Abstract
Rheumatic heart disease is an autoimmune sequela of group A streptococcal infection. Previous studies have established that streptococcal M protein is structurally and immunologically similar to cardiac myosin, a well-known mediator of inflammatory heart disease. In this study, we investigated the hypothesis that streptococcal M protein could produce inflammatory valvular heart lesions similar to those seen in rheumatic fever (RF). Fifty percent (3 of 6) of Lewis rats immunized with recombinant type 6 streptococcal M protein (rM6) developed valvulitis as well as focal lesions of myocarditis. Valvular lesions initiated at the valve surface endothelium spread into the valve. Anitschkow cells and verruca-like lesions were present. T cells from rM6-immunized rats proliferated in the presence of purified cardiac myosin, but not skeletal myosin. A T-cell line produced from rM6-treated rats proliferated in the presence of cardiac myosin and rM6 protein. The study demonstrates that the Lewis rat is a model of valvular heart disease and that streptococcal M protein can induce an autoimmune cell-mediated immune attack on the heart valve in an animal model. The data support the hypothesis that a bacterial antigen can break immune tolerance in vivo, an important concept in autoimmunity.
Rheumatic fever (RF) is an inflammatory disease that may result in immune attack of the heart following group A streptococcal pharyngitis (18, 26). In susceptible individuals, an immune response to a streptococcal antigen appears to initiate events that result in development of RF. Reports of increased incidence of RF continue in the United States (3, 28, 29; E. L. Kaplan, Editorial, Eur. J. Clin. Microbiol. Infect. Dis. 10:55–57, 1991), and it remains a major cause of heart disease in children worldwide (14). The new and continued outbreaks of RF have kindled new interest in elucidating mechanisms involved in the pathogenesis of the disease. The pathogenesis of rheumatic valvular heart disease is thought to be mediated by autoimmune mechanisms induced by streptococcal components, such as streptococcal M proteins and group A carbohydrate. The streptococcal antigens immunologically mimic heart antigens, such as cardiac myosin (1, 5, 6, 8, 10, 11, 21–23). Our previous work suggests that antibodies against cardiac myosin and N-acetyl-glucosamine, the dominant epitope of the group A carbohydrate, may play a role in injury to the valve surface endothelium (9). However, the valve lesions observed in RF contain large numbers of T cells infiltrating through the valve endothelium (20), and T cells from RF valves have been shown to proliferate in the presence of peptides from the A and B repeat regions of streptococcal M protein (11). In our study, evidence suggests that the Lewis rat is a model of valvular heart disease and supports the hypothesis that M protein-responsive T cells may be responsible in part for the pathogenesis of valvular heart disease in RF.
Although it is well established that the streptococcal M protein extends from the surface of the streptococcal cell as an alpha-helical coiled-coil dimer with structural homology to myosin and other alpha-helical coiled-coil molecules (16, 17), no studies have investigated the role of M protein in an animal model of valvular heart disease, the most serious sequela of RF. To our knowledge, no animal models of valvular heart disease have been reported. For these reasons, we investigated whether intact streptococcal rM6 protein could induce rheumatic-like inflammatory heart disease in the Lewis rat, an established model of cardiac myosin-induced myocarditis (15). In Lewis rats, streptococcal rM6 protein was shown to induce valvular heart lesions similar to those observed in rheumatic heart disease. Study of concomitant T-lymphocyte responses and a T-cell line from the M protein-immunized rats suggested that T cells responsive to M protein and cardiac myosin were present in the model and may be responsible for lymphocytic infiltration of the heart.
MATERIALS AND METHODS
Antigens.
Rabbit skeletal myosin , mouse laminin, rabbit skeletal tropomyosin, actin, and lysozyme were all purchased from Sigma Chemical Co., St. Louis, Mo.
Preparation of purified human cardiac myosin.
