–e66. doi: 10.1097/MPA.0000000000001883. [DOI] [PubMed] [Google Scholar]
- 126.Ghazale A, Chari ST. Optimising corticosteroid treatment for autoimmune pancreatitis. Gut. 2007;56:1650–1652. doi: 10.1136/gut.2007.129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Topazian M, Witzig TE, Smyrk TC, Pulido JS, Levy MJ, Kamath PS, Chari ST. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2008;6:364–366. doi: 10.1016/j.cgh.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 128.Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 Levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62:1755–1762. doi: 10.1002/art.27435. [DOI] [PubMed] [Google Scholar]
- 129.Raina A, Yadav D, Krasinskas AM, McGrath KM, Khalid A, Sanders M, Whitcomb DC, Slivka A. Evaluation and management of autoimmune pancreatitis: experience at a large US center. Am J Gastroenterol. 2009;104:2295–2306. doi: 10.1038/ajg.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naitoh I, Nakazawa T, Ohara H, Sano H, Ando T, Hayashi K, Tanaka H, Okumura F, Miyabe K, Yoshida M, Takahashi S, Joh T. Autoimmune pancreatitis associated with various extrapancreatic lesions during a long-term clinical course successfully treated with azathioprine and corticosteroid maintenance therapy. Intern Med. 2009;48:2003–2007. doi: 10.2169/internalmedicine.48.2695. [DOI] [PubMed] [Google Scholar]
- 131.Nikolic S, Maisonneuve P, Dahlman I, Löhr JM, Vujasinovic M. Exocrine and Endocrine Insufficiency in Autoimmune Pancreatitis: A Matter of Treatment or Time? J Clin Med. 2022;11 doi: 10.3390/jcm11133724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ito T, Kawabe K, Arita Y, Hisano T, Igarashi H, Funakoshi A, Sumii T, Yamanaka T, Takayanagi R. Evaluation of pancreatic endocrine and exocrine function in patients with autoimmune pancreatitis. Pancreas. 2007;34:254–259. doi: 10.1097/01.mpa.0000250127.18908.38. [DOI] [PubMed] [Google Scholar]
- 133.Löhr JM, Panic N, Vujasinovic M, Verbeke CS. The ageing pancreas: a systematic review of the evidence and analysis of the consequences. J Intern Med. 2018;283:446–460. doi: 10.1111/joim.12745. [DOI] [PubMed] [Google Scholar]
- 134.Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European Gastroenterol J. 2017;5:153–199. doi: 10.1177/2050640616684695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maruyama M, Arakura N, Ozaki Y, Watanabe T, Ito T, Yoneda S, Maruyama M, Muraki T, Hamano H, Matsumoto A, Kawa S. Type 1 autoimmune pancreatitis can transform into chronic pancreatitis: a long-term follow-up study of 73 Japanese patients. Int J Rheumatol. 2013;2013:272595. doi: 10.1155/2013/272595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maruyama M, Arakura N, Ozaki Y, Watanabe T, Ito T, Yoneda S, Maruyama M, Muraki T, Hamano H, Matsumoto A, Kawa S. Risk factors for pancreatic stone formation in autoimmune pancreatitis over a long-term course. J Gastroenterol. 2012;47:553–560. doi: 10.1007/s00535-011-0510-y. [DOI] [PubMed] [Google Scholar]
- 137.Shiokawa M, Kodama Y, Yoshimura K, Kawanami C, Mimura J, Yamashita Y, Asada M, Kikuyama M, Okabe Y, Inokuma T, Ohana M, Kokuryu H, Takeda K, Tsuji Y, Minami R, Sakuma Y, Kuriyama K, Ota Y, Tanabe W, Maruno T, Kurita A, Sawai Y, Uza N, Watanabe T, Haga H, Chiba T. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 2013;108:610–617. doi: 10.1038/ajg.2012.465. [DOI] [PubMed] [Google Scholar]
