Abstract
Among the rheumatic diseases whose symptoms are more often associated with the possibility of cancer and other malignancies are systemic sclerosis, dermatomyositis and rheumatic polymyalgia. However, a differential diagnosis should be performed in each case of non-typical rheumatic disease and/or other neoplastic disease risk factors. The article’s aim was based on a literature review of this subject and presentation own a case description and discussion about arthritis as a paraneoplastic syndrome.
The conclusions of our analysis were as follows: more often paraneoplastic arthritis occurs in men, in ages higher than 50 years old, in patients who poorly respond to treatment of arthritis with polyarticular symmetrical involvement of the limbs, seronegative type of inflammatory joint disease. In this group of patients, complete remission after treatment of the primary tumor and recurrence of the symptoms in the presence of metastasis was observed.
Keywords: rheumatoid arthritis, psoriatic arthritis, paraneoplastic arthritides, neoplasia
Introduction
Paraneoplastic syndromes are heterogeneous and difficult to diagnose entities, especially in rheumatological practice where they act as mimickers of commoner clinical conditions. The inflammatory joint manifestations are the result of complex immunological mechanisms given the presence of the neoplasm in the absence of evident tumoral localization in the affected joint.
Herein we present the case of oligo- and seronegative arthritis with hand and atlantoaxial joint involvement in a patient with cutaneous psoriasis subsequently diagnosed with a neuroendocrine tumor of the ileocecal valve.
Furthermore, we perform a systematic review on paraneoplastic arthritis mimicking other rheumatological conditions pointing out the most relevant clinical criteria to distinguish them from non-paraneoplastic disorders.
Material and methods
We extensively searched via PubMed articles using as key words “arthritis”, “rheumatoid arthritis”, “psoriatic arthritis”, “spondylarthritis” each combined with “tumor”, “cancer”, “paraneoplastic”. Given the rarity of the clinical manifestation, we decided to also include case reports in this review as well.
Studies that were not written in the English language and that were present only in the form of abstracts were not included. We set as reference the latest classification criteria for rheumatoid arthritis (RA) [1], psoriatic arthritis (PSA) [2] and axial spondylarthritis (AxSPA) [3].
We included studies with clinical and imaging proven arthritis. We excluded RS3PE, palmar fasciitis and digital clubbing from this review since in the literature are reported numerous evidences that correlate these pathologies to neoplasms [4]. Moreover, papers reporting cases of patients in treatment with checkpoint inhibitors were excluded beforehand. Informed written consent was obtained from the patient.
Results
We found 35 articles, for a total of 134 patients affected by a neoplasm presenting with articular inflammatory manifestations, most frequent type of tumor concomitant to an articular paraneoplastic manifestation was lung adenocarcinoma, followed by hematological malignancies and urinary tract cancers. Patients mean age was 53.6 ±17.23 years old, 60% males and 40% females, mean anti-citrullinated protein antibodies (ACPA) levels 134.8 ±70 IU/ml (6), mean rheumatoid factor (RF) 378.9 ±395.6 IU/ml (6), mean anti-nuclear antibody (ANA) titres 1 : 160 (only 3 positive), mean C-reactive protein (CRP) 8.72 ±7.01 mg/dl, mean erythrocyte sedimentation rate (ESR) 73.06 ±34.43 mm/h.
Moreover, 6 patients had an asymmetrical polyarthritis, 72 a symmetrical polyarthritis, 18 monoarthritis and 35 oligoarthritis, 76.4% displayed a poor or partial response to non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticosteroids (GCs) or conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), 17.6% displayed a good response.
Conversely, 39.47% of patients experienced a full remission after chemotherapy o surgery or both, 21% displayed an improvement of symptoms while 18.4% had a poor response to tumor treatment. In the examined papers only 26 patients had a definitive diagnosis of rheumatic disease: 20 RA, 3 definet as a pseudogout, 2 AxSPA, 1 reactive arthritis. Detailed results are displayed in Tables I–IV.
Table I.
Epidemiological features of paraneoplastic patients’ phenotype
| Overall patients | Male/female | Mean age | Mimicker | ACPA | RF | ANA | CRP | ESR | Involvement | Response to AT(NSAIDs/GCs/csDmards) | Response to TT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 134 | 81 M (60%)/47 F (35%)6 unknown (5%) | 53.6 ±17.23 | 20 RA 3 pseudogout 2 AxSPA 1 reactive arthritis |
134.8 ±70 UI/ml(6) |
378.9 ±395.6 UI/ml(6) |
1 : 160(3) | 8.72 ±7.01* mg/dl | 73.06 ±34.43* | 72 symmetrical polyarthritis 35 oligoarhtiris 18 monoarhtritis 6 asymmetrical polyarthritis |
76.4% partial/poor 17.6% good 8.8% n.a. |
39.47% remission 21% improvement 18.4% poor 18% n.a. |
Approximately.
ACPA – anti-citrullinated protein antibodies, ANA – anti-nuclear antibodies, AxSPA – axial spondylarthritis, AT – arthritis treatment, CRP – C-reactive protein, csDmards – conventional synthetic disease-modifying antirheumatic drugs, ESR – erythrocyte sedimentation rate, GCs – glucocorticosteroids, NSAIDs – non-steroidal anti-inflammatory drugs, RA – rheumatoid arthritis, RF – rheumatoid factor, TT – tetanus toxoid.
Table IV.