Cardiac myosin was purified from human heart tissue according to the method of Tobacman et al. (27), with slight modifications. Briefly, heart tissue was homogenized in a low-salt buffer (40 mM KCl, 20 mM imidazole [pH 7.0], 5 mM EGTA, 5 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg of leupeptin per ml) for 15 s on ice. The washed myofibrils were collected by centrifugation at 16,000 × g for 10 min. The pellet was then resuspended in high-salt buffer (0.3 M KCl, 0.15 M K2HPO4, 1 mM EGTA, 5 mM DTT, 0.5 mM PMSF, 1 μg of leupeptin per ml) and homogenized for three 30-s bursts on ice. The homogenized tissue was further incubated on ice with stirring for 30 min to facilitate actin-myosin extraction. Following clarification by centrifugation, actin-myosin was precipitated by addition of 10 volumes of cold water, followed by a pH adjustment to 6.5. DTT was added to 5 mM, and the precipitation was allowed to proceed for 30 min. The acto-myosin was then pelleted by centrifugation at 16,000 × g. The actin-myosin pellet was then resuspended in high-salt buffer, ammonium sulfate was increased to 33%, and the KCl concentration was increased to 0.5 M. After the actin-myosin pellet and salts were dissolved, ATP was added to 10 mM and MgCl2 was added to 5 mM, and then the solution was centrifuged at 20,000 × g for 15 min to remove actin filaments. The supernatant was removed and stored at 4°C in the presence of the following inhibitors: 0.5 mM PMSF, 5 μg of TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone) per ml, and 1 μg of leupeptin per ml. Human skeletal myosin was also purified by a similar procedure.
Immunization of Lewis rats.
Eight-week-old Lewis rats (Harlan Sprague-Dawley, Indianapolis, Ind.) were immunized intraperitoneally with 500 μg of purified recombinant type 6 M protein (provided by V. A. Fischetti) in complete Freund's adjuvant supplemented with 5 mg of heat-killed mycobacteria H37RA per ml as previously described for cardiac myosin-induced myocarditis (15). The rats received an intraperitoneal injection of 2 × 1010 B. pertussis cells as an additional adjuvant (15). Seven days later, the rats were boosted with 500 μg of the rM6 antigen in incomplete Freund's adjuvant. Negative control animals were immunized with phosphate-buffered saline (PBS) plus adjuvants. All rats were sacrificed 17 days after the initial immunization.
Histological examination of tissues.
Heart, liver, and kidneys were fixed in 10% buffered formalin and imbedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin for microscopic histological examination. Myocarditis and valvulitis lesions were scored as 1+ for 10% of tissue affected with focal lesions, 2+ for 25% of tissue affected with focal lesions, 3+ for 50% of tissue affected with lesions, or 4+ for confluent lesions affecting the majority of the tissue.
Lymphocyte proliferation assays.
The proliferative response of lymphocytes was measured in a tritiated [3H]thymidine incorporation assay as described previously (4). Lymphocytes were cultured in 96-well flat-bottom plates at 5 × 105 cells per well with 25 μg of antigen per ml and 2.5 × 105 mitomycin-treated spleen cells or 5 μg of phytohemagglutinin (PHA) per ml for 3 days. Lymphocyte samples were tested in triplicate, and the results were recorded as cpm with the background subtracted. Proliferation medium consisted of Iscoves's modified Dulbecco's medium (IMDM), 2% rat serum, 50 mM 2-mercaptoethanol, 100 U of penicillin per ml, and 100 mg of streptomycin per ml. Wells were pulsed with 1.0 μCi of tritiated thymidine (ICN, Irvine, Calif.) 18 h before being harvested onto filters with a cell harvester. Tritiated thymidine incorporation was measured in a liquid scintillation counter. Values represent the stimulation index (SI = mean of test cpm/mean of media control cpm). Medium controls in the proliferation assays ranged from 2,000 to 5,000 cpm.
Production and proliferation of T-cell lines.
Seventeen days after the initial immunization, popliteal and inguinal lymph nodes were asceptically removed and minced into a single-cell suspension. The cell suspension was washed and cultured for 3 days (2 × 106 cells/ml) with 25 μg of human cardiac myosin per ml in proliferation medium. Lymphocytes were recovered and resuspended in medium with 10% fetal bovine serum and 20 U of recombinant human interleukin-2 (IL-2) per ml (Cetus). The T-cell line was maintained by repeated cycles with antigen stimulation utilizing either rM6 protein or human cardiac myosin as the antigen and mitomycin C-treated syngeneic spleen cells. The T-cell line was cloned by limiting dilution at 0.5 cell/well in 96-well round-bottom plates. To measure the proliferative response of rat T-cell line M6.8, it was cultured at 5 × 104 cells per well in 96-well round-bottom plates with 20 μg of antigen and mitomycin C-treated spleen cells. Forty-eight hours later, tritiated thymidine was added, and the plates were harvested as described above for the lymph node assay.
