Abstract
Antibody–drug conjugates (ADCs) is a fast moving class of targeted biotherapeutics that currently combines the selectivity of monoclonal antibodies with the potency of a payload consisting of cytotoxic agents. For many years microtubule targeting and DNA-intercalating agents were at the forefront of ADC development. The recent approval and clinical success of trastuzumab deruxtecan (Enhertu®) and sacituzumab govitecan (Trodelvy®), two topoisomerase 1 inhibitor-based ADCs, has shown the potential of conjugating unconventional payloads with differentiated mechanisms of action. Among future developments in the ADC field, payload diversification is expected to play a key role as illustrated by a growing number of preclinical and clinical stage unconventional payload-conjugated ADCs. This review presents a comprehensive overview of validated, forgotten and newly developed payloads with different mechanisms of action.
Keywords: Antibody–drug conjugates, Payload, Topoisomerase 1 inhibitor, Cytotoxic molecule
Statement of significance
The ability to cure cancer depends on the diversification of the mechanisms of action of the therapeutic compounds used. A number of compounds have been abandoned due to excessive toxicity. The conjugation of such agents with antibodies might provide a better therapeutic index and our presentation may therefore be of interest for a broad scientific community.
Introduction
The use of monoclonal antibodies as therapeutic vector was hypothesized by Paul Ehrlich within the concept of the “Magic Bullet” [1]. Remarkable improvements in biology and chemistry led to the development of antibody–drug conjugates (ADCs), a new generation of biotherapeutics that combines the high specificity of antibodies to the potency of cytotoxic small molecules, with the aim to deliver highly potent payloads within the targeted cell. These weaponized antibodies could in certain cases significantly improve the therapeutic index of cytotoxic molecules and reduce their off-target toxicity, a major issue of conventional cytotoxic chemotherapies. Anticancer ADCs are comprised of three parts, a monoclonal antibody (mAb) which specifically recognizes an antigen on the target cell, a potent cytotoxic small molecule that triggers cell death when released, and a linker that binds these two elements together [2].
The complexity of these agents explains their arduous development, best exemplified by the chaotic history of gemtuzumab ozogamicin, which was first approved in 2000, removed from most markets in 2010 and then reapproved by the FDA in 2017 [3, 4]. Major improvements in mAb design, target selection, conjugation technologies, payload selection and standardization of quality controls have allowed this family to evolve into a mature component of the anticancer pharmacopeia with 13 agents currently approved for the treatment of cancer, among which seven have been approved during these past three years [5, 6].
Given the potency of the payload, the antibody must be highly selective for its target and maintain its half-life and biological properties after conjugation. Antibody engineering, such as Fc (fragment crystallizable) silencing or Fc capacity enhancement, is a potent tool that is developed to, respectively, balance antibody off-target toxicity induced by T-cell targeting or increase antibody functions such as ADCC and ADCP, and both methods have proven their clinical benefits in the context of therapeutic mAbs [2, 7, 8]. In the context of ADCs, it has been hypothesized that Fc silencing would considerably reduce off-target toxicity. Thrombocytopenia and neutropenia are common adverse effects observed in patients treated with ADCs that could relate to the expression of FcγRIIa receptor at the surface of platelets [9]. MEDI4276, an analogue of trastuzumab emtansine (T-DM1, Kadcyla®) with reduced FcγR binding, has been explored in clinical trials, aiming to reduce thrombocytopenia observed with T-DM1 [10] (NCT02576548). Surprisingly this ADC has demonstrated significant toxicity in a first-in-human trial [11]. In contrast, Fc capacity enhancement has proven its benefits with the approval of brentuximab vedotin (Blenrep®), whose mAb is afucosylated. The recent development of mAb derivatives widens vector possibilities while aiming to improve essential characteristics of ADC carriers. Smaller formats such as mAb fragments (scFv, single-chain variable fragment, and Fabs, fragment antigen-binding) have been explored in this context (small-format drug conjugates) with the aim to enhance solid tumor penetration and cell internalization [12, 13]. However, to date, no such candidates have entered clinical trials as they were found to face rapid elimination and may consequently not present benefits over classical mAb formats [14]. In contrast, another family of small-format drug conjugates based on bicycle peptides seems to meet the challenge of competing with mAb format conjugates with competitive uptake efficiency, as illustrated with the three bicycle peptide conjugates currently in clinical trials (NCT04561362, NCT04180371 and NCT03486730). Other important parameters than the size impact on pharmacokinetics (PK) and circulating half-life of these macromolecules, including chemical modifications, affecting their retention by the kidney tubular epithelium, as well as their recycling rate through the neonatal Fc receptor (FcRn) [15, 16]. Small-format conjugates may benefit from the modulation of these parameters. Multivalent binding entities such as diabodies or bispecifics are being developed to improve antigen affinity, selectivity, or internalization and could constitute promising vectors [17].
The most widely applied bioconjugation methods use lysine side-chain amines and cysteine interchain thiols [18]. However, the heterogeneity of the resulting mixture has been a major issue in ADC failure. Other conjugation strategies have been developed, including site-specific conjugation through specific or engineered amino acids, but failed to demonstrate improved outcome in clinical trials at this time. Iladatuzumab vedotin (DCDS0780A), a THIOMAB™ version of the FDA-approved polatuzumab vedotin (Polivy®), was explored in clinical trials but failed to reach phase II due to excessive ocular toxicity at the tested doses [19]. This THIOMAB™-drug conjugate’s technology demonstrated a great potential, but the difference in the two mAbs and/or in the clinical design, including the selected doses, indication and patient population may have resulted in the approval of one and not the other. It is important to highlight in this context that it is not only the technology that matters. Novel conjugation strategies such as glycans and short peptide tags (enzyme-assisted ligation) or more recently through ADP-ribosyl cyclase are being explored with the aim to generate homogeneous and physically stable ADCs [20–25].
The linker plays a major role in ADC design since it strongly impacts on the safety, potency and activity of the ADC. Most importantly, the linker is expected to remain stable in circulation to avoid premature detachment of the drug while allowing its release within the targeted cell. Two categories of linkers have been developed and can be distinguished based on their cleavability. Cleavable linkers are either sensitive to pH for hydrazone linkers, to glutathione or disulfide isomerase for disulfide linkers and to proteases such as cathepsin B for dipeptide bonds. Non-cleavable linkers rely on lysosomal degradation of the antibody moiety, thereby conserving at least one amino acid, most commonly lysine or cysteine, attached to the payload-linker complex. This approach improves the linkage stability since antibody digestion is required for payload release. While it is emphasized that the more stable the linker is, the less off-target toxicity it triggers, these technologies were often found to be too stringent to support anti-tumoral activity. Safety of ADCs remains a major challenge in their design and on-target in addition to off-target toxicity is not only driven by the instability of the linker-payload. On-target toxicity is rather always driven by the mAb and its affinity/avidity to the target, together with the payloads’ mechanism of action, again illustrating how important the match between tumor type, target antigen and ADC construction is recent interest in linker design improvement has led to the development of hydrophilic linkers to balance payload hydrophobicity [26–29]. Sulfonate, polyethylene glycol (PEG), polysarcosine (PSAR) or more recently DNA-based linkers have significantly improved ADC stability and pharmacokinetics, leading to less toxic and more active ADCs [30–37].
The drug-to-antibody ratio (DAR) has until recently been maintained under a value of four to avoid mAb aggregation and limit the overall hydrophobicity of ADCs, which has been reported to be correlated with toxicity, reduced half-life and a narrow therapeutic index. Increased DAR is therefore more suitable for less hydrophobic, or well-compensated hydrophobic payloads, as illustrated with novel linker technologies including masking entities, or linker hydrophilic inserts, whose efficiency directly determines the capacity of DAR increase [35, 38]. Restoration of hydrophilicity and naked-like mAb pharmacokinetic profile in addition to a high DAR considerably increase the payload’s exposure to the tumor. These new linker technologies have enabled the development of less potent payloads than DNA-intercalating or microtubule-disrupting agents, as illustrated with the approval of two topoisomerase 1-based ADCs conjugated at DAR8, trastuzumab deruxtecan (Enhertu®) and sacituzumab govitecan (Trodelvy®). These highly loaded ADCs with unconventional payloads could also potentially widen the therapeutic indications of ADCs by addressing tumors with high or low target expression levels, as illustrated with the recent approval of Enhertu® in HER2-low-expressing tumors based on the results of DESTINY-Breast04. In contrast, in the context of highly potent payloads, a lower DAR has so far been preferable, as illustrated with the recent approval of loncastuximab tesirine (Zynlonta®), a DAR2 of extremely potent pyrrolobenzodiazepines (PBDs) payload. Of note, unlike trastuzumab deruxtecan and analogues evaluated in clinical trials that are conjugated at DAR8, datopotamab deruxtecan’s DAR is lowered to 4 to reduce the toxicity driven by its target [39], illustrating the multidimensional design of ADCs.
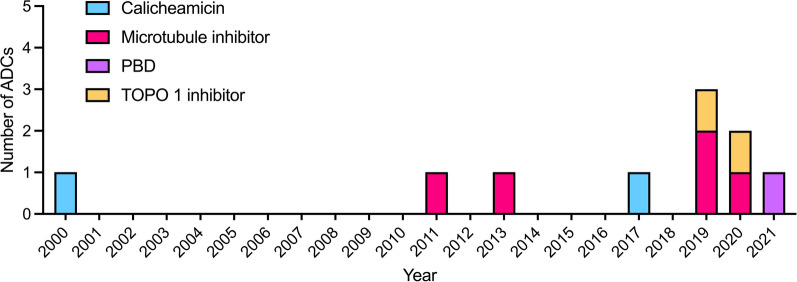
The payload (also designated as warhead) exerts the ADC’s intracellular cytotoxic activity. The nature of the cytotoxic agent covalently bound to the antibody through the linker moiety is of great importance since its mechanisms of action will determine the resulting ADC's potency as an anticancer compound and its possible indications. First-generation ADCs, coupled to conventional chemotherapeutics (taxoids, anthracyclines), lacked efficacy as the payloads were not potent enough since only a small fraction of the total conjugates administrered successfully delivered their payload within the target cell [2, 40]. Tumor penetration, target copy number at the cell surface and ADC internalization and degradation strongly impact the intracellular concentration of the free payload. The payload must therefore be highly potent at low concentration, with 50% inhibitory concentrations (IC50s) in the low to sub-nanomolar range [41]. Other factors such as molecule stability in plasma and under acidic conditions, accessibility of a conjugation site or solubility are crucial [42]. For many years, payloads were essentially represented by 2 categories: microtubule inhibitors, including maytansinoids and auristatins and DNA-alkylating agents, such as calicheamicins. These payloads lead to the approval of eight ADCs (Fig. 1).
Fig. 1.
FDA approval of anticancer ADCs. ADCs are identified according to the nature of their payload: Microtubule-disrupting agents; DNA-targeting agents: Calicheamicin, pyrrolobenzodiazepine (PBD), topoisomerase 1 (TOPO 1) inhibitor
Newer and more potent DNA-alkylating agents such as PBD monomers and dimers, indolino-benzodiazepines (IGNs) or cyclopropabenzindolone (CBI) monomers and dimers, with IC50 values in the picomolar range, have been at the forefront of ADC design [43, 44]. These molecules are among the most potent anti-tumor chemicals ever synthesized, and their specific targeting to tumor cells through an ADC construction was investigated to generate highly potent “magic bullets.” However, strong dose-limiting toxicities have limited their clinical development [45, 46] and currently only loncastuximab tesirine has obtained FDA approval in 2021 (Fig. 1) [47].
Efforts have been made to diversify payload families to molecules with original mechanisms of action, including several which do not directly target DNA or microtubules. Less potent molecules have benefited from major breakthroughs in ADC construction, through improved linker design allowing higher DAR values, more stable payload attachment or enhanced bystander killing activity. A recent and spectacular success has been the development of topoisomerase 1 (topo-1) inhibitors, which represented a turning point in payload selection, with the approval of two topo-1 inhibitor-based ADCs since 2019 (Fig. 1), trastuzumab deruxtecan (Enhertu®, DS-8201a) and sacituzumab govitecan (Trodelvy®) [48, 49]. Recent reviews have described the landscape of validated and exploratory therapeutic targets for ADCs [50, 51]. This review aims to describe the landscape of validated, forgotten and newly developed payloads in ADC context with diverse mechanisms of action, excluding microtubule inhibitors and DNA-alkylating agents.
A successful payload family: Topoisomerase 1 inhibitors
Topoisomerase 1 inhibitors constitute the most recent antibody–drug conjugate payload family to be approved by the FDA, first driven by trastuzumab deruxtecan, followed by sacituzumab govitecan (Table 1, Fig. 2A) [48, 49]. The recent development of these conjugates based on moderately potent payloads has been enabled by the production of highly loaded ADCs with DAR values of 8 [52, 53].
Table 1.