Clinical, hematological and anthropological features of paraneoplastic arthritides
| Author | CRP | ESR | Mimicker | Age | Arthritis treatment | Response to AT | Tumor medical treatment | Tumor surgical treatment | Response of articular manifestations to tumor treatments |
|---|---|---|---|---|---|---|---|---|---|
| Longley et al. 1986 [26] | n.a. | n.a. | n.a. | 49 (g.u.) | NSAIDs, GCs | Partial | n.a. | n.a. | n.a. |
| Lambert, Nuki 1992 [39] | n.a. | 43 | RA | 63 M | NSAIDs, cyclophosphamide | Partial | CHT (nos) | Penis amputation | n.a. |
| Cohen et al. 1993 [27] | n.a. | 73 | AxSPA | 61 F | Piroxicam, SSZ, prednisone, AZT | n.a. | n.a. | n.a. | n.a. |
| Drenth et al. 1995 [38] | n.a. | 135 | RA | 49 F | Naproxen, indometacin | Poor | Oral cyclophosphamide (100 mg/m2 days 1–14), 5-fluorouracil (600 mg/m2 days 1 and 8), methotrexate (40 mg/m2 days 1 and 8), and prednisone (30 mg/day) | n.p. | Remission after 3 months of CHT |
| Stummvoll et al. 2001 [5] | 4.6 (1), 2.9 (2) | 53 (1), 35 (2) | RA (1) | 60 M | Diclofenac (1), Diclofenac (2) | Poor (1) Partial (2) | Cisplatin and etoposide soldesam, 5HT3 inhibitors (1), 5-fluorouracil and leucovorin (2) | Lobectomy and lymphadenectomy (1), surgery (2) | Response after chemotherapy (1), response after surgery (2) |
| Lima et al. 2002 [31] | n.a. | n.a. | n.a. | 35 F | GCs | Poor | Cyclophosphamide, doxorubicin, vincristine, and prednisone | n.ap. | Improvement |
| Glinkov et al. 2003 [6] | n.a. | n.a. | n.a. | 23 F | GCs, NSAIDs | Poor | n.a. | Resectomy | Full remission |
| Mok, Kwan 2003 [7] | 2.2 | 80 | RA | 69 F | NSAIDs, SSZ, GCs | Poor to NSAIDs, partial to SSZ and GCs | n.p. | n.p. | Partial improement |
| Wiese et al. 2004 [40] | n.a. | 26 | Reactive arthritis | 34 F | NSAIDs | Poor | n.p. | Resectomy | Full remission |
| Ardalan, Shoja 2007 [28] | n.a. | 53 | RA | 47 M | MPPT, PDN | Poor | VAD | n.a. | Full remission |
| Bivalacqua et al. 2007 [21] | n.a. | n.a. | RA | 52 M | n.a. | n.a. | n.a. | Lobectomy + cystectomy | Full remission |
| Cantini et al. 2007 [16] | n.a. | n.a. | n.a. | 57 M | NSAIDs | Poor | n.a. | Resectomy | Full remission |
| Morel et al. 2008 [15] | ↑ | n.a. | RA | 57 (16 M/10 F) | NSAIDs, GCs, csDMARDs | NSAIDS – good (45%) GCs –excellent (91%) csDMARDs (poor) | n.a. | n.a. | n.a. |
| Tedeschi et al. 2017 [29] | 19.1 | 115 | Pseudo gout | 75 M | Naproxen, MPPT colchicine, GCs injections | Partial | Azacitidine | n.a. | Poor |
| Zupancic et al. 2008 [17] | 1.5 | 106 | n.a. | 43 M | Naproxen, prednisone | Poor | Cisplatin + etoposide | n.p. | Good |
| Bahat et al. 2009 [9] | n.a. | n.a. | RA | 72 M (1), 63 F (2) | NSAIDS, PDN, MTX, plaquenil (1) | Good (1), poor (2) | COP (1), 5-FU (2) | n.a. (1), palliative (2) | Partial (1), good (2) |
| Kumar et al. 2009 [8] | 19.1 | 115 | RA | 58M | Naproxen, MPPT, colchicine, GCs injections | Partial | Azacitidine | n.a. | Poor |
| Larson et al. 2011 [19] | 3.4 | 35 | RA | 45 F | Ibuprofen, GCs | Poor | n.a. | n.a. | n.a. |
| Kobak 2013 [25] | 1.5 | 106 | n.a. | 43 M | Naproxen, prednisone | Poor | Cisplatin + etoposide | n.p. | Good |
| Raja et al. 2010 [37] | n.a. | n.a. | RA | 40 F | NSAIDS, PDN, MTX, plaquenil (1) | Good (1), poor (2) | COP (1), 5-FU (2) | n.a. (1), palliative (2) | Partial (1), good (2) |
| Han et al. 2012 [18] | Normal | 44 | RA | 55 F | n.a. | n.a. | Gefitinib | n.a. | Good |
| Aruch et al. 2013 [32] | n.a. | n.a. | n.a. | 38 M | Dexamethasone | Poor | ABVD | n.a. | Full remission |
| Ochi et al. 2012 [10] | 15.6 | 104 | RA | 71 M | n.a. | n.a. | n.a. | Total gastrectomy | Full remission after surgery |
| Prashanth et al. 2013 [33] | Normal | Normal | n.c. | 10 M | Aspirin, naproxen, MPPT | Poor | n.a. | n.a. | Full remission with therapy |
| Handy et al. 2015 [34] | 7.1 | 122 | RA | 61 F | NSAIDs, GCs | Poor | CYC, vincristine, doxorubicin, and dexamethasone | n.ap. | Poor |
| Kisacik et al. 2014 [22] | 65.1 ±85.5 | 58.5 ±31.9 | RA | 58.02 ±15.3 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Watson et al. 2015 [20] | 11.8 (1), 1.2 (2) | 21 (1), n.a. (2) | RA (1 + 2) | 80 F (1), 71 F (2) | NSAIDs, GCs | Poor to NSAIDs, good to MPPT (1), poor to NSAIDs and GCs (2) | RT and CHT nos (1) | n.a. (1), lobectomy (2) | Remission (1), Remission after surgery (2) |
| Erlij et al. 2016 [35] | 110 | RA | 46 M | MPPT, prendisolone | Partial | Doxorubicin, bleomycin, vinblastine and dacarbazine | n.ap. | Full remission | |
| Levi Sandri et al. 2016 [13] | n.a. | n.a. | RA | 19 M | n.a. | n.a. | n.a. | n.a. | n.a. |
| Gamage et al. 2018 [36] | ↑ (n.r.) | ↑ (n.r.) | RA | 45 M | NSAIDs, colchicine | Poor | RCHOP | n.i. | Full remission |
| Iqbal et al. 2018 [30] | ↑ (n.r.) | ↑ (n.r.) | Pseudo-gout | 83 M | GCs | Good | Azacitidine | n.ap. | Full remission |
| Eidenschink et al. 2019 [24] | 15.6 | 69 | n.a. | 73 M | Naproxen | Partial | n.a. | n.a. | Improvement |
| Briones-Figueroa et al. 2019 [14] | 9.5 | 51 | n.a. | 69 M | GCs (prednisone) | Poor | RT and CHT | n.p. | Improvement |
| Rabah et al. 2020 [11] | 10.43 | 120 | RA | 83 M | NSAIDs, PDN | Poor | n.a. | n.a. | n.a. |
| Sachdev Manjit Singh et al. 2021[23] | n.a. (1) | 61 (1), raised (2) | SPA (1), RA (2) | 65 F (1), 64 M (2) | PDN, SSZ (1 + 2) | Poor (1) | Gefitinib (1), carboplatin + paclitaxel + gemcitabine (2) | n.p. | Improvement (1), n.a. (2) |
| Silvèrio-Antònio et al. 2021 [12] | 17.3 | 94 | RA | 64 M | Dexamethasone, HCQ, PDN | Good | Cisplatin, 5-FU, trastuzumab | n.p. | Poor |
ABVD – doxorubicin, bleomycin, vinblastine, and dacarbazine, CHT – choline transporter, CYC – cyclophosphamide, GCs – glucocorticosteroids, F – female, HCQ – hydroxychloroquine, M – male, MPPT – methylprednisolone, MTX – methotrexate, n.a. – not assessed, n.p. – not performed, NSAIDs – non-steroidal anti-inflammatory drugs, PDN – prednisone, RA – rheumatoid arthritis, RCHOP – rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone, SPA – spondyloarthropathy, SSZ – salazopyrin, 5-FU – fluoropyrimidine 5-fluorouracil.
Table III.