RESULTS
Lewis rats develop focal myocarditis and valvulitis following immunization with streptococcal recombinant M6 protein (rM6).
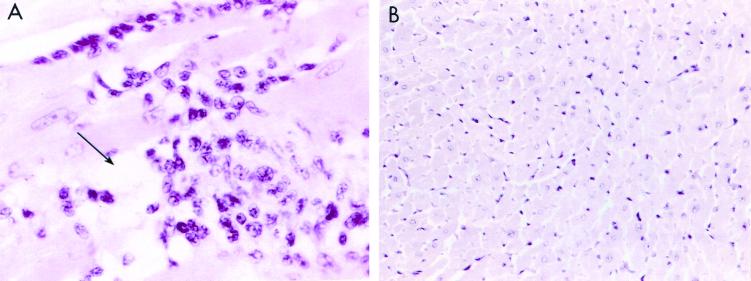
Examination of heart sections from the rM6-immunized rats revealed regions of focal myocarditis in 3 of 6 animals (Fig. 1A and Table 1). Figure 1 illustrates an example of a focal lesion with cellular infiltrate in a section of rat myocardium stained with hematoxylin and eosin. Focal lesions were scattered throughout the rat myocardium containing interstitial accumulations of mononuclear cells intermixed with a lesser number of neutrophils. Myocyte necrosis (arrow in Fig. 1A) is noted in the central lesion. Heart tissue sections from control rats immunized with PBS and adjuvants had no cellular infiltrate and illustrate normal myocardium (Fig. 1B).
FIG. 1.
Illustration of myocarditis in Lewis rat heart sections stained with hematoxylin and eosin. (A) Myocarditis is shown in rats immunized with streptococcal rM6 protein (magnification, ×400). Focal lesions were observed scattered throughout the rat myocardium, which contained interstitial accumulations of mononuclear cells intermixed with a lesser number of neutrophils. Myocyte necrosis (arrow) is noted in the central lesion. (B) Heart tissue sections from control rats immunized with PBS and adjuvants contained no lesions (magnification, ×400). Although not shown, lesions were not found in kidneys and livers of any of the animals.
TABLE 1.
Myocarditis and valvulitis in Lewis rats immunized with streptococcal M6 proteina
| Antigen | No. of rats with/no. tested
|
|
|---|---|---|
| Myocarditis | Valvulitis | |
| rM6b | 3/6 | 3/6 |
| PBS | 0/6 | 0/6 |
| Cardiac myosin | 6/6 | 10/23 |
All rats were sacrificed on day 17.
Recombinant type 6M protein.
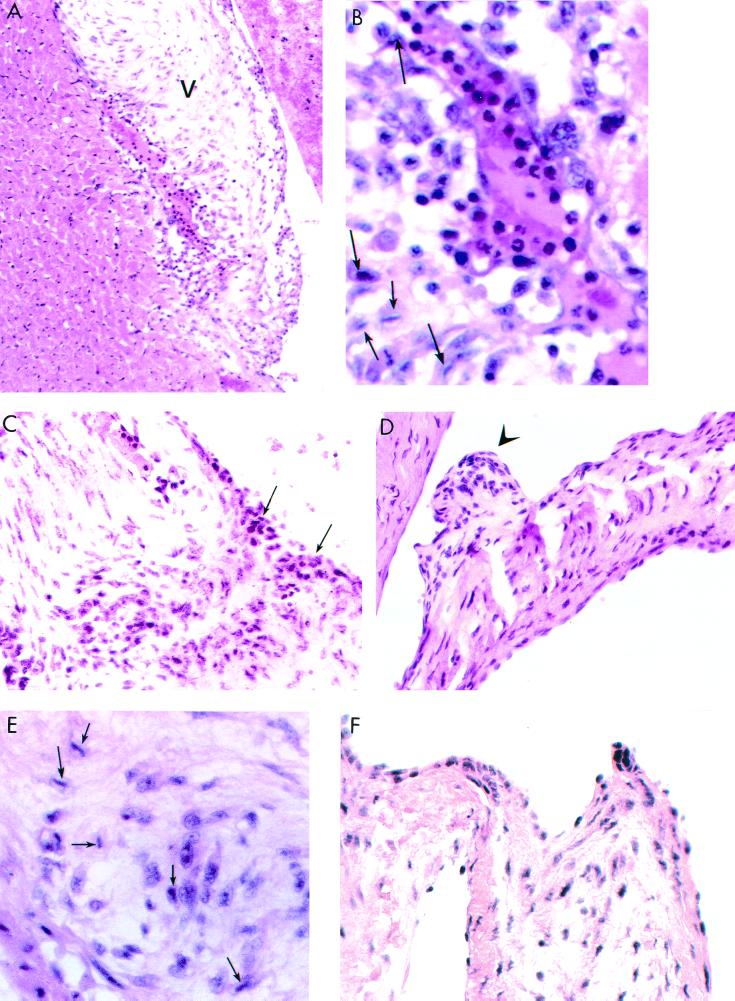
Most interesting was the observation of valvulitis in the mitral valves of rM6-immunized rats. Sections of rat hearts were stained with hematoxylin and eosin and microscopically evaluated for cellular infiltrates in the valves. Cardiac valvulitis seen in 3 of 6 rats was characterized by infiltrating mononuclear cells and neutrophils (Fig. 2A to F and Table 1). Figures 2A and B demonstrate valvulitis in the base of the mitral valve (V) adjacent to myocardium, which did not contain lesions. Figure 2A illustrates a lower magnification for the orientation of the valve and myocardium. The enlargement in Fig. 2B shows the presence of mononuclear and Anitschkow cells (arrows). Anitschkow