Abstract
Background
Receptor activator of nuclear factor κ B ligand (RANKL), osteoprotegerin (OPG), cartilage oligomeric matrix protein (COMP), bone morphogenetic protein (BMP-2), and fibroblast growth factor 23 (FGF-23) are involved in inflammation, calcium deposition, and fibrosis of blood vessels. Acute changes in these factors may contribute to the progression of arteriosclerosis, especially if their elevated serum levels persist postoperatively.
Material/Methods
A total of 90 patients (79 White, 4 African American, and 7 Other) undergoing elective heart surgery were enrolled in the study. Blood was collected before surgery and after surgery at 24 hours, 7 days, and 3 months to allow for longitudinal comparisons. After the plasma isolation, several biomarkers levels were studied using an enzymatic-linked assay. Demographic and clinical information were obtained from electronic health records.
Results
At 24 hours after surgery, RANKL (RANKLbaseline=248.7±215.7 vs RANKLt24h=376.4±329.7; P=0.035), and BMP-2 (BMP-2baseline=283.7±255.4 vs BMP-2t24h=482.4; P=0.015) were significantly elevated compared to baseline, with levels returning to baseline at 7 days. FGF-23 increased significantly from baseline (FGF-23baseline=1020±1210) to 7 days (FGF-237d=2191±5188; P=0.029) and remained significantly higher than baseline at 3 months (FGF-233m=2041±3521; P=0.044). White blood cells (WBC) remained elevated at discharge (WBCbaseline=6.8±2.1 vs WBC24h=15.0±5.3 vs WBCdischarge =8.8±3.4). IL-8 and C-reactive protein normalized at 3 months. Estimated blood loss was significantly correlated with RANKL at 24 hours (r2=0.33; P=0.035). Serum creatinine levels after surgery at 24 hours (r2=0.41; p=0.008) and 7 days (r2=0.59; P=0.000) was strongly correlated with COMP.
Conclusions
Persistent elevation of serum FGF-23 indicates a potential for accelerated arteriosclerosis after cardiac surgery.
Keywords: Arteriosclerosis; BMP2 Protein, Human; Fibroblast Growth Factor 23; Receptor Activator of Nuclear Factor-kappa B; Thoracic Surgery
Background
Cardiac surgery activates general and vascular inflammation secondary to iatrogenic damage to myocardium and endothelium [1–5]. Additionally, non-biological materials deployed during coronary artery bypass grafting (CABG) or valve replacement will further fuel inflammation [3,6,7]. Subsequently, complement consumption, intravascular coagulation activation, and free radical production are observed. Together, these factors lead to accumulation of modified apolipoproteins and increased calcification, which are key factors in the progression of arteriosclerosis [8,9]. A Disintegrin and Metalloproteinase 17 (ADAM-17) enzyme is pivotal in fibrosis and arteriosclerosis acceleration via modulation of TNFα, which is a key cytokine in inflammatory response [10,11]. Other elements of nonspecific inflammation, C-reactive protein (CRP) and interleukin-6 (IL-6) are also involved in remodeling blood vessels and endothelial function47]. Consequently, perioperative inflammation can accelerate arteriosclerosis, with the subsequent decline in cardiac output, increased risk of aneurysm formation, and end-organ malfunction [8,9,12–14]. However, it is unclear if, to what extent, and for how long perioperative inflammation results in endothelial abnormalities and vascular remodeling after cardiac surgery.
In addition to nonspecific markers, perioperative inflammation also affects several specific factors critical in the natural history of arteriosclerosis [9]. Receptor Activator of the Nuclear Factor κB Ligand (RANKL) translates the inflammatory process into calcified deposits in the smooth muscle of blood vessels via Nuclear Factor kappa-light-chain-enhancer of activated B cells (NFκB) and c-Jun-N-terminal Kinase (JNK) upon binding to its receptor (RANK) [15,16]. This action is synergistic with TNFα. Furthermore, RANKL/RANK increases serum Bone Morphotic Protein 2 (BMP-2) levels, another pivotal factor in calcifying blood vessels [15]. RANKL may persistently activate during cardiac surgery vie monocyte colony stimulation factor (M-CSF) for several weeks into recovery [16–18]; therefore, cardiac surgery can create conditions for sustained and persistent activation of NFκB, RANKL, and BMP-2 [15,18]. The physiological effect of RANK/RANKL is countered by osteoprotegerin (OPG), a decoy receptor for RANKL [19,20]. Thus, it is not surprising to observe that OPG levels are inversely correlated with the acceleration and magnitude of arteriosclerosis and subsequent organ failure [21–23]. Conversely, supplementing OPG reverses calcification and atherosclerosis [24]. This fine-tuned system can become imbalanced secondary to perioperative inflammation, ischemia, and free radical release, resulting in depletion of OPG, increasing the risk of unbalanced RANK/RANKL influence [19,25]. Like the RANK/RANKL/OPG system, Cartilage Oligomeric Matrix Protein (COMP) and BMP-2 create another balanced interaction between pro- and anti-arteriosclerotic factors [25,26]. COMP induces vascular remodeling and senescence and co-localizes with calcifications but is also critical in maintaining smooth vasculature phenotype [27,28]. It exerts its action via BMP-2-integrin interactions and the ADAM-17 system [29]. COMP co-localization within calcified lesions is linked to interference with BMP-2 and acceleration of arteriosclerosis [30,31]. Additionally, arteriosclerosis may be accelerated by fibroblast growth factor 23 (FGF-23), an inflammatory marker of chronic inflammation and kidney failure. Specific mechanisms include fibrosis, calcium deposition, thickening of carotid intima, and ADAM-17 modulation [11,32–35]. Subsequently, FGF-23 was demonstrated to be a risk factor for accelerated coronary artery disease and post-cardiac surgery complications [32,34,36,37].
