Abstract
Background
Early hematoma expansion (HE) occurs in 20 to 40% of spontaneous intracerebral hemorrhage (ICH) patients and is a primary determinant of early deterioration and poor prognosis. Previous studies have shown that inflammation is a major pathological feature of ICH, and the neutrophil-to-platelet ratio (NPR) is a marker of systemic inflammation. Therefore, we aimed to assess the association between the NPR and HE in ICH patients.
Methods
We retrospectively collected and analyzed data from ICH patients who received treatment at our institution from January 2018 to November 2019. The NPR was calculated from the admission blood test. Brain computed tomography (CT) scans were performed at admission and repeated within 24 h. Hematoma growth was defined as relative growth > 33% or absolute growth > 6 ml.
Results
A total of 317 patients were enrolled in our study. Multivariate logistic regression analysis indicated that the NPR was an independent predictor of HE [odds ratio (OR) = 1.742; 95% CI: 1.508–2.012, p < 0.001]. Receiver operating characteristic (ROC) curve analysis revealed that the NPR could predict HE, with an area under the curve of 0.838 (95% CI, 0.788–0.888, p < 0.001). The best predictive cut-off of the NPR for HE was 5.47 (sensitivity, 75.3%; specificity, 77.6%).
Conclusions
A high NPR was associated with an increased risk of HE in patients with ICH.
Keywords: Hematoma expansion, Inflammation, Intracerebral hemorrhage, Neutrophil-to-platelet ratio, Predictor
Background
Spontaneous intracerebral cerebral hemorrhage (ICH) comprises approximately 10 to 20% of all strokes, with high rates of disability and mortality [1]. Early hematoma expansion (HE) occurs in 20 to 40% of ICH patients and is a primary determinant of early deterioration and poor prognosis [2]. Previous studies have shown that inflammation is a major pathological feature of ICH [3–5]. In addition, systemic inflammatory responses have been found to be associated with the pathological process of active bleeding in ICH patients [6]. The neutrophil-to-platelet ratio (NPR) is a marker of systemic inflammation. A previous study indicated that the interaction between neutrophils and platelets plays a role in vascular injury after cerebral infarction [7]. Moreover, He et al. conducted a retrospective study of 279 ischemic stroke patients with hemorrhagic transformation and found that a high NPR was an independent predictor of hemorrhagic transformation [8].
However, the literature regarding the relation between the NPR and HE in ICH patients is infrequent. Therefore, this study aimed to explore the association between the NPR at admission and early HE after spontaneous ICH.
Methods
Patients and definitions
We performed a retrospective review of patients with ICH who visited West China Hospital from January 2018 to November 2019. The study protocol was approved by the ethics committee of our hospital. Informed consent was obtained from all patients or family members. We defined the inclusion criteria as follows: 1) a diagnosis of intracranial hemorrhage by computed tomography (CT); 2) patient underwent the first CT scan on admission and the second CT scan at 24 h after the onset of symptoms; 3) routine examinations and laboratory blood tests were conducted within 24 h after admission; and 4) patient age ≥ 18 years. We excluded patients with 1) ICH attributable to aneurysm, arteriovenous malformation or moyamoya disease; 2) ICH attributable to acute cerebral infarction, thrombolysis of cerebral or myocardial infarction; 3) prior systemic diseases such as cancer, hematological diseases, immunological disease, neurological disease, recent infectious disease, severe hepatic dysfunction, renal dysfunction and coagulation dysfunction; 4) a medical history of anticoagulant use or antiplatelet treatments; 5) patients who underwent surgery before the 24-h CT; and 6) isolated intraventricular hemorrhage.
Clinical manifestation assessment
Baseline clinical and demographic parameters were collected at hospital arrival, including age; sex; Glasgow Coma Scale (GCS) score on admission; National Institutes of Health Stroke Scale (NIHSS) score on admission; blood pressure; cigarette consumption and alcohol use; and the medical history, including that of ischemic stroke, ICH, hypertension and diabetes mellitus.