Clinical and immunological features of paraneoplastic inflammatory manifestations
| Author | Study type | Tumor(s) | N | TNM | Arthritis | Inolved joints | ACPA | RF | ANA | ENA | Neo-plastic markers | Other signs | Outcome/median survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Longley et al. 1986 [26] | CS | Myeloproliferative disease | 2 | n.a. | Asymmetrical polyarthritis | Wrist, MCP, IFJ, knee, ankle | n.a. | Neg. | Neg. | Neg. | n.a. | Cutaneous vasculitis | Death |
| Lambert, Nuki 1992 [39] | CR | Penis squamous carcinoma | 1 | n.a. | Symmetrical polyarthritis | Hands, wrist, knees | n.a. | Neg. | n.a. | n.a. | n.a. | Multicentric reticulohystiocytosis | Death |
| Cohen et al. 1993 [27] | CR | Lymphocytic lymphoma | 1 | IV | Axial arthritis | Bilaterla SIJ, hips, lumbar and cervical spine, sternoclavear | n.a. | Neg. | Neg. | n.a. | n.a. | Laterocervical lymphoadenophaty, nephrolitiasis | n.a. |
| Drenth et al. 1995 [38] | CR | Breast ductal carcinoma | 1 | pT2N1M0 | Polyarthritis | Knee, ankle, wrist, elbow, and shoulders | n.a. | Neg | n.a. | n.a. | Fever, erythematous rash | Survived | |
| Stummvoll et al. 2001 [5] | CR | SCLC (1), colon adenocarcinoma (2) | 2 | pT2pN0 (1, SCLC), pT3pN1, G2 (2, colon) | Symmetrical polyarthritis | MCP and IFJ, shoulder, knee (1), joints hands (2) | n.a. | Neg (1) | 1 : 80 (1) | n.a. | n.a. | n.a. | Survived (1) |
| Lima et al. 2002 [31] | CR | CML | 1 | n.a. | Polyarthritis | n.a. | n.a. | n.a. | Neg. | n.a. | n.a. | Weakness, weight loss, diarrhea, myalgias | Death |
| Glinkov et al. 2003 [6] | CR | HCC | 1 | n.a. | Polyarthritis | n.a. | n.a. | n.a. | n.a. | n.a. | AFP | Erythema nodosum | Survived |
| Mok, Kwan 2003 [7] | CR | Adenocarcinoma of uknown origin | 1 | n.a. | Symmetrical polyarthritis | Shoulders, elbows, wrists, MCPs, PIPs, knee and ankle | n.a. | + | Neg. | n.a. | n.a. | Peritoneal carcinosis, ascites | Death |
| Wiese et al. 2004 [40] | CR | Ovarian teratoma | 1 | n.a. | Polyarthritis | Knees, wrists | n.a. | Neg. | Neg. | n.a. | n.a. | Hypersomnolence, fatigability, night sweats, and periodic mouth ulcers | Survived |
| Ardalan, Shoja 2007 [28] | CR | MM | 1 | n.a. | Symmetrical polyarthritis | Hands and feets | n.a. | Neg. | Neg. | n.a. | n.a. | AKI | Survivied |
| Bivalacqua et al. 2007 [21] | CR | NSCLC + bladder cancer | 1 | T2N0 (Mx ?) | Symmetrical polyarthritis | Ankles, knees, hands, elbow, shoulder | n.a. | n.a. | n.a. | n.a. | n.a. | Shortness of breath and wheezing | Survived |
| Cantini et al. 2007 [16] | CS | NSCLC | 5 | n.a. | Mononarthritis | Knee | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Survived |
| Morel et al. 2008 [15] | CS | solid cancer (20 [13, 60% lung adenocarcinoma]), haematological disease (6) | 26 | T2 (50%) N1 (46.1%) M0 (80%) | Symmetric polyarthritis | Wrists, hands, knees | n.a. | Neg. (78.3%) | Neg. (70.8%) | n.a. | n.a. | Weight loss (42%), fatigue (46%), fever (27%) | 1.2 years |
| Tedeschi et al. 2017 [29] | CR | MDS | 1 | n.a. | Asymmetrical oligoarthritis | Left ankle, atlanto-epistrhopheal, right knee | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Death |
| Zupancic et al. 2008[17] | CR | SCLC | 1 | n.a. | Asymmetrical polyarthritis | Left ankle, left wrist, shoulder | Neg. | n.a. | Neg. | Neg. | n.a. | n.a. | Death |
| Bahat et al. 2009 [9] | CS | AIL (1), colon adenocarcinoma (2) | 2 | n.a. (1) n.a. | Symmetrical polyarthritis | Hands, wrists (1 + 2) | Neg. (1 +2) | n.a. | n.a. | n.a. | n.a. | Inguinal and axillary lymphoadenopathies, splenomegaly (1) | Death (1), survived (2) |
| Kumar et al. 2009 [8] | CR | Pancreatic adenocarcinoma | 1 | n.a. | Asymmetrical polyarthritis | Wrist, hand, elbow, shoulder | 154.6 IU/ml | 419.5 IU/ml | Neg. | n.a. | 373 U/ml CA 19.9 | Nausea, vomiting, diarrhea, weight loss | Death |
| Larson et al. 2011 [19] | CR | Lung adenocarcinoma | 1 | IV | Symmetrical polyarthritis | Elbows, PIPs, MCP, knees | 86 IU/ml | 199 IU/ml | Neg. | n.a. | n.a. | Cough, shortness of breath, pulmonary embolism | Death |
| Kobak 2013 [25] | CR | Prostatic cancer | 1 | n.a. | Monoarthritis | Left ankle | n.a. | Neg. | Neg. | n.a. | PSA | Foot drop | Survived |
| Raja et al. 2010 [37] | CR | Lymphoid granulomatosis | 1 | IV | Symmetrical polyarthritis | Knees, ankles, wrists | 133 IU/ml | > 250 IU/ml | n.a. | n.a. | n.a. | Maculopapular rash, fever, weight loss | Death |
| Han et al. 2012 [18] | CR | Lung adenocarcinoma | 1 | n.a. | Symmetrical polyarthritis | Wrists, shoulder, knee, elbows, MCPs | n.a. | Neg. | Neg. | n.a. | CEA 11.9 ng/ml | Cough, fever, anorexia, weight loss | n.a. |
| Aruch, Mims 2013[32] | CR | Hodgkin lymphoma | 1 | 3A | Oligoarthritis | Ankle, knee | n.a. | n.a. | n.a. | n.a. | n.a. | Lymphadenopathies, nephrotic syndrome | Survived |
| Ochi et al. 2012 [10] | CR | Gastric cancer | 1 | n.a. | Symmetrical polyarthritis | Shoulders, knees, wrists | Neg. | Neg. | n.a. | n.a. | CA 19.9 197.4 U/ml | Weight loss | n.a. |
| Prashanth et al. 2013 [33] | CR | Leukemia | 1 | n.a. | Asymmetrical polyarthritis | Left knee, ankles | Neg. | n.a. | Neg. | n.a. | n.a. | n.a. | n.a. |
| Handy et al. 2015[34] | CR | T-ALL | 1 | n.a. | Symmetrical Polyarthritis | Wrists, MCPs, knees, left ankle | 242 IU/ml | 1.148 IU/ml | 1 : 80 | n.a. | n.a. | Fever, tachycardia, tachypnea, adenopathies | Death |