Despite the volume of indirect evidence for accelerated arteriosclerosis in patients after cardiac surgery, markers of arteriosclerosis have been little studied in these patients [2,6,7,23,33,35]. Prior data suggest that pre-existing ischemic conditions in the heart may result in an imbalance between pro- versus anti-arteriosclerotic of RANK/RANKL/OPG and COMP/BMP-2 systems [19,26]. Furthermore, sustained expression of M-CSF after cardiac surgery provides a mechanism for overexpression of RANK/RANKL, potentially modifying the risk of arteriosclerosis [18,20,25]. Progression of arteriosclerosis in patients with end-stage renal disease is fueled by an imbalance of these factors [4,12,34,35]. If the post-surgical serum milieu exhibits a misbalance towards pro-arteriosclerotic factors, the benefits of cardiac surgery may be minimized as protracted elevation of these markers accelerates arteriosclerosis [12,38].
Here, we hypothesize that perioperative inflammation will disrupt RANK/RANKL/OPG and/or COMP/BMP-2 balance during the acute period, with recovery to baseline levels at 3 months. Furthermore, we hypothesize that FGF-23 will be transiently elevated as a part of vascular inflammation and progressive arteriosclerosis, considering that the changes in serum arteriosclerosis markers will follow the dynamics of inflammatory markers (CRP, IL-8). This study addresses a gap in the knowledge concerning the dynamics of arteriosclerotic serum markers after cardiac surgery.
Material and Methods
Ethics and Consent
The University of Pennsylvania Institutional Review Board approved the study (#815686). All patients scheduled for non-emergent cardiac surgery were approached. Patients were enrolled in the study after consent was secured.
Patient Population
In this observational study, a total of 90 patients admitted for elective cardiac surgery at the University of Pennsylvania Health System were approached between 2015 and 2020 and consented. The specific types of cardiac surgery type are detailed in Table 1. Inclusion criteria were adult patients (≥18 years) undergoing elective cardiac surgery. The exclusion criteria included patients <18 years, emergent surgery, immunosuppression, history of transplant, and lack of consent.
Table 1.
Patient characteristics and surgical details.
| Demographics (N=90) | [n/N (%)] |
|---|---|
| Age [years±SD] | 64.1±12.15 |
| Over 60 | 67/90 (74.44%) |
| Sex | |
| Male | 69/90 (76.67%) |
| Female | 21/90 (23.33%) |
| Race | |
| Black | 4/90 (4.44%) |
| White | 79/90 (87.87%) |
| Other/Asian/Unknown | 7/90 (7.69%) |
| Pre-existing conditions | [n/N (%)] |
| Weight [kg] (mean±SD) | 83.7±20.06 |
| BMI (mean±SD) | 27.5±5.71 |
| Charleston Comorbidity Index [χ±SD] | 4.0±2.02 |
| Acute coronary syndrome | 13/90 (14.44%) |
| Chronic heart failure | 14/90 (15.56%) |
| Peripheral vascular disease | 8/90 (8.89%) |
| Cerebrovascular accident/transient ischemic attack | 10/90 (11.11%) |
| Chronic obstructive pulmonary disorder | 8/90 (8.99%) |
| Diabetes melitus | 26/90 (28.89%) |
| Anesthesia & surgery data | [n/N (%)] |
| Duration of anesthesia; mean±SD [min] | 3745±98.8 |
| Duration of surgery; mean±SD [min] | 265.9±92.95 |
| Duration of cardiopulmonary bypass; mean±SD [min] | 131.6±58.73 |
| Coronary artery bypass surgery | 44/90 (48.89%) |
| Mitral valvuloplasty & replacement | 14/90 (15.56%) |
| Aortic valvuloplasty & replacement | 24/90 (26.67%) |
| Aortic aneurysm repair | 6/90 (6.67%) |
| Others | 3/90 (3.33%) |
| Estimated Blood Loss mean±SD [mL] | 202±270.9 |
| Perioperative management | [n/N (%)] |
| Transfused Packed Red Blood Cells, mean; (IQ25; IQ75) [mL] | 85±236.1 |
| Transfused Fresh Frozen Plasma, mean; (IQ25; IQ75) [mL] | 51±186 |
| Ketorolac administration | 7/90 (7.87%) |
| Acetaminophen administration | 72/90 (79.78%) |
| Acetylsalicylic acid administration | 67/90 (74.16%) |
| Total opioids administration [mg] | 88.82±9.01 |
| Total benzodiazepines administration [mg] | 3.7±1.71 |
| Functional outcomes | mean±SD |
| APACHE score at 1 h | 17.4±5.85 |