Laboratory examinations
Radiological data were recorded, including hematoma location, hematoma size and the presence of intraventricular hemorrhage (IVH). Laboratory variables were also recorded, including red blood cell (RBC) count, hemoglobin, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), platelet count, prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR) and blood glucose level. Admission NPR was calculated as the ratio of the ANC × 100 to the platelet count.
Outcome assessments
Two reviewers independently evaluated all the head CT scans. Any disagreement between the two reviewers was resolved by consensus. All patients underwent the first CT scan at admission, and the follow-up CT scan was performed within 24 h of symptom onset. Hematoma volume was measured by the ABC/2 method as described previously [9]. HE was defined as absolute growth of > 6 ml or relative growth of > 33% from the first CT to the follow-up CT [10].
Statistical analysis
The clinical data, laboratory parameters and imaging characteristics of ICH patients with and without HE were compared. Continuous variables are expressed as mean ± standard deviation or median with interquartile range (IQR) for normally distributed and non-normally distributed variables, respectively, whereas categorical variables are expressed as frequency and percentage. Univariate analyses were conducted by independent t-test, Mann–Whitney U-test, chi-square (χ2) test, or Fisher’s exact test. Independent t-tests or Mann–Whitney U-tests were applied to compare continuous variables. The chi-square (χ2) test or Fisher’s exact test was used to compare categorical data. Variables with a p-value < 0.10 on univariate analysis were entered into a multivariate regression model. To facilitate interpretation, some variables were classified as follows: GCS score as “13–15 points”, “9–12 points” and “3–8 points” and hematoma location as “infratentorial hematoma” and “supratentorial hematoma”. Receiver operating characteristic (ROC) curve analysis was performed to indicate the value of the NPR for the prediction of HE in ICH patients. The cut-off value of the NPR was set by the Youden index from the ROC curve. A value of p < 0.05 was considered to be significant. All the above mentioned statistical analyses were carried out with SPSS version 21.0 (SPSS, Chicago, IL, USA).
Results
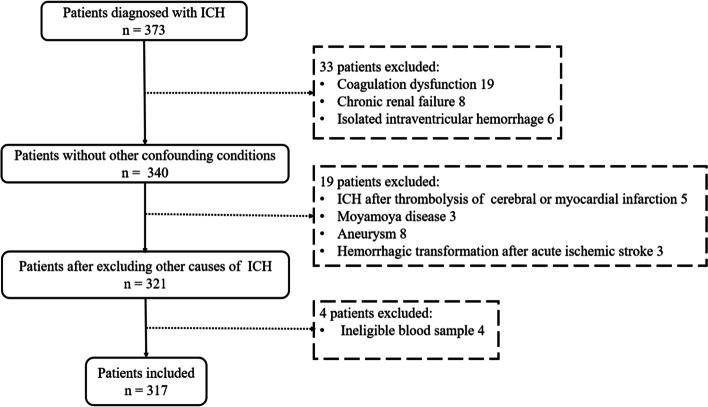
A total of 317 consecutive ICH patients (230 males and 87 females) meeting the inclusion criteria were enrolled in this study (Fig. 1). The median age, median baseline hematoma volume and median NPR were 56 years (IQR, 45–67.5), 24.00 ml (IQR, 10.15–41.84) and 4.79 (IQR, 2.70–6.63), respectively. HE was detected in 89 (28%) patients. For these patients, the median age was 58 (IQR, 49–70) years, 61 (68.5%) were men and 28 (31.5%) were women. The median NPR of the HE group was significantly higher than that of the group without HE (p < 0.001) (Table 1).
Fig. 1.
Flowchart of study enrollment
Table 1.
