Abstract
Lower extremity peripheral artery disease (PAD) affects >230 million adults worldwide and is associated with increased risk of various adverse clinical outcomes (other cardiovascular diseases such as coronary heart disease and stroke and leg outcomes such as amputation). Despite its prevalence and clinical importance, PAD has been historically underappreciated by health care professionals and patients. This underappreciation seems multifactorial (eg, limited availability of the first-line diagnostic test, the ankle-brachial index, in clinics; incorrect perceptions that a leg vascular disease is not fatal and that the diagnosis of PAD would not necessarily change clinical practice). In the past several years, a body of evidence has indicated that these perceptions are incorrect. Several studies have consistently demonstrated that many patients with PAD are not receiving evidence-based therapies. Thus, this scientific statement provides an update for health care professionals regarding contemporary epidemiology (eg, prevalence, temporal trends, risk factors, and complications) of PAD, the present status of diagnosis (physiological tests and imaging modalities), and the major gaps in the management of PAD (eg, medications, exercise therapy, and revascularization). The statement also lists key gaps in research, clinical practice, and implementation related to PAD. Orchestrated efforts among different parties (eg, health care providers, researchers, expert organizations, and health care organizations) will be needed to increase the awareness and understanding of PAD and improve the diagnostic approaches, management, and prognosis of PAD.
Keywords: AHA Scientific Statements, diagnosis, epidemiology, peripheral artery disease, prognosis, risk factors
Lower extremity peripheral artery disease (PAD) is atherosclerotic disease of the arteries supplying the legs.1 Despite its prevalence and impact on adverse clinical outcomes, impaired physical function, and reduced physical activity, PAD has been understudied and underrecognized compared with other atherosclerotic diseases such as myocardial infarction and stroke.2 The lack of awareness has led to underdiagnosis and undertreatment of PAD in the United States and around the world.2
There appear to be several reasons for this underrecognition of PAD. For example, the first-line method to diagnose PAD, the ankle-brachial index (ABI), is not readily available in most clinics in the United States.3 Also, many people think that leg diseases cannot be fatal, whereas myocardial infarction has been recognized as a leading cause of sudden cardiac death for a long time. Similarly, PAD is not widely recognized as a disabling condition, whereas stroke is established as the leading cause of disability. Difficulty walking, a hallmark of PAD-related disability, may be considered normal aging by clinicians, and the protean nature of ischemic leg symptoms can be mistaken for other diseases like arthritis or spinal degenerative disease. Also, some experts think that the specific investigation of PAD is not important, because evidence from myocardial infarction and stroke can be extrapolated to PAD.
The key question is whether these perceptions about PAD (eg, PAD does not lead to mortality) are accurate or not. A body of evidence has been indicating that PAD is strongly associated with mortality, primarily as a strong predictor of future myocardial infarction and stroke. Moreover, limb-related complications attributable to PAD, such as lower extremity amputation and acute limb ischemia (ALI), are devastating. Also, the causes of PAD and the other atherosclerosis diseases are not identical, despite considerable overlap, indicating the need for specific diagnostic and treatment approaches for PAD. To help clinicians grasp the contemporary epidemiology of PAD and the major gaps in the management of PAD, a working group with expertise in cardiology, general internal medicine, geriatrics, epidemiology, vascular surgery, and vascular medicine has compiled this scientific statement.
DEFINITION OF PAD
Historically, the terms peripheral artery (or arterial) disease and peripheral vascular disease have been used loosely.4 These terminologies have often included any or all atherosclerotic disease separate from cardiac disease, including carotid artery, renal artery, leg artery, and aortic diseases.5 Peripheral vascular disease may additionally include peripheral venous and lymphatic disease. In an era of precision medicine, we believe that precise definitions should be used.6 For the purpose of this scientific statement, we define PAD as “lower extremity PAD.” Specifically, we are referring to atherosclerotic obstruction from the aortoiliac segments to the pedal arteries.