| APACHE score at 24 h | 9.7±4.93 |
| APACHE score at 48 h | 9.5±5.31 |
| Length of Stay (LOS) at 28 days | mean±SD |
| LOS ICU | 8.7±43.2 |
| LOS hospital | 10.7±22.3 |
| Discharged home | 75/90 (83.33%) |
| Discharged to healthcare facility | 14/90 (15.56%) |
| Death | 1/90 (1.11%) |
APACHE – Acute Physiology and Chronic Health Evaluation; LOS – length of stay; ICU – Intensive Care Unit.
Clinical Data Collection and Outcomes
Demographic and clinical data were obtained from electronic health records (EHR), including surgical, anesthesia, perioperative, and length of stay (LOS) records (Table 1). Morphine equivalents were calculated for opioids in the first 24 hours following surgery. APACHE II scores were calculated upon admission to the Intensive Care Unit (ICU) and 24 hours later [39,40]. The diagnoses of deep venous thrombosis, pulmonary embolisms, and cerebrovascular events pre- and peri-operatively were extracted from medical records by manual chart review. Mortality and disposition were defined at the time of hospital discharge.
Study Procedure
After patient consent was secured, blood was collected before the onset of surgery (t0). Subsequent blood procurements took place 24 hours (t24hr) and 7 days (t7d) after surgery, with the final follow-up occurring at 3 months (t3m).
Blood was collected one of 3 ways: 1) from arterial lines during the hospital stay, 2) from the venous system using central lines, or 3) manually drawn using the Vacutainer™ system (BD; Franklin Lakes, NJ). Blood was collected and stored at 4°C with processing within 2–4 hours after collection. Plasma was isolated by centrifuging blood for 10 min at 1200×g, 4°C. The isolated plasma was aliquoted and stored at −80°C.
Assessment of Biomarkers and Biological Variables
RANKL, OPG, COMP, and BMP-2 were assessed using a multiplex system (Thermofisher, Philadelphia, PA). FGF-23 was tested in serum using an enzyme-linked immunosorbent assay (ELISA) kit (R&D; Minneapolis, MN). Markers of inflammation (CRP, IL-8, CD40L, P-selectin) were measured using a multiplex system (Thermofisher, Philadelphia, PA).
White blood cells, serum creatinine, and platelet count were obtained from medical records as part of lab orders.
Statistical Analysis
Parametric variables were presented as mean with standard deviation (SD) and compared using the t test for 2 variables, while ANOVA was used for multiple comparisons with Duncan’s post hoc analysis. For non-parametric variables, median (Me) and interquartile ranges (IR) were utilized and the Mann-Whitney U test was used. Pearson r correlation coefficients were used to assess relationships between variables. Two-sided P values less than 0.05 were considered statistically significant for all tests unless a specific null hypothesis was formulated first. Statistical analyses were performed with Graph (GraphPad; GraphPad Software LLC, San Diego, CA).
Results
Patient Baseline Data
Neither sex nor age affected the levels of RANKL, OPG, COMP, or BMP-2 at baseline. Pre-surgical serum FGF-23 levels were significantly higher in males than females (FGF-23male=3059.1±6924.8 vs FGF-23female=2267±71664; t[57;18]=15.25; P=0.00000) (data not shown).
Patients with pre-existing moderate to severe renal disease had significantly higher levels of COMP compared to patients without severe renal disease (COMPhealthy=11.9±13.2 vs COMPrenal failure=36.5±51.6; t[36;10]=15.25; P=0.00000), while the other biomarker levels were not significantly different between these 2 groups. Pre-existing peripheral artery disease, heart failure, renal failure, or liver failure did not affect baseline levels of RANKL, OPG, BMP-2, COMP, or FGF-23. Patients with diabetes had significantly higher levels of BMP-2 (BMP-2healthy=229.9±219 vs BMP-2Diabetes=1388±2325; t[21;6]=15.25; P=0.026) and FGF-23 (FGF-23healthy=2184±4056 vs BMP-2Diabetes=21773±66450; t[73]=2.17; P=0.03) compared with patients without diabetes, while the other biomarker levels were not significantly different between these 2 groups (data not shown).