cells are characteristically seen in hematoxylin- and eosin-stained sections of rheumatic hearts, and they are termed “owl-eye” cells because of their appearance due to a condensed nucleus. Figure 2C shows the infiltration of the mitral valve through the endothelium at the valve surface. Apparently, lymphocytes and neutrophils infiltrated the valve through the endothelial surface and not from the myocardium, since no lesions were found in the myocardium adjacent to the valve. Figure 2D illustrates a verruca-like nodule on the valve surface, while Fig. 2E shows Anitschkow cells (arrows). Verrucae are raised nodular lesions seen around the base of the valve in rheumatic carditis. Figure 2F shows a hematoxylin- and eosin-stained heart valve tissue section from control rats immunized with PBS and adjuvants. Figure 2F shows that the valve from adjuvant-immunized rats was normal with no cellular infiltrates. The myocardial and valve lesions appeared to be heart specific, since no lesions were present in hematoxylin- and eosin-stained tissue sections from livers and kidneys of rM6-immunized rats. In the histopathologic evaluation, it was noted that only the mitral valve was affected and that the other valves viewed were normal. Due to the small size of the rat hearts, it was impossible to view all valves in a single animal.
FIG. 2.
Illustration of valvulitis and cellular infiltration in hematoxylin- and eosin-stained mitral valves from Lewis rats immunized with streptococcal rM6 protein. (A and B) Valvulitis in the base of the mitral valve (V) adjacent to myocardium, which did not contain lesions. The lower magnification in panel A orients the valve (V) adjacent to the myocardium. The enlargement in panel B shows the presence of mononuclear and Anitschkow (arrows) cells. (C) Arrows indicate disruption of endocardial (endothelial) surface with infiltrating cells. (D) Verruca-like nodule (arrow) on the valve surface. (E) Anitschkow cells (arrows). (F) Hematoxylin- and eosin-stained normal heart valve tissue section from control rats immunized with PBS and adjuvants. Magnifications, ×200 and ×400.
In summary, 50% of the rats immunized with rM6 protein developed myocardial and valvular lesions, while no histological changes were observed in any tissues of the PBS-adjuvant control animals (Fig. 1B and 2F and Table 1). As a positive control, Lewis rats immunized with 500 μg of human cardiac myosin and adjuvants developed 3+ to 4+ myocarditis in 100% of the immunized animals (Table 1).