| Kisacik et al. 2014 [22] | CS | Solid tumors (39), haematological malignancies (26) | 65 | n.a. | Polyarthritis (22), oligoarthritis (31), monoarthritis (12) | Hands and wrists (28), ankle (35), knee (39) | 7/65 | 15/65 | 10/65 | n.a. | n.a. | n.a. | n.a. |
| Watson et al. 2015[20] | CR | Papillary breast carcinoma (1), lung adenocarcinoma (2) | 2 | n.a. (1), n.a. (2) | Polyarthritis (1 + 2) | Knee (1), shoulder (1), wrist (1), hands, feets (2) | 36 IU/ml (1), neg. (2) | 42 IU/ml (1), neg. (2) | n.a. (1 + 2) | n.a. (1 + 2) | n.a. (1 + 2) | n.a. (1 + 2) | Survived (1 + 2) |
| Erlij et al.2016 [35] | CR | Hodking lymphoma | 1 | n.a. | Symmetrical polyarthritis | MCP, PIJ | Neg. | Neg. | Neg. | n.a. | n.a. | Membranopro-liferative glomerulonephritis + AKI + B symptoms | Survived |
| Levi Sandri et al. 2016 [13] | CR | Follicular dendritic cell liver sarcoma | 1 | n.a. | Polyarthritis | Wrists and knees | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Gamage et al. 2018 [36] | CR | DLBCL | 1 | IV | Polyarthritis | n.a. | Neg. | Neg. | Neg. | Neg. | n.a. | Fever, night sweats, weight loss, generalized lymphoadenopathy | Survived |
| Iqbal et al. 2018 [30] | CR | MDS | 1 | n.a. | Polyarthritis | Left wrist, knee | Neg. | Neg. | Neg. | n.a. | n.a. | n.a. | Survived |
| Eidenschink et al. 2019 [24] | CR | Mesothelioma | 1 | IV | Oligoarthritis | Wrist, shoulder, knee | Neg. | Neg. | Neg. | Neg. | n.a. | Fever, hypertension, tachypnoea | n.a. |
| Briones-Figueroa et al. 2019 [14] | CR | Lingual squamous cell carcinoma | 1 | n.a. | Symmetrical polyarthritis | Wrists, proximal inter-phalangeal joints, knees and elbows | Neg. | Neg. | Neg. | n.a. | Neg. | Asthenia, hyporexia, and weight loss | Survived |
| Rabah et al. 2020 [11] | CR | Gastric cancer | 1 | T3N1mx | Symmetrical polyarthritis | Hands,elbows, feets | Neg. | Neg. | n.a. | n.a. | n.a. | Weight loss, fatigue | n.a. |
| Sachdev Manjit Singh et al.2021 [23] | CR | Lung adenocarcinoma (1), ovarian cystadeno-carcinoma (2) | 2 | n.a. (1), IIIc (2) | Asymmetrical oligoarthritis (1 + 2) | Ankles (1), wrist (2, 3), MCP (1–5) PIP (2) | n.a. (1) | Neg. (1 + 2) | 1 : 320 (1), 1 : 160 (2) | n.a. (1) | n.a. (1) | Weight loss and anorexia(2) | n.a. (1), death (2) |
| Silvèrio-Antonio et al. 2021[12] | CR | Gastric cancer | 1 | T3N2M0, G3 | Symmetric polyarthritis | PIP, MCP, wrists, hands | 156.9 IU/ml | 215 IU/ml | n.a. | n.a. | n.a. | Vomiting, progressive fatigue, and weight loss | 18 months |
ACPA – anti-citrullinated protein antibodies, AFP – α-fetoprotein, AKI – acute kidney injury, AIL – aintegumenta-like, ANA – anti-nuclear antibody, CAE – caprine arthritis-encephalitis syndrome, CML – chronic myeloid leukemia, DLBCL – diffuse large B-cell lymphoma, ENA – 2’-O,4’-C-Ethylene-bridged nucleic acid, HCC – hepatocellular carcinoma, n.a. – not applicable, MCP – metacarpophalangeal, MDS – myelodysplastic syndrome, Neg. – negative, NSCLC – non-small cell lung cancer, PIJ – proximal interphalangeal joint, PIP – proximal interphalangeal, SIJ – sacroiliac joint, RF – rheumatoid factor, SCLC – small-cell lung cancer, T-ALL – T cell acute lymphoblastic leukemia, TNM – tumor-node-metastasis.
Case description
The patient was a Caucasian 67-year-old white man referred to our outpatient clinic in March 2021 for stiffness and pain of the right hand. At presentation the wrist appeared to be swollen, fovea sign was elicitable at palpation and the extremity was painful at extension and dorsiflexion. The patient a had history of cutaneous psoriasis, hypertension and, recently, had undergone Bentall’s surgery.
The following laboratory investigations were required: ESR, CRP, ANA, ACPA, RF, hepato-renal function, uric acid, serum protein electrophoresis, anti-HBV antibodies, anti-HCV, HIV test, QuantiFERON and Mantoux.
Moreover, during the first visit, a musculoskeletal ultrasound was performed using a linear probe of 6–18 MHz which highlighted the presence of synovial hypertrophy of the radio-carpal and mid-carpal joints, associated with moderate joint effusion, marked inflammatory pattern at power Doppler (PD) and tenosynovitis of common extensor tendons with an inflammatory patter at PD. Flexor tendon sheaths appeared within normal limits and hyperechoic deposits suggestive for crystal arthropathy were absent.
During the first visit an intra-articular injection of the affected wrist was practiced with 20 mg of triamcinolone acetonide and the patient was prescribed with hand X-rays and a therapeutic cycle of methylprednisolone 16 mg/day to be tapered in 1 month.
The laboratory investigations were negative for RF, ACPA and ANA, exception made for ESR and CRP which appeared elevated. In the meantime, the intra-articular injection carried out at the wrist did not produce any improvement, nor did oral GCs therapy.
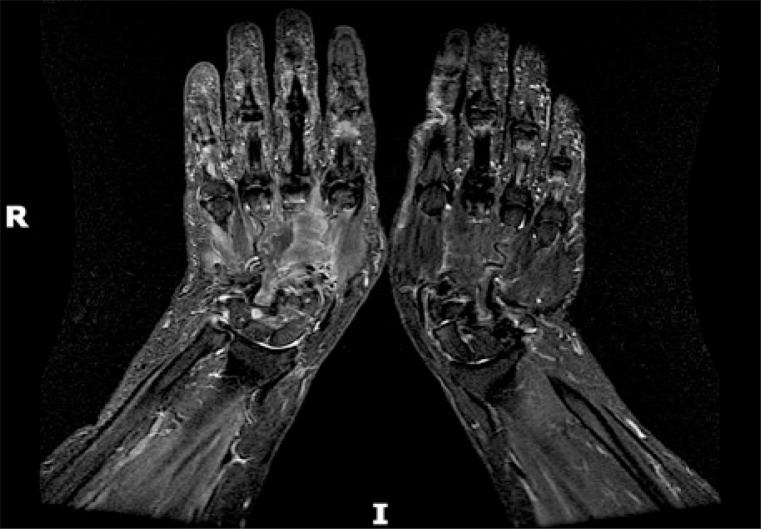
Hands X-rays were unremarkable for erosions, joint space narrowing or any sign of juxta-articular demineralization, therefore it was decided to subject the patient’s limb to a nuclear magnetic resonance (MRI). The magnetic resonance confirmed the presence of severe synovitis of the radiocarpal and midcarpal joints, as well as common extensor tendon sheaths tenosynovitis (Fig. 1).