Biomarker Dynamics After Surgery
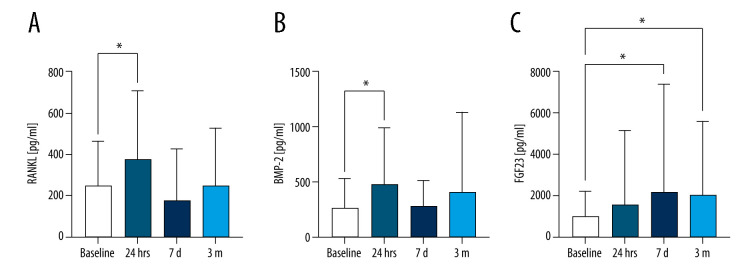
When assessing biomarker levels for the entire patient population, RANKL levels were significantly increased from baseline to 24 hours (RANKLbaseline=248.7±215.7 vs RANKLt24h=376.4±329.7; P=0.035), with levels returning to baseline at 7 days and remaining there at 3 months (Figure 1A). COMP serum level did not change significantly after surgery (data not shown). OPG levels were significantly different from baseline to t24hr (F[3,10]=8.87; P=0.048) when paired time series were compared, but no pairwise comparisons revealed any significant difference (data not shown). Serum BMP-2 levels were significantly increased 24 hours after surgery (BMP-2baseline=283.7±255.4 vs BMP-2t24h=482.4; P=0.015) and normalized afterward (Figure 1B). Serum COMP levels were highly variable at all time points, with no statistically significant changes (data not shown). FGF-23 increased significantly from baseline (FGF-23baseline=1020±1210) to 7 days (FGF-237d=2191±5188; P=0.029) and remained significantly higher than baseline at 3 months (FGF-233m=2041±3521; P=0.044) (Figure 1C). There was not a significant increase in FGF-23 at 24 hours (add p=0.45). RANKL levels at 24 hours correlated strongly with COMP (r=0.42; P=0.009) and FGF-23 (r=0.57; P=0.02).
Figure 1.
Biomarker levels after surgery.
Biomarker levels were measured with RT-PCR from patient blood samples taken at baseline (pre-surgery), 24 hours after surgery, 7 days after surgery, and 3 months after surgery. Biomarker levels are presented as mean±SD. (A) RANKL, (B) BMP-2, (C) FGF-23. * P<0.05. SD – standard deviation.
Duration of anesthesia, surgery, cardiopulmonary bypass, or cross-clamp did not significantly affect levels of measured factors at any time points. However, estimated blood loss during surgery was significantly correlated with serum RANKL at 24 hours (r=0.33; P=0.035) (data not shown).
OPG serum levels 24 hours after surgery were correlated with the total amount of morphine (r=0.34; P=0.026) and benzodiazepines (r=0.44; P=0.02) received during anesthesia. Perioperative intake of acetylsalicylic acid (ASA) was related to a significantly decreased level of OPG at 24 hours (U[51]=2.07; P=0.038), but no other markers were affected, even though the data were highly non-parametric. Perioperative intake of acetaminophen, ketorolac, or steroids did not affect any arteriosclerosis markers at 24 hours or 7 days (data not shown).
Inflammatory Markers After Surgery
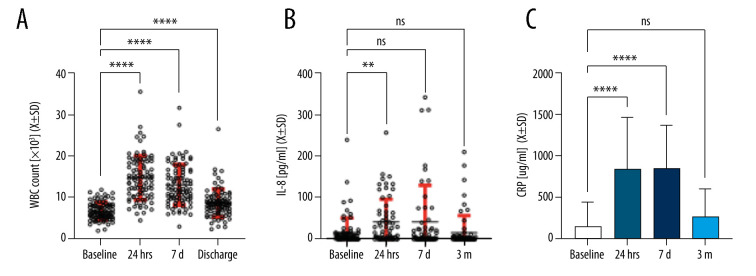
Nonspecific inflammation was measured via white blood cells (WBC) levels, IL-8, P-selectin, and CD40L. After a significant increase in WBCs and IL-8 at 24 hours, WBC levels remained significantly higher at 7 days and 3 months, while IL-8 levels returned to baseline at 7 days and remained at baseline at 3 months (Figure 2A, 2B). Serum P-selectin (F[3;127]=0.48; P=ns) and CD40L (F[4;95]=0.99; P=ns) levels remained unchanged throughout the observation period (data not shown). Baseline CRP serum levels (MeCRPbaseline=27[0;120]) were significantly elevated at 24 hours (MeCRP24hr=763[231;1502]) and 7 days (MeCRP7d=685[343;1360]), with levels returning to baseline at 3 months (MeCRP3m=110[48;331]; P=0.13) (Figure 2C).