Landscape of topoisomerase 1 inhibitors antibody–drug conjugates
| Payload name | ADC name | Antibody | Antibody target | linker | Conjugation site | DAR | Development status | Indication | Last publication date or clinical trial status | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Dxd | DsS8201a, trastuzumab deruxtecan, Enhertu | trastuzumab | HER2 | mc-GGFG-AM cleavable | cysteine | 8 | FDA approved | unresectable or metastatic HER2 + BC, gastric or gastroesophageal carcinoma | N/A | 48 |
| U3-1402, patritumab deruxtecan | patritumab | HER3 | mc-GGFG-AM cleavable | cysteine | 8 | phase II | NSCLC, metastatic colorectal, metastatic BC and BC | Recruiting or active | NCT04479436, NCT03260491, NCT02980341, NCT04619004, NCT04676477, NCT04699630, NCT04610528, NCT0496576 | |
| Ds-1062a, datopotamab deruxtecan | datopotamab | TROP2 | mc-GGFG-AM cleavable | cysteine | 4 | phase III | NSCLC, metastatic lung cancer, TNBC, HR + BC, BC | Recruiting or active | NCT02923115, NCT03742102, NCT04526691, NCT04612751, NCT05104866, NCT03401385, NCT04940325, NCT04656652, NCT04484142, NCT05215340 | |
| Ds-7300a | B7-H3 antibody | B7-H3 | mc-GGFG-AM cleavable | cysteine | 4 | phase I/II | Advanced solid malignant tumors | Recruiting or active | NCT04145622 | |
| Ds-6157a | GPR20 antibody | GPR20 | mc-GGFG-AM cleavable | cysteine | 8 | Phase I | GIST | Recruiting or active | NCT04276415 | |
| SN-38 | IMMU-132, sacituzumab govitecan, Trodelvy TM | sacituzumab (hRS7) | TROP2 | Cleavable CL2A (hydrolyzable) | cysteine | 7.6 | FDA approved | HER + /HER-Metastatic BC (mTNBC) | N/A | 49 |
| IMMU-130 | labetuzumab (hMN-14) | CEACAM5 | Cleavable CL2A (pH-sensitive) | cysteine | 7.6 | Phase II (withdrawn) | Solid tumors (metastatic colorectal; colon cancer; rectal cancer) | 2020 (Phase II withdrawal) | NCT01270698, NCT01605318, NCT01915472 | |
| IMMU-140 | IMMU–112 (hL243) | HLA-DR | Cleavable CL2A (pH-sensitive) | cysteine | 6.1 | late preclinical | ALL, CLL, MM, AML, DLBCL, HL and melanoma | 2018 | 87 | |
| Epratuzumab (hLL2) | CD22 | Cleavable CL2A (pH-sensitive) or CL2E (cathepsin cleavable) | cysteine | 6 | preclinical | B cell malignancy, Lymphoma and leukemia | 2012 | 88 | ||
| Veltuzumab | CD20 | Cleavable CL2A (pH-sensitive) or CL2E (cathepsin cleavable) | cysteine | 6 | preclinical | B cell malignancy | 2012 | 88 | ||
| Milatuzumab | CD74 | Cleavable CL2A (pH-sensitive) or CL2E (cathepsin cleavable) | cysteine | 6.5–6.6 | Preclinical | solid cancers (and lymphoma) | 2013 | 89 | ||
| Trastuzumab | HER2 | ester bond or carbonate cleavable (± PEG4) | Cysteine | 3.2–3.7 | preclinical | ovarian | 2015 | 90 | ||
| A7R | IL-7R | Carbamate bond + PEG12 | Cysteine | 4 to 6 | late preclinical | IL-7R + Lymphoid malignancies, autoimmune diseases, metastatic solid tumors | 2018 | 93 | ||
| rituximab | CD20 | Carbamate or ester cleavable + PEG27 | cysteine | 7 | preclinical | Lymphoma | 2013 | 91 | ||
| B8-4 | EpCam | Carbamate or ester cleavable + PEG27 | cysteine | 8.5 | preclinical | Pancreatic cancer | 2013 | 91 | ||
| 35–4 | Collagen-4 | Carbamate or ester cleavable + PEG27 | cysteine | 7.5 | preclinical | hypovascular stroma-rich tumor (pancreatic) | 2013 | 91 | ||
| AZ'0132 (exatecan derivative) | AZD8205 | B7-H4 antibody | n.d | n.d | n.d | 8 | Phase I/Phase II | breast, ovarian and endometrial cancers and cholangiocarcinoma | 2021 | NCT05123482, 69 |
| KL610023 (belotecan derivative) | SKB-264 | n.d | TROP2 | n.d | n.d | 7.4 | Phase I/II | ovarian epithelial and breast cancer, gastric and urothelial carcinoma, NSCLC, SCLC | Recruiting or active | NCT04152499 |
| Exatecan mesylate | Trastuzumab | HER2 | cleavable glucuronide + PSARLink | cysteine | 8 | preclinical | HER2 + solid tumors | 2021 | 36 | |
| PRO1184 | Fra antibody | Fra | hydrophilic protease cleavable | cysteine | 8 | late preclinical | NSCLC, ovarian | 2022 | 77 | |
| PRO1102 | Trastuzumab | HER2 | hydrophilic protease cleavable | cysteine | 8 | POC | 2022 | 76 | ||
| PRO1160 | CD70 antibody | CD70 | hydrophilic protease cleavable | cysteine | 8 | preclinical | 2022 | 78 | ||
| 7-n-butyl-10-amino-CPT | cAC10 | CD30 | mc-Val-cit-PAB or glucuronide cleavable linkers | cysteine | 4 to 8 | preclinical | hematologic malignancies | 2009 | 94 | |
| h1F6 | CD70 | mc-Val-cit-PAB or glucuronide cleavable linkers | cysteine | 4 to 8 | preclinical | hematologic malignancies and renal cell carcinoma | 2009 | 94 | ||
| cBR96 | LeY | mc-Val-cit-PAB or glucuronide cleavable linkers | cysteine | 4 to 8 | preclinical | carcinoma | 2009 | 94 | ||
| 7-n-butyl-9-amino-10, 11-MDO-CPT | cAC10 | CD30 | mc-Val-cit-PAB or glucuronide cleavable linkers | cysteine | 4 to 8 | preclinical | hematologic malignancies | 2009 | 94 | |
| h1F6 | CD70 | mc-Val-cit-PAB or glucuronide cleavable linkers | cysteine | 4 to 8 | preclinical | hematologic malignancies and renal cell carcinoma | 2009 | 94 | ||
| cBR96 | LeY | mc-Val-cit-PAB or glucuronide cleavable linkers | cysteine | 4 to 8 | preclinical | carcinoma | 2009 | 94 | ||
| CPT | trastuzumab | HER2 | Pt–PEG, ester cleavage | cysteine | 2.5–4 | POC | Solid tumors | 2017 | 95 | |
| cetuximab | EGFR | Pt–PEG, ester cleavage | cysteine | 2.5–4 | POC | Solid tumors | 2017 | 95 | ||
| rituximab | CD20 | Pt–PEG, ester cleavage | cysteine | 2.5–4 | POC | N/A | 2017 | 95 | ||
| AMDCPT | SGN-CD30c | cAC10 | CD30 | maleimidopropionyl-PEG7-valine-lysine-glycine | cysteine | 8 | late preclinical | Relapse and refractory lymphoma | 2020 | 97, 98 |
| cAC10 | CD30 | val-lysine-glycine tripeptide ± PEG4 or PEG8 | cysteine | 8 | preclinical | lymphoma | 2021 | 96 | ||
| other CPT derivative | EGFR or Fra | mc-AAA-AM cleavable ± polyhydroxyl moiety | cysteine | 6.4–7.5 | preclinical | solid tumors | 2019 | 79 |
FDA-approved (Bold), clinically evaluated (Italic) and preclinically developed ADCs are described. ADC Antibody–drug conjugate, DAR drug-to-antibody ratio, FDA Food and Drug Administration
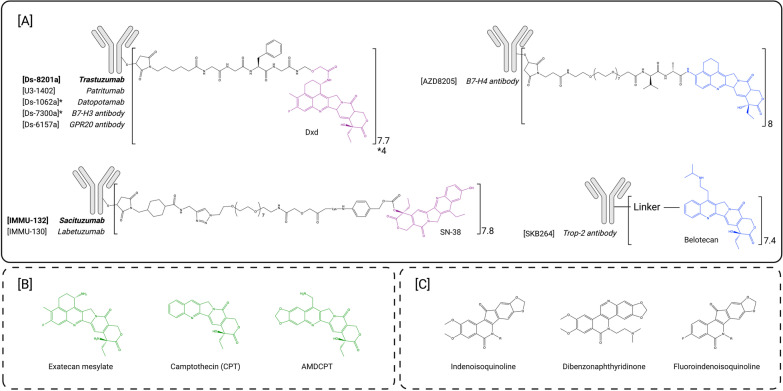
Fig. 2.
Structure of topoisomerase I inhibitors-based ADCs. A FDA-approved ADCs and payloads (purple) and ADCs and payloads under clinical evaluation (blue). B Topoisomerase 1 inhibitors used in preclinical development (green). C Next-generation topoisomerase I inhibitors as potential payloads for ADCs. Notations within the figure: [ADC name], antibody, payload
Topoisomerase enzymes are located within the cell nucleus. Their role is to control and repair DNA supercoiling and entanglements occurring during DNA opening, upstream transcription and replication. These catalytic enzymes cleave, repair supercoils and re-ligate DNA strands. Topoisomerases are divided into two families differentiated by their cleavage activity: topoisomerases I cleave single-stranded DNA while topoisomerases II cleave double-stranded DNA. Topoisomerase inhibitors specifically bind to the interface of DNA-topoisomerase complexes, thereby inhibiting the topoisomerase repair machinery and leading to DNA damage and consequently cell apoptosis [54–56]. The most potent topoisomerase inhibitors, however, are 100 to 1000-fold less potent than maytansines or calicheamicin, explaining the initial lack of interest for this payload class in initial ADC design [57].
This payload class includes camptothecin- and non-camptothecin-based compounds. Camptothecin (CPT) is a natural plant alkaloid composed of five chemical rings which is poorly water-soluble (Fig. 2B). Several derivatives that present improved bioavailability have been approved by regulatory authorities, namely topotecan, irinotecan and belotecan [58, 59]. These agents have been approved in several indications including ovarian, lung, cervical and colon cancers. A liposomal formulation of irinotecan has also been approved for the treatment of advanced pancreatic cancer. Several other CPT derivatives have been synthetized such as gimatecan which is currently in phase II evaluation for the treatment of ovarian, fallopian tube or peritoneal cancers (NCT04846842). The most significant severe adverse events (SAEs) of CPT-based molecules include severe watery diarrhea, neutropenia and thrombocytopenia [60].
CPT derivatives have recently been used as ADC payloads due to their intermediate cytotoxic potency, with IC50 values in the low nanomolar range. Their potency lies in between those of very potent (picomolar IC50s) anti-microtubule/DNA-targeting agents and those of the conventional (micromolar IC50s) chemotherapy agents that were initially used in the very first ADC programs and failed for lack of efficacy reasons (methotrexate and doxorubicin). To date, two CPT derivatives have been successfully conjugated to antibodies and approved: DXd, and the active metabolite of irinotecan, SN-38 (Table 1).
Exatecan and derivatives
DXd is a derivative of exatecan (also known as DX8951f), a compound with increased activity and improved solubility compared to CPT, and described not to be an ABCC2 or ABCG1 substrate [61]. Unconjugated exatecan was evaluated in several clinical trials but its poor therapeutic window, with dose-limiting neutropenia and thrombocytopenia and strong gastrointestinal toxicity, did not allow an improvement in survival rates [62]. In a first attempt, bioconjugation of exatecan onto antibodies led to partial success with significant aggregation of the conjugate. This issue was solved by Daiichi Sankyo scientists by using a slightly modified glycolic acid derivative of exatecan, named DXd. It was found that this new compound retained exatecan potency, while enabling the successful bioconjugation of up to 8 DXd molecules per antibody without significant aggregation. This deruxtecan drug-linker was used in several proprietary ADC programs, such as DS-8201a (Enhertu®), U3-1402 and DS-6157a, conjugated at DAR8 and DS-1062a and DS-7300a conjugated at lower DAR (4) to limit their toxicity [39], that are either approved by the FDA (DS-8201a) or currently under clinical evaluation (Table 1, Fig. 2A). Although the DXd payload presented lower passive membrane permeability than exatecan mesylate, it was found to be less myelotoxic and was therefore also selected for its improved safety profile [63].
Trastuzumab deruxtecan (DS-8201a or Enhertu®) is composed of the already approved HER2-targeting antibody trastuzumab, attached to 8 DXd payloads through a maleimide-based mc-GGFG-am protease cleavable linker (Fig. 2A). This innovative DAR8 ADC demonstrated an improved preclinical therapeutic window compared to first-generation ADCs, thanks to its optimized linker and payload [52, 61, 64–67]. Following two large phase 3 studies (DESTINY-Breast03, NCT03529110, DESTINY-Gastric01, NCT03329690), trastuzumab deruxtecan has been clinically approved by the FDA in 2019 for the treatment of unresectable or metastatic HER2 + breast cancer (BC) and in 2021 for the treatment of advanced or metastatic HER2 + gastric or gastroesophageal carcinoma and later in 2022 for the treatment of unresectable or metastatic HER2 + non-small cell lung cancer (DESTINY-Lung02) [68–70]. Importantly trastuzumab deruxtecan demonstrated strong antitumor activity in breast cancer patients who relapsed after treatment with trastuzumab emtansine and displayed more potent activity than irinotecan in patients with gastric cancer and durable anticancer activity in non-small cell lung cancer (NSCLC) and colorectal cancers [71–73]. Another breakthrough in ADC approval is illustrated by its clinical evaluation in the DESTINY-Breast04 trial that lead to its approval in 2022 for the treatment of unresectable or metastatic HER2-low breast cancer [74]. Several other clinical trials are currently ongoing, including DESTINY-breast05 and DESTINY-breast09 that, respectively, evaluate Enhertu in patients with residual disease after neo-adjuvant therapy in HER2 + BC or versus current first-line standard of care regimen in HER2 + BC, again illustrating its success. Four other ADCs that contain this promising linker-payload are currently under clinical evaluation for the treatment of solid tumors, by targeting either HER3 in NSCLC, metastatic colorectal and breast cancers, TROP2 in NSCLC and triple negative breast cancer (TNBC), B7-H3 in advanced solid tumors or GPR20 in gastrointestinal stromal tumors (GIST) (Table 1).
More recently, exatecan (Fig. 2B) has been preclinically explored as a potential ADC payload thanks to the development of hydrophilic cleavable linker architectures that are able to circumvent the hydrophobic and pro-aggregation characteristics of the compound. This allowed the conjugation of exatecan at elevated DAR values without disturbing the ADCs’ pharmacokinetic properties [36, 75, 76] (Table 1). These ADCs demonstrated strong antitumor activity in tumor xenografts and displayed a stronger bystander killing effect compared to deruxtecan-based ADCs thanks to the improved passive cell permeability of exatecan compared to DXd [36]. Two ADCs are being developed using this drug-linker strategy: PRO1184 and PRO1160 containing a hydrophilic exatecan-based linker are conjugated at DAR8 to, respectively, anti-FRa and anti-CD70 antibodies and are expected to enter clinical trials in 2023 [77, 78]. Recent in vivo studies have also demonstrated that exatecan does not require the fluorine ring function to exert its anti-tumoral activity thus widening the functionalization possibilities of the molecule to generate linkable derivatives (Table 1). The most promising ADC developed using this strategy (mAbE-21a, derivative 11, DAR7.5) demonstrated remarkable anti-tumor activity with complete remissions at 0.25 mg/kg in an EGFR+ model [79].
A novel proprietary exatecan derivative, AZ’0132, was disclosed this year and is being investigated as the payload of the ADC AZD8205 targeting B7-H4 [80] (Table 1, Fig. 2A). AZD8205 is currently undergoing phase I/phase II investigation for the treatment of breast, ovarian and endometrial cancers as well as cholangiocarcinoma (NCT05123482).
Irinotecan
Irinotecan has been approved by the FDA for the treatment of various solid tumors such as gastrointestinal malignancies, glioblastomas and cervical cancer and is a pro-drug of the topoisomerase 1 inhibitor SN-38 [59]. SN-38 is water insoluble and causes severe toxicity including strong myelosuppression and high-grade diarrhea [81, 82]. Irinotecan was therefore developed to improve bioavailability and to obtain an acceptable therapeutic index. IMMU-132 (Trodelvy®) is an anti-TROP2 antibody conjugated to a SN-38 based drug-linker (Table 1, Fig. 2A) [49]. This ADC has been approved by the FDA in 2020 for the treatment of triple negative metastatic breast cancer [83] and metastatic urothelial cancer and is currently in clinical trials for the treatment of HR+ /HER2-, prostate and endometrial cancers (NCT03725761 and NCT04251416). Other ADCs have been developed with this SN-38-based linker including IMMU-130 (labetuzumab govitecan) [84–86] and IMMU-140 [87] targeting, respectively, CEACAM5 and HLA-DR (Table 1). Labetuzumab govitecan demonstrated acceptable toxicity and activity in phase I (NCT01270698), however, the phase II evaluation has been terminated in 2020 for undeclared reasons (NCT01915472). IMMU-140 is directed against HLA-DR and has shown promising preclinical activity both in hematological malignancies and melanoma [87]. SN-38 payload is also under preclinical evaluation against various liquid tumors (Table 1). To the best of our knowledge, despite promising preclinical results, none of these ADCs have entered clinical trials and the last related publications are over seven years old [88–92]. More recently, an A7R-SN-38 ADC has been developed for the treatment of autoimmune diseases, to circumvent steroid resistance (Table 1) [93].
Belotecan derivative
Another topoisomerase 1 inhibitor, KL610023, which is a derivative of the FDA-approved molecule belotecan is being investigated as an ADC payload. This topoisomerase I inhibitor was developed to generate an anti-TROP2 ADC (SKB-264), currently in a phase I/II clinical trial (NCT04152499) in patients with various solid tumors (Table 1, Fig. 2A).
Other topoisomerase 1 inhibitors
One of the limitations of camptothecin-based derivatives as ADC payloads is the lack of a linkable chemical amine group within the molecule. Other CPT derivatives have been synthetized to insert a linkable function within the payload without altering its anti-tumor properties (Table 1) [79, 94–96]. Among these derivatives, preclinical studies of cAC10, an anti-CD30 antibody conjugated to 8 AMDCPT molecules, have shown very promising results (Fig. 2B) [97, 98]. Several non-camptothecin derivatives have recently been developed, including indenoisoquinolines [99, 100], dibenzonaphthyridinone [101, 102] and fluoroindenoisoquinolines [103] (Fig. 2C). These molecules were shown to present several advantages compared to CPT derivatives including higher cytotoxicity, improved stability or prolonged activity and are currently in early phase clinical trials as small molecules. LMP-517, conjugated to a fluoroindenoisoquinoline, is being investigated; however, to the best of our knowledge, no data have been disclosed yet [104].