AWARENESS
Despite its high prevalence and the morbid and mortal outcomes detailed later in this statement, overall awareness regarding PAD is limited. In the PARTNERS study (Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival) conducted in primary care practices throughout the United States in 1999, among patients with a prior PAD diagnosis, 83% of patients, yet only 49% of their physicians, were aware of the PAD diagnosis.2 Even in a recent systematic review of PAD knowledge and awareness, 61% of general practitioners reported screening patients for PAD, whereas only 6% were aware of guidance regarding evidence-based therapies for PAD.7 In this same study, the results of formal examinations of medical students and trainee physicians demonstrated poor to modest overall knowledge regarding PAD-related data gathering and its interpretation. Among patients and the general public, PAD awareness ranged from 21% to 61%. Lack of patient and health care professional awareness contributes to delayed or underused treatment, as detailed later in this statement.
Multiple factors likely contribute to this lack of PAD awareness. First, as noted earlier, there has been variability in the nomenclature and definition of PAD, which presents a challenge to effective communication about the disease. Second, variation in the clinical presentation of PAD is also likely a source of confusion regarding PAD.5,8 Only 10% to 30% have typical intermittent claudication (ie, exertional calf pain resolving within 10 minutes of rest), with the remaining having either no exertional leg symptoms (20%–50%) or atypical leg symptoms (40%–50%).2 Atypical leg symptoms may include pain/discomfort that begins at rest or pain/discomfort that does not cause the patient to stop walking, pain/discomfort that does not consistently resolve with rest1; these symptoms can be confused with symptoms of lower extremity arthritis or degenerative spinal disease. Last, an important component of PAD unawareness is the lack of knowledge and appreciation among health care professionals and patients of the poor prognosis of PAD. In this context, the terminology of “peripheral” in PAD may provide an impression that this clinical condition is not critical.10 However, as noted later in this statement, this notion is clearly incorrect, because PAD is associated with various adverse outcomes. Thus, increasing awareness of all aspects of PAD, including the definition, diagnosis, clinical manifestation, and complications, is critical to improving overall outcomes among this growing and undertreated population.
PREVALENCE AND TEMPORAL TRENDS
Overall PAD
PAD is the third leading cause of atherosclerotic morbidity, following coronary heart disease and stroke. A systematic review of 34 studies (22 from high-income countries and 12 from low- and middle-income countries) demonstrated that the prevalence of PAD was ≈5% at 40 to 44 years of age and ≈12% at 70 to 74 years of age in both men and women in high-income countries (Figure 1).11 The prevalence of PAD in women in low- and middle-income countries was very similar to that in high-income countries, but the corresponding estimates for men in low- and middle-income countries compared with high-income countries were ≈2% and ≈8%, respectively. Between the years 2000 and 2010, the number of persons living with PAD increased by 13.1% in high-income countries and 28.7% in low- and middle-income countries.
Figure 1. Prevalence of peripheral artery disease by age in men (A and B) and women (C and D) in high-income countries (A and C) and low- and middle-income countries (B and D).11.