Figure 2.
Inflammatory markers after surgery.
White blood cells, IL-8 and CRP from patient blood samples taken at baseline (pre-surgery) and at 24 hours, 7 days, and 3 months after surgery. Levels are presented as mean±SD. (A) WBC count, (B) IL-8, (C) CRP. Confidence intervals are indicated in red bars. ** P<0.01; **** P<0.0001. ns – not significant; SD – standard deviation; WBC – white blood cells.
Patient Outcomes
Serum creatinine levels at 24 hours (r=0.41; P=0.008) and 7 days after surgery (r=0.59; P=0.000) (Figure 3) were strongly correlated with COMP at the same follow-up times. APACHE scores at 1 and 24 hours after was surgery did not correlate with any of the markers. However, COMP level at 24 hours strongly correlated with length of stay in the ICU (r=0.39; P=0.000).
Figure 3.
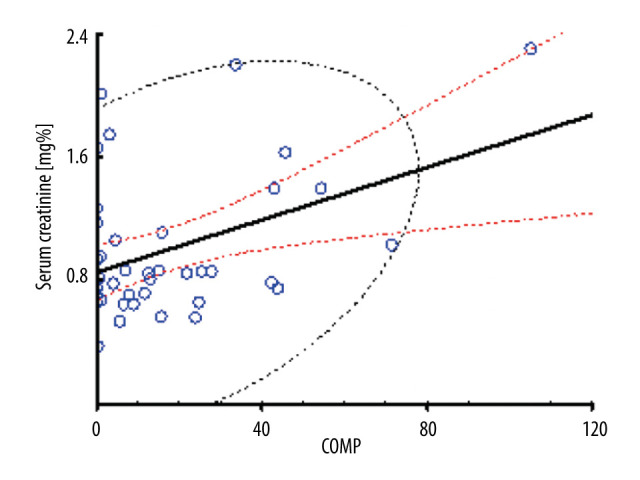
Correlation of serum creatinine with COMP.
Serum creatinine levels at 24 hours were significantly correlated with serum COMP serum levels. Red lines indicate 95% confidence intervals.
The incidence of deep venous thrombosis (n=2) and pulmonary embolism (n=2) was too low to calculate meaningful statistics. Patients with stroke after admission to the hospital had elevated levels of BMP-2, but that difference was present even before surgery (data not shown).
Discussion
This is the first long-term observational study of serum markers related to the progression of arteriosclerosis and arterial stiffness in patients undergoing cardiac surgery. We hypothesized that imbalance within dyads of pro- versus anti-arteriosclerotic serum biomarkers (RANK/RANKL/OPG and COMP/BMP-2) would result in significant abnormalities secondary to peri-surgical inflammation and that FGF-23 levels would be elevated after surgery. We demonstrated that RANKL and BMP-2 were elevated during the acute perioperative period. Significantly, FGF-23 persisted in serum for 3 months after elective heart surgery. Prolonged FGF-23 activation could accelerate arteriosclerosis, potentially limiting the long-term benefits of cardiac surgery. These changes were accompanied by the resolution of some but not all inflammatory markers.
Dynamic changes in RANK/RANKL/OPG, COMP/BMP-2, and FGF-23 in the acute perioperative period are expected as they play an essential role in the inflammatory response [19,21–23,32,34,41]. Additionally, they are significantly involved in kidney function, which may be negatively affected by surgery [16,19–21,28,29]. The COMP/BMP-2 levels we observed were correlated with perioperative kidney function and length of stay, which is most likely a reflection that patients with renal failure stay longer in the hospital. Abnormalities in kidney function via klotho and the vitamin D system can also affect long-term bone metabolism and FGF-23 levels [14,32,36]. Interestingly, vitamin D levels are often disturbed during cardiac surgery and are linked to increased adverse events [42–44].
The only correlation between arteriosclerosis markers and the level of insult was for RANKL and the estimated blood loss. The lack of correlation between APACHE and several measures of inflammation suggests that changes in RANKL and COMP are part of a nonspecific inflammatory response. In preliminary data, due to the high variability, type of surgery did not significantly differentiate factors. The lack of differences between types of surgery may be a result of markers responding to insult non-specifically, the degree of insult exceeding any threshold for a differential response, or inadequate coding for surgical procedures. In this study, we failed to demonstrate a relationship between serum creatinine postoperatively and FGF-23 levels, despite some studies suggesting a strong correlation [34,36]. This correlation is likely the result of abnormal calcium metabolism, a common occurrence in end-stage renal disease patients [21,32,34,45]. Our patient population had few patients with kidney failure. Also, the observed release of FGF-23 is most likely directly related to cardiac damage, as the vast majority of our patients had normal creatinine levels and were not on dialysis [45].