Payloads that have reached clinical trials: promises and failures
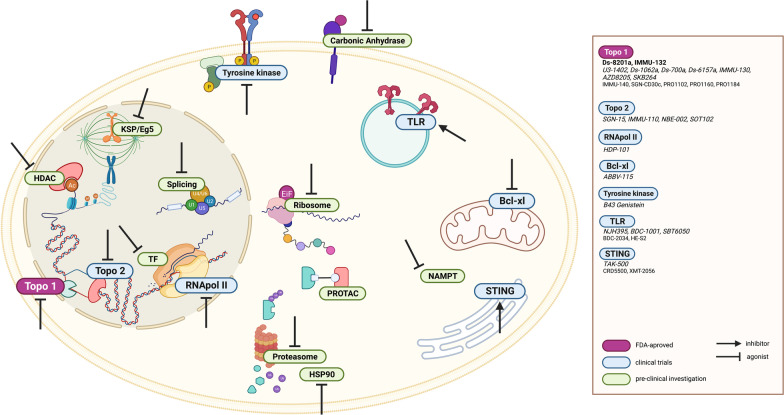
While topoisomerase 1 inhibitors have profoundly modified the ADC payload landscape, several other agents have been evaluated in clinical trials. Table 2 summarizes original payloads which have been evaluated in patients. The main categories include topoisomerase 2 inhibitors, RNA polymerase inhibitors, Bcl-xL inhibitors and immune stimulants. In addition, glucocorticoids are now emerging as ADC payloads for indications beyond oncology.
Table 2.
Clinical landscape of unconventional ADC payloads
| ADC description | Last known clinical development (starting–ending date) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Payload mechanism | Payload name | ADC name | Antibody (or vector) name | Target | Phase I | Phase I/II | Phase II | Phase III | Indication | Sponsor | NCT number |
| Oncology | |||||||||||
| Topoisomerase II inhibitors | Doxorubicin | SGN-15 (BMS-182248) | BR-96 | Lewis-Y | 1999–2005 (DISCONTINUED) | Le-Y expressing epithelial tumors, Hormone refractory prostate carcinoma, mBC, NSCLC | Seattle Genetics | NCT00086333, NCT00051571, NCT00031187, NCT00051584, NCT00028483 | |||
| IMMU-110 (hLL1-DOX) | Milatuzumab | CD74 | July 2010–July 2013 | multiple myeloma | Immunomedics (Gilead Science) | NCT01101594 | |||||
| PNU-159682 | NBE-002 | ROR1 | June 2020 | TNBC, advanced solid tumor, advanced cancer | NBE therapeutics (Boehringer Ingelheim) | NCT04441099 | |||||
| SOT102 | CLDN18.2 | April 2022 | Gastric and pancreatic cancer | SOTIO | NCT05525286 | ||||||
| RNApolII inhibitors | alpha-amanitin | HDP-101 | J22.9-ISY | BCMA (CD-269) | May 2021 | Multiple myeloma | Heidelberg Pharma | NCT04879043 | |||
| Bcl-xL inhibitors | Clezutoclax | ABBV-155 (mirzotamab clezutoclax) | mirzotamab | B7-H3 (CD276) | July 2018 | Advanced solid tumors | AbbVie | NCT03595059 | |||
| DHFR inhibitors | Methotrexate | N/A | KS1/4 | 1990 | NSCLC | 105 | |||||
| TK inhibitors | Genistein | B43 genistein | B43 | CD19 | March 2000 (DISCONTINUED) | ALL and NHL | Parker Hughes Cancer Center | NCT00004858 | |||
| Immune stimulants | TLR7/8 agonist | NJH395 | Unknown | HER2 (ErbB2) | December 2018–2021 (COMPLETED) | Non-Brest HER2 + advanced malignancies | Novartis Pharmaceuticals | NCT03696771 | |||
| BDC-1001 | Trastuzumab (biosimilar) | HER2 (ErbB2) | February 2020 | Advanced and HER2-expressing solid tumors | Bolt Biotherapeutics | NCT04278144 | |||||
| TLR8 agonist (zuvotolimod) | SBT6050 | Pertuzumab | HER2 (ErbB2) | October 2021 (DISCONTINUED) | HER2-positive tumors | Silverback Therapeutics | NCT05091528, NCT04460456 | ||||
| STING agonist (TAK-676) | TAK-500 | CCR2 antibody | CCR2 | October 2021 | advanced or metastatic solid tumors | Takeda | NCT05070247, 188 | ||||
| Beyond oncology | |||||||||||
| Glucocorticoids | Glucocorticoid receptor modulator | ABBV-3373 | adalimumab (Humira®) | TNF-a | March 2019–Aug 2020 (COMPLETED) | Rheumatoid Arthritis | AbbVie | NCT03823391 | |||
| ABBV-154 | n.d | TNF-a | May 2021 | Rheumatoid Arthritis, Severely active Crohn's disease and Polymyalgia Rheumatica | AbbVie | NCT04888585, NCT05068284, NCT04972968 | |||||
| RNApolII inhibitors | dmDNA31 | DSTA4637S (Anti-S. aureus TAC, RG-7861) | Human THIOMAB™ anti-S. aureus | Staphylococcus aureus | May 2017–Jan. 2020 (COMPLETED) | Bacteremia | Genentech, Inc | NCT03162250 | |||
ADC Antibody–drug conjugate, DAR drug-to-antibody ratio
Topoisomerase 2 inhibitors
Topoisomerase 2 inhibitors are widely used in anticancer therapy in hematological malignancies and in solid tumors. Their mechanisms of action are complex and may involve not only direct inhibition of topoisomerase 2 activity but also DNA intercalation, ROS induction and mitochondrial disruption. Their toxicity profile includes myelosuppression, gastrointestinal toxicity and in some cases high-grade cardiotoxicity.
Doxorubicin has been used as first-line therapy for several decades for the treatment of breast, bladder and thyroid cancers, as well as lymphomas and multiple myeloma. Doxorubicin was also among the very first class of payloads used in ADC development, when conventional chemotherapeutic small molecules were first conjugated. The first ADC containing a topoisomerase 2 inhibitor (SGN-15, BMS-182248) was comprised of doxorubicin conjugated to the mouse BR-96 antibody, targeting Le-Y antigen (Table 2, Fig. 3). SGN-15 was developed at the very beginning of ADC discovery along with KS1/4-methotrexate (Table 2) [105], in the 1980s, for the treatment of prostate, breast and NSCL cancers. Its phase I clinical trial demonstrated acceptable tolerability, however the phase II led to off-target toxicities due to the instability of the linker and the expression of the Le-Y target in normal tissues. The ADC therefore lacked efficacy at the tolerated dose [106, 107]. Disappointing outcomes observed with the conjugation of already approved chemotherapies forged the consensus that an ADC payload should be much more potent than conventional chemotherapeutic agents. This led to the development of second-generation ADCs, conjugated to far more potent payloads, such as microtubule inhibitors and DNA-damaging agents [2].
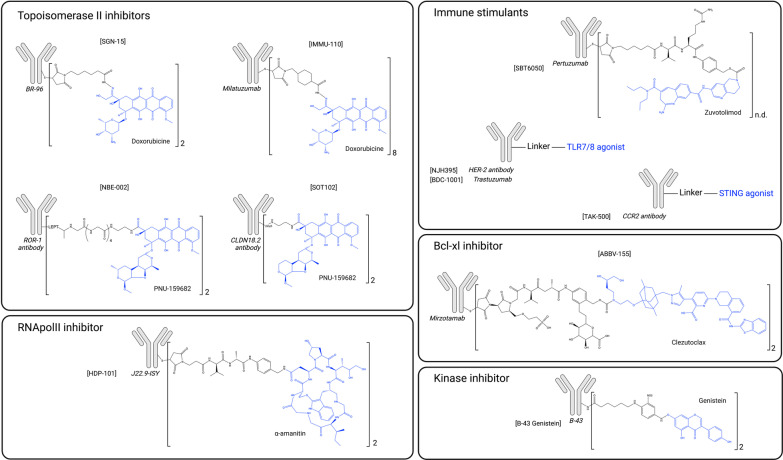
Fig. 3.
Structure of antibody–drug conjugates that have reach clinical trials and their payloads (blue) classified regarding their mechanism of action. Notations within the figure: [ADC name], antibody, payload
In spite of these early unsatisfactory developments, doxorubicin was later conjugated to the CD74-targeting antibody milatuzumab (IMMU-110) for the treatment of multiple myeloma (Table 2, Fig. 3) [108]. This ADC was brought to clinical trials but demonstrated disappointing efficacy and its development was discontinued in 2013 (NCT01101594). Additionally, doxorubicin was used as an ADC payload in a preclinical linker proof of concept study (Table 3), having been conjugated either to a new cleavable linker (NEBI) [109] or a non-cleavable linker (SMAC) [110]. Results of the SMAC study showed that a non-cleavable linker may be too stringent for the development of a doxorubicin-based ADC, since no cytotoxicity was observed.
Table 3.
Landscape of unconventional ADC payloads investigated at the preclinical stage
| Payload mechanism | Payload family | Payload name {ADC name} | Target antigen | DAR | First publication date | Reference(s) |
|---|---|---|---|---|---|---|
| Topoisomerase II inhibitors | Anthracyclines | Doxorubicin | N/A | N/A | 2010 | 109 |
| HER2 | 4 | 2017 | 110 | |||
| CD30 | 4 | 2017 | 110 | |||
| PNU-159682 | LGR5 | 2 | 2015 | 111 | ||
| CD22 | 2 | 2015 | 114 | |||
| CD30 | 4 | 2017 | 109 | |||
| tenascin-C | 2 | 2017 | 116 | |||
| HER2 | 4 | 2019 | 115 | |||
| CD46 | 2 | 2020 | 117 | |||
| EREG | n.d | 2022 | 118 | |||
| PNU-159682 + MMAF | HER2 | 2 + 2 | 2019 | 119 | ||
| Daunorubicin | alpha-fetoprotein | 1.2 | 1984 | 120 | ||
| ganglioside | 20 | 1991 | 121 | |||
| Idarubicin | Ly2.1 | 2 to 4 | 1988 | 122 | ||
| CD19 | 3.2 | 1993 | 123 | |||
| HER2 | 1 | 2019 | 124 | |||
| Transcription inhibitors | RNApolII inhibitors | Beta-amanitin | Albumin | 1.9 | 1973 | 130 |
| MUC1 | n. d | 2006 | 131 | |||
| PSMA | 2 | 2014 | 132, 133 | |||
| Alpha-amanitin and derivatives | Thy 1.2 | 3.6–6.3 | 1981 | 134 | ||
| EpCam | 4 to 8 | 2012 | 137 | |||
| HER2 | 2 | 2018 | 138 | |||
| PSMA | 2 | 2018 | 138 | |||
| CD19 | 2 | 2018 | 138 | |||
| Amanitin + MMAE | FGFR1 | 1 + 1 | 2018 | 139 | ||
| Phalloidin | Albumin | n. d | 1986 | 140 | ||
| Trichothecene T-2 | murine EL-4 lymphoma | n.d | 1991 | 141 | ||
| Verrucarin A | oncofetal glycoprotein | n.d | 1991 | 141 | ||
| Roridin A | oncofetal glycoprotein | n.d | 1991 | 141 | ||
| Transcription Factor inhibitors | Triptolide | CD26 | 6.5 | 2019 | 145 | |
| EGFR | 5.5 | 2020 | 146 | |||
| HER2 | 2 to 3 | 2021 | 147 | |||
| HDAC inhibitors | ST7464AA1 | EGFR | 4.5 | 2018 | 150 | |
| HER2 | 5 | 2020 | 151 | |||
| Vorinostat (SAHA) | EGFR, HER2 | 4 to 6 | 2021 | 152 | ||
| Dacinostat (VP-LAQ824) | EGFR, HER2 | 3 | 2021 | 152 | ||
| Kinase inhibitors | PTK inhibitors | Genistein | EGFR | n.d | 1998 | 168 |
| 17.1A mAb | 3 | 2003 | 169 | |||
| PIKK inhibitors | Neolymphostin | HER2 | 2 | 2019 | 170 | |
| multi-kinase inhibitors | Dasatinib | CXCR4 | 3 | 2015 | 171 | |
| Staurosporine | EGFR | n.d | 2018 | 172 | ||
| Immune stimulants | TLR7/8 agonists | UC-1V150 | CD20 | 1 to 3 | 2015 | 184 |
| CL264 | HER2 | 2 | 2020 | 185 | ||
| D18 | PDL1 | 2 | 2021 | 186 | ||
| n.d | PDL1 | 2 | 2022 | 181 | ||
| n.d {BDC-2034} | CEACAM5 | 2.5 | 2021 | 179, 180 | ||
| T785 | HER2 | 2 | 2020 | 185 | ||
| TLR7 agonists | AmberX ADC | HER2 | n.d | 2022 | 187 | |
| STING agonists | {CRD5500} | HER2 | n.d | 2019 | 189 | |
| XMT-1621 | FcyR | 2 | 2022 | 192 | ||
| XMT-1621 {XMT-2056} | HER2 | 8 | 2021 | 190, 191 | ||
| Protein synthesis inhibitors | HSP90 inhibitors | Geldanamycin | HER2 | n.d | 2000 | 195, 196, 197 |
| CD70 | 4 | 2009 | 198 | |||
| HER2 | n.d | 2021 | 199 | |||
| Splicing inhibitors | Thailanstatin A | HER2 | 2 to 3 | 2016 | 25, 202 | |
| Translation inhibitors | Psymberin (irciniastatin A) | CD30, CD70 | 5.4 | 2010 | 204 | |
| Proteasome inhibitors | carmaphycin B analogues | HER2 | 1 to 2 | 2019 | 205 | |
| PROTACs/glue degraders | BET/BRD4 degraders | GNE-987 | CCL1/HER2 | 6 | 2019 | 209 |
| MZ1 analogue | HER2 | 4 | 2020 | 212 | ||
| STEAP1 | 2 or 6 | 2021 | 210, 211 | |||
| BRD4/VHL | STEAP1/CCL1 | 6 | 2021 | 213 | ||
| BRD4/CRBN | HER2 | 2 | 2019 | 213 | ||
| ERa degraders | ERa/XIAP | HER2/CD22/B7-H4 | 2 to 6 | 2020 | 214 | |
| ERa/VHL | HER2/CD22/B7-H4 | 2 to 6 | 2020 | 214 | ||
| TGFbR2 degraders | TGFbR2/VHL | HER2 | 2 to 4 | 2020 | 208 | |
| BRM degraders | BRL/VHL | CD22 | 6 | 2020 | 208 | |
| GSTP1 degraders | Smol006 {ORM-5029} | HER2 | 4 | 2022 | 215 | |
| Others | NAMPT inhibitors | FK-866 analogues | c-kit or HER2 | 2 to 4 | 2018 | 217 |
| CD30 | 8 to 10 | 2018 | 216 | |||
| KSP inhibitors | Filanesib derivative | HER2, c-kit | 3 to 4.5 | 2019 | 218 | |
| HER‐2, TWEAKR/Fn14 | 2 to 4 | 2018 | 219, 202 | |||
| carbonic anhydrase inhibitors | CA IX and XII peptides | CA IX and XII | n.d | 2022 | 230 | |
| Beyond cytotoxic payloads | lipid homeostasis and inflammation | LXR agonist | CD11a | 2 | 2016 | 235 |
| Enzymatic activity inhibition | CGS27023A | MMP9 | 4 to 8 | 2019 | 229 |
ADC Antibody–drug conjugate, DAR drug-to-antibody ratio
Given the lack of efficacy of doxorubicin as an ADC payload, another anthracycline, PNU-159682, which is 100-fold more cytotoxic than doxorubicin, was later explored. Besides being much more potent than other topoisomerase 2 inhibitors, PNU-159682 is not an efflux pump substrate. Payloads that are substrates for efflux pumps have been found to be limiting factors in ADC development [111]. In 2020, a novel PNU-159682-based ADC, NBE-002 (Table 2, Fig. 3), which targets ROR1, entered a phase I/II clinical trial (NCT04441099). Interestingly, NBE-002 induced long-term immune protection, which suggests that it could successfully be combined with immune checkpoint inhibitors (ICIs) [112]. SOT102 (formerly SO-N102) is another promising PNU-159682-based ADC that targets CLDN18.2 (Table 2, Fig. 3). SOT102 demonstrates a large therapeutic window in low-expressing tumors and has entered phase I clinical trials in April 2022 (EudraCT Number 2021–005,873-25) [113]. Many preclinical uses of PNU-159682 were also reported and results demonstrated its ability to by-pass mechanisms of resistance of usual payloads such as MMAE or DM1 (Table 3) [110, 111, 114–118]. The PNU-159682 payload was also conjugated alongside with MMAE to form a dual drug ADC (Table 3). However, while both mechanisms of action were simultaneously observed in vitro, no synergy was observed [119].