Univariate analysis of clinical characteristics related to HE in patients with ICH
| Characteristic | Total (n = 317) |
HE (n =89) |
No HE (n =228) |
p-Value |
|---|---|---|---|---|
| Age(years) | 56(45,67.50) | 58(49,70) | 54(43,66) | 0.028* |
| Sex (male) | 230(72.6%) | 61(68.5%) | 169(74.1%) | 0.329 |
| Hypertension | 258(81.4%) | 76(85.4%) | 182(79.8%) | 0.335 |
| Diabetes mellitus | 26(8.2%) | 6(6.7%) | 20(8.8%) | 0.653 |
| Prior ischemic stroke | 10(3.2%) | 2(2.2%) | 8(3.5%) | 0.826 |
| Prior ICH | 11(3.5%) | 4(4.5%) | 7(3.1%) | 0.779 |
| Smoking | 98(30.9%) | 23(25.8%) | 75(32.9%) | 0.279 |
| Alcohol consumption | 83(26.2%) | 19(21.3%) | 64(28.1%) | 0.256 |
| Onset-to-first-CT time(hours) | 7(4,15) | 8(5,14.5) | 7(4,15) | 0.249 |
| SBP(mmHg) | 163 ± 27 | 162 ± 26 | 164 ± 27 | 0.664 |
| DBP(mmHg) | 96 ± 18 | 96 ± 16 | 97 ± 19 | 0.578 |
| GCS score on admission | - | - | - | < 0.001** |
| 13-15 | 114(36.0%) | 15(16.9%) | 99(43.4%) | - |
| 9-12 | 104(32.8%) | 29(32.6%) | 75(32.9%) | - |
| 3-8 | 99(31.2%) | 45(50.6%) | 54(23.7%) | - |
| NIHSS on admission | 12(9,14) | 12(10,14) | 10(9,13) | 0.019* |
| Infratentorial hematoma | 67(21.1%) | 20(22.5%) | 47(20.6%) | 0.760 |
| Hematoma size (ml) | 24.00(10.15,41.84) | 35.24(14.98,52.20) | 21.23(8.36,38.18) | <0.001** |
| Presence of IVH | 133(42.0%) | 42(47.2%) | 91(39.9%) | 0.256 |
| RBC, x1012 | 4.58 ± 0.72 | 4.44 ± 0.75 | 4.63 ± 0.70 | 0.031* |
| Hemoglobin(g/L) | 139 ± 20 | 135 ± 21 | 140 ± 19 | 0.061 |
| Neutrophil, x109 | 8.12(5.25,11.33) | 10.86(8.56,13.74) | 6.85(4.74,9.93) | <0.001** |
| Lymphocyte, x109 | 1.22(0.79,1.83) | 0.91(0.65,1.29) | 1.31(0.87,1.93) | <0.001** |
| Monocyte, x109 | 0.51(0.38,0.73) | 0.63(0.40,0.81) | 0.50(0.35,0.69) | 0.003* |
| Platelet, x109 | 185 ± 64 | 159 ± 60 | 195 ± 62 | <0.001** |
| NPR | 4.79(2.70,6.63) | 7.37(5.46,9.67) | 3.85(2.50,5.40) | <0.001** |
| PT (s) | 10.9(10.5,11.4) | 11.1(10.7,11.5) | 10.9(10.4,11.3) | 0.009* |
| APTT (s) | 26.1(24.4,27.6) | 25.4(24.2,27.6) | 26.3(24.6,27.6) | 0.224 |
| INR | 0.93(0.89,0.97) | 0.95(0.91,1.00) | 0.92(0.89,0.97) | 0.005* |
| Blood glucose (mmol/L) | 7.85(6.37,9.69) | 8.68(7.08,11.87) | 7.60(6.31,9.23) | <0.001** |
Values are n (%) and median (25,75%)
HE hematoma expansion, ICH intracerebral hemorrhage, SBP systolic blood pressure, DBP diastolic blood pressure, GCS Glasgow coma scale, NIHSS National Institutes of Health stroke scale, IVH intraventricular hemorrhage, RBC red blood cells, NPR neutrophil to platelet ratio, PT prothrombin time, APTT activated partial thromboplastin time, INR international normalized ratio
*p < 0.05. **p < 0.001
Univariate analysis showed significant associations between HE with age (p = 0.028), GCS score on admission (p < 0.001), NIHSS score on admission (p = 0.019), baseline hematoma volume (p < 0.001), RBC count (p = 0.03), ANC (p < 0.001), ALC (p < 0.001), AMC (p = 0.003), Platelet (p < 0.001), NPR (p < 0.001), PT (p = 0.009), INR (p = 0.005) and blood glucose (p < 0.001) (Table 1).