Arteriosclerosis can be accelerated by nonspecific inflammation or general vascular inflammation [4,20,38,46]. Here, we observed perseverance of leukocytes, but CRP and IL-8 levels normalized by 3 months. Also, markers of vascular inflammation (P-selectin, CD40L) remained at baseline for the duration of the study [47–50]. This suggests that patients may be at higher risk of atherosclerosis over time, considering the role of CRP in this process [7,12]. Notably, elevated CRP would work synergistically with FGF-23 to accelerate arteriosclerosis [9,28,33,34,41].
The study has several limitations. The pilot nature of this project precludes in-depth analysis of several demographic and clinical factors, potentially affecting levels of measured samples [38,51]. We did not measure KLOTHO activation or vitamin D, which are key components in calcium metabolism and arteriosclerosis [11,32,41,51]. We demonstrated that FGF-23 is elevated at 3 months, but we did not determine the duration of FGF-23 prolongation. Establishing the duration of persistence of serum FGF-23 is critical to determining the potential impact of FGF-23 on the acceleration of arteriosclerosis. Additionally, we did not directly measure the level of arteriosclerosis acceleration using ultrasound or invasive methods. This pilot study was not designed to measure clinical outcomes, as no prior study has examined the longevity of the studied markers past the perioperative period. The significance of FGF-23 elevation needs to be established in a larger patient population, as the incidence of stroke, post-operative coronary syndrome, and death was low in our group. Our study did not account for pre-existing conditions that often lead to increased stiffness of the arteries [8,12,28], such as hypertension and smoking, or current medications that patients are taking, such as statins, which can change the natural history of arterial stiffness progression [52].
Despite these limitations, this study has strengths that include a relatively homogenous patient population and longitudinal design, allowing the comparison of data across time for the same individuals. In addition, the population size was within acceptable limits for an exploratory study, and the methodology for ascertaining the serum level of the markers was robust. We also accounted for several variables critical for the serum level of the studied markers, even though some have complex relationships to age, diabetes, and sex [20,27,36,38,51].
In summary, this pilot study indicates that some factors favoring arteriosclerosis can persist for several months after cardiac surgery. Assessing the clinical correlates of this finding is the next step.
Conclusions
We demonstrated that RANKL and BMP-2 are elevated during the acute perioperative period and that significantly elevated levels of FGF-23 persist in serum even 3 months after non-emergent heart surgery. The prolonged period of FGF-23 activation could lead to an acceleration of arteriosclerosis.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Ethics Approval and Consent to Participate
The Institutional Review Board approved the study at the University of Pennsylvania (#815686).
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Giacinto O, Satriano U, Nenna A, et al. Inflammatory response and endothelial dysfunction following cardiopulmonary bypass: Pathophysiology and pharmacological targets. Recent Pat Inflamm Allergy Drug Discov. 2019;13:158–73. doi: 10.2174/1872213X13666190724112644. [DOI] [PubMed] [Google Scholar]
- 2.Mach L, Bedanova H, Soucek M, et al. Impact of cardiopulmonary bypass surgery on cytokines in epicardial adipose tissue: Comparison with subcutaneous fat. Perfusion. 2017;32:279–84. doi: 10.1177/0267659116683791. [DOI] [PubMed] [Google Scholar]
- 3.McGuinness J, Bouchier-Hayes D, Redmond JM. Understanding the inflammatory response to cardiac surgery. Surgeon. 2008;6:162–71. doi: 10.1016/s1479-666x(08)80113-8. [DOI] [PubMed] [Google Scholar]
- 4.de Vries MR, Simons KH, Jukema JW, et al. Vein graft failure: From pathophysiology to clinical outcomes. Nat Rev Cardiol. 2016;13:451–70. doi: 10.1038/nrcardio.2016.76. [DOI] [PubMed] [Google Scholar]