Fig. 1.
Magnetic resonance T2-weighted sequences revealing mid-carpal and radio-carpal joint effusion as well as extensor tendons sheaths tenosynovitis of the right wrist.
Psoriatic arthropathy was therefore diagnosed, and given the negativity of screening for occult infectious diseases, methotrexate 10 mg subcutaneously once a week, followed by 5 mg of folic acid within 24–48 hours was started.
No sooner did the patient performed the first methotrexate’s syringe, that was admitted to the emergency department for gastrointestinal bleeding and anemia. An abdominal computed tomography (CT) scan was performed and revealed the presence of a solid, space-occupying lesion at the ileocecal valve with suspicious hepatic metastasis.
Therefore immunosuppressive therapy was interrupted, the patient underwent intestinal resection, from the subsequent and the histopathological analysis of the lesion the specimens revealed the presence of a neuroendocrine tumor (NET) with glandular pattern expressing chromogranin, synaptophysin, neuron-specific enolase (NSE), with a Ki-67 of 2% (G1 stage according to WHO) and a pathological staging of pT3pN2aM0. Serological chromogranin and NSE at time of the surgery were respectively 592 ng/ml (normal values < 109 ng/ml) and 24.3 (normal values < 15).
The patient subsequently returned to our attention, reporting a clear improvement of symptoms: clinical and ultrasound examination were at this time negative for any synovitis sign. Therefore the patient was entrusted to the care of the oncologist who prescribed somatostatin analogues (once monthly) and scheduled the follow-up of the other heterotopic lesions.
Three months later the patient returned to the emergency-urgency department complaining of cervical-brachialgia, thus we decided to perform a CT study of the cervical spine that subsequently revealed the presence of lesions suspicious for bone secondarisms at vertebral level.
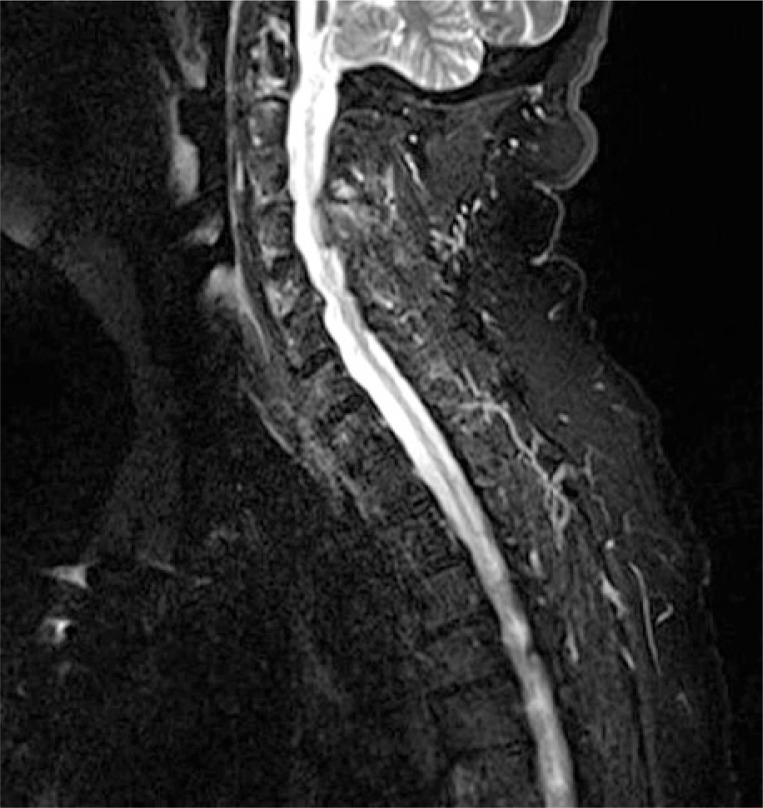
From the subsequent MRI study, the presence of osteolytic lesions was excluded, however the presence of edema and a slight slipping of the axial tooth was evidenced, consistent with the presence of synovitis and bone marrow edema (BME) at the level of the atlantoaxial joint and extending to the anterior surface of vertebrae from C1 to C5 (Fig. 2).
Fig. 2.
Magnetic resonance T2-weighted images showing BME of C1–C5 as well as synovitis of C1–C2.
As soon as the inflammatory arthropathy affecting the atlantoaxial joint was identified, a semi-rigid collar was recommended, and GCs bolus therapy was scheduled. Nevertheless, the patient denied the intravenous bolus therapy with GCs. The subsequent PET/TC DOTATATE did not reveal any new anomalous uptake; therefore, he has been currently attaining to the follow up schedule and sticking to the oncologist therapy.
The latest head-neck MRI after 3 months revealed a spontaneous reduction of BME at atlantoaxial and cervical level. Last serological evaluation of chromogranin documented a significant reduction of chromogranin levels (134 ng/ml).
Discussion
According to results and conclusions of searching of literature which we described above and our case description we may discuss some of define cancers associated with rheumatic diseases.
Gastrointestinal cancers
Stumvoll et al. [5] was the first to report the case report of a patient presenting with symmetrical polyarthritis subsequently diagnosed with a colon adenocarcinoma. Several years later Glinkov et al. [6] described the case of a young women with a two year history erythema nodosum and polyarhtirits subsequently diagnosed with an hepatocellular carcinoma.
Mok et Kwan [7] in 2003 described the case of a patients developing a severe symmetrical polyarthritis with RF positivity in a patient soon after diagnosed with peritoneal carcinomatosis; subsequent investigations revealed the presence of a space occupying lesion in the liver, however a clearer diagnosis was not obtained due to the unwillingness of the patients to perdue other invasive maneuvers.
Subsequently, Kumar et al. [8] described the case of a patient presenting with asymmetric polyarthritis and elevated RF with an underlying pancreatic adenocarcinoma. Bahat et al. [9] the same year presented the case of 3 patients (2 with colon adenocarcinoma and 1 with acute immunoblastic leukemia – AIL) with a symmetric polyarthritis and negative RF as well as ACPA, mimicking a seronegative RA.
Ochi et al. [10], Rabah et al. [11], Silvèrio-Antònio et al. [12] described patients affected by gastric cancers developing a symmetrical polyarthritis mimicking a traditional rheumatoid pattern. To date, Levi Sandri et al. [13] are the only group of study who have described an RA-like polyarthritis in a 19 year old boy diagnosed with a follicular dendritic cell sarcoma of the liver.
Finally, Briones-Figueroa et al. [14] in 2019 described the case of a 69 years old man presenting with a symmetrical paraneoplastic arthritis associated with weight loss in a patient subsequently with a squamous cell carcinoma of the tongue.
Pulmonary cancers
From the results of our review (Table II) pulmonary cancers appear as the main kind of tumors linked to the subsequent development of an inflammatory articular disease; histological specimens reveal that the most frequent type of neoplasia is non-small cell lung cancer (NSCLC).