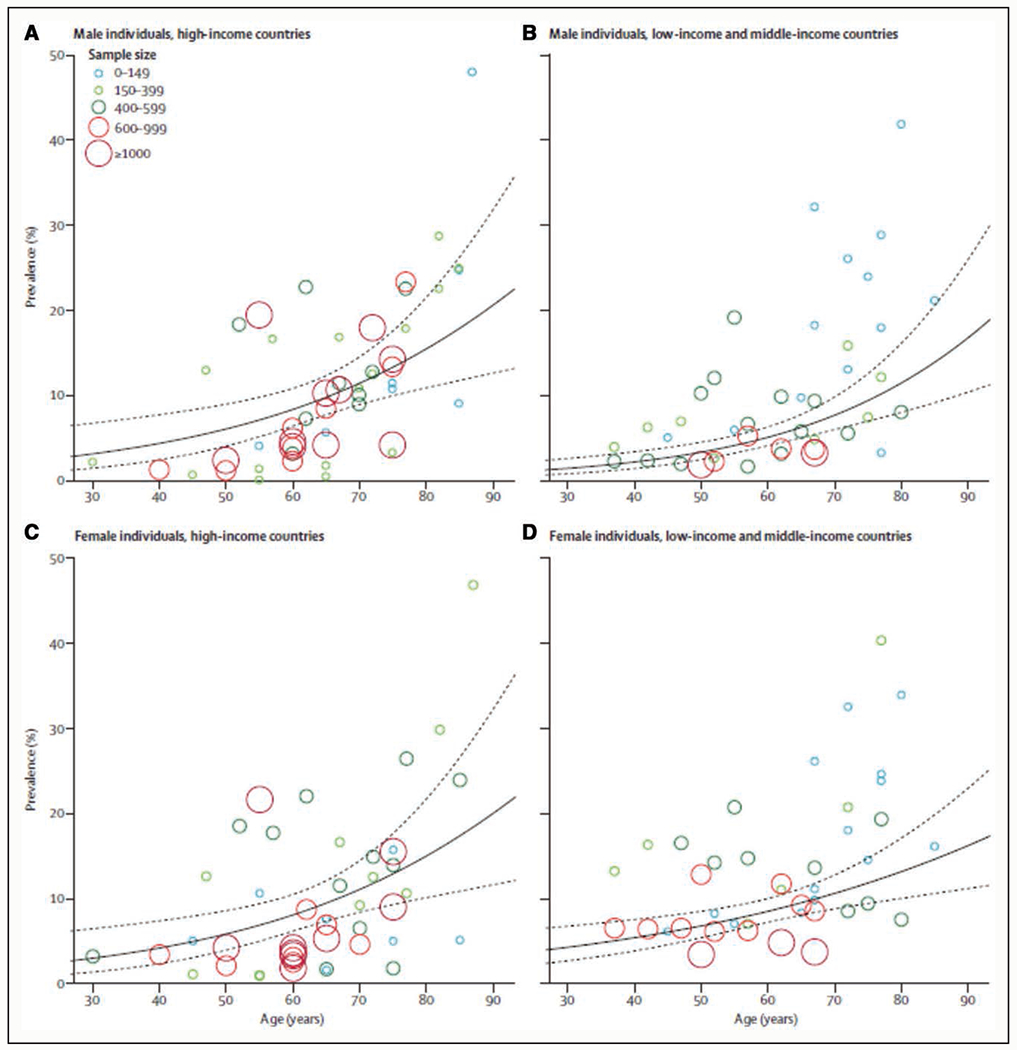
Reprinted from Fowkes et al. Copyright ©2013, with permission from Elsevier.
Another recent systematic review estimated that 238 million people were living with PAD in 2015: 64 million living in high-income countries and 172 million living in low- and middle-income countries.12 Thus, PAD should be recognized as an increasingly global problem. A recent publication from the Global Burden of Disease study also indicates that PAD cases have risen each year since 1990.13 Similarly, disability-adjusted life-years, years of life lost, and years lived with disability increased over this period. These changes represent population growth rather than a change in age-specific incidence. Worldwide, the age-specific prevalence has been largely steady.
The prevalence of PAD in the United States at ≥40 years of age is estimated to be ≈7%, corresponding to 8.5 million adults, from a study pooling 7 community-based studies.14 However, these studies were conducted in the late 1990s or in the beginning of 2000. For example, the National Health and Nutrition Examination Survey, a nationally representative cohort, has not measured ABI since 2004. Especially given the obesity and diabetes epidemic,15 a contemporary estimate of PAD prevalence in the United States is needed.