The multivariate analysis indicated that GCS score (3–8 points) [OR = 3.387, 95% CI: 1.443–7.949, p = 0.005], NPR (OR = 1.742; 95% CI: 1.508–2.012, p < 0.001) and larger hematoma size (OR = 1.015, 95% CI: 1.002–1.028, p = 0.022) were significantly correlated with HE (Table 2).
Table 2.
Multivariate analysis of predictors for HE
| Predictors | OR (95% CI) | p-Value |
|---|---|---|
| Age(years) | 0.998 (0.975-1.021) | 0.857 |
| NPR(per 1 increase) | 1.742 (1.508-2.012) | <0.001** |
| GCS score on admission | - | - |
| GCS (13-15 points) | Reference | - |
| GCS (9-12 points) | 1.796 (0.772-4.183) | 0.174 |
| GCS (3-8 points) | 3.387 (1.443-7.949) | 0.005* |
| NIHSS on admission | 1.020(0.927-1.122) | 0.688 |
| PT (s) | 0.929 (0.354-2.441) | 0.882 |
| RBC, x1012 | 0.967 (0.446-2.098) | 0.932 |
| Hemoglobin(g/L) | 1.003 (0.985-1.020) | 0.772 |
| Lymphocyte, x109 | 0.727 (0.457-1.158) | 0.180 |
| Monocyte, x109 | 2.003 (0.616-6.508) | 0.248 |
| Neutrophil, x109 | 0.926 (0.715-1.199) | 0.561 |
| Platelet, x109 | 1.001 (0.994-1.007) | 0.842 |
| INR | 3.230 (0.024-430.0) | 0.639 |
| Blood glucose (mmol/L) | 1.073 (0.981-1.174) | 0.125 |
| Hematoma size (per 1 ml increase) | 1.015 (1.002-1.028) | 0.022* |
OR odds ratio, CI confidence interval, HE hematoma expansion, NPR neutrophil to platelet ratio, GCS Glasgow coma scale, NIHSS National Institutes of Health stroke scale, PT prothrombin time, RBC red blood cells, INR international normalized ratio
*P < 0.05. **P < 0.001
The area under the ROC curve was 0.838 (95% CI, 0.788–0.888, p < 0.001) for HE (Fig. 2). The best predictive cut-off value of HE was 5.47 (sensitivity, 75.3%; specificity, 77.6%).
Fig. 2.
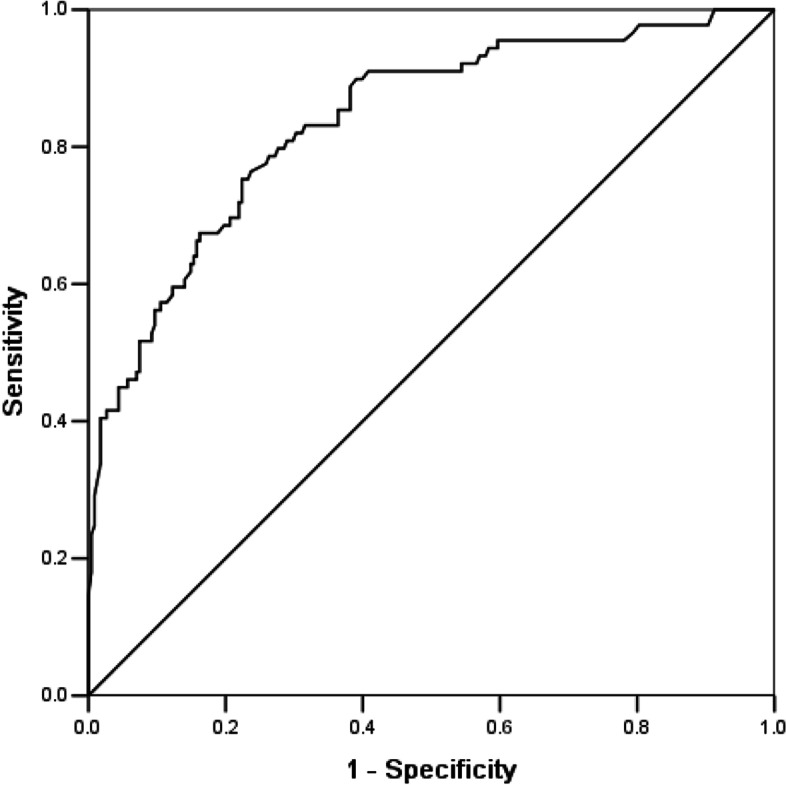
Receiver operating characteristic curve for the value of the NPR for the prediction of HE in ICH patients
Discussion
This study, to the best of our knowledge, is the first to analyze the relationships between the NPR and HE in spontaneous ICH patients. The present study found that a high NPR was related to HE after spontaneous ICH.