- 5.Ruaengsri C, Schill MR, Khiabani AJ, et al. The Cox-maze IV procedure in its second decade: Still the gold standard? Eur J Cardiothorac Surg. 2018;53:i19–i25. doi: 10.1093/ejcts/ezx326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornowski R, Hong Mun K, Tio Fermin O, et al. In-stent restenosis: Contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31:224–30. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 7.Dibra A, Mehilli J, Braun S, et al. Inflammatory response after intervention assessed by serial C-reactive protein measurements correlates with restenosis in patients treated with coronary stenting. Am Heart J. 2005;150:344–50. doi: 10.1016/j.ahj.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 8.von Rossum A, Laher I, Choy JC. Immune-mediated vascular injury and dysfunction in transplant arteriosclerosis. Front Immunol. 2015;5:684. doi: 10.3389/fimmu.2014.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loupy A, Vernerey D, Viglietti D, et al. Determinants and outcomes of accelerated arteriosclerosis. Circ Res. 2015;117:470–82. doi: 10.1161/CIRCRESAHA.117.306340. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Oka T, Chow FL, et al. Tumor necrosis sactor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–82. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 11.Donate-Correa J, Mora-Fernández C, Martínez-Sanz R, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2013;165:179–83. doi: 10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]
- 12.Fredman G, Spite M. Specialized pro-resolving mediators in cardiovascular diseases. Mol Aspects Med. 2017;58:65–71. doi: 10.1016/j.mam.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jefferson AL, Cambronero FE, Liu D, et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation. 2018;138:1951–62. doi: 10.1161/CIRCULATIONAHA.118.032410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Wan X, Laudanski K, et al. Left-sided ventricular-arterial coupling and volume responsiveness in septic shock patients. Shock. 2019;52:577–82. doi: 10.1097/SHK.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 15.Panizo S, Cardus A, Encinas M, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4 dependent pathway. Circ Res. 2009;104:1041–48. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami R, Nakagami H, Noma T, et al. RANKL system in vascular and valve calcification with aging. Inflamm Regen. 2016;36:10. doi: 10.1186/s41232-016-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsubaki M, Satou T, Itoh T, et al. Bisphosphonate- and statin-induced enhancement of OPG expression and inhibition of CD9, M-CSF, and RANKL expressions via inhibition of the Ras/MEK/ERK pathway and activation of p38MAPK in mouse bone marrow stromal cell line ST2. Mol Cell Endocrinol. 2012;361:219–31. doi: 10.1016/j.mce.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Laudanski K, Zawadka M, Polosak J, et al. Acquired immunological imbalance after surgery with cardiopulmonary bypass due to epigenetic over-activation of PU.1/M-CSF. J Transl Med. 2018;16:143. doi: 10.1186/s12967-018-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimamura M, Nakagami H, Osako MK, et al. OPG/RANKL/RANK axis is a critical inflammatory signaling system in ischemic brain in mice. Proc Natl Acad Sci. 2014;111:8191–96. doi: 10.1073/pnas.1400544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndip A, Wilkinson FL, Jude EB, et al. RANKL-OPG and RAGE modulation in vascular calcification and diabetes: Novel targets for therapy. Diabetologia. 2014;57:2251–60. doi: 10.1007/s00125-014-3348-z. [DOI] [PubMed] [Google Scholar]
- 21.Nitta K, Akiba T, Uchida K, et al. Serum osteoprotegerin levels and the extent of vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1886–89. doi: 10.1093/ndt/gfh263. [DOI] [PubMed] [Google Scholar]
- 22.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95:1046–57. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 23.Kaden JJ, Bickelhaupt S, Grobholz R, et al. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Weiss RM, Lund DD, Chu Y, et al. Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice. PLoS One. 2013;8:e65201. doi: 10.1371/journal.pone.0065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimamura M, Nakagami H, Osako MK, et al. OPG/RANKL/RANK axis is a critical inflammatory signaling system in ischemic brain in mice. Proc Natl Acad Sci USA. 2014;111:8191–96. doi: 10.1073/pnas.1400544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Jia Q, Feng X, et al. Hypoxia decrease expression of cartilage oligomeric matrix protein to promote phenotype switching of pulmonary arterial smooth muscle cells. Int J Biochem Cell Biol. 2017;91:37–44. doi: 10.1016/j.biocel.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Fu Y, Gao C, et al. Cartilage oligomeric matrix protein prevents vascular aging and vascular smooth muscle cells senescence. Biochem Biophys Res Commun. 2016;478:1006–13. doi: 10.1016/j.bbrc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Canfield AE, Farrington C, Dziobon MD, et al. The involvement of matrix glycoproteins in vascular calcification and fibrosis: An immunohistochemical study. J Pathol. 2002;196:228–34. doi: 10.1002/path.1020. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Gao C, Liang Y, et al. Shift of macrophage phenotype due to cartilage oligomeric matrix protein deficiency drives atherosclerotic calcification. Circ Res. 2016;119:261–76. doi: 10.1161/CIRCRESAHA.115.308021. [DOI] [PubMed] [Google Scholar]