Critical Limb Ischemia/Amputation
Critical limb ischemia (CLI) (or chronic limb-threatening ischemia16) is a severe form of PAD and usually defined as PAD with rest pain, nonhealing wounds, or tissue loss.1,17 A systematic review has reported that the 1-year cumulative incidence for each of mortality and amputation is ≈20% among patients with CLI.18 Because few population-based studies have investigated CLI, the epidemiology of CLI is not well understood. Using data from the Marketscan database, which includes medical records from large employers’ health plans, Medicare, and Medicaid, Nehler et al19 reported that the prevalence of CLI is 1.3%, accounting for 11% of diagnosed PAD cases, among the eligible study population ≥40 years of age. The rate of CLI admission in the United States was constant between 2003 and 2011, with ≈150 per 100 000 population.20
The rate of lower extremity amputation declined from 2000 to 2009, but since has started to increase in people with diabetes.21 Specifically, the total annual amputation rate per 1000 individuals with diabetes was 3 in 2009 but exceeded 4.5 in 2015. Although the exact reasons behind this increase in lower extremity amputation in diabetes are unclear, it is important to note that the increase was consistently observed in both major (above ankle) and minor (below ankle) amputations. In the same period, the annual amputation rate in people without diabetes was constant at ≈0.17 per 1000 individuals.
Lifetime Risk
Lifetime risk estimate is a useful parameter to communicate long-term risk, especially among younger adults whose 10-year risk estimate is low and thus cannot inform long-term decision-making of preventive therapies. The American Heart Association (AHA) and the American College of Cardiology (ACC) 2018 Guideline on the Management of Blood Cholesterol provides a lifetime risk algorithm for people 20 to 59 years of age but does not take into account PAD.22 In this regard, a recent US study estimated lifetime risk of PAD by pooling 6 community-based US cohorts.23 According to that study, the lifetime risk of PAD was estimated to be ≈30% in Black men and women and ≈20% in White and Hispanic women and men. The study demonstrated that, for a given age, sex, and race/ethnicity, the lifetime risk estimate of PAD can vary by 3- to 5-fold depending on the status of the traditional risk factors for PAD such as smoking and diabetes. The calculator to predict lifetime risk of PAD is available online.24
DIAGNOSIS
Physiological Testing
ABI, the ratio of ankle-to-brachial systolic blood pressure, is the first-line noninvasive diagnostic method for PAD,1,25 requiring standardized measurement methodology.26 An ABI ≤0.90 is considered PAD.1,25,26 The diagnostic performance of ABI to detect PAD, with >50% stenosis based on imaging modalities as the gold standard, is reasonably good, with sensitivity and specificity, respectively, at 61% to 73% and 83% to 96%.27–29
Several studies have shown that women tend to have lower ABIs than men, potentially because of shorter height.30,31 A population-based study specifically explored this issue and found that, after accounting for demographic and clinical factors (eg, age and height), healthy women had on average an ABI 0.017 lower than healthy men.32 Nonetheless, given the small difference from a clinical perspective for individual diagnosis, major clinical guidelines use the same ABI threshold of 0.90 in both sexes.1,25
The ABI can be falsely high in the presence of stiffened ankle arteries related to medial artery calcification, a condition mostly observed in patients with diabetes or chronic kidney disease (CKD).33,34 In this scenario, it is recommended to measure the toe-brachial index (TBI), the ratio of the toe-to-brachial systolic blood pressure,1,25,34 because medial calcification rarely affects digital arteries (detailed techniques to measure TBI can be found elsewhere35). In general, a TBI ≤0.70 is accepted as diagnostic for PAD.1 A recent study including 1162 patients from a US vascular laboratory demonstrates that the overall accuracy (the proportion of tests that were either true positive or true negative) of the TBI to detect PAD was consistent in patients with diabetes versus those without diabetes (74% versus 78%). However, this was not the case for ABI, which had an accuracy of 66% in patients with diabetes versus 81% in patients without diabetes.34 This study reported a similar pattern for CKD versus non-CKD. Of importance, a few studies have recently demonstrated that TBI may predict poor outcomes beyond ABI in patients with diabetes or CKD.36–38 This evidence supports the simultaneous measurement of ABI and TBI in patients with diabetes or CKD for detecting PAD.