The occurrence of HE could be detected within 3 h of symptom onset in approximately 73% of ICH patients, and clinically obvious expansion was present in 35% of patients [11–13]. Accumulating evidence suggests that inflammation caused by HE accelerates brain injury in patients with ICH [4, 14–16]. Although the relationship between WBC count and outcome in ICH patients has been well demonstrated [3, 17], the correlation between early HE and leukocyte subsets remains disputed [4, 16, 18, 19]. In our study, the univariate analysis showed significant associations between HE and ANC, ALC, AMC and NPR, and multivariate logistic regression analysis showed that all those biomarkers mentioned above could not independently predict early HE, except for the NPR. However, the exact underlying mechanism of the associations between routine blood variables and HE remains unclear and needs further study.
Neutrophils play a basic defensive role in both infection-related diseases and aseptic inflammation, which are indicators of inflammation and immune response [20]. Neutrophils appear first in the hematoma [21], delivering pro-inflammatory factors, oxygen free radicals and proteases, which could have an effect on blood–brain barrier (BBB) disruption and brain damage [22, 23]. Previous studies have reported that neutrophils are the primary cellular source of metalloproteinases (MMPs), specifically MMP-9, working on the BBB [24]. Moreover, it has been shown that in ischemia–reperfusion injury, the increase in BBB permeability induced by WBC-derived MMP-9 is associated with peak neutrophil infiltration [25]. Furthermore, accumulating studies have reported that HE is associated with changes in the basal membrane of the BBB induced by MMP [4, 26, 27]. Therefore, it is reasonable to believe that neutrophils are associated with HE.
Several studies have reported the relationship between neutrophil count and hematoma size. Neutrophil count at admission was positively associated with intracerebral hemorrhage volume [28], and it was found in another study that the inhibition of neutrophil recruitment could reduce the amount of bleeding [29]. One study reported that neutrophil count was negatively related to an increased risk of HE during the hyperacute phase of ICH [19]. One possible explanation for this paradoxical finding is that the injury to blood vessels caused by neutrophils may be mediated by platelets, and neutrophil-platelet interactions may play different roles in vascular inflammation at different stages of ICH [30]. Despite activated neutrophils having a procoagulant role [31], the mutual relationship between neutrophils and platelets could increase the formation of reactive oxygen species and aggravate vascular damage [32]. In addition, platelets, as a considerable contributor to some pro-inflammatory factors, could enhance the aggregation of activated neutrophils [30, 33].
In a review of neutrophil-platelet interplay, activated platelets were found to be connected with the release of inflammatory mediators, the accumulation of neutrophils, and increased vascular permeability [7]. By locally releasing soluble vascular protective factors, platelet-endothelial interplay may prevent or treat neutrophil-induced vascular damage [34]. The hemostatic role of platelets depends on embolization and coagulation at the location of vascular injury [35], which contributes to the preservation the integrity of the BBB [36]. Systemic inflammation is often accompanied by thrombocytopenia, which may be attributed to the immune response in the blood circulation [30]. According to the above, it can be speculated that in patients with ICH, the higher the NPR, the more serious the BBB damage may be, resulting in the higher occurrence of HE.