Daunorubicin and idarubicin conjugates were also developed at the preclinical stage in the 1990s (Table 3, Fig. 4) but displayed reduced efficacy [120–123]. An anti-HER2 affibody-idarubicin conjugate has more recently been evaluated in vitro with specificity to HER2-positive head and neck squamous cell carcinoma (HNSCC) cells rather than to HER2-positive BC cells (Table 3) [124].
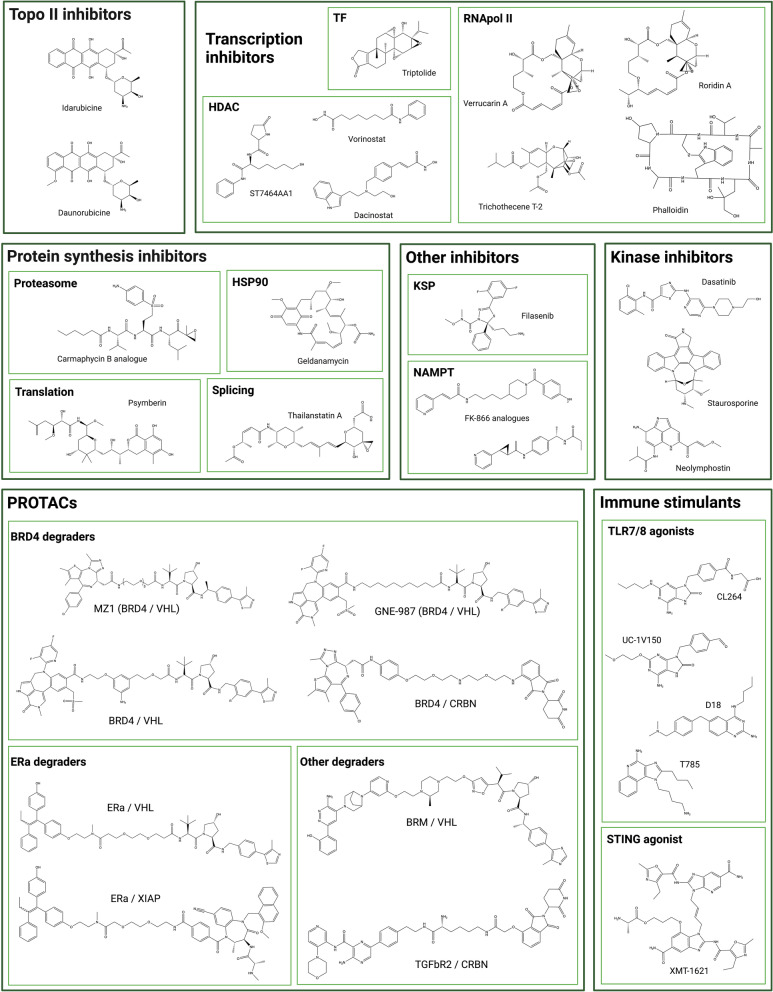
Fig. 4.
Chemical structure of unconventional ADC payloads conjugated at the preclinical stage
Transcription inhibitors
Transcription has a fundamental role in cell development, activity and proliferation and could therefore constitute an innovative and original target for an ADC payload. Transcription is regulated by RNA polymerase II (RNApolII) that directly binds to DNA and involves transcription factors that form complexes with RNApolII to initiate transcription (such as TFIIH) and co-regulators (such as histone deacetylases, HDAC) that mediate chromatin structure and accessibility. While some HDAC inhibitors have gained approval, there are currently no approved RNApolII inhibitors due to poor tolerability [125].
Amatoxins are natural and highly potent RNApolII inhibitors derived from the Amanita mushroom [126]. Alpha-amanitin and beta-amanitin together with seven other macrocyclic derivatives constitute the amatoxin family. Despite their extensive use as laboratory reagents to explore transcription mechanisms, alpha-amanitin proved to be far too toxic, particularly to the liver, to be further developed as an anticancer agent [127, 128]. However, this molecule presents numerous advantages as a potential ADC payload, including its original intracellular target, its favorable physicochemical properties (including hydrophilicity), its insensitivity to efflux pumps, and its ability to generate cytotoxicity in quiescent cancer cells [129]. By contrast amanitin’s hydrophilicity is expected to prevent neighbor cell killing via the bystander effect, which may restrict its use to homogeneously distributed targets. And even in those cases, complete lack of bystander effect may lead to a lack of efficacy as target distribution vary from a patient to another.
The amanitin derivative beta-amanitin was first conjugated in 1973 to albumin, and this ADC precursor demonstrated selective killing of macrophages (Table 3) [130]. This derivative was later conjugated to anti-MUC1 and anti-PSMA antibodies and demonstrated strong selective cytotoxicity in preclinical models (Table 3) [131–133]. Its analogue alpha-amanitin and its derivative azo-amanitin were also very early used as ADC payloads (Table 3) [134]. The azo-amanitin-ADC demonstrated approximately 500-fold higher cytotoxicity than the unconjugated molecule. This is explained by the hydrophilicity of the molecule that reduces cell membrane permeability, while it is efficiently internalized as an ADC construct. As of May 2021, the first amanitin-antibody conjugate (ATAC®) candidate, HDP-101, has entered an early phase clinical trial (Table 2, Fig. 3). HDP-101 is a BCMA-targeting ADC currently being evaluated in patients with multiple myeloma and plasma cell disorders (NCT04879043) [135]. ATACs were recently characterized as immune activating drugs. They were found to induce immunogenic cell death (ICD) and to exhibit synergy with ICI which opens new horizons for combination possibilities in the clinical setting [136]. Of note, a number of alpha-amanitin ADCs directed against other targets (EpCam, HER2, PSMA, CD19) have displayed potent anti-tumor activity both in vitro and in vivo (Table 3) [137, 138]. Alpha-amanitin has also been conjugated as a dual warhead alongside with MMAE (Table 3) [139]. This DAR 1 + 1 ADC targets FGFR1 and has demonstrated a potent in vitro cytotoxicity. Other highly potent RNApolII inhibitors were conjugated in the 1990s, such as phalloidin, and the mycotoxins trichothecene, verrucarin A and roridin A (Table 3, Fig. 4) [140, 141]. Considering how much ADC design has progressed since the 90s, and the nanomolar cytotoxicity of these compounds in various cell lines, these molecules may be the object of further exploration in the coming years [142].
Another strategy to stop DNA transcription is to inhibit transcription factors (TFs). TFs are essential to RNApolII attachment to the DNA at the initiation step [143]. TF inhibitors (TFi) have already demonstrated their anti-tumor activity in clinical trials with the water-soluble pro-drug minnelide, currently in phase II evaluation (NCT04896073). Triptolide, a natural compound derived from the Chinese medicinal herb called "thunder god vine", is highly cytotoxic but is also hydrophobic, presents poor bioavailability and high toxicity (Fig. 4). Efforts are therefore being made to develop analogues with better pharmacochemical properties [144]. Another strategy would be to conjugate this molecule to a targeting entity, thus by-passing these issues. Triptolide was recently conjugated for the first time to an anti-CD26 antibody to target mesotheliomas and lymphomas (Table 3) [145]. This non-cleavable ADC efficiently stopped mRNA synthesis in targeted cells and presented promising in vitro and in vivo antitumor activity. A cetuximab–triptolide ADC was also developed for the treatment of EGFR-positive lung cancers (Table 3) [146]. This ADC presented selectivity toward EGFR-overexpressing models and lower toxicity than unconjugated triptolide. Cetuximab–triptolide efficiently induced transcription inhibition, with potent in vitro and in vivo anti-tumor activity. A HER2-targeting triptolide ADC was also evaluated with similar results [147]. However, for each triptolide-based ADC, high doses were required to observe anti-tumor activity in xenograft models and no maximum tolerated dose was reported in these papers, thus questioning the width of the therapeutic index.
HDACs (histone deacetylases) impact on transcription factors and are therefore involved in various cellular processes including transcription. They have been found to be overexpressed or overactivated in cancer cells and are thought to be involved in increased proliferation, migration and invasion [148, 149]. Vorinostat and dacinostat are two examples of FDA-approved HDAC inhibitors (HDACi). These molecules however present strong risks of systemic side effects such as thrombocytopenia and gastrointestinal toxicity, and a poor PK profile. They have been studied in ADC design since 2018: ST74612AA1 is the first bioconjugated HDAC inhibitor (Table 3, Fig. 4). This relatively non-toxic molecule is a second-generation pan-HDACi. This molecule was conjugated to cetuximab and trastuzumab, and both ADCs presented a safer profile than unconjugated HDACi, while being active in cell-line-derived xenograft (CDX) and patient-derived xenograft (PDX) models [150, 151]. However, as was observed with TFi-based ADCs, xenograft models were treated with the high dosing of 30 mg/kg. In 2020, vorinostat and dacinostat were also conjugated to cetuximab and trastuzumab with interesting anti-proliferative activity in vitro (Table 3, Fig. 4) [152].
Bcl-xL inhibitors
Bcl-2 family members can either be pro (Bad, Bim, PUMA, Bik, Bak, Bax, etc.)- or anti-apoptotic proteins (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, etc.). In cancer cells, the equilibrium between these proteins is generally tilted toward survival, making anti-apoptotic proteins interesting and original targets for an innovative ADC payload [153].
Bcl-xL and Bcl-2 inhibitors are classified according to their chemical function scaffold into 4 major families: N-acylsulfonamides (navitoclax, venetoclax), indoles (obatoclax), gossypol acetic acid (AT-101, sabutoclax) and benzothiazole hydrazones (such as WEHI-539) [154–159]. Inhibition of Bcl-xL has been associated with profound thrombocytopenia, justifying the search for highly specific Bcl-2 inhibitors such as venetoclax [160]. Currently venetoclax is approved in a subgroup of patients with chronic lymphocytic leukemia and in acute myeloid leukemia [161].
ABBV-155 (mirzotamab clezutoclax) is an anti-B7-H3 antibody conjugated to the Bcl-xL inhibitor clezutoclax (Table 2, Fig. 3). This innovative ADC entered an ongoing phase I/II clinical trial in 2018 for the treatment of advanced solid tumors as a single agent and in combination with paclitaxel in patients with advanced non-small cell lung cancer and breast cancer (NCT03595059). No dose-limiting toxicities were reported in the first 31 patients included in the single agent phase 1 cohort, with SAEs consisting of anemia, decreased lymphocyte count, fatigue and diarrhea. Partial responses were observed in the paclitaxel combination arm in 21% of patients.
Tyrosine kinase inhibitors
The human kinome comprises over 500 kinases, among which more than 150 are associated with various diseases including cancers. Protein kinases are enzymes that catalyze phosphorylation and are divided into 3 categories: serine, threonine or tyrosine kinases. Over a quarter of small molecules currently being investigated in clinical trials are protein kinase inhibitors and more than 30 FDA-approved molecules for cancer treatment are kinase inhibitors. In cancer a variety of kinase families are involved in cell cycle progression, cell proliferation, motility and angiogenesis. Since the approval of the first kinase inhibitor imatinib in 2001, kinase inhibitors have been classified into 5 categories: Types I and II are ATP competitive, respectively targeting the active or inactive form of the kinase; type III binds to an allosteric pocket of ATP; type IV to an allosteric pocket of the kinase, and type V combines multiple binding modes [162].
While being largely explored for cancer treatment, protein kinase inhibitors have not been extensively explored as ADC payloads presumably because of their low potency. The anti-CD19 antibody B43 has been conjugated to genistein, an isoflavone phytoestrogen contained in soybean, which was found to induce apoptosis and cell proliferation inhibition via the inhibition of epidermal growth factor receptor (EGFR), a tyrosine kinase receptor (Table 2, Fig. 3) [163]. Preclinical studies in vitro and in vivo (mouse, rat, non-human primates: NHP) demonstrated no toxicity at cumulative doses of 100 mg/kg and stronger anti-tumor effects than standard chemotherapies in murine models [164, 165]. These promising results led to its first-in-human study in 1999 for the treatment of ALL and NHL. Apart from presenting a favorable pharmacokinetic profile in humans, no toxicity and a promising anti-tumor activity were reported [166, 167]. Unfortunately, the status of this compound has not been further reported (NCT00004858). Two additional studies investigated the antitumor activity of genistein conjugated to either anti-EGFR or 17.1A mAb, which targets an epithelial membrane antigen (Table 3). The anti-EGFR-genistein ADC demonstrated good tolerability up to 140 mg/kg, and significant anti-tumor activity at 1 mg/kg in preclinical models. 17.1A-genistein was found to be more active than unconjugated genistein in colon cancer models [168, 169].
More recently three other kinase inhibitors have been evaluated as ADC payloads. These molecules include neolymphostin (a PIKK inhibitor), and dasatinib and staurosporine, two multi-kinase inhibitors (Table 3, Fig. 4). Trastuzumab neolymphostin demonstrated selectivity and in vitro cytotoxicity despite being less potent than other usual trastuzumab-based ADCs [170]. An anti CXCR4 mAb coupled to dasatinib selectively delivered dasatinib to targeted T-cells and presented a strong immunosuppressive effect [171]. Lastly, the widely used laboratory reagent and multi-kinase inhibitor staurosporine was conjugated to cetuximab for the treatment of KRAS/BRAS mutated colon cancer cells [172]. Overall, the efficacy of tyrosine kinase inhibitors in ADC format is found to be limited and this family may not succeed in more advanced settings.
Immune-stimulating antibody conjugates
Immune-stimulating antibody conjugates represent a new category of antibody–drug conjugates, with 2 ADCs currently in clinical trials (Table 2, Fig. 3) (NJH395, BDC-1001), and one, SBT6050, whose clinical evaluation has been terminated due to the sponsor’s strategic decision (NCT05091528). STING agonists and TLR agonist constitute the two main categories of conjugated immune stimulants.
The success of immune checkpoint inhibitors which target the adaptive immune system has greatly enhanced efforts to harness the stimulation of the innate immune system. However the systemic administration of the most potent agents such as STING and TLR agonists is associated with severe systemic toxicity, caused by a cytokine release syndrome, thereby restricting current studies to intra-tumor injections [173, 174]. Their conjugation to proteins or mAbs thus appears to be a promising means to exploit their strong antitumor potential while improving the tolerance profile.
Several immune-stimulating ADCs containing TLR agonists are currently being evaluated in the clinical setting. NJH395, which combines a small molecule TLR7/8 agonist with an anti-HER2 mAb is the first to have reached clinical evaluation (Table 2, Fig. 3). A phase I clinical trial in 18 patients with non-breast HER2 + malignancies (NCT03696771) showed severe toxicity including cytokine release syndrome, and lymphocyte depletion, in the absence of significant antitumor activity [175]. Similarly, BDC-1001, an immune-stimulating conjugate comprising an anti-HER2 antibody conjugated to a TLR7/8 agonist is currently under phase I/II evaluation for the treatment of patients with solid HER2 + tumors as a single agent or in combination with nivolumab (NCT04278144, Table 2, Fig. 3). Its preclinical evaluation demonstrated potent and durable immune-mediated antitumor efficacy and the clinical evaluation shows promising outcomes including no toxicity at the tested dose and evidence of clinical activity [176–178]. The analogue BDC-2034, targeting CEACAM5, has shown anti-tumor activity in vivo at low dose (0.5 mg/kg), activation of the innate immune system and reprogramming of intra-tumor myeloid, thus supporting its clinical development (Table 3) [179, 180]. The above-mentioned TLR7/8 analogue was also conjugated to an anti-PD-L1 antibody, aiming to combine immune checkpoint inhibition, antibody-dependent cellular phagocytosis (ADCP) and intra-tumor myeloid reprogramming [181]. This immune-stimulating ADC outperformed anti-PD-L1 anti-tumoral activity in preclinical models. SBT6050 is a pertuzumab-TLR8 agonist conjugate which is currently being evaluated as a single agent as well as in combination with anti PD1 inhibitors (NCT04460456) and with trastuzumab deruxtecan for the treatment of HER2-positive solid cancers (NCT05091528). Pertuzumab does not bind to the same HER2 epitope as trastuzumab and studies have demonstrated a synergistic potential between trastuzumab and SBT6050 [182, 183].