In a separate study, we found that valvulitis occurred in 10 of 23 and 6 of 13 (approximately 45%) Lewis rats immunized with human or rat cardiac myosin, respectively. The valvulitis caused by cardiac myosin was locally severe in the valve, with an average of grade 3+ lesions containing mononuclear cells and macrophages. However, Lewis rats immunized with human skeletal myosin or rabbit skeletal tropomyosin did not develop myocarditis or valvultitis in 0 of 6 rats. In addition, 0 of 3 rats given murine laminin did not develop myocarditis or valvulitis. The data show that only human and rat cardiac myosins and recombinant streptococcal M protein produced inflammatory heart disease. Other alpha-helical coiled-coil proteins, such as human skeletal myosin, rabbit skeletal tropomyosin, or mouse laminin, did not produce inflammatory heart disease in the Lewis rat model. Therefore, the disease-producing factor was not the alpha-helical coiled-coil structure, but epitopes related to the heart in cardiac myosins and streptococcal M protein.
Proliferative responses of lymphocytes from rM6-immunized rats to cardiac myosin and rM6 protein.
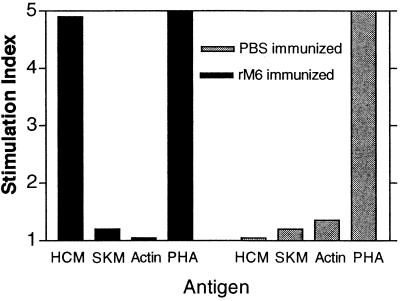
Cardiac myosin-reactive lymphocytes were present in Lewis rats after immunization with streptococcal rM6 protein. Lymph node cells of rM6-immunized rats were reacted with human cardiac myosin, and the proliferative response was measured with the tritiated thymidine incorporation assay. The lymphocytes from rM6-immunized rats proliferated in response to human cardiac myosin, but not to rabbit skeletal myosin or to actin (Fig. 3). Lymphocytes from adjuvant control animals did not proliferate in response to any of the three antigens. Interestingly, cardiac myosin and skeletal myosin share extensive regions of sequence identity, but only cardiac myosin has been shown to induce myocarditis in animals (19, 25).
FIG. 3.
Lymphocytes from rM6-immunized rats proliferate in the presence of human cardiac myosin. Lymphocytes from inguinal and popliteal lymph nodes of rats immunized and boosted with 500 μg of rM6 were reacted with human cardiac myosin (HCM), rabbit skeletal myosin (RSM), and actin in the tritiated thymidine incorporation assay as described in Materials and Methods. Values represent the SI (mean of test cpm/mean of medium control cpm). Medium controls in the proliferation assays ranged from 2,000 to 5,000 cpm.
Characterization of T cells from rM6-immunized Lewis rats.
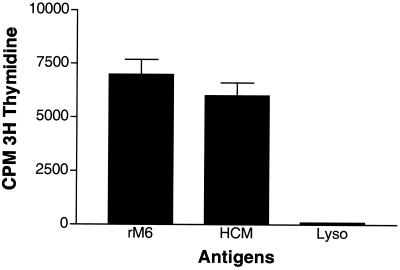
To further demonstrate that streptococcal M protein and cardiac myosin-responsive T cells were present in the rM6-immunized rats, a T-cell line was produced from lymph node T cells of rats immunized with rM6 protein. T-cell line M6.8 was maintained in culture by cycles of antigenic stimulation with human cardiac myosin or rM6 protein followed by expansion in IL-2-containing medium. The line was subcloned and kept in culture for more than 6 weeks before being characterized. T-cell line M6.8 proliferated in the presence of streptococcal rM6 protein and human cardiac myosin, but was not stimulated by lysozyme or medium alone (Fig. 4). In further experiments, T-cell line M6.8 was shown to proliferate to cardiac myosin in a dose-dependent fashion (data not shown). Analysis by flow cytometry revealed that line M6.8 was CD3+, alpha-beta T-cell receptor positive (TCR-αβ+) and CD4+ (Table 2). In conclusion, a CD4+ T-cell line from rM6-immunized rats was responsive to both M protein and cardiac myosin.
FIG. 4.
Response of rat T-cell line M6.8 to rM6 protein and human cardiac myosin. The proliferative response of line M6.8 was measured with a tritiated [3H] thymidine uptake assay. T-cell line M6.8 was cultured as 5 × 104 cells per well with 20 μg of antigen per ml. rM6, purified type 6 M protein; HCM, human cardiac myosin; Lyso, lysozyme plus 2.5 × 105 mitomycin-treated spleen cells. Samples were tested in triplicate, and the results were recorded as cpm with the background subtracted. Error bars are shown.
TABLE 2.