The NPR may be more stable as a ratio than individual blood parameters, such as neutrophils or thrombocytes, because of the mutual relationship between neutrophils and platelets. Recent studies have shown a correlation between the NPR and other diseases, such as ischemic stroke [8, 37–40], ST-elevation myocardial infarction [41] and infective endocarditis [42]. For example, a high NPR was related to an increased risk of hemorrhagic transformation in acute ischemic stroke patients [8]; the platelet-to-neutrophil ratio (PNR) was found to be an independent protective predictor of 90-day prognosis in patients with acute ischemic stroke [38]. Moreover, the PNR on admission could independently predict poor functional prognosis in ischemic stroke patients undergoing intravenous thrombolysis [39]. Consistent with these findings, we found a significant association between the NPR and ICH, and a high NPR on admission was an independent predictor of HE in ICH patients.
In addition, previous studies have reported that a larger baseline ICH volume and lower GCS score on admission were associated with HE [43, 44]. In our study, the initial hematoma volume and GCS score on admission were predictors of HE in both univariate analysis and multivariate regression analysis. The results were consistent with Zhang’s study [44] and Li’s study [45]. On the other hand, an onset-to-first-CT time of less than 6 h was independently associated with HE [19, 46]. However, in our study, the median onset-to-first-CT time was 7 h and the median for the group with HE was 8 h. One possible explanation may be that West China Hospital of Sichuan University is an upper-level hospital in Southwest China, and many patients request to be transferred to our hospital for treatment from lower-level hospitals in this region or other regions. Therefore, the median onset-to-first-CT time in our study was longer.
Given HE being the independent risk factor for disability and death in ICH patients, it is crucial to timely identify HE [2, 13]. In this case, NPR has its clinical implications. Firstly, in clinical practice, as a routine indicator of blood test, it is easy and convenient to obtain NPR. Moreover, the predictability of NPR here can help clinicians initially estimate the risk of HE in ICH patients, and then conduct appropriate treatment and follow-up CT for that population. In addition, since single predictor for HE has its limitation, there are currently a variety of prediction scores containing several predictors [47]. Similarly, the combination of NPR and other predictive factors can form new prediction scores with higher specificity and sensitivity. However, the potential clinical implications of NPR should be further investigated.
Several limitations should be noted in this study. First, the data were recorded in a single center, and the sample size was limited. Second, although the results of this retrospective study may be influenced by confounding factors, multivariate analysis was used to address this problem. Third, given the complex role of inflammation in HE, more studies that record more inflammatory biomarkers are necessary in future studies.
Conclusions
This present study showed that a high NPR was related to the risk of HE in ICH patients. These findings may assist clinicians in identifying ICH patients who have increased risk for HE and then in conducting appropriate treatment and follow-up CT for that population. In view of the limitations of the study, future well-designed studies are needed to confirm our findings.
Acknowledgements
Not applicable.
Abbreviations
- ICH
Intracerebral cerebral hemorrhage
- HE
Hematoma expansion
- NPR
Neutrophil-to-platelet ratio
- CT
Computed tomography
- GCS
Glasgow Coma Scale
- NIHSS
National Institutes of Health Stroke Scale
- IVH
Intraventricular hemorrage
- RBC
Red blood cell
- AMC
Absolute monocyte count
- ANC
Absolute neutrophil count
- ALC
Absolute lymphocyte count
- PT
Prothrombin time
- APTT
Activated partial thromboplastin time
- INR
International normalized ratio
- IQR
Interquartile range
- ROC
Receiver operating characteristic
- BBB
Blood–brain barrier
- MMPs
Metalloproteinases
- PNR
Platelet-to-neutrophil ratio
Authors' contributions
Y.L. and J.Z. designed the project. Y.L. and X.Y. did the statistical analyses and wrote the manuscript draft. Y.L., H.Z., J.Z., X.Y., X.H. and H.L. prepared Figures 1, 2 and Tables 1, 2. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China grant number 81801186, Science and Technology Department of Sichuan Provence grant number 2020YFQ0009 and Outstanding Subject Development 135 Project of West China Hospital, Sichuan University grant number ZY2016102.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving the patient included were in accordance with the ethical standards of the institutional board and with the 1964 Helsinki Declaration. This study is approved by the Medical Ethics Committee of the West China Hospital of Sichuan University. At admission, the subjects or the guardians of patients with cognitive impairment provided written informed consent for research and publication.