- 30.Du Y, Wang Y, Wang L, et al. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ Res. 2011;108:917–28. doi: 10.1161/CIRCRESAHA.110.234328. [DOI] [PubMed] [Google Scholar]
- 31.Boström K, Watson K, Horn S, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coban M, Inci A, Yılmaz U, Asilturk E. The association of fibroblast growth factor 23 with arterial stiffness and atherosclerosis in patients with autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2018;43:1160–73. doi: 10.1159/000492244. [DOI] [PubMed] [Google Scholar]
- 33.Masai H, Joki N, Sugi K, Moroi M. A preliminary study of the potential role of FGF-23 in coronary calcification in patients with suspected coronary artery disease. Atherosclerosis. 2013;226:228–33. doi: 10.1016/j.atherosclerosis.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 34.Turan MN, Kircelli F, Yaprak M, et al. FGF-23 levels are associated with vascular calcification, but not with atherosclerosis, in hemodialysis patients. Int Urol Nephrol. 2016;48:609–17. doi: 10.1007/s11255-016-1231-1. [DOI] [PubMed] [Google Scholar]
- 35.Alonso A, Misialek JR, Eckfeldt JH, et al. Circulating fibroblast growth factor-23 and the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3:e001082. doi: 10.1161/JAHA.114.001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuñón J, Fernández-Fernández B, Carda R, et al. Circulating fibroblast growth factor-23 plasma levels predict adverse cardiovascular outcomes in patients with diabetes mellitus with coronary artery disease. Diabetes Metab Res Rev. 2016;32:685–93. doi: 10.1002/dmrr.2787. [DOI] [PubMed] [Google Scholar]
- 37.McKay TB, Rhee J, Colon K, et al. Preliminary study of serum biomarkers associated with delirium after major cardiac surgery. J Cardiothorac Vasc Anesth. 2022;36:118–24. doi: 10.1053/j.jvca.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vikulova DN, Grubisic M, Zhao YL, et al. Premature atherosclerotic cardiovascular disease: trends in incidence, risk factors, and sex-related differences, 2000 to 2016. J Am Heart Assoc. 2019;8:e012178. doi: 10.1161/JAHA.119.012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 40.Peres Bota D, Melot C, Lopes Ferreira F, et al. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002;28:1619–24. doi: 10.1007/s00134-002-1491-3. [DOI] [PubMed] [Google Scholar]
- 41.Vázquez-Sánchez S, Poveda J, Navarro-García JA, et al. An overview of FGF-23 as a novel candidate biomarker of cardiovascular risk. Frontiers in Physiology. 2021;12:632260. doi: 10.3389/fphys.2021.632260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zittermann A, Kuhn J, Dreier J, et al. Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery. Eur Heart J. 2013;34:1358–64. doi: 10.1093/eurheartj/ehs468. [DOI] [PubMed] [Google Scholar]
- 43.Turan A, Grady M, You J, et al. Low vitamin D concentration is not associated with increased mortality and morbidity after cardiac surgery. PLoS One. 2013;8:e63831. doi: 10.1371/journal.pone.0063831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soh V, Tan SJX, Sehgal R, et al. The relationship between vitamin D status and cardiovascular diseases. Curr Probl Cardiol. 2021;46:100836. doi: 10.1016/j.cpcardiol.2021.100836. [DOI] [PubMed] [Google Scholar]
- 45.Seiler S, Heine GH, Fliser D. Clinical relevance of FGF-23 in chronic kidney disease. Kidney Int Suppl. 2009;76:S34–42. doi: 10.1038/ki.2009.405. [DOI] [PubMed] [Google Scholar]
- 46.Roman N, Shanker B, Davis AB, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:23999–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 47.Papi M, Didona B, De Pita O, et al. Livedo vasculopathy vs small vessel cutaneous vasculitis: Cytokine and platelet P-selectin studies. Arch Dermatol. 1998;134:447–52. doi: 10.1001/archderm.134.4.447. [DOI] [PubMed] [Google Scholar]
- 48.Kuret T, Lakota K, Žigon P, et al. Insight into inflammatory cell and cytokine profiles in adult IgA vasculitis. Clin Rheumatol. 2019;38:331–38. doi: 10.1007/s10067-018-4234-8. [DOI] [PubMed] [Google Scholar]
- 49.Cieślik P, Hrycek A. Pentraxin 3 as a biomarker of local inflammatory response to vascular injury in systemic lupus erythematosus. Autoimmunity. 2015;48:242–50. doi: 10.3109/08916934.2014.983264. [DOI] [PubMed] [Google Scholar]
- 50.Wang C-L, Wu Y-T, Liu C-A, et al. Expression of CD40 ligand on CD4+ T-cells and platelets correlated to the coronary artery lesion and disease progress in Kawasaki disease. Pediatrics. 2003;111:e140–47. doi: 10.1542/peds.111.2.e140. [DOI] [PubMed] [Google Scholar]
- 51.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60:200–7. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upala S, Wirunsawanya K, Jaruvongvanich V, Sanguankeo A. Effects of statin therapy on arterial stiffness: A systematic review and meta-analysis of randomized controlled trial. Int J Cardiol. 2017;227:338–41. doi: 10.1016/j.ijcard.2016.11.073. [DOI] [PubMed] [Google Scholar]