Characterization of T-cell line M6.8
| Surface phenotype | % Positivea |
|---|---|
| CD3 | 92.0 |
| CD4 | 89.4 |
| CD8 | 7.8 |
| TCR-αβ | 93.3 |
| TCR-γδ | 2.4 |
| Immunoglobulin control | 0.0 |
Flow cytometry.
DISCUSSION
Our report describes an animal model of valvular heart disease, which to our knowledge has not been described previously. In addition, our novel observations in the Lewis rat show that streptococcal M protein induced valvular heart disease that strongly resembled valve disease in RF. Following immunization with rM6 protein, striking cellular infiltration of the valve was observed with focal lesions in myocardium. The cellular infiltrate entered through the valve surface (Fig. 2C), suggesting that the valve endothelium is an important location for entry of inflammatory cells rather than entry from myocardium. The sensitivity of Lewis rats to valvular heart disease is novel and will be a potentially useful and powerful tool with which to study rheumatic and immune-mediated valvular heart disease.
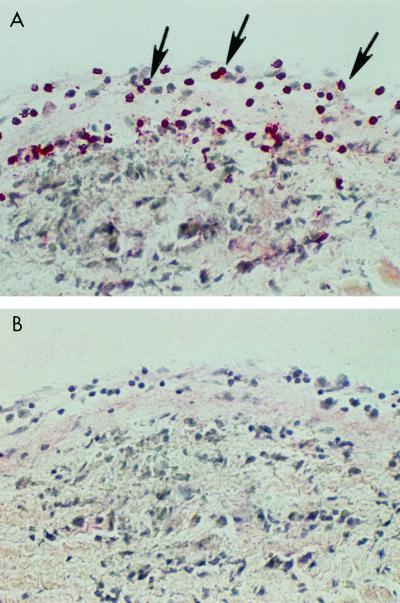
Histologic evaluation of rat myocardium in valvular heart disease revealed normal myocardium adjacent to the diseased valve as shown in Fig. 2A. In human rheumatic heart disease, entry through the valve surface was also seen, as shown in Fig. 5 and reference 20, and it was comparable to that seen in our study of the Lewis rat model. In Fig. 5, a human rheumatic heart valve section was reacted with anti-CD4+ antibody and is shown for comparison with the rat valve sections. The human rheumatic valve section illustrates the infiltration of CD4+ lymphocytes (stained with fast red) through the endothelium into the valve and the presence of a necrotic Aschoff body in the valve tissue. We have found CD4+ and CD8+ T cells present in valves in PepM5-induced rat valvulitis (unpublished data). We wanted to examine the CD4+ and CD8+ lymphocytes in rM6-induced rat valvulitis. However, limitations due to formalin-fixed tissues in the rM6 study prevented this analysis.
FIG. 5.
Human rheumatic heart valve section reacted with anti-CD4+ antibody shown for comparison with the rat valve sections. The human rheumatic valve section (A) illustrates the infiltration of CD4+ lymphocytes (stained with fast red) through the endothelium into the valve and the presence of a necrotic Aschoff body in the valve tissue. A rheumatic valve section was reacted with a control immunoglobulin G1 antibody and did not show any reactivity (B). Arrows point to CD4+ T lymphocytes entering the valve.
Our data continue to confirm a relationship between epitopes of streptococcal M protein and cardiac myosin. The similarity between M protein and cardiac myosin is significant enough to produce inflammatory heart disease in the Lewis rats. In a separate study in our laboratory, we have identified A repeat region sequences that produce valvulitis in Lewis rats, while B and C repeat region sequences did not produce valvular heart disease (manuscript in preparation). Furthermore, in studies by Guilherme et al., sequences in the A and B repeat regions of M5 protein were reported to stimulate T cells from rheumatic valves (11). Conclusions from tests of M protein sequences indicate that only A and B repeat regions contain pathogenic epitopes responsible for valvular or myocardial disease.
Previous studies with mice have also identified myocarditic epitopes of M5 protein in the A and B repeat regions of M protein (8). Mice developed focal myocarditis but no valvular heart disease from immunization with specific M5 peptides located within the A and B repeat regions of the M5 protein molecule. However, C repeat region sequences of the streptococcal M protein did not produce myocardial lesions, most likely due to the protein's homology with skeletal myosins, which do not produce heart disease (8). Although many M protein epitopes have been shown to be cross-reactive with myosin, only peptides sharing sequence homology with cardiac myosins produced myocardial lesions in mice (8, 12). Repeated regions of M proteins that share homology with only cardiac myosins may break tolerance to cardiac myosin and induce myocardial disease (12).