Table II.
Frequency of primary tumors and histotypes associated to an inflammatory articular involvement
| Primary tumors | Frequency | Histotypes |
|---|---|---|
| Lung cancers | 44 | NSCLC (26), SCLC (3), epidermoidis (6), mesothelioma (1), bronchogenic (1), unknown (7) |
| Hematological malignancies | 45 | Chronic myeloproliferative disease (4), lymphocytic lymphoma (1), multiple myeloma (4), non-Hodgkin lymphoma (8), Hodgkin lymphoma (3), acute myeloblastic leukemia (8), myelodysplastic syndrome (6), acute immunoblastic leukemia (1), lymphoid granulomatosis (1), T-ALL (1), acute lymphoblastic leukemia (4), chronic lymphoblastic leukemia (1), leukemia n.o.s. (1) |
| Urinary tract cancers | 9 | Bladder cancer (4), prostatic cancer (3), renal cancer (2) |
| Gastrointestinal tumors | 16 | Pancreatic adenocarcinoma (3), gastric cancer (5), cilindric epithelioma (1), lingual squamous cell carcinoma (1), coledoc cancer (1), liver sarcoma (1), colon cancers (3), HCC (1) |
| Breast cancers | 11 | Galactophoric adenocarcinoma (1), papillary breast adenocarcinoma (1), breast ductal carcinoma (1), unknown (8) |
| Reproductive apparatus cancers | 4 | Penis squamous cell carcinoma (1), ovarian teratoma (1), endometrial carcinoma (1), ovarian cystoadenocarcinoma (1) |
| Thyroid cancers | 1 | Papillary carcinoma (1) |
| Bone cancers | 1 | Sternal condrosarcoma (1) |
| Cancer of unknown origin | 3 | n.a. |
HCC – hepatocellular carcinoma, n.a. – not assessed, NSCLC – non-small cell lung cancer, SCLC – small-cell lung cancer, T-ALL – T cell acute lymphoblastic leukemia.
Morel et al. [15] reported 26 patients affected by solid neoplasia presenting with a symmetrical polyarthritis mimicking RA, most of them were affected by lung cancer. Subsequently, the group of study of Cantini et al. [16] in 2007 reported the case of 5 patients affected by NSCLC presenting with a paraneoplastic knee monoarthritis.
The year later Zupancic et al. [17] described the case of an asymmetrical polyarthritis occurring in a patient subsequently diagnosed with small-cell lung cancer (SCLC). Han et al. [18] in 2011, Larson et al. [19], Watson et al. [20] and Bivalacqua et al. [21] in different case reports described the case of patients developing symmetrical polyarthritis mimicking RA subsequently diagnosed with a lung adenocarcinoma.
Kisacik et al. [22] in 2014, presenting the largest case series related to this topic, described 65 paraneoplastic arthritis associated to lung adenocarcinoma as the most frequent type of solid tumor linked to an articular inflammatory involvement.
Sachdev Manjit Singh et al. [23] in 2021 described one of the few cases of paraneoplastic arthritis mimicking an AxSPA in a patient subsequently diagnosed with lung adenocarcinoma and the case of a seronegative symmetrical polyarthritis mimicking RA subsequently diagnosed with ovarian cystoadenocarcinoma.
The only case of a patients diagnosed with pleural mesothelioma developing a paraneoplastic asymmetrical oligoarthritis is described by Eidenschink et al. [24] in 2019.
Urinary tract cancers
The only patients with a prostatic cancer presenting with a paraneoplastic arthritis is described by Kobak [25]. Other patients presenting with articular inflammatory manifestation relatable to the presence of a neoplasia are reported in the case series of Morel et al. [15] and Kisacik et al. [22].
Hematological malignancies
Hematological malignancies are the most frequent type of tumors associated to an inflammatory articular disease after lung cancer. Main kind of hematological diseases held responsible for this paraneoplastic manifestation are: non-Hodgkin lymphoma (NHL) (8), acute myeloblastic leukemia (AML) (8) and multiple myeloma (MM) (4).
Since Longley et al. [26] who in 1986 were the first authors to diagnose a myeloproliferative disorder in 2 patients with cutaneous angiitis and polyarthritis, many literature evidence has been collected in these years; Cohen et al. 1993 [27] described one of the few cases reported in literature of an AxSPA manifesting as a paraneoplastic syndrome concomitant to a well differentiated lymphocytic lymphoma.
Then, Ardalan et Shoja [28] in 2007 presented the case of a patient with acute kidney injury (AKI) and RA-like symmetrical polyarthritis subsequently diagnosed with MM.
Tedeschi et al. [29] and Iqbal et al. [30] were the only authors to report a case of paraneoplastic arthritis mimicking pseudogout correlated with an underlying myelodysplastic syndrome. Lima et al. [31] described the case of a paraneoplastic polyarthritis, polymyositis and hypercalcemia developing in a patient an acute lymphoblastic leukemia on a chronic myeloid leukemia (CML).
Aruch et Mims [32] in 2013 described the case of a patient presenting with nephrotic syndrome, Hodgkin lymphoma and oligoarthritis. The year later Prashanth et al. [33] described the case of a child presenting with polyarthritis and acute leukemia (not otherwise specified).
In one of the largest case series ever published on the topic, Kisacik et al. [22] in his case series reported 26 hematological malignancies; in this study, the most frequent hematological disease linked to an articular inflammatory involvement was the AML.
Concomitantly, Handy et al. [34] described the only case of a RA-like polyarthritis developing in a patient with a T cell ALL. Furthermore, Erlij et al. [35], reported one of the few cases of Hodgkin lymphoma held responsible for articular paraneoplastic manifestations.
The latest report found in literature lay to Gamage et al. [36] who described the case of paraneoplastic polyarthritis in a patient with diffuse large B cell lymphoma, and Raja et al. [37] who described the only case of a lymphoid granulomatosis responsible of articular paraneoplastic involvement.
Breast cancers
The only two case reports documenting a paraneoplastic polyarthritis in women diagnosed with breast cancer are of Drenth et al. [38] in 1995 and Watson et al. [20] several years later: both documented a paraneoplastic symmetrical polyarthritis mimicking RA in patients with a breast ductal carcinoma.
The largest amount of evidences correlating mammary cancers to inflammatory articular involvement are reported in the case series of Morel et al. [15] and Kisacik et al. [22].
Other neoplastic arthritis
To the best of our knowledge Lambert et Nuki [39] in 1992 was the only to report the case of a patient with a multicentric reticulohistiocytosis presenting concomitantly to a symmetrical polyarthritis affecting hands, feet and wrists.
Wiese et al. [40] in 2004 and subsequently Sachdev Manjit Singh et al. [23] in 2021 are the only authors to have described an articular inflammatory involvement associated to the presence of ovarian tumors: the former identified a patient with an ovarian teratoma, the latter one described a subject with cystadenocarcinoma.
Moreover, in the case series of Kisacik et al. [22] are reported few cases of reproductive apparatus tumors correlated with paraneoplastic articular manifestations.