- 138.Schneider A, Hirth M, Münch M, Weiss C, Löhr JM, Ebert MP, Pfützer RH. Risk of Cancer in Patients with Autoimmune Pancreatitis: A Single-Center Experience from Germany. Digestion. 2017;95:172–180. doi: 10.1159/000455963. [DOI] [PubMed] [Google Scholar]
- 139.Hart PA, Law RJ, Dierkhising RA, Smyrk TC, Takahashi N, Chari ST. Risk of cancer in autoimmune pancreatitis: a case-control study and review of the literature. Pancreas. 2014;43:417–421. doi: 10.1097/MPA.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 140.Okamoto A, Watanabe T, Kamata K, Minaga K, Kudo M. Recent Updates on the Relationship between Cancer and Autoimmune Pancreatitis. Intern Med. 2019;58:1533–1539. doi: 10.2169/internalmedicine.2210-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Asano J, Watanabe T, Oguchi T, Kanai K, Maruyama M, Ito T, Muraki T, Hamano H, Arakura N, Matsumoto A, Kawa S. Association Between Immunoglobulin G4-related Disease and Malignancy within 12 Years after Diagnosis: An Analysis after Longterm Followup. J Rheumatol. 2015;42:2135–2142. doi: 10.3899/jrheum.150436. [DOI] [PubMed] [Google Scholar]
- 142.Kurita Y, Fujita Y, Sekino Y, Watanabe S, Iwasaki A, Kagawa K, Tanida E, Yagi S, Hasegawa S, Sato T, Hosono K, Kato S, Kobayashi N, Ichikawa Y, Endo I, Nakajima A, Kubota K. IgG4-related sclerosing cholangitis may be a risk factor for cancer. J Hepatobiliary Pancreat Sci. 2021;28:524–532. doi: 10.1002/jhbp.957. [DOI] [PubMed] [Google Scholar]
- 143.Hirano K, Tada M, Sasahira N, Isayama H, Mizuno S, Takagi K, Watanabe T, Saito T, Kawahata S, Uchino R, Hamada T, Miyabayashi K, Mohri D, Sasaki T, Kogure H, Yamamoto N, Nakai Y, Yoshida H, Ito Y, Akiyama D, Toda N, Arizumi T, Yagioka H, Takahara N, Matsubara S, Yashima Y, Koike K. Incidence of malignancies in patients with IgG4-related disease. Intern Med. 2014;53:171–176. doi: 10.2169/internalmedicine.53.1342. [DOI] [PubMed] [Google Scholar]
- 144.Shinozaki H, Sasakura Y, Shinozaki S, Terauchi T, Matsui J, Kobayashi K, Lefor AK, Ogata Y. Cholangiocarcinoma Presenting after Eight Years of Treatment of IgG4-Related Autoimmune Pancreatitis with Steroids. Case Rep Gastroenterol. 2021;15:154–162. doi: 10.1159/000512402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ghazale A, Chari S. Is autoimmune pancreatitis a risk factor for pancreatic cancer? Pancreas. 2007;35:376. doi: 10.1097/MPA.0b013e318073ccb8. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto M, Takahashi H, Tabeya T, Suzuki C, Naishiro Y, Ishigami K, Yajima H, Shimizu Y, Obara M, Yamamoto H, Himi T, Imai K, Shinomura Y. Risk of malignancies in IgG4-related disease. Mod Rheumatol. 2012;22:414–418. doi: 10.1007/s10165-011-0520-x. [DOI] [PubMed] [Google Scholar]
- 147.Wallace ZS, Wallace CJ, Lu N, Choi HK, Stone JH. Association of IgG4-Related Disease With History of Malignancy. Arthritis Rheumatol. 2016;68:2283–2289. doi: 10.1002/art.39773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ahn SS, Song JJ, Park YB, Lee SW. Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis. 2017;20:1028–1035. doi: 10.1111/1756-185X.13093. [DOI] [PubMed] [Google Scholar]