In the Lewis rat model of M protein-induced heart disease, we were able to establish a T-cell line that proliferated in response to both human cardiac myosin and M protein. The T-cell line gives an important demonstration of potential T-cell cross-reactivity between M protein and cardiac myosin and its link with production of heart disease by group A streptococci. In support of cross-reactive T cells in valve lesions, T cells from valves of RF patients have been shown to proliferate in the presence of peptides of streptococcal M5 protein and heart tissue antigens (11).
Since cardiac myosin is not thought to be present in the valve, how mimicry between M protein and cardiac myosin produces valvular heart disease is an important question. Our studies suggest that laminin links myosin with the valve. Recently, a cytotoxic antimyosin and antistreptococcal monoclonal antibody from rheumatic carditis was shown to recognize laminin, an extracellular matrix alpha-helical coiled-coil protein that is an integral part of the valve structure (2, 7, 9). Evidence presented in previous work supports the hypothesis that laminin, present in the basement membrane of the valve and secreted by endothelial cells, is a target of cross-reactive antimyosin, antistreptococcal antibody. Laminin present in valves may cross-react with antimyosin T cells and antibody that recognizes M protein, myosin, and laminin. Because cardiac myosin is an intracellular molecule, peptides of it may be presented to T cells during turnover in cardiac tissues (24).
In summary, this work is important in establishing a model of autoimmune valvulitis whereby rheumatic heart disease and its parameters can be investigated. The evidence supports molecular mimicry as a potential model of inflammatory heart disease following group A streptococcal infection. Finally, the investigation of an animal model of rheumatic carditis and molecular mimicry is vital to our understanding of how infectious agents contribute to autoimmune disease.
ACKNOWLEDGMENTS
We thank Mark Hemric for purification of human cardiac and skeletal myosin and Janet Heuser for expert technical assistance.
This work was supported by grants HL35280 and HL56267 to M.W.C. from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Adderson E E, Shikhman A R, Ward K E, Cunningham M W. Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetyl-glucosamine/anti-myosin antibody V region genes. J Immunol. 1998;161:2020–2031. [PubMed] [Google Scholar]
- 2.Antone S M, Adderson E E, Mertens N M J, Cunningham M W. Molecular analysis of V gene sequences encoding cytotoxic anti-streptococcal/anti-myosin monoclonal antibody 36.2.2 that recognizes the heart cell surface protein laminin. J Immunol. 1997;159:5422–5430. [PubMed] [Google Scholar]
- 3.Ayoub E M. Resurgence of rheumatic fever in the United States. Postgrad Med. 1992;92:133–142. doi: 10.1080/00325481.1992.11701445. [DOI] [PubMed] [Google Scholar]
- 4.Coligan J E, Kruisbeck A D, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. 1, section 3.12. New York, N.Y: Greene Publishing and Wiley-Interscience; 1994. [Google Scholar]
- 5.Cunningham M W. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham M W. Streptococci and rheumatic fever. In: Rose N R, Friedman H, editors. Microorganisms and autoimmune disease. New York, N.Y: Plenum Publishing Corp.; 1996. pp. 13–66. [Google Scholar]
- 7.Cunningham M W, Antone S M, Gulizia J M, McManus B M, Fischetti V A, Gauntt C J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham M W, Antone S M, Smart M, Liu R, Kosanke S. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. 1997;65:3913–3923. doi: 10.1128/iai.65.9.3913-3923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvin J E, Hemric M E, Ward K, Cunningham M W. Cytotoxic monoclonal antibody from rheumatic carditis reacts with human endothelium: implications in rheumatic heart disease. J Clin Investig. 2000;106:470–511. doi: 10.1172/JCI7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibofsky A, Kerwar S, Zabriskie J B. Rheumatic fever: the relationships between host, microbe and genetics. Rheum Dis Clin N Am. 1998;24:237–259. doi: 10.1016/s0889-857x(05)70007-7. [DOI] [PubMed] [Google Scholar]