Paraneoplastic arthritis are an heterogenous group of disorders challenging to rule out in everyday clinical practice since there is a lack of well-structured guidelines to assist the physician during the diagnostic process. Physiopathology of paraneoplastic arthritides remains poorly understood and it seems to involve formation of immunocomplexes and a T cell participation [4].
From the results of our systematic review, we identified that the most frequent phenotype of a paraneoplastic arthritis is represented by a middle-aged man (> 50 years old) with a seronegative symmetrical polyarthritis mimicking RA, often related to the presence of an underlying lung cancer.
That lung tumors are frequently linked to paraneoplastic syndromes is not a novelty in literature and probably this is given to a higher immunogenicity created by the neoplastic epithelial invasion and tumoral microenvironment; however, an interesting observation is that despite the high prevalence of cancers worldwide, very few seems relatable to the presence of paraneoplastic inflammatory joint involvement.
Moreover, rheumatological diseases display a higher prevalence in female gender, conversely in our review we documented that paraneoplastic articular involvement occur more frequently in male subjects. In line with what is already reported, we found that majority of patients affected by a paraneoplastic arthritis did not respond to GCs, NSAIDs and csDMARDs.
Furthermore, in 39.4% of those treated with chemotherapy or surgery, thus reducing or eliminating neoplastic burden, full remission of articular inflammation was achieved. These observations allow to strengthen the hypothesis that paraneoplastic arthritis is immunologically correlated with neoplasm and that tumoral control may mitigate paraneoplastic symptoms.
In our clinical experience, the scarce responsiveness to GCs therapy and the disappearance of inflammatory joint involvement after the gastrointestinal neuroendocrine tumors (GI-NET) removal allowed us to suspect the articular symptoms as being of paraneoplastic nature.
An interesting paper of Hagiwara et al. [41] studied the prevalence of paraneoplastic arthritis in a cohort of patients classifiable as psoriatic according to CASPAR criteria and demonstrated that 19 out of 115 patients had developed arthritis shortly before the tumor was discovered.
Given the challenging clinical assessment in diagnosis of paraneoplastic arthritis, evaluation of neoplastic markers may be of some utility, in fact our patient had abnormally elevated chromogranin levels which dropped near normality after the excision of the primary tumor.
According to the paper of Parperis et al. [42], which proposed an interesting diagnostic flow chart, neoplastic markers are involved during the clinical investigations, nevertheless their research should be done clearly bearing in mind what the physician is trying to rule out.
After several weeks from the GI-NET removal our patient developed a severe cervical-brachialgia which brought to the diagnosis of C1–C2 synovitis with bone marrow edema extending to C5, a condition ever previously described as being of paraneoplastic nature. The involvement of the axial tooth is a typical and rare manifestation of RA which hardly fit the history of our patient.
Study limitations
Main limit of this study lay in the high number of case series and case report included, given the rarity of the disorder.
Conclusions
To date, this is the most up-to-date review regarding paraneoplastic arthritis; bullet points that we found to correctly identify a paraneoplastic disorder are:
lack of/poor response to NSAIDs, GCs and csDMARD therapy,
polyarticular symmetrical involvement of limbs,
absence of RF and ACPA,
complete remission of paraneoplastic symptoms after treatment of primary tumor,
recurrence of the symptoms in presence of metastasis,
age higher than 50 years old,
major involvement of male subjects.
Footnotes
The authors declare no conflict of interest.
References
- 1.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581, DOI: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 2.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665–2673, DOI: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 3.Rudwaleit M, van Der Heijde D, Landewé R, et al. the development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783, DOI: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 4.Manger B, Schett G. Paraneoplastic syndromes in rheumatology. Nat Rev Rheumatol 2014; 10: 662–670, DOI: 10.1038/nrrheum.2014.138. [DOI] [PubMed] [Google Scholar]
- 5.Stummvoll GH, Aringer M, Machold KP, et al. Cancer polyarthritis resembling rheumatoid arthritis as a first sign of hidden neoplasms. Scand J Rheumatol 2001; 30: 40–44, DOI: 10.1080/030097401750065319. [DOI] [PubMed] [Google Scholar]
- 6.Glinkov S, Krasnaliev I, Atanassova M, et al. Hepatocellular carcinoma associated with paraneoplastic erythema nodosum and polyarthritis. J Hepatol 2003; 39: 656–657, DOI: 10.1016/s0168-8278(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 7.Mok CC, Kwan YK. Rheumatoid-like polyarthritis as a presenting feature of metastatic carcinoma: a case presentation and review of the literature. Clin Rheumatol 2003; 22: 353–354, DOI: 10.1007/s10067-003-0741-2. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Sethi S, Irani F, Bode BY. Anticyclic citrullinated peptide antibody-positive paraneoplastic polyarthritis in a patient with metastatic pancreatic cancer. Am J Med Sci 2009; 338: 511–512, DOI: 10.1097/MAJ.0b013e3181b0babe. [DOI] [PubMed] [Google Scholar]
- 9.Bahat G, Kamali S, Saka B, et al. Paraneoplastic arthritis may mimic rheumatoid arthritis with symmetrical and upper extremity predilecting presentation. J Clin Rheumatol 2009; 15: 319–320, DOI: 10.1097/RHU.0b013e3181b18ebd. [DOI] [PubMed] [Google Scholar]
- 10.Ochi K, Horiuchi Y, Seki M, et al. Polyarthritis and posterior interosseous nerve palsy associated with gastric carcinoma. Rheumatol Int 2012; 32: 2557–2559, DOI: 10.1007/s00296-011-2047-z. [DOI] [PubMed] [Google Scholar]
- 11.Rabah S, Bani Hani D, Jilani N. Paraneoplastic arthritis in a patient with gastric cancer: a case report. Cureus 2020; 12: 10–13, DOI: 10.7759/cureus.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvério-António M, Parlato F, Martins P, et al. Gastric adenocarcinoma presenting as a rheumatoid factor and anti-cyclic citrullinated protein antibody-positive polyarthritis: a case report and review of literature. Front Med (Lausanne) 2021; 8: 627004, DOI: 10.3389/fmed.2021.627004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi Sandri GB, Colasanti M, Vennarecci G, Ettorre GM. Paraneoplastic arthritis as first symptom of a liver inflammatory pseudotumor-like follicular dendritic cell sarcoma. Liver Int 2016; 36: 1392, DOI: 10.1111/liv.13148. [DOI] [PubMed] [Google Scholar]