Promising preclinical results have been reported for other immune-stimulating ADCs, conjugated to either UC-1V150, CL264 or T785 TLR 7/8 agonists (Table 3, Fig. 4) [184, 185]. An anti-PD-L1 conjugated to the TLR7/8 agonist D18 has very recently been disclosed with promising preliminary results, including a potent anti-tumor activity in the B16 melanoma model which is a PD1-resistant model (Table 3, Fig. 4) [186]. Recently, a more selective agonist, i.e., TLR7 agonist has been investigated as immune-stimulating ADC payload [187]. The other emerging family of immune stimulant payloads are STING agonists. TAK-500 constitute the first STING agonist immune activating ADC to enter clinical trials and is currently recruiting patients (NCT05070247) [188]. This CCR2 directed ADC (TAK-676) is being evaluated for the treatment of solid tumors (Table 2, Fig. 3). In addition, three STING-conjugated ADCs are being developed at the preclinical stage: CDR-550, XMT-2056 and more recently a FcγR-targeting immune-stimulating ADC conjugated to the STING agonist XMT-1621 [189–192] (Table 3). The most advanced, XMT-2056 (STING agonist: XMT-1621, Fig. 4) leds to complete remissions of tumors at 1 mg/kg in mouse xenografts and demonstrated a synergistic activity with ICI while being tolerated in NHP with no clinical signs nor adverse histopathological findings. This promising ADC should enter clinical trials for a first-in-human study in 2022.
Unconventional payloads at the preclinical stage
Since tumor cells have increased anabolic activity, several payload candidates have targeted various steps of protein synthesis, including transcription, splicing and translation inhibitors as well as protein catabolism. Another approach would be to target other ubiquitous cellular processes which are excessively active in neoplastic cells. However, only carefully designed ADCs may support this category of payload since even though the concentration of ADC is higher in tumors than in surrounding tissues, most of the compound administered intravenously does not localize to the tumor [193].
HSP90 inhibitors
HSP90 (heat-shock protein 90) is a major chaperone protein which has been shown to be abnormally expressed in a variety of tumors. Several HSP90 inhibitors, derived from the geldanamycin (GA, Fig. 4) backbone, have been developed and tested in clinical settings. Upon binding to HSP90, inhibitors prevent its ability to protect its client proteins from proteasomal degradation [194]. Major limitations identified to date are significant dose-limiting toxicities and poor pharmacokinetic profiles. In the early 2000s, efforts have been made to chemically modify GA to synthesize a maleimide cleavable drug-linker suitable for bioconjugation (Table 3) [195–197]. The resulting trastuzumab-GA ADC demonstrated an increase of overall tumor-bearing mice survival compared to mice treated with trastuzumab. Streptonigrin and 17-amino-geldanamycin were used to generate anti-CD70 and anti-CD30 cleavable ADCs at a DAR4 and were found to be active in preclinical models (Table 3) [198]. A recent resurgence of GA in the payload landscape repositioned this molecule, by generating a HER2 scFv HBD/GA ADC that demonstrated anti-tumoral activity in a HER2-positive lung preclinical model (Table 3) [199].
Splicing inhibitors
After transcription, pre-mRNA undergoes processing into mature mRNA by the removal of introns, achieved by the spliceosome. snRNPs (small nuclear ribonucleoproteins) U1, 2 4, 5 and 6 constitute the major snRNPs of the spliceosome. These complexes are essential to the generation of mature mRNA and are commonly deregulated in cancer cells. Targeting the SF3B1 subunit of U2 has been shown to efficiently inhibit splicing [200]. Several agents have been shown to be potent splicing inhibitors, including pladienolides, spliceostatins and thailanstatins. However, these highly cytotoxic molecules with IC50s in the nanomolar range were not further developed due to chemical instability. E7107, a pladienolide analogue, was evaluated in clinical trials (NCT00499499), but discontinued due to safety concerns, in particular severe ocular toxicity [201]. Thailanstatin A-trastuzumab conjugates were shown to be highly active in preclinical models, with greater potency than T-DM1 in certain in vivo models (Table 3, Fig. 4) [25, 202].
Translation inhibitors
The development of tolerable translation inhibitors has proven to be challenging given the universal importance of translation in healthy tissues. Omacetaxine (previously designated homoharringtonine) is the first FDA-approved translation inhibitor and interferes with the initial elongation step of protein synthesis [203]. Several other translation inhibitors have been developed for the treatment of various cancers, targeting ribosomes, EIFs (eukaryotes translation initiation factors) or mTOR. To date only psymberin has been used as a potential ADC payload (Table 3, Fig. 4). Psymberin, also known as irciniastatin A, is a natural carbohydrate isolated from a marine sponge. Its conjugation to anti-CD30 and anti-CD70 antibodies through a beta-glucuronide linker demonstrated selectivity and anti-proliferative activity in vitro with IC50s in the sub-nanomolar range [204].
Proteasome inhibitors
Proteasome inhibitors are an extremely potent class of anticancer agents. Bortezomib was approved in 2003 for the treatment of patients with multiple myeloma and has since then significantly improved the outcome of patients. Several other inhibitors have been developed, with reduced neurotoxic effects and/or allowing oral administration. Epoxyketone derivatives such as carmaphycin B analogues which strongly inhibit the 20S proteasome, have been conjugated to trastuzumab (Table 3, Fig. 4) [205]. Despite satisfactory in vitro cytotoxicity of the unconjugated payload, the corresponding ADC proved to be less potent than the corresponding MMAE-based ADC.
PROTACS
Proteolysis Targeting Chimeric Molecules (PROTACs) are bifunctional molecules that bring together the E3 ligase with the target protein thus allowing its ubiquitination and degradation by the proteasome [206, 207]. Instead of directly inhibiting its target protein, PROTACs trigger its degradation with several potential clinical advantages such as prolonged effect, catalytic activity and therefore very potent cytotoxicity. Degrader-antibody conjugates (DACs) constitute an exciting emerging family in the ADC landscape. In DAC design, PROTACs can benefit from being transported by the mAb inside the cell to overcome their limited cell permeability. Current DACs constructions, biological activities and challenges have been reported in a recent review [208]. The BRD4/BET degraders, GNE-987, was conjugated to an anti-CLL1 antibody leading to a restored pharmacokinetic profile and potent in vivo activity in mice xenografts (Table 3, Fig. 4) [209]. MZI analogues conjugated to trastuzumab or an anti-STEAP1 antibody were also evaluated in vitro, demonstrating selective BRD4 degradation and cell cytotoxicity (Table 3, Fig. 4) [210–212]. Other BRD4 degrader-antibody conjugates, either comprising VHL or CRBN ligands have also recently been generated [213] (Table 3, Fig. 4). Similarly, Estrogen Receptor (ER), TGFbR2 and BRM degraders, are being investigated as DAC payloads by being conjugated to anti-HER2, anti-B7-H4 and/or anti-CD22 antibodies (Table 3, Fig. 4) [208, 214]. ORM-5029, the latest disclosed DAC or Antibody neoDegrader Conjugate (AnDC™), aims to deliver a GSPT1 degrader (Smol006) to HER2-expressing cells via pertuzumab. This AnDC™ has demonstrated stronger cytotoxicity than other GSPT1 degraders and anti-tumor activity comparable to that of DS-8201a [215]. The toxicity of ORM-5029 is currently under investigation and results will constitute the first report regarding the therapeutic window of DACs.
Other molecules
Efforts in payload diversification have led to the recent preclinical development of unconventional antibody–drug conjugates delivering payloads with unique mechanism of action. Alteration of cellular metabolism by targeting nicotinamide phosphoribosyltransferase inhibitor (NAMPTs) constitutes a novel and original ADC technology. FK-866 analogues were conjugated to an anti-CD30 antibody and the subsequent ADC selectively depleted NAD in vitro and in vivo [216] (Table3, Fig. 4). CD30-NAMPTi demonstrated promising in vivo anti-tumor activity in xenografts with complete remissions at 3 mg/kg in the L540cy model. A favorable therapeutic index was outlined with a MTD greater than 100 mg/kg in rat. Other NAMPTis were also synthesized and conjugated to a c-Kit targeting mAb [217]. Despite selective and potent cytotoxicity in vitro (sub-nanomolar IC50s), these non-cleavable ADCs were moderately active in vivo with only partial responses at 20 mg/kg.
KSP (kinesin spindle protein) inhibitors, also named Eg5 inhibitors, constitute an emerging family of ADC payloads. Eg5 is a promising target for antitumor therapy since its expression is specific to proliferating cells and it is not expressed in cells of the nervous system. These payloads should therefore not present the neurological side effects classically associated with microtubule targeting agents. KSP inhibition prevents centrosome separation during cell division thus leading to mitotic arrest [218]. KSP inhibitor derivatives, with sub-nanomolar potency, were conjugated to HER2 and TWEAKR/Fn14 targeting antibodies (Table 3, Fig. 4) [219, 220]. The TWEAKR-KSPi ADC allowed a complete remission in a urothelial PDX while ispinesib, a small molecule KSP inhibitor, only delayed tumor growth in this model. ADCs have also been produced by conjugation of filanesib with an acceptable PK profile and good in vivo potency [221].
Conclusions
Antibody–drug conjugates have become an important component in the treatment of a growing number of cancer indications, and several hundred clinical trials are ongoing to explore novel targets and indications. The spectacular progress achieved in the ADC field is mainly supported by the tailoring of their design to a particular target. This has been rendered feasible thanks to several achievements including (1) the exploration and validation of a growing number of targets [222], (2) proper screening of mAbs specifically for ADC design, with a focus on cross-reactivities, preferential tumor binding helped by pH variations, decreased affinity to low nanomolar range to avoid stickiness and facilitate internalization and FcRn recycling, (3) improvements in conjugation technology enabling a higher drug-to-antibody ratio, and/or the restoration of the naked mAb-like pharmacokinetic profile [223] and (4) diversification of payloads (pictured in Fig. 5), as recently exemplified by the breakthroughs achieved with topoisomerase 1 inhibitors.
Fig. 5.
Schematic representation of the ADC payload’s target landscape beyond microtubules and DNA-intercalating agents. Notations: FDA-approved ADCs, ADCs in clinical trials
Among future developments, the continued diversification of payloads with original mechanisms of action is expected to play a key role. In advanced disease, cure is most generally achieved by combining agents with complementary mechanisms of action and, whenever possible, non-redundant toxicities. While the currently approved ADCs possess mechanisms of action which are similar to those of conventional chemotherapeutic agents, it is possible that future payloads will target vital cellular phenomena which have until now been intractable due to excessive toxicity. As previously observed with auristatins and maytansinoids conjugated with cleavable linkers, the bystander effect of topo-1 ADCs has demonstrated its effectiveness for the treatment of low or heterogeneous tumors and should be of growing interest in later development. Another major advantage of these novel payloads may rely on their ability to target quiescent tumor cells, which constitute the bulk of the tumor reservoir in patients. In addition, among the emerging payload families, several kinase inhibitors currently associated with severe side effects would benefit from a larger therapeutic index. For their part, PROTACs benefit from a substoichiometric activity that would in theory reduce the payload threshold for cytotoxicity.
Payload diversification also promises an opening of the ADC therapeutic arsenal to other cancers that do not yet benefit from targeted therapy. Sacituzumab govitecan led to the validation of TROP2 as a target in TNBC, and SN-38-based ADCs in clinical evaluation highlight the potential of new targets in cancer types that were poorly represented in the ADC landscape (HER3, CEACAM5, B7-H3 and GPR20). Interestingly, clinical trials against TNBC exhibit a growing number of antibody–drug conjugates comprising original payloads, including Dxd, PNU-159682 and SN-38 [224].
As we have aimed to describe in this review, several potential payloads have been identified and many have shown promising preclinical results. Some of these compounds have entered clinical trials but have not been pursued because of an unsatisfactory toxicity profile. In this regard, it should be emphasized that major technological advances, in particular the possibility to safely obtain ADCs with high drug-to-antibody ratios, support the fact that many of these payloads which were explored at a time when ADC production and characterization were suboptimal should be reconsidered with currently available technologies.
New ADC formats that integrate original payloads, such as dual payloads (Table 3), theranostic [225] and non-internalizing [226] conjugates have shown great potential in recent preclinical studies and may constitute growing fields in ADC research. Non-internalizing ADCs would particularly benefit from newer payloads that present a strong bystander killing effect [227] or that are directed against extracellular or stromal targets [228], as exemplified by the PNU-159682-based ADC targeting tenascin-C, the inhibition of matrix metalloproteinase extracellular protein or more recently the inhibition of carbonic anhydrases [116, 229, 230] (Table 3). Interestingly ADC technology is also being explored in non-oncological indications [231, 232]. Two original ADCs (ABBV-3373 and ABBV-154) containing a glucocorticoid receptor modulator (GRM) are being clinically evaluated for the treatment of rheumatoid arthritis and Crohn’s disease (Table 2, NCT03823391, NCT04888585, NCT05068284 and NCT04972968). Other immunology ADC payloads are being investigated in preclinical settings and could constitute an emerging class in ADC design [233, 234]. In addition, an A-rifamycin derivative, that demonstrated promising results in preclinical evaluation [235], has been investigated in phase 1 clinical trials in patients with Staphylococcus aureus bacteremia (NCT03162250). Non-cytotoxic payloads are also entering the payload landscape with the example of intracellular targeting of lipid metabolism by conjugating a Liver X Receptor (LXR) agonist to anti-CD11b antibody for the treatment of atherosclerosis (Table 3) [236].
Despite the broadened landscape of eligible diseases, a key issue for the development of these novel payloads will be to mitigate their side effects. Currently approved ADCs have shown that they are associated with expected (myelosuppression, neurotoxicity) or unexpected (such as ocular [237, 238] or pulmonary [68, 239]) toxicities. Obtaining a satisfactory therapeutic index will thus be an essential property for the future development of innovative ADC payloads.
Acknowledgements
This work has been supported in part by a CIFRE grant from the French Ministry of Education, Research and Innovation.
Author contributions
LC designed and wrote the manuscript. CD designed and reviewed the manuscript. LS and WV reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Agence Nationale de la Recherche (ANR) and Mablink Bioscience.
Availability of data and materials
None. This manuscript is a review.
Declarations
Ethics approval and consent to participate
None. This manuscript is a review.
Consent for publication
None. This manuscript is a review.