- 11.Guilherme L, Cunha-Neto E, Coelho V, Snitcowsky R, Pomerantzeff P M A, Assis R V, Pedra F, Neumann J, Goldberg A, Patarroyo M E, Pileggi F, Kalil J. Human heart-filtrating T cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation. 1995;92:415–420. doi: 10.1161/01.cir.92.3.415. [DOI] [PubMed] [Google Scholar]
- 12.Huber S A, Cunningham M W. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsackieviral myocarditis. J Immunol. 1996;156:3528–3534. [PubMed] [Google Scholar]
- 13.Hu-Li J, Ohara J, Watson C, Tsang W, Paul W E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 14.Kaur S, Kumar D, Grover A, Khanduja K L, Kaplan E L, Gray E D, Ganguly N K. Ethnic differences in expression of susceptibility marker(s) in rheumatic fever/rheumatic heart disease patients. Int J Cardiol. 1998;64:9–14. doi: 10.1016/s0167-5273(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 15.Kodama M, Matsumoto Y, Fujiwara M, Massani M, Izumi T, Shibota A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1991;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 16.Manjula B N, Fischetti V A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980;151:695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manjula B N, Trus B L, Fischetti V A. Presence of two distinct regions in the coiled-coil structure of the streptococcal Pep M5 protein: relationship to mammalian coiled-coil proteins and implications to its biological properties. Proc Natl Acad SciUSA. 1985;82:1064–1068. doi: 10.1073/pnas.82.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minich L L, Tani L Y, Pagotto L T, Shaddy R E, Veasy L G. Doppler echocardiography distinguishes between physiologic and pathologic “silent”mitral regurgitation in patients with rheumatic fever. Clin Cardiol. 1997;20:924–926. doi: 10.1002/clc.4960201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neu N, Rose N R, Beisel K W, Herskowitz A, Gurri-Glass G, Craig S W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 20.Roberts S, Kosanke S, Dunn S T, Jankelow D, Duran C M G, Cunningham M W. Immune mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis. 2001;183:507–511. doi: 10.1086/318076. [DOI] [PubMed] [Google Scholar]
- 21.Shikhman A R, Cunningham M W. Immunological mimicry between N-acetyl-beta-D-glucosamine and cytokeratin peptides. Evidence for a microbially driven anti-keratin antibody response. J Immunol. 1994;152:4375–4387. [PubMed] [Google Scholar]
- 22.Shikhman A R, Greenspan N S, Cunningham M W. Cytokeratin peptide SFGSGFGGGY mimics N-acetyl-beta-D-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. J Immunol. 1994;153:5593–5606. [PubMed] [Google Scholar]
- 23.Shikhman A R, Greenspan N S, Cunningham M W. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-D-glucosamine. J Immunol. 1993;151:3902–3913. [PubMed] [Google Scholar]
- 24.Smith S C, Allen P M. Expression of myosin-class II major histocompatibility complexes in the normal myocardium occurs before induction of autoimmune myocarditis. Proc Natl Acad Sci USA. 1992;89:9131–9135. doi: 10.1073/pnas.89.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith S C, Allen P M. Myosin-induced acute myocarditis is a T cell mediated disease. J Immunol. 1991;147:2141–2147. [PubMed] [Google Scholar]
- 26.Stollerman G H. Rheumatic and heritable connective tissue diseases of the cardiovascular system. In: Braunmald E, editor. Heart disease: a textbook of cardiovascular medicine. Vol. 11. Philadelphia, Pa: W. B. Saunders; 1988. pp. 1706–1734. [Google Scholar]
- 27.Tobacman L S, Adelstein R S. Enzymatic comparisons between light chain isozymes of human cardiac myosin subfragment-1. J Biol Chem. 1984;259:11226–11230. [PubMed] [Google Scholar]
- 28.Veasy L G, Tani L Y, Hill H R. Persistence of acute rheumatic fever in the intermountain area of the United States. J Pediatr. 1994;124:9–16. doi: 10.1016/s0022-3476(94)70247-0. [DOI] [PubMed] [Google Scholar]
- 29.Veasy L G, Wiedmeier S E, Orsmond G S. Resurgence of acute rheumatic fever in the intermountain area of the United States. N Engl J Med. 1987;316:421–427. doi: 10.1056/NEJM198702193160801. [DOI] [PubMed] [Google Scholar]