- 14.Briones-Figueroa A, Sifuentes-Giraldo WA, Carrillo-Gijón R, Morell-Hita JL. Paraneoplastic polyarthritis as the first manifestation of lingual carcinoma. Eur J Rheumatol 2019; 6: 52–53, DOI: 10.5152/eurjrheum.2018.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morel J, Deschamps V, Toussirot E, et al. Characteristics and survival of 26 patients with paraneoplastic arthritis. Ann Rheum Dis 2008; 67: 244–247, DOI: 10.1136/ard.2007.070086. [DOI] [PubMed] [Google Scholar]
- 16.Cantini F, Niccoli L, Nannini C, et al. Isolated knee monoarthritis heralding resectable non-small-cell lung cancer. a paraneoplastic syndrome not previously described. Ann Rheum Dis 2007; 66: 1672–1674, DOI: 10.1136/ard.2007.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zupancic M, Annamalai A, Brenneman J, Ranatunga S. Migratory polyarthritis as a paraneoplastic syndrome. J Gen Intern Med 2008; 23: 2136–2139, DOI: 10.1007/S11606-008-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han YM, Fang LZ, Zhang XH, et al. Polyarthritis as a prewarning sign of occult lung cancer. Kaohsiung J Med Sci 2012; 28: 54–56, DOI: 10.1016/j.kjms.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson E, Etwaru D, Siva C, Lawlor K. Report of anti-CCP antibody positive paraneoplastic polyarthritis and review of the literature. Rheumatol Int 2011; 31: 1635–1638, DOI: 10.1007/s00296-009-1294-8. [DOI] [PubMed] [Google Scholar]
- 20.Watson GA, O’Neill L, Law R, et al. Case report migrating polyarthritis as a feature of occult malignancy: 2 case reports and a review of the literature. Case Rep Oncol Med 2015; 2015: 934039, DOI: 10.1155/2015/934039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bivalacqua TJ, Alphs H, Aksentijevich I. Paraneoplastic polyarthritis from non-small-cell lung cancer metastatic to the bladder. J Clin Oncol 2007; 2621–2623, DOI: 10.1200/JCO.2007.11.5600. [DOI] [PubMed] [Google Scholar]
- 22.Kisacik B, Onat AM, Kasifoglu T, et al. Diagnostic dilemma of paraneoplastic arthritis: case series. Int J Rheum Dis 2014; 17: 640–645, DOI: 10.1111/1756-185X.12277. [DOI] [PubMed] [Google Scholar]
- 23.Sachdev Manjit Singh B, Wan SA, Cheong YK, et al. Arthritis as an initial presentation of malignancy: two case reports. J Med Case Rep 2021; 15: 1–5, DOI: 10.1186/s13256-020-02642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eidenschink M, Beard A, Ewart D. Paraneoplastic migratory oligoarthritis in a patient with malignant mesothelioma. Am J Med 2019; 132: e801–e802, DOI: 10.1016/j.amjmed.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Kobak S. Chronic monoarthritis and foot-drop as a paraneoplastic syndrome in prostate cancer. Rheumatol Int 2013; 33: 223–225, DOI: 10.1007/s00296-010-1564-5. [DOI] [PubMed] [Google Scholar]
- 26.Longley S, Caldwell JR, Panush RS. Paraneoplastic vasculitis. Unique syndrome of cutaneous angiitis and arthritis associated with myeloproliferative disorders. Am J Med 1986; 80: 1027–1030, DOI: 10.1016/0002-9343(86)90660-1. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MR, Carrera GE, Lundberg J. Rapidly progressive sacroiliitis in a patient with lymphocytic lymphoma. Ann Rheum Dis 1993; 52: 239–240, DOI: 10.1136/ard.52.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ardalan MR, Shoja MM. Multiple myeloma presented as acute interstitial nephritis and rheumatoid arthritis-like polyarthritis. Am J Hematol 2007; 82: 309–313, DOI: 10.1002/ajh.20796. [DOI] [PubMed] [Google Scholar]
- 29.Tedeschi SK, Stone RM, Helfgott SM. Calcium pyrophosphate crystal inflammatory arthritis (pseudogout) with myelodysplastic syndrome: a new paraneoplastic syndrome? J Rheumatol 2017; 44: 1101–1102, DOI: 10.3899/jrheum.170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iqbal SM, Aslam HM, Faizee F, et al. Pseudogout: an autoimmune paraneoplastic manifestation of myelodysplastic syndrome. Cureus 2018; 10: e3372, DOI: 10.7759/cureus.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima M, Coutinho J, Bernardo L, et al. Philadelphia-positive T cell acute lymphoblastic leukemia with polymyositis, migratory polyarthritis and hypercalcemia following a chronic myeloid leukemia. Ann Hematol 2002; 81: 174–177, DOI: 10.1007/s00277-001-0422-7. [DOI] [PubMed] [Google Scholar]
- 32.Aruch DB, Mims MP. Paraneoplastic nephrotic syndrome and inflammatory arthritis at diagnosis in Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 2013; 13: 77–79, DOI: 10.1016/j.clml.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Prashanth GP, Bhandankar M, Patil VD. Migratory polyarthritis as a paraneoplastic syndrome in childhood leukemia. Rheumatol Int 2013; 33: 1647–1648, DOI: 10.1007/s00296-011-2345-5. [DOI] [PubMed] [Google Scholar]
- 34.Handy CE, Robles G, Haque U, Houston B. T cell ALL presenting as seropositive rheumatoid arthritis: case report and review of the literature on seropositive paraneoplastic arthritis. Clin Rheumatol 2015; 34: 1647–1650, DOI: 10.1007/s10067-014-2697-9. [DOI] [PubMed] [Google Scholar]
- 35.Erlij D, Calderón B, Rivera A, et al. Polyarthritis and membranoproliferative glomerulonephritis as paraneoplastic manifestation of Hodgkin’s lymphoma: a case report and literature review. Reumatol Clin 2016; 12: 282–284, DOI: 10.1016/j.reuma.2015.10.009 [Article in English, Spanish]. [DOI] [PubMed] [Google Scholar]
- 36.Gamage KKK, Rifath MIM, Fernando H. Migratory polyarthritis as a paraneoplastic syndrome in a patient with diffuse large B cell lymphoma: a case report. J Med Case Rep 2018; 12: 189, DOI: 10.1186/s13256-018-1700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raja R, Lamont D, Yung A, Solanki K. A can of red herrings. Int J Rheum Dis 2010; 13: e46–e50, DOI: 10.1111/j.1756-185X.2010.01535.x. [DOI] [PubMed] [Google Scholar]
- 38.Drenth JP, de Kleijn EH, de Mulder PH, van der Meer JW. Metastatic breast cancer presenting as fever, rash, and arthritis. Cancer 1995; 75: 1608–1611, DOI: . [DOI] [PubMed] [Google Scholar]
- 39.Lambert CM, Nuki G. Multicentric reticulohistiocytosis with arthritis and cardiac infiltration: Regression following treatment for underlying malignancy. Ann Rheum Dis 1992; 51: 815–817, DOI: 10.1136/ard.51.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiese W, Alansari H, Tranchida P, Madrid FF. Paraneoplastic polyarthritis in an ovarian teratoma. J Rheumatol 2004; 3: 1854–1857. [PubMed] [Google Scholar]
- 41.Hagiwara K, Suyama Y, Fukuda K. Clinical experience in 115 patients with arthritis and/or enthesitis who met the classification criteria for psoriatic arthritis (CASPAR) within the last two years – possible association with malignant disorders. Mod Rheumatol 2016; 26: 625–629, DOI: 10.3109/14397595.2015.1097000. [DOI] [PubMed] [Google Scholar]
- 42.Parperis K, Constantinidou A, Panos G. Paraneoplastic arthritides: insights to pathogenesis, diagnostic approach, and treatment. J Clin Rheumatol 2016; 27: e505–e509, DOI: 10.1097/RHU.0000000000001202. [DOI] [PubMed] [Google Scholar]