Competing interests
LC, LS and WV are employees of Mablink Bioscience. CD and WV are shareholders of Mablink Bioscience.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 2.Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 3.Bross PF, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 4.Petersdorf SH, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327–344. doi: 10.1038/s41571-021-00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.do Pazo C, Nawaz K, Webster RM. The oncology market for antibody–drug conjugates. Nat Rev Drug Discov. 2021;20:583–584. doi: 10.1038/d41573-021-00054-2. [DOI] [PubMed] [Google Scholar]
- 7.Arlotta KJ, Owen SC. Antibody and antibody derivatives as cancer therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1556. doi: 10.1002/wnan.1556. [DOI] [PubMed] [Google Scholar]
- 8.Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-engineering for modulated effector functions—improving antibodies for cancer treatment. Antibodies. 2020;9(4):64. doi: 10.3390/antib9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiao J, Al-Tamimi M, Baker RI, Andrews RK, Gardiner EE. The platelet Fc receptor. FcγRIIa Immunol Rev. 2015;268:241–252. doi: 10.1111/imr.12370. [DOI] [PubMed] [Google Scholar]
- 10.Uppal H, et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1) Clin Cancer Res. 2015;21:123–133. doi: 10.1158/1078-0432.CCR-14-2093. [DOI] [PubMed] [Google Scholar]
- 11.Pegram MD, et al. First-in-human, phase 1 dose-escalation study of biparatopic anti-HER2 antibody-drug conjugate MEDI4276 in patients with HER2-positive advanced breast or gastric cancer. Mol Cancer Ther. 2021;20:1442–1453. doi: 10.1158/1535-7163.MCT-20-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deonarain MP. Miniaturised ’antibody’-drug conjugates for solid tumours? Drug Discov Today Technol. 2018;30:47–53. doi: 10.1016/j.ddtec.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Deonarain MP, Yahioglu G. Current strategies for the discovery and bioconjugation of smaller, targetable drug conjugates tailored for solid tumor therapy. Expert Opin Drug Discov. 2021;16:613–624. doi: 10.1080/17460441.2021.1858050. [DOI] [PubMed] [Google Scholar]
- 14.Deonarain MP, et al. Small-format drug conjugates: a viable alternative to ADCs for solid tumours? Antibodies. 2018;7(2):16. doi: 10.3390/antib7020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rypáček F, Drobník J, Chmelař V, Kálal J. The renal excretion and retention of macromolecules. Pflugers Arch. 1982;392:211–217. doi: 10.1007/BF00584298. [DOI] [PubMed] [Google Scholar]
- 16.Pyzik M, et al. The neonatal Fc receptor (FcRn): a misnomer? Front Immunol. 2019;10:1540. doi: 10.3389/fimmu.2019.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat Rev Drug Discov. 2018;17:197–223. doi: 10.1038/nrd.2017.227. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9:33–46. doi: 10.1007/s13238-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera AF, et al. Anti-CD79B antibody-drug conjugate DCDS0780A in patients with B-cell non-hodgkin lymphoma: phase 1 dose-escalation study. Clin Cancer Res. 2022;28:1294–1301. doi: 10.1158/1078-0432.CCR-21-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q. Site-specific antibody conjugation for ADC and beyond. Biomedicines. 2017;5:E64. doi: 10.3390/biomedicines5040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duivelshof BL, et al. Glycan-mediated technology for obtaining homogeneous site-specific conjugated antibody-drug conjugates: synthesis and analytical characterization by using complementary middle-up LC/HRMS analysis. Anal Chem. 2020;92:8170–8177. doi: 10.1021/acs.analchem.0c00282. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, et al. A simple and efficient method to generate dual site-specific conjugation ADCs with cysteine residue and an unnatural amino acid. Bioconjugate Chem. 2021;32:1094–1104. doi: 10.1021/acs.bioconjchem.1c00134. [DOI] [PubMed] [Google Scholar]
- 23.Hussain AF, et al. Toward homogenous antibody drug conjugates using enzyme-based conjugation approaches. Pharmaceuticals. 2021;14(4):343. doi: 10.3390/ph14040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Z, et al. Synthesis of site-specific antibody-drug conjugates by ADP-ribosyl cyclases. Sci Adv. 2020;6(23):eaba6752. doi: 10.1126/sciadv.aba6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puthenveetil S, et al. Multivalent peptidic linker enables identification of preferred sites of conjugation for a potent thialanstatin antibody drug conjugate. PLoS ONE. 2017;12:e0178452. doi: 10.1371/journal.pone.0178452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buecheler JW, Winzer M, Tonillo J, Weber C, Gieseler H. Impact of payload hydrophobicity on the stability of antibody-drug conjugates. Mol Pharm. 2018;15:2656–2664. doi: 10.1021/acs.molpharmaceut.8b00177. [DOI] [PubMed] [Google Scholar]
- 27.Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;11:99–109. doi: 10.3109/1547691X.2013.821564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyon RP, et al. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat Biotechnol. 2015;33:733–735. doi: 10.1038/nbt.3212. [DOI] [PubMed] [Google Scholar]
- 29.Hamblett KJ, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 30.Simmons JK, Burke PJ, Cochran JH, Pittman PG, Lyon RP. Reducing the antigen-independent toxicity of antibody-drug conjugates by minimizing their non-specific clearance through PEGylation. Toxicol Appl Pharmacol. 2020;392:114932. doi: 10.1016/j.taap.2020.114932. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, et al. PEG linker improves antitumor efficacy and safety of affibody-based drug conjugates. Int J Mol Sci. 2021;22:1540. doi: 10.3390/ijms22041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao S, et al. Site-specific and hydrophilic ADCs through disulfide-bridged linker and branched PEG. Bioorg Med Chem Lett. 2018;28:1363–1370. doi: 10.1016/j.bmcl.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Burke PJ, et al. Optimization of a PEGylated glucuronide-monomethylauristatin E linker for antibody-drug conjugates. Mol Cancer Ther. 2017;16:116–123. doi: 10.1158/1535-7163.MCT-16-0343. [DOI] [PubMed] [Google Scholar]
- 34.Sonzini S, et al. Improved physical stability of an antibody-drug conjugate using host-guest chemistry. Bioconjug Chem. 2020;31:123–129. doi: 10.1021/acs.bioconjchem.9b00809. [DOI] [PubMed] [Google Scholar]
- 35.Viricel W, et al. Monodisperse polysarcosine-based highly-loaded antibody-drug conjugates. Chem Sci. 2019;10:4048–4053. doi: 10.1039/C9SC00285E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conilh L, et al. Exatecan antibody drug conjugates based on a hydrophilic polysarcosine drug-linker platform. Pharmaceuticals. 2021;14(3):247. doi: 10.3390/ph14030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dovgan I, et al. On the use of DNA as a linker in antibody-drug conjugates: synthesis, stability and in vitro potency. Sci Rep. 2020;10:7691. doi: 10.1038/s41598-020-64518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yurkovetskiy AV, et al. A polymer-based antibody-Vinca drug conjugate platform: characterization and preclinical efficacy. Cancer Res. 2015;75:3365–3372. doi: 10.1158/0008-5472.CAN-15-0129. [DOI] [PubMed] [Google Scholar]
- 39.Okajima D, et al. Datopotamab Deruxtecan, a novel TROP2-directed antibody–drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20:2329–2340. doi: 10.1158/1535-7163.MCT-21-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teicher BA, Chari RVJ. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:6389–6397. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 41.Anderl J, Faulstich H, Hechler T, Kulke M. Antibody–drug conjugate payloads. In: Ducry L editor. Antibody-Drug Conjugates, 2013. pp. 51–70. 10.1007/978-1-62703-541-5_4. [DOI] [PubMed]
- 42.Widdison WC, Chari RVJ. Factors involved in the design of cytotoxic payloads for antibody–drug conjugates. In: Phillips GL editor Antibody-drug conjugates and immunotoxins: from pre-clinical development to therapeutic applications. Springer; 2013. pp. 93–115. 10.1007/978-1-4614-5456-4_6.
- 43.Mantaj J, Jackson PJM, Rahman KM, Thurston DE. From anthramycin to pyrrolobenzodiazepine (PBD)-containing antibody-drug conjugates (ADCs) Angew Chem Int Ed Engl. 2017;56:462–488. doi: 10.1002/anie.201510610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaghoubi S, et al. Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy. J Cell Physiol. 2020;235:31–64. doi: 10.1002/jcp.28967. [DOI] [PubMed] [Google Scholar]
- 45.Saber H, Simpson N, Ricks TK, Leighton JK. An FDA oncology analysis of toxicities associated with PBD-containing antibody-drug conjugates. Regul Toxicol Pharmacol. 2019;107:104429. doi: 10.1016/j.yrtph.2019.104429. [DOI] [PubMed] [Google Scholar]
- 46.Hartley JA. Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy. Expert Opin Biol Ther. 2021;21:931–943. doi: 10.1080/14712598.2020.1776255. [DOI] [PubMed] [Google Scholar]
- 47.Lee A. Loncastuximab tesirine: first approval. Drugs. 2021;81:1229–1233. doi: 10.1007/s40265-021-01550-w. [DOI] [PubMed] [Google Scholar]
- 48.Keam SJ. Trastuzumab deruxtecan: first approval. Drugs. 2020;80:501–508. doi: 10.1007/s40265-020-01281-4. [DOI] [PubMed] [Google Scholar]
- 49.Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020;80:1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Criscitiello C, Morganti S, Curigliano G. Antibody–drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol. 2021;14:20. doi: 10.1186/s13045-021-01035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moek KL, de Groot DJA, de Vries EGE, Fehrmann RSN. The antibody-drug conjugate target landscape across a broad range of tumour types. Ann Oncol. 2017;28:3083–3091. doi: 10.1093/annonc/mdx541. [DOI] [PubMed] [Google Scholar]
- 52.Ogitani Y, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 53.Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) Oncotarget. 2015;6:22496–22512. doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 55.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanizawa A, Fujimori A, Fujimori Y, Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994;86:836–842. doi: 10.1093/jnci/86.11.836. [DOI] [PubMed] [Google Scholar]
- 58.Zunino F, Pratesi G. Camptothecins in clinical development. Expert Opin Investig Drugs. 2004;13:269–284. doi: 10.1517/13543784.13.3.269. [DOI] [PubMed] [Google Scholar]
- 59.Bailly C. Irinotecan: 25 years of cancer treatment. Pharmacol Res. 2019;148:104398. doi: 10.1016/j.phrs.2019.104398. [DOI] [PubMed] [Google Scholar]
- 60.Thomas A, Pommier Y. Targeting topoisomerase I in the era of precision medicine. Clin Cancer Res. 2019;25:6581–6589. doi: 10.1158/1078-0432.CCR-19-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takegawa N, et al. DS-8201a, a new HER2-targeting antibody–drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141:1682–1689. doi: 10.1002/ijc.30870. [DOI] [PubMed] [Google Scholar]
- 62.Abou-Alfa GK, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 63.Ogitani Y, et al. Wide application of a novel topoisomerase I inhibitor-based drug conjugation technology. Bioorg Med Chem Lett. 2016;26:5069–5072. doi: 10.1016/j.bmcl.2016.08.082. [DOI] [PubMed] [Google Scholar]
- 64.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takegawa N, et al. DS-8201a, a new HER2-targeting antibody–drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141:1682–1689. doi: 10.1002/ijc.30870. [DOI] [PubMed] [Google Scholar]
- 66.Iwata TN, et al. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17:1494–1503. doi: 10.1158/1535-7163.MCT-17-0749. [DOI] [PubMed] [Google Scholar]
- 67.Nagai Y, Oitate M, Shiozawa H, Ando O. Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica. 2019;49:1086–1096. doi: 10.1080/00498254.2018.1531158. [DOI] [PubMed] [Google Scholar]
- 68.Modi S, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shitara K, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 70.Research, C. for D. E. and. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer. FDA. 2022.
- 71.Cortés J, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 72.Li BT, et al. Trastuzumab deruxtecan in HER2-mutant non–small-cell lung cancer. N Engl J Med. 2022;386:241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siena S, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779–789. doi: 10.1016/S1470-2045(21)00086-3. [DOI] [PubMed] [Google Scholar]
- 74.Commissioner, O. of the. FDA Approves First Targeted Therapy for HER2-Low Breast Cancer. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer. 2022.
- 75.Liu H, et al. Abstract P196: novel hydrophilic drug linkers enable exatecan-based antibody-drug conjugates with promising physiochemical properties and in vivo activity. Mol Cancer Ther. 2021;20:P196. doi: 10.1158/1535-7163.TARG-21-P196. [DOI] [Google Scholar]
- 76.AACR Annual Meeting 2022-The preclinical pharmacology of PRO1102, a novel exatecan-based HER2-directed antibody-drug conjugate with robust anti-tumor activity. https://cattendee.abstractsonline.com/meeting/10517/Presentation/12222.
- 77.AACR Annual Meeting 2022 - PRO1184, a novel folate receptor alpha-directed antibody-drug conjugate, demonstrates robust anti-tumor activity in mouse carcinoma models. https://cattendee.abstractsonline.com/meeting/10517/Presentation/12260.
- 78.AACR Annual Meeting 2022 - PRO1160, a novel CD70-directed antibody-drug conjugate, demonstrates robust anti-tumor activity in mouse models of renal cell carcinoma and non-Hodgkin lymphoma. https://cattendee.abstractsonline.com/meeting/10517/Presentation/12232.
- 79.Li W, et al. Synthesis and evaluation of camptothecin antibody-drug conjugates. ACS Med Chem Lett. 2019;10:1386–1392. doi: 10.1021/acsmedchemlett.9b00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.AACR Annual Meeting 2022-Discovery and first disclosure of AZD8205, a B7-H4-targeted antibody-drug conjugate utilizing a novel topoisomerase I linker-warhead. https://cattendee.abstractsonline.com/meeting/10517/Presentation/12224.
- 81.Gupta E, et al. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res. 1994;54:3723–3725. [PubMed] [Google Scholar]
- 82.Haaz MC, Rivory L, Riché C, Vernillet L, Robert J. Metabolism of irinotecan (CPT-11) by human hepatic microsomes: participation of cytochrome P-450 3A and drug interactions. Cancer Res. 1998;58:468–472. [PubMed] [Google Scholar]
- 83.Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020;80:1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moon S-J, et al. Antibody conjugates of 7-ethyl-10-hydroxycamptothecin (SN-38) for targeted cancer chemotherapy. J Med Chem. 2008;51:6916–6926. doi: 10.1021/jm800719t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Govindan SV, Cardillo TM, Moon S-J, Hansen HJ, Goldenberg DM. CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res. 2009;15:6052–6061. doi: 10.1158/1078-0432.CCR-09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Govindan SV, et al. Improving the therapeutic index in cancer therapy by using antibody-drug conjugates designed with a moderately cytotoxic drug. Mol Pharm. 2015;12:1836–1847. doi: 10.1021/mp5006195. [DOI] [PubMed] [Google Scholar]
- 87.Cardillo TM, et al. IMMU-140, a novel SN-38 antibody-drug conjugate targeting HLA-DR, mediates dual cytotoxic effects in hematologic cancers and malignant melanoma. Mol Cancer Ther. 2018;17:150–160. doi: 10.1158/1535-7163.MCT-17-0354. [DOI] [PubMed] [Google Scholar]
- 88.Sharkey RM, Govindan SV, Cardillo TM, Goldenberg DM. Epratuzumab-SN-38: a new antibody-drug conjugate for the therapy of hematologic malignancies. Mol Cancer Ther. 2012;11:224–234. doi: 10.1158/1535-7163.MCT-11-0632. [DOI] [PubMed] [Google Scholar]
- 89.Govindan SV, et al. Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol Cancer Ther. 2013;12:968–978. doi: 10.1158/1535-7163.MCT-12-1170. [DOI] [PubMed] [Google Scholar]
- 90.Yao Y, et al. Synthesis, characterization and targeting chemotherapy for ovarian cancer of trastuzumab-SN-38 conjugates. J Control Release. 2015;220:5–17. doi: 10.1016/j.jconrel.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 91.Yasunaga M, Manabe S, Tarin D, Matsumura Y. Tailored immunoconjugate therapy depending on a quantity of tumor stroma. Cancer Sci. 2013;104:231–237. doi: 10.1111/cas.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yasunaga M, Manabe S, Tarin D, Matsumura Y. Cancer-stroma targeting therapy by cytotoxic immunoconjugate bound to the collagen 4 network in the tumor tissue. Bioconjugate Chem. 2011;22:1776–1783. doi: 10.1021/bc200158j. [DOI] [PubMed] [Google Scholar]
- 93.Yasunaga M, Manabe S, Matsumura Y. Immunoregulation by IL-7R-targeting antibody-drug conjugates: overcoming steroid-resistance in cancer and autoimmune disease. Sci Rep. 2017;7:10735. doi: 10.1038/s41598-017-11255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burke PJ, et al. Design, synthesis, and biological evaluation of antibody−drug conjugates comprised of potent camptothecin analogues. Bioconjugate Chem. 2009;20:1242–1250. doi: 10.1021/bc9001097. [DOI] [PubMed] [Google Scholar]
- 95.Gupta N, et al. Development of a facile antibody–drug conjugate platform for increased stability and homogeneity. Chem Sci. 2017;8:2387–2395. doi: 10.1039/C6SC05149A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lyski RD, et al. Development of novel antibody-camptothecin conjugates. Mol Cancer Ther. 2021;20:329–339. doi: 10.1158/1535-7163.MCT-20-0526. [DOI] [PubMed] [Google Scholar]
- 97.Ryan M, et al. SGN-CD30C, an investigational CD30-Directed camptothecin antibody-drug conjugate (ADC), Shows strong anti tumor activity and superior tolerability in preclinical studies. Blood. 2020;136:41–42. doi: 10.1182/blood-2020-136577. [DOI] [Google Scholar]
- 98.Lyski R, et al. Abstract 2885: discovery of a tripeptide-based camptothecin drug-linker for antibody-drug conjugates with potent antitumor activity and a broad therapeutic window. Can Res. 2020;80:2885. doi: 10.1158/1538-7445.AM2020-2885. [DOI] [Google Scholar]
- 99.Kummar S, et al. Clinical and pharmacologic evaluation of two dosing schedules of indotecan (LMP400), a novel indenoisoquinoline, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;78:73–81. doi: 10.1007/s00280-016-2998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lv P-C, et al. Design, synthesis, and biological evaluation of potential prodrugs related to the experimental anticancer agent indotecan (LMP400) J Med Chem. 2016;59:4890–4899. doi: 10.1021/acs.jmedchem.6b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurtzberg LS, et al. Genz-644282, a novel non-camptothecin topoisomerase I inhibitor for cancer treatment. Clin Cancer Res. 2011;17:2777–2787. doi: 10.1158/1078-0432.CCR-10-0542. [DOI] [PubMed] [Google Scholar]
- 102.Sooryakumar D, Dexheimer TS, Teicher BA, Pommier Y. Molecular and cellular pharmacology of the novel noncamptothecin topoisomerase I inhibitor Genz-644282. Mol Cancer Ther. 2011;10:1490–1499. doi: 10.1158/1535-7163.MCT-10-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marzi L, et al. Novel fluoroindenoisoquinoline non-camptothecin topoisomerase I inhibitors. Mol Cancer Ther. 2018;17:1694–1704. doi: 10.1158/1535-7163.MCT-18-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Our Pipeline | Gibson Oncology. https://gibsononcology.com/our-pipeline/.