Consent for publication
We have obtained consent to publish from the participant to report individual patient data.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yujian Li and Xiang Yang contributed to the work equally and should be listed as co-first authors.
References
- 1.Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Dowlatshahi D, Demchuk AM, Flaherty ML, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–467. doi: 10.1212/01.WNL.0000133204.81153.AC. [DOI] [PubMed] [Google Scholar]
- 4.Silva Y, Leira R, Tejada J, et al. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36:86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]
- 5.Wang KW, Cho CL, Chen HJ, et al. Molecular biomarker of inflammatory response is associated with rebleeding in spontaneous intracerebral hemorrhage. Eur Neurol. 2011;66:322–327. doi: 10.1159/000332027. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Qian J, Tao C, et al. Neutrophil to lymphocyte ratio predicts island sign in patients with intracranial hemorrhage. Medicine (Baltimore) 2018;97:e13057. doi: 10.1097/MD.0000000000013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Culebras A, Durán-Laforet V, Peña-Martínez C, et al. Myeloid cells as therapeutic targets in neuroinflammation after stroke: Specific roles of neutrophils and neutrophil-platelet interactions. J Cereb Blood Flow Metab. 2018;38:2150–2164. doi: 10.1177/0271678X18795789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, Ruan Y, Yuan C, et al. High Neutrophil-to-Platelet Ratio Is Associated With Hemorrhagic Transformation in Patients With Acute Ischemic Stroke. Front Neurol. 2019;10:1310. doi: 10.3389/fneur.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.STR.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 10.Wada R, Aviv RI, Fox AJ, et al. CT angiography "spot sign" predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 11.Fujii Y, Tanaka R, Takeuchi S, et al. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. 1994;80:51–57. doi: 10.3171/jns.1994.80.1.0051. [DOI] [PubMed] [Google Scholar]
- 12.Fujii Y, Takeuchi S, Sasaki O, et al. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–1166. doi: 10.1161/01.STR.29.6.1160. [DOI] [PubMed] [Google Scholar]
- 13.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 14.Lee KR, Colon GP, Betz AL, et al. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84:91–96. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlmann CR, Librizzi L, Closhen D, et al. Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke. 2009;40:1458–1466. doi: 10.1161/STROKEAHA.108.535930. [DOI] [PubMed] [Google Scholar]
- 16.Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-Lymphocyte Ratio Predicts the Outcome of Acute Intracerebral Hemorrhage. Stroke. 2016;47:1654–1657. doi: 10.1161/STROKEAHA.116.013627. [DOI] [PubMed] [Google Scholar]
- 17.Sun W, Peacock A, Becker J, et al. Correlation of leukocytosis with early neurological deterioration following supratentorial intracerebral hemorrhage. J Clin Neurosci. 2012;19:1096–1100. doi: 10.1016/j.jocn.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S, Kelley RE, Dandapani BK, et al. Acute leukocyte and temperature response in hypertensive intracerebral hemorrhage. Stroke. 1995;26:1020–1023. doi: 10.1161/01.STR.26.6.1020. [DOI] [PubMed] [Google Scholar]
- 19.Morotti A, Phuah CL, Anderson CD, et al. Leukocyte Count and Intracerebral Hemorrhage Expansion. Stroke. 2016;47:1473–1478. doi: 10.1161/STROKEAHA.116.013176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leliefeld PH, Wessels CM, Leenen LP, et al. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016;20:73. doi: 10.1186/s13054-016-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Jickling GC, Liu D, Ander BP, et al. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35:888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Xu H, Du X, et al. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab. 2006;26:1089–1102. doi: 10.1038/sj.jcbfm.9600264. [DOI] [PubMed] [Google Scholar]