An ABI 0.90 to 1.0 is considered as borderline low ABI and cannot rule out PAD.26 As detailed later in the statement, a body of evidence indicates that borderline low ABI is associated with increased risk of mortality and reduced physical function. In the case of borderline low ABI, particularly if symptoms suspect for exertional leg ischemia are present, the sensitivity to detect PAD can be improved by measuring ABI after a treadmill test (heel raise is an alternative method).26 Although the criteria to evaluate postexercise ABI have not been standardized,39 postexercise ABI <0.90 or a drop of ABI >20% or ankle pressure drop >30 mm Hg are usually considered as diagnostic.26 Postexercise ABI should be also considered in patients with potential intermittent claudication with normal ABI.
Another option to overcome ABI limitations is to study the ankle arteries’ Doppler flow pattern and velocities. In the San Diego Population Study, the addition of tibial artery Doppler assessment identified 20% additional diseased legs missed by the ABI.40 Waveform analysis enables us to detect occlusive disease despite calcified arteries in patients with diabetes, and to identify those at high risk of cardiovascular disease (CVD) and limb events.41
Besides ABI and TBI, other physiological tests include segmental perfusion pressure and transcutaneous oxygen pressure. The former enables us to localize the pressure drop downstream of a significant stenosis, whereas the latter assesses the tissue oxygenation, not only useful to quantify the consequence of malperfusion, but also to identify viable tissue after revascularization, and a contrario for delimitation of the amputation line in severe cases.42
Imaging
Noninvasive imaging for the assessment of anatomy and severity of arterial stenosis for patients with PAD has evolved over the past decade because of technical improvements.42 These include the ability to image distal vessels with calcification, lower contrast dose, and higher spatial resolution. The selection of imaging modalities to diagnose PAD should depend on several factors, including the patients’ symptoms (eg, claudication versus CLI), kidney function, and ABIs.
Computed Tomographic Angiography
Multidetector computed tomography scanners, including helical and multistation axial acquisitions, have now enabled the rapid scanning of the entire arterial system.43 For evaluating the indication of revascularization in patients with PAD, both computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) are accepted as appropriate imaging tests.44 The sensitivity and specificity of multidetector CTA compared with angiography is ≈90% for detecting PAD.45 CTA uses iodinated contrast and ionizing radiation to visualize pathology from the aorta to the lower extremity. The scan times take a few seconds, but diagnosis can be difficult in small tibial vessels with calcification and multiple occlusions. The recent development of 256-row CTA has made detecting stenosis in the tibial location possible,46 except in patients with calcified disease. New imaging techniques are being developed, including computed tomography perfusion to allow visualization of hypoxic regions of the lower extremity,47,48 which can also demonstrate the effect of interventional treatment.49,50
Magnetic Resonance Angiography
The sensitivity and specificity of MRA in detecting PAD with stenosis >50% is the same as CTA, 90% to 100%.51 MRA has several advantages in diagnosing PAD over CTA. MRA requires no radiation, calcium does not interfere with the diagnosis, and it can be helpful in evaluating for bone marrow edema in patients who have ulcers with possible osteomyelitis. However, the procedure time is considerably longer. Also, there is a concern of gadolinium-induced nephrogenic systemic fibrosis in patients with decreased kidney function. However, a recent systematic review including 4931 patients with a glomerular filtration rate <30 mL·min−1·1.73 m−2showed that there were zero cases of nephrogenic systemic fibrosis when a group II gadolinium-based contrast agent (gadobenate dimeglumine, gadobutrol, gadoterate meglumine, or gadoteridol) was used.52 Also, noncontrast MRA can be an option in some patients in capable facilities.53 Another advantage of MRA is that it allows for hemodynamic measurements. Advanced techniques such as blood oxygenation level-dependent imaging and arterial spin labeling allow for assessing changes in perfusion to the calf muscle without gadolinium.54,55
Duplex Ultrasound
This modality is safe to all patients but is operator dependent. The sensitivity and specificity depend on several factors, including the presence of calcium in the arterial wall, the location or depth of the vessel, and the presence of multiple occlusions at different locations.56 The femoral and popliteal arteries can usually be assessed well, whereas the iliac vessels and aorta can be challenging because of the presence of bowel gas and body habitus. This modality can also take some time to perform a complete examination. Ultrasound is often used to assess the effectiveness and patency after endovascular and surgical treatment.57 New advances using contrast-mediated ultrasound are being developed to evaluate perfusion to the lower extremity.58
Catheter-Based Angiography
Catheter-based angiography remains the gold standard for diagnosing PAD but is now limited to patients receiving endovascular revascularization.1 New techniques are available that help to reduce the use of iodinated contrast, where CTA and MRA imaging can be fused to the angiogram, which has the potential to reduce the use of contrast and radiation.59 Also, in some institutions, CO2 angiography is used as a replacement or supplement (to reduce contrast) of conventional contrast-based angiography. Other new innovations to assess perfusion in CLI are discussed in a separate AHA scientific statement.42
SCREENING
In 2018, the US Preventive Services Task Force stated that the evidence regarding a recommendation for screening for PAD among individuals without symptoms indicative of PAD as part of cardiovascular risk assessment was inconclusive.60 On the other hand, some expert organizations, including the AHA, recommend screening of PAD with ABI in adults at high risk (eg, adults ≥65 years of age and those 50–64 years of age with traditional risk factors).1,61 A recent Danish trial20 demonstrated that the comprehensive screening of PAD (using ABI), hypertension, and abdominal aortic aneurysm (using ultrasound) among men 65 to 74 years of age, followed by optimization of statin therapy, aspirin, blood pressure control, and surgical aneurysm repair, as appropriate, reduced mortality by 7% over 5 years of followup.62 Accordingly, AHA, together with a few other organizations, urges a wider implementation of PAD screening with ABI in high-risk populations.63
PAD has been traditionally thought of as affecting men more than women.64 However, more recent population-based studies show conflicting results about the association of sex with PAD.65–67 Based on ABI measurements, women appear to have an equal or higher prevalence of PAD than men.31,68 For example, a study pooling data from 6 US community-based cohorts estimated that the prevalence of PAD is 5.0% in men and 5.9% in women ≥40 years of age.30 We should interpret these results in the context of ABI tending to be lower with shorter height.31 Nonetheless, men present more frequently with claudication symptoms, whereas women have a higher prevalence of asymptomatic PAD or CLI.65 Consistent with this notion, women with symptomatic PAD tend to have more femoropopliteal occlusive disease and multilevel disease than men based on angiographic imaging.69,70
There are significant race-based differences in the prevalence and incidence of PAD. The aforementioned study pooling 6 US cohorts reported that the prevalence of PAD was 11.6% in Black individuals and 5.5% in non-Hispanic White individuals.30 This discrepancy persists after adjustment for traditional risk factors.71 Black patients also tend to present with more severe disease than White patients and have a higher risk of major amputation.72,73 Although not specified in major clinical guidelines,1 health care professionals should recognize the elevated risk of PAD in Black individuals. Also, we should try to understand the social constructs behind this observation better.
In this context, several socioeconomic factors are related to PAD. Adults with low household income, low education levels, and higher neighborhood deprivation have >2-fold increase in the risk of PAD even after adjustment for CVD risk factors.74,75 These associations are consistent across races.75 There are some observational data to suggest that the socioeconomic associations with PAD may be related to treatment differences with secondary preventative medications,76 although further research into this concept is necessary.
Although the sex differences in PAD prevalence and severity can be ascribed, at least in part, to hormonal differences,77 race differences are likely related to social determinants of health.78 Disparities in access to care, mistrust of the medical system, and structural racism may contribute to reduced risk factor treatment, a delayed diagnosis of PAD, and, subsequently, a higher risk of major amputation in diverse populations.79–82 Further research investigating structural inequities as a fundamental driver of health disparities, as recently called for by the AHA,83 will be critical for better understanding racial inequities in the prevalence, incidence, treatment, and complications of PAD.
RISK FACTORS
Conventional Risk Factors
Evidence has supported traditional cardiovascular risk factors in PAD such as diabetes, smoking, dyslipidemia, and hypertension. A sedentary lifestyle also increases the risk in the development of PAD.84 The Edinburg study reported that the risk of PAD is inversely related to physical activity.85 Of these conventional risk factors, diabetes and smoking are particularly strongly related to the development of PAD.86,87
Individuals with diabetes are at an increased risk of developing asymptomatic or symptomatic PAD, with an increase in claudication of 2- to 3-fold greater compared with individuals without diabetes.88–91 Diabetes worsens outcomes in patients with PAD, by mostly affecting infrapopliteal arteries, increasing risk of CLI, amputation, and mortality.92–94 Accordingly, ≈70% of nontraumatic lower extremity amputations in the United States occur in patients with diabetes,21 disproportionally to its overall prevalence of 12%.95
From another perspective, PAD is an important contributor to diabetes-related foot ulcer, a devastating condition with a high mortality risk and high medical cost affecting ≈13% of patients with diabetes in the United States.96–98 Up to half of patients with diabetes-related foot ulcer have PAD.99 The presence of PAD significantly worsens the prognosis in patients with diabetes-related foot ulcer with decreased healing rates, recurrence of ulceration, major limb amputation, and long-term survival.100,101
Like diabetes, cigarette smoking doubles the risk of PAD compared with nonsmoking.102,103 The risk increases cumulatively with the number of cigarettes smoked and the start age of tobacco use, with starting before 16 years of age having the greatest risk.104,105 Although smoking cessation decreases the risk of PAD, a recent community-based cohort study demonstrated that it takes up to 30 years for the risk for PAD of the individuals who stopped smoking to reach that of individuals who do not smoke, whereas the risk for coronary heart disease returns to the baseline within 20 years (Figure 2).87
Figure 2. Adjusted hazard ratio of 3 major atherosclerotic diseases according to time since quitting smoking.87.
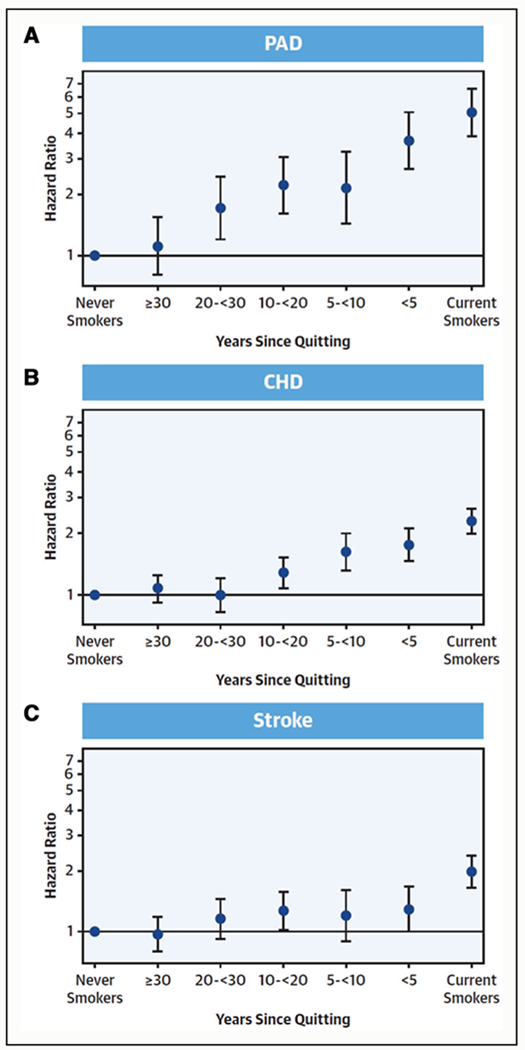
A, Peripheral artery disease (PAD). B, Coronary heart disease (CHD). C, Stroke. Reprinted from Ding et al.87 Copyright ©2019, with permission from the American College of Cardiology Foundation.
Several studies have demonstrated total cholesterol and low levels of high-density lipoprotein cholesterol to be associated with PAD.106,107 In addition, apolipoprotein B and lipoprotein(a) levels have been shown as independent risk factors.108–110 A recent trial in patients with established CVD treated with hepatocyte-directed antisense oligonucleotide revealed a dose-dependent reduction of lipoprotein(a),111 although the risk reduction of CVD including PAD is yet to be determined. A recent analysis from the Women’s Health Study has reported that small low-density lipoprotein particle, rather than low-density lipoprotein cholesterol, was associated with incident PAD.112 This study also has shown that triglyceride-rich lipoproteins may be especially important in the development of PAD. This observation has a clinical implication because icosapent ethyl, a triglyceride-lowering medication, has reduced major adverse cardiovascular events in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial),113 although this trial has not reported results for PAD as an outcome.
Evidence supports the robust association of elevated blood pressure with PAD.106,114 The association is more evident for systolic blood pressure than diastolic blood pressure.115 Specifically, in the ARIC study (Atherosclerosis Risk in Communities), systolic blood pressure demonstrated a graded association with PAD, with an adjusted hazard ratio of 2.6 at ≥140 mm Hg and 1.6 at 120 to 139 mm Hg, whereas the significantly elevated risk of PAD was only observed above 90 mm Hg for diastolic blood pressure.115 This observation has clinical implications regarding how blood pressure should be interpreted for PAD risk.
Nonconventional Risk Factors
PAD develops as an inflammatory cascade within arterial walls leading to atherosclerosis. In the Edinburgh Artery Study, inflammatory markers such as CRP (Creactive protein) and IL-6 (interleukin-6) were found to be elevated in patients with symptomatic PAD.109 Studies have found that elevated levels of these inflammatory markers are associated with the most severe form of PAD and at the highest risk for CVD events. Hemostatic factors such as fibrinogen have been associated as an independent risk factor114 and a strong predictor for the development of PAD.116,117
Some studies suggest HIV as a risk factor for PAD.118,119 A US study including veterans showed that individuals with a sustained CD4 cell count <200 cells/mm3 had nearly 2-fold higher risk of PAD than individuals without HIV. There was no excess risk among individuals with a CD4 cell count ≥500 cells/mm3.118
There is evidence demonstrating an association between metals and cardiovascular disease.120–125 Despite mounting evidence, the relationship is underappreciated. For instance, lead exposure has been shown to contribute to 10 times the number of cardiovascular deaths originally estimated.120 The association of blood lead and PAD in National Health and Nutrition Examination Survey 1999 to 2000, revealed that blood lead levels were 14% higher in cases with PAD than without.126 The Strong Heart Study evaluated the association of urine cadmium concentrations with the incidence of PAD, showing a prospective association between PAD and urine cadmium, independent from smoking.127 Higher urine cadmium levels have been associated with an increase in PAD severity, with no PAD having the lowest urine cadmium concentration and CLI with the highest levels of urine cadmium.128
Air pollution exposure is linked with CVD, including PAD.129–131 A population-based study of 18 000 individuals, associated urban living with a 2- to 3-fold increased risk of PAD compared with individuals living in rural areas.132 Similarly, those living near major roadways demonstrated a decrease in ABI.131
Depression has emerged as a risk factor for the incidence and progression of PAD.133,134 This may be attributable to medication noncompliance or a decrease in physical activity. The Heart and Soul study revealed a hazard ratio of 2.09 (95% CI, 1.09–4.00) of developing PAD in patients with depressive symptoms after adjustment for sex and age.135 Individuals with depression and PAD had worse functional outcomes, greater need for revascularization, and worse quality of life.136,137
Microvascular Abnormalities
PAD is usually recognized as a manifestation of macrovascular disease. However, several recent studies have indicated the potential involvement of microvascular disease in the progression of PAD. For example, an international consortium of individual-level data including 0.8 million adults has shown that albuminuria, a representative measure of microvascular disease, is more strongly associated with leg amputation than overall PAD (eg, adjusted hazard ratio ≈6 versus ≈3 in urinary albumin-to-creatinine ratio >300 versus <10 mg/g).138 Moreover, a community-based cohort has demonstrated that the presence of any retinopathy (eg, hemorrhage or