- 105.Elias DJ, et al. Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunconjugate in patients with non-small cell lung carcinoma1. Can Res. 1990;50:4154–4159. [PubMed] [Google Scholar]
- 106.Saleh MN, et al. Phase I trial of the anti-Lewis Y drug immunoconjugate BR96-doxorubicin in patients with lewis Y-expressing epithelial tumors. J Clin Oncol. 2000;18:2282–2292. doi: 10.1200/JCO.2000.18.11.2282. [DOI] [PubMed] [Google Scholar]
- 107.Tolcher AW, et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17:478–484. doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- 108.Sapra P, et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11:5257–5264. doi: 10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
- 109.Luong A, Issarapanichkit T, Kong SD, Fong R, Yang J. pH-Sensitive, N-ethoxybenzylimidazole (NEBI) bifunctional crosslinkers enable triggered release of therapeutics from drug delivery carriers. Org Biomol Chem. 2010;8:5105–5109. doi: 10.1039/c0ob00228c. [DOI] [PubMed] [Google Scholar]
- 110.Stefan N, et al. Highly potent, anthracycline-based antibody-drug conjugates generated by enzymatic, Site-specific Conjugation. Mol Cancer Ther. 2017;16:879–892. doi: 10.1158/1535-7163.MCT-16-0688. [DOI] [PubMed] [Google Scholar]
- 111.Junttila MR, et al. Targeting LGR5+ cells with an antibody-drug conjugate for the treatment of colon cancer. Sci Transl Med. 2015;7(314):314ra186. doi: 10.1126/scitranslmed.aac7433. [DOI] [PubMed] [Google Scholar]
- 112.Tolcher AW, et al. NBE-002: a novel anthracycline-based antibody-drug conjugate (ADC) targeting ROR1 for the treatment of advanced solid tumors—a phase 1/2 clinical trial. JCO. 2021 doi: 10.1200/JCO.2021.39.15_suppl.TPS1108. [DOI] [Google Scholar]
- 113.Sadilkova LK, et al. Abstract 1204: SO-N102, a novel CLDN182-targeting antibody-drug conjugate with strong anti-tumor effect in various solid tumors expressing low target levels. Cancer Res. 2021;81:1204. doi: 10.1158/1538-7445.AM2021-1204. [DOI] [Google Scholar]
- 114.Yu S-F, et al. A novel anti-CD22 anthracycline-based antibody-drug conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. 2015;21:3298–3306. doi: 10.1158/1078-0432.CCR-14-2035. [DOI] [PubMed] [Google Scholar]
- 115.D’Amico L, et al. A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J Immunother Cancer. 2019;7:16. doi: 10.1186/s40425-018-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dal Corso A, Gébleux R, Murer P, Soltermann A, Neri D. A non-internalizing antibody-drug conjugate based on an anthracycline payload displays potent therapeutic activity in vivo. J Control Release. 2017;264:211–218. doi: 10.1016/j.jconrel.2017.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holte D, et al. Evaluation of PNU-159682 antibody drug conjugates (ADCs) Bioorg Med Chem Lett. 2020;30:127640. doi: 10.1016/j.bmcl.2020.127640. [DOI] [PubMed] [Google Scholar]
- 118.AACR Annual Meeting 2022-Development and characterization of an epiregulin antibody-drug conjugate for targeting colorectal cancer cell plasticity. https://cattendee.abstractsonline.com/meeting/10517/Presentation/16961.
- 119.Nilchan N, et al. Dual-mechanistic antibody-drug conjugate via site-specific selenocysteine/cysteine conjugation. Antib Ther. 2019;2:71–78. doi: 10.1093/abt/tbz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kato Y, et al. A novel method of conjugation of daunomycin with antibody with a poly-L-glutamic acid derivative as intermediate drug carrier. An anti-alpha-fetoprotein antibody-daunomycin conjugate. J Med Chem. 1984;27:1602–1607. doi: 10.1021/jm00378a013. [DOI] [PubMed] [Google Scholar]
- 121.Lavie E, et al. Monoclonal antibody L6-daunomycin conjugates constructed to release free drug at the lower pH of tumor tissue. Cancer Immunol Immunother. 2005 doi: 10.1007/BF01744941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pietersz GA, Smyth MJ, McKenzie IF. Immunochemotherapy of a murine thymoma with the use of idarubicin monoclonal antibody conjugates. Cancer Res. 1988;48:926–931. [PubMed] [Google Scholar]
- 123.Rowland AJ, Pietersz GA, McKenzie IF. Preclinical investigation of the antitumour effects of anti-CD19-idarubicin immunoconjugates. Cancer Immunol Immunother. 1993;37:195–202. doi: 10.1007/BF01525435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghanemi M, et al. Specific targeting of HER2-positive head and neck squamous cell carcinoma line HN5 by idarubicin-ZHER2 affibody conjugate. Curr Cancer Drug Targets. 2019;19:65–73. doi: 10.2174/1568009617666170427105417. [DOI] [PubMed] [Google Scholar]
- 125.Laham-Karam N, Pinto GP, Poso A, Kokkonen P. Transcription and translation inhibitors in cancer treatment. Front Chem. 2020;8:276. doi: 10.3389/fchem.2020.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lindell TJ, Weinberg F, Morris PW, Roeder RG, Rutter WJ. Specific inhibition of nuclear RNA polymerase II by α-amanitin. Science. 1970;170:447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- 127.Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- 128.Garcia J, et al. Amanita phalloides poisoning: mechanisms of toxicity and treatment. Food Chem Toxicol. 2015;86:41–55. doi: 10.1016/j.fct.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 129.Pahl A, Lutz C, Hechler T. Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov Today Technol. 2018;30:85–89. doi: 10.1016/j.ddtec.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 130.Barbanti-Brodano G, Fiume L. Selective killing of macrophages by amanitin-albumin conjugates. Nat New Biol. 1973;243:281–283. doi: 10.1038/newbio243281a0. [DOI] [PubMed] [Google Scholar]
- 131.Danielczyk A, et al. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol Immunother. 2006;55:1337–1347. doi: 10.1007/s00262-006-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hechler T, Kulke M, Mueller C, Pahl A, Anderl J. Abstract 664: amanitin-based antibody-drug conjugates targeting the prostate-specific membrane antigen. Can Res. 2014;74:664. doi: 10.1158/1538-7445.AM2014-664. [DOI] [Google Scholar]
- 133.Gallo F, et al. Enhancing the pharmacokinetics and antitumor activity of an α-amanitin-based small-molecule drug conjugate via conjugation with an Fc domain. J Med Chem. 2021;64:4117–4129. doi: 10.1021/acs.jmedchem.1c00003. [DOI] [PubMed] [Google Scholar]
- 134.Davis MT, Preston JF. A conjugate of alpha-amanitin and monoclonal immunoglobulin G to Thy 1.2 antigen is selectively toxic to T lymphoma cells. Science. 1981;213:1385–1388. doi: 10.1126/science.6115471. [DOI] [PubMed] [Google Scholar]
- 135.Figueroa-Vazquez V, et al. HDP-101, an anti-BCMA antibody-drug conjugate, safely delivers amanitin to induce cell death in proliferating and resting multiple myeloma cells. Mol Cancer Ther. 2021;20:367–378. doi: 10.1158/1535-7163.MCT-20-0287. [DOI] [PubMed] [Google Scholar]
- 136.AACR Annual Meeting 2022-Amatoxin-based antibody-drug conjugates induce immunogenic cell death and improve the anti-tumor efficacy of immune checkpoint inhibitors in humanized mouse models. https://cattendee.abstractsonline.com/meeting/10517/Presentation/12238.
- 137.Moldenhauer G, et al. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J Natl Cancer Inst. 2012;104:622–634. doi: 10.1093/jnci/djs140. [DOI] [PubMed] [Google Scholar]
- 138.Kulke M, et al. Abstract 735: SAR of amanitin and optimization of linker-amanitin derivatives for solid tumors. Can Res. 2018;78:735. doi: 10.1158/1538-7445.AM2018-735. [DOI] [Google Scholar]
- 139.Świderska KW, Szlachcic A, Opaliński Ł, Zakrzewska M, Otlewski J. FGF2 dual warhead conjugate with monomethyl auristatin E and α-amanitin displays a cytotoxic effect towards cancer cells overproducing FGF receptor 1. Int J Mol Sci. 2018;19:2098. doi: 10.3390/ijms19072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barbanti-Brodano G, Derenzini M, Fiume L. Toxic action of a phalloidin-albumin conjugate on cells with a high protein uptake. Nature. 1974;248:63–65. doi: 10.1038/248063a0. [DOI] [PubMed] [Google Scholar]
- 141.Hinman, L. M. & Yarranton, G. Chapter 25. New approaches to non-immunogenic monoclonal antibody cancer therapies. In: Bristol JA editor Annual Reports in Medicinal Chemistry, 1993. vol. 28, pp. 237–246.
- 142.Nielsen C, Casteel M, Didier A, Dietrich R, Märtlbauer E. Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Res. 2009;25:77–84. doi: 10.1007/s12550-009-0011-5. [DOI] [PubMed] [Google Scholar]
- 143.Liu X, Bushnell DA, Kornberg RD. RNA polymerase II transcription: structure and mechanism. Biochim Biophys Acta. 2013;1829:2–8. doi: 10.1016/j.bbagrm.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Noel P, et al. Triptolide and its derivatives as cancer therapies. Trends Pharmacol Sci. 2019;40:327–341. doi: 10.1016/j.tips.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Hayashi M, et al. Novel antibody-drug conjugate with anti-CD26 humanized monoclonal antibody and transcription factor IIH (TFIIH) inhibitor, triptolide, inhibits tumor growth via impairing mRNA synthesis. Cancers. 2019;11:E1138. doi: 10.3390/cancers11081138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang K, et al. Cetuximab-triptolide conjugate suppresses the growth of EGFR-overexpressing lung cancers through targeting RNA polymerase II. Mol Ther Oncolytics. 2020;18:304–316. doi: 10.1016/j.omto.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wei D, et al. Site-specific construction of triptolide-based antibody-drug conjugates. Bioorg Med Chem. 2021;51:116497. doi: 10.1016/j.bmc.2021.116497. [DOI] [PubMed] [Google Scholar]
- 148.Hideshima T, Richardson P, Anderson K. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther. 2011 doi: 10.1158/1535-7163.MCT-11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McClure JJ, Li X, Chou CJ. Advances and challenges of HDAC inhibitors in cancer therapeutics. Adv Cancer Res. 2018;138:183–211. doi: 10.1016/bs.acr.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 150.Cini E, et al. Antibody drug conjugates (ADCs) charged with HDAC inhibitor for targeted epigenetic modulation †Electronic supplementary information (ESI) available: experimental procedures, biological activity data, NMR spectra for characterisation. Chem Sci. 2018;9:6490–6496. doi: 10.1039/c7sc05266a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Milazzo FM, et al. ErbB2 targeted epigenetic modulation: anti-tumor efficacy of the ADC trastuzumab-HDACi ST8176AA1. Front Oncol. 2020;9:1534. doi: 10.3389/fonc.2019.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cianferotti C, et al. Antibody drug conjugates with hydroxamic acid cargos for histone deacetylase (HDAC) inhibition. Chem Commun. 2021;57:867–870. doi: 10.1039/D0CC06131J. [DOI] [PubMed] [Google Scholar]
- 153.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 155.Souers AJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 156.Trudel S, et al. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 157.Baggstrom MQ, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol. 2011;6:1757–1760. doi: 10.1097/JTO.0b013e31822e2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu Y, et al. Sabutoclax, pan-active BCL-2 protein family antagonist, overcomes drug resistance and eliminates cancer stem cells in breast cancer. Cancer Lett. 2018;423:47–59. doi: 10.1016/j.canlet.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 159.Lessene G, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9:390–397. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 160.Zhang H, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 161.Deeks DE. Venetoclax: first global approval. Drugs. 2016;76:979–987. doi: 10.1007/s40265-016-0596-x. [DOI] [PubMed] [Google Scholar]
- 162.Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharmacol Res. 2020;152:104609. doi: 10.1016/j.phrs.2019.104609. [DOI] [PubMed] [Google Scholar]
- 163.Jaiswal N, Akhtar J, Singh SP, Badruddeen, Ahsan F. An overview on genistein and its various formulations. Drug Res. 2019;69:305–313. doi: 10.1055/a-0797-3657. [DOI] [PubMed] [Google Scholar]
- 164.Messinger Y, et al. In vivo toxicity and pharmacokinetic features of B43 (anti-CD19)-genistein immunoconjugate in nonhuman primates. Clin Cancer Res. 1998;4:165–170. [PubMed] [Google Scholar]
- 165.Ek O, et al. In vivo toxicity and pharmacokinetic features of B43(Anti-CD19)-Genistein immunoconjugate. Leuk Lymphoma. 1998;30:389–394. doi: 10.3109/10428199809057550. [DOI] [PubMed] [Google Scholar]
- 166.Chen C-L, et al. Clinical pharmacokinetics of the CD19 receptor-directed tyrosine kinase inhibitor B43-genistein in patients with B-lineage lymphoid malignancies. J Clin Pharmacol. 1999;39:1248–1255. doi: 10.1177/00912709922012051. [DOI] [PubMed] [Google Scholar]
- 167.Uckun FM, et al. Treatment of therapy-refractory B-lineage acute lymphoblastic leukemia with an apoptosis-inducing CD19-directed tyrosine kinase inhibitor. Clin Cancer Res. 1999;5:3906–3913. [PubMed] [Google Scholar]
- 168.Uckun FM, et al. In vivo toxicity, pharmacokinetics, and anticancer activity of Genistein linked to recombinant human epidermal growth factor. Clin Cancer Res. 1998;4:1125–1134. [PubMed] [Google Scholar]
- 169.Gentile MS, et al. Targeting colon cancer cells with genistein-17.1A immunoconjugate. Int J Oncol. 2003;22:955–959. [PubMed] [Google Scholar]
- 170.Zhou D, et al. Novel PIKK inhibitor antibody-drug conjugates: synthesis and anti-tumor activity. Bioorg Med Chem Lett. 2019;29:943–947. doi: 10.1016/j.bmcl.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 171.Wang RE, et al. An immunosuppressive antibody-drug conjugate. J Am Chem Soc. 2015;137:3229–3232. doi: 10.1021/jacs.5b00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chao W-T, et al. Abstract 3729: developing cetuximab-staurosporine conjugate as the therapeutic medicine in KRAS/BRAF mutated colon cancer cells. Can Res. 2018;78:3729. doi: 10.1158/1538-7445.AM2018-3729. [DOI] [Google Scholar]
- 173.Amouzegar A, Chelvanambi M, Filderman JN, Storkus WJ, Luke JJ. STING Agonists as Cancer Therapeutics. Cancers. 2021;13:2695. doi: 10.3390/cancers13112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Y, et al. Small-molecule modulators of toll-like receptors. Acc Chem Res. 2020 doi: 10.1021/acs.accounts.9b00631. [DOI] [PubMed] [Google Scholar]
- 175.Janku F, et al. 378 A first in-human, multicenter, open-label, dose-finding phase 1 study of the immune stimulator antibody conjugate NJH395 in patients with nonbreast HER2+ advanced malignancies. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-SITC2020.0378. [DOI] [Google Scholar]
- 176.Ackerman SE, et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat Cancer. 2021;2:18–33. doi: 10.1038/s43018-020-00136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ackerman SE, et al. Abstract 1559: TLR7/8 immune-stimulating antibody conjugates elicit robust myeloid activation leading to enhanced effector function and anti-tumor immunity in pre-clinical models. Can Res. 2019;79:1559. doi: 10.1158/1538-7445.AM2019-1559. [DOI] [Google Scholar]
- 178.LeBlanc H, et al. 605 Systemically administered HER2-targeted ISACs provoke a rapid, local response that engages the innate and adaptive arms of the immune system to eradicate tumors in preclinical models. J Immunother Cancer. 2020 doi: 10.1136/jitc-2020-SITC2020.0605. [DOI] [Google Scholar]
- 179.Mallet W, et al. 784 BDC-2034: discovery of a CEA-targeting immune-stimulating antibody conjugate (ISAC) for solid tumors. J Immunother Cancer. 2021 doi: 10.1136/jitc-2021-SITC2021.784. [DOI] [Google Scholar]
- 180.AACR Annual Meeting 2022-The CEA-targeted ISAC, BDC-2034, shows preclinical efficacy associated with innate immune activation, phagocytosis, and myeloid reprogramming. https://cattendee.abstractsonline.com/meeting/10517/Presentation/16947.
- 181.AACR Annual Meeting 2022-PD-L1-targeted ISAC combines myeloid cell activation, immune-checkpoint inhibition and ADCP to improve anti-tumor efficacy over anti-PD-L1 antibodies in preclinical models. https://cattendee.abstractsonline.com/meeting/10517/Presentation/16962.
- 182.Metz H, et al. SBT6050, a HER2-directed TLR8 therapeutic, as a systemically administered, tumor-targeted human myeloid cell agonist. JCO. 2020;38:3110–3110. doi: 10.1200/JCO.2020.38.15_suppl.3110. [DOI] [Google Scholar]
- 183.Comeau MR, et al. Abstract 4537: SBT6050, a HER2-directed TLR8 ImmunoTACTMtherapeutic, is a potent human myeloid cell agonist that provides opportunity for single agent clinical activity. Can Res. 2020;80:4537. doi: 10.1158/1538-7445.AM2020-4537. [DOI] [Google Scholar]
- 184.Gadd AJR, Greco F, Cobb AJA, Edwards AD. Targeted activation of toll-like receptors: conjugation of a toll-like receptor 7 agonist to a monoclonal antibody maintains antigen binding and specificity. Bioconjugate Chem. 2015;26:1743–1752. doi: 10.1021/acs.bioconjchem.5b00302. [DOI] [PubMed] [Google Scholar]
- 185.Ackerman SE, et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat Cancer. 2021;2:18–33. doi: 10.1038/s43018-020-00136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.He L, et al. Immune modulating antibody-drug conjugate (IM-ADC) for cancer immunotherapy. J Med Chem. 2021;64:15716–15726. doi: 10.1021/acs.jmedchem.1c00961. [DOI] [PubMed] [Google Scholar]
- 187.AACR Annual Meeting 2022-Tumor-targeted immune activation via a site-specific TLR7-agonist antibody-drug conjugate. https://cattendee.abstractsonline.com/meeting/10517/Presentation/21768.
- 188.AACR Annual Meeting 2022-First-in-human study of TAK-500, a novel STING agonist immune stimulating antibody conjugate (ISAC), alone and in combination with pembrolizumab in patients with select advanced solid tumors. https://cattendee.abstractsonline.com/meeting/10517/Presentation/20324.
- 189.Banerjee M, et al. Abstract LB-061: CRD5500: a versatile small molecule STING agonist amenable to bioconjugation as an ADC. Cancer Research. 2019;79:LB-061. doi: 10.1158/1538-7445.AM2019-LB-061. [DOI] [Google Scholar]
- 190.AACR Annual Meeting 2022-XMT-2056, a HER2-targeted Immunosynthen STING-agonist antibody-drug conjugate, binds a novel epitope of HER2 and shows increased anti-tumor activity in combination with trastuzumab and pertuzumab. https://cattendee.abstractsonline.com/meeting/10517/Presentation/17073.
- 191.Duvall JR, et al. Abstract 1738: XMT-2056, a well-tolerated, immunosynthen-based STING-agonist antibody-drug conjugate which induces anti-tumor immune activity. Can Res. 2021;81:1738. doi: 10.1158/1538-7445.AM2021-1738. [DOI] [Google Scholar]
- 192.AACR Annual Meeting 2022 - Tumor cell-targeted STING-agonist antibody-drug conjugates achieve potent anti-tumor activity by delivering STING agonist specifically to tumor cells andFcγRI-expressing subset of myeloid cells. https://cattendee.abstractsonline.com/meeting/10517/Presentation/14046.
- 193.Scott AM, et al. A phase I trial of humanized monoclonal antibody A33 in patients with colorectal carcinoma: biodistribution, pharmacokinetics, and quantitative tumor uptake. Clin Cancer Res. 2005;11:4810–4817. doi: 10.1158/1078-0432.CCR-04-2329. [DOI] [PubMed] [Google Scholar]
- 194.Park H-K, et al. Unleashing the full potential of Hsp90 inhibitors as cancer therapeutics through simultaneous inactivation of Hsp90, Grp94, and TRAP1. Exp Mol Med. 2020;52:79–91. doi: 10.1038/s12276-019-0360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mandler R, Dadachova E, Brechbiel JK, Waldmann TA, Brechbiel MW. Synthesis and evaluation of antiproliferative activity of a geldanamycin-Herceptin immunoconjugate. Bioorg Med Chem Lett. 2000;10:1025–1028. doi: 10.1016/S0960-894X(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 196.Mandler R, Kobayashi H, Davis MY, Waldmann TA, Brechbiel MW. Modifications in synthesis strategy improve the yield and efficacy of geldanamycin−herceptin immunoconjugates. Bioconjugate Chem. 2002;13:786–791. doi: 10.1021/bc010124g. [DOI] [PubMed] [Google Scholar]
- 197.Mandler R, Kobayashi H, Hinson ER, Brechbiel MW, Waldmann TA. Herceptin-geldanamycin immunoconjugates: pharmacokinetics, biodistribution, and enhanced antitumor activity. Cancer Res. 2004;64:1460–1467. doi: 10.1158/0008-5472.CAN-03-2485. [DOI] [PubMed] [Google Scholar]
- 198.Burke PJ, et al. Novel immunoconjugates comprised of streptonigrin and 17-amino-geldanamycin attached via a dipeptide-p-aminobenzyl-amine linker system. Bioorg Med Chem Lett. 2009;19:2650–2653. doi: 10.1016/j.bmcl.2009.03.145. [DOI] [PubMed] [Google Scholar]
- 199.Lim KS, Lee DY, Han S, Bull DA, Won Y-W. Targeted delivery of heat shock protein 90 inhibitors prevents growth of HER2-positive tumor. Biomaterials. 2021;273:120817. doi: 10.1016/j.biomaterials.2021.120817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Effenberger KA, Urabe VK, Jurica MS. Modulating splicing with small molecular inhibitors of the spliceosome. Wiley Interdiscip Rev RNA. 2017;8(2):e1381. doi: 10.1002/wrna.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee SC-W, Abdel-Wahab O. Therapeutic targeting of splicing in cancer. Nat Med. 2016;22:976–986. doi: 10.1038/nm.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Puthenveetil S, et al. Natural product splicing inhibitors: a new class of antibody-drug conjugate (ADC) payloads. Bioconjug Chem. 2016;27:1880–1888. doi: 10.1021/acs.bioconjchem.6b00291. [DOI] [PubMed] [Google Scholar]
- 203.Gandhi V, Plunkett W, Cortes JE. Omacetaxine: a protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin Cancer Res. 2014;20:1735–1740. doi: 10.1158/1078-0432.CCR-13-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jeffrey SC, De Brabander J, Miyamoto J, Senter PD. Expanded utility of the β-glucuronide linker: ADCs that deliver phenolic cytotoxic agents. ACS Med Chem Lett. 2010;1:277–280. doi: 10.1021/ml100039h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Almaliti J, et al. Exploration of the carmaphycins as payloads in antibody drug conjugate anticancer agents. Eur J Med Chem. 2019;161:416–432. doi: 10.1016/j.ejmech.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Stanton BZ, Chory EJ, Crabtree GR. Chemically induced proximity in biology and medicine. Science. 2018;359:eaao5902. doi: 10.1126/science.aao5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022 doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dragovich PS. Degrader-antibody conjugates. Chem Soc Rev. 2022 doi: 10.1039/d2cs00141a. [DOI] [PubMed] [Google Scholar]
- 209.Pillow TH, et al. Antibody Conjugation of a chimeric BET degrader enables in vivo activity. ChemMedChem. 2020;15:17–25. doi: 10.1002/cmdc.201900497. [DOI] [PubMed] [Google Scholar]
- 210.Dragovich PS, et al. Antibody-mediated delivery of chimeric BRD4 degraders. Part 1: exploration of antibody linker, payload loading, and payload molecular properties. J Med Chem. 2021;64:2534–2575. doi: 10.1021/acs.jmedchem.0c01845. [DOI] [PubMed] [Google Scholar]
- 211.Dragovich PS, et al. Antibody-mediated delivery of chimeric BRD4 degraders. Part 2: improvement of in vitro antiproliferation activity and in vivo antitumor efficacy. J Med Chem. 2021;64:2576–2607. doi: 10.1021/acs.jmedchem.0c01846. [DOI] [PubMed] [Google Scholar]
- 212.Maneiro M, et al. Antibody–PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem Biol. 2020;15:1306–1312. doi: 10.1021/acschembio.0c00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chuang SH et al. Conjugués Anticorps-Protac. 2019
- 214.Dragovich PS, et al. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERα) Bioorg Med Chem Lett. 2020;30:126907. doi: 10.1016/j.bmcl.2019.126907. [DOI] [PubMed] [Google Scholar]
- 215.AACR Annual Meeting 2022-ORM-5029: A first-in-class targeted protein degradation therapy using antibody neodegrader conjugate (AnDC) for HER2-expressing breast cancer. https://cattendee.abstractsonline.com/meeting/10517/Presentation/15093.
- 216.Neumann CS, et al. Targeted delivery of cytotoxic NAMPT inhibitors using antibody-drug conjugates. Mol Cancer Ther. 2018;17:2633–2642. doi: 10.1158/1535-7163.MCT-18-0643. [DOI] [PubMed] [Google Scholar]
- 217.Karpov AS, et al. Nicotinamide phosphoribosyltransferase inhibitor as a novel payload for antibody-drug conjugates. ACS Med Chem Lett. 2018;9:838–842. doi: 10.1021/acsmedchemlett.8b00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.El-Nassan HB. Advances in the discovery of kinesin spindle protein (Eg5) inhibitors as antitumor agents. Eur J Med Chem. 2013;62:614–631. doi: 10.1016/j.ejmech.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 219.Lerchen H-G, et al. Antibody-drug conjugates with pyrrole-based KSP inhibitors as the payload class. Angew Chem Int Ed Engl. 2018;57:15243–15247. doi: 10.1002/anie.201807619. [DOI] [PubMed] [Google Scholar]
- 220.Lerchen H-G, et al. Tailored linker chemistries for the efficient and selective activation of ADCs with KSPi payloads. Bioconjugate Chem. 2020;31:1893–1898. doi: 10.1021/acs.bioconjchem.0c00357. [DOI] [PubMed] [Google Scholar]
- 221.Karpov AS, et al. Discovery of potent and selective antibody-drug conjugates with Eg5 inhibitors through linker and payload optimization. ACS Med Chem Lett. 2019;10:1674–1679. doi: 10.1021/acsmedchemlett.9b00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Criscitiello C, Morganti S, Curigliano G. Antibody-drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol. 2021;14:20. doi: 10.1186/s13045-021-01035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Su Z, et al. Antibody–drug conjugates: recent advances in linker chemistry. Acta Pharmaceutica Sinica B. 2021;11:3889–3907. doi: 10.1016/j.apsb.2021.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Trapani D, Curigliano G. Accelerating progress in early triple-negative breast cancer: a viewpoint on antibody-drug conjugates, back from St Gallen breast cancer conference 2021. The Breast. 2021 doi: 10.1016/j.breast.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xiao D, et al. A bifunctional molecule-based strategy for the development of theranostic antibody-drug conjugate. Theranostics. 2021;11:2550–2563. doi: 10.7150/thno.51232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Joubert N, Denevault-Sabourin C, Bryden F, Viaud-Massuard M-C. Towards antibody-drug conjugates and prodrug strategies with extracellular stimuli-responsive drug delivery in the tumor microenvironment for cancer therapy. Eur J Med Chem. 2017;142:393–415. doi: 10.1016/j.ejmech.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 227.Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117:1736–1742. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Szot C, et al. Tumor stroma-targeted antibody-drug conjugate triggers localized anticancer drug release. J Clin Invest. 2018;128:2927–2943. doi: 10.1172/JCI120481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Love EA, et al. Developing an antibody-drug conjugate approach to selective inhibition of an extracellular protein. ChemBioChem. 2019;20:754–758. doi: 10.1002/cbic.201800623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Testa C. et al. First studies on tumor associated carbonic anhydrases IX and XII monoclonal antibodies conjugated to small molecule inhibitors. J Enzyme Inhib Med Chem. 10.1016/j.breast.2021.12.012 [DOI] [PMC free article] [PubMed]
- 231.Theocharopoulos C, Lialios P-P, Samarkos M, Gogas H, Ziogas DC. Antibody-drug conjugates: functional principles and applications in oncology and beyond. Vaccines. 2021;9:1111. doi: 10.3390/vaccines9101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dragovich PS. Antibody-drug conjugates for immunology. J Med Chem. 2022;65:4496–4499. doi: 10.1021/acs.jmedchem.2c00339. [DOI] [PubMed] [Google Scholar]
- 233.Hobson AD, et al. Design and development of glucocorticoid receptor modulators as immunology antibody-drug conjugate payloads. J Med Chem. 2022;65:4500–4533. doi: 10.1021/acs.jmedchem.1c02099. [DOI] [PubMed] [Google Scholar]
- 234.Dragovich PS. Antibody-drug conjugates for immunology. J Med Chem. 2022;65:4496–4499. doi: 10.1021/acs.jmedchem.2c00339. [DOI] [PubMed] [Google Scholar]
- 235.Lehar SM, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527:323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 236.Lim RKV, et al. Targeted delivery of LXR agonist using a site-specific antibody-drug conjugate. Bioconjug Chem. 2015;26:2216–2222. doi: 10.1021/acs.bioconjchem.5b00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Trudel S, et al. Targeting B-Cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma: a dose-escalation and expansion phase 1 trial (BMA117159) Lancet Oncol. 2018;19:1641–1653. doi: 10.1016/S1470-2045(18)30576-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lonial S, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–221. doi: 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 239.Swain SM, et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev. 2022;106:102378. doi: 10.1016/j.ctrv.2022.102378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None. This manuscript is a review.