- 25.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Sabín J, Delgado P, Abilleira S, et al. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 28.Adeoye O, Walsh K, Woo JG, et al. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23:e107–111. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Z, Yu S, Chen X, et al. Suppression of NLRP3 attenuates hemorrhagic transformation after delayed rtPA treatment in thromboembolic stroke rats: Involvement of neutrophil recruitment. Brain Res Bull. 2018;137:229–240. doi: 10.1016/j.brainresbull.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Rossaint J, Margraf A, Zarbock A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front Immunol. 2018;9:2712. doi: 10.3389/fimmu.2018.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald B, Davis RP, Kim SJ, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanichakarn P, Blair P, Wu C, et al. Neutrophil CD40 enhances platelet-mediated inflammation. Thromb Res. 2008;122:346–358. doi: 10.1016/j.thromres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Bhasym A, Annarapu GK, Saha S, et al. Neutrophils develop rapid proinflammatory response after engulfing Hb-activated platelets under intravascular hemolysis. Clin Exp Immunol. 2019;197:131–140. doi: 10.1111/cei.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho-Tin-Noé B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011; 56–65. [DOI] [PMC free article] [PubMed]
- 35.Stalker TJ, Welsh JD, Brass LF. Shaping the platelet response to vascular injury. Curr Opin Hematol. 2014;21:410–417. doi: 10.1097/MOH.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones LD, Jackson JW, Maggirwar SB. Modeling HIV-1 Induced Neuroinflammation in Mice: Role of Platelets in Mediating Blood-Brain Barrier Dysfunction. PLoS ONE. 2016;11:e0151702. doi: 10.1371/journal.pone.0151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long H, Qin K, Chen J, et al. Biomarkers of gastric cancer-related ischemic stroke and its underlying pathogenesis. Medicine (Baltimore) 2018;97:e0493. doi: 10.1097/MD.0000000000010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin P, Li X, Chen J, et al. Platelet-to-neutrophil ratio is a prognostic marker for 90-days outcome in acute ischemic stroke. J Clin Neurosci. 2019;63:110–115. doi: 10.1016/j.jocn.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Pan H, Fu M, Ge W, et al. The effects of changes in platelet-to-neutrophil ratios 24 hours after intravenous thrombolysis on prognosis in acute ischemic stroke patients. Clin Neurol Neurosurg. 2020;190:105739. doi: 10.1016/j.clineuro.2020.105739. [DOI] [PubMed] [Google Scholar]
- 40.Wang MQ, Sun YY, Wang Y, et al. Platelet-to-neutrophil ratio after Intravenous Thrombolysis Predicts Unfavorable Outcomes in Acute Ischemic Stroke. Curr Neurovasc Res. 2020. [DOI] [PMC free article] [PubMed]
- 41.Somaschini A, Cornara S, Demarchi A, et al. Neutrophil to platelet ratio: A novel prognostic biomarker in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Eur J Prev Cardiol. 2019; : 2047487319894103. [DOI] [PubMed]
- 42.Wei XB, Liu YH, He PC, et al. The impact of admission neutrophil-to-platelet ratio on in-hospital and long-term mortality in patients with infective endocarditis. Clin Chem Lab Med. 2017;55:899–906. doi: 10.1515/cclm-2016-0527. [DOI] [PubMed] [Google Scholar]
- 43.Kazui S, Minematsu K, Yamamoto H, et al. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–2375. doi: 10.1161/01.STR.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 44.Zhang F, Zhang S, Tao C, et al. Association between serum glucose level and spot sign in intracerebral hemorrhage. Medicine. 2019;98:e14748. doi: 10.1097/MD.0000000000014748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Huang YJ, Zhang G, et al. Intraventricular Hemorrhage and Early Hematoma Expansion in Patients with Intracerebral Hemorrhage. Sci Rep. 2015;5:11357. doi: 10.1038/srep11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71:158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yogendrakumar V, Moores M, Sikora L, et al. Evaluating hematoma expansion scores in acute spontaneous intracerebral hemorrhage: A systematic scoping review. Stroke. 2020;51:1305–1308. doi: 10.1161/STROKEAHA.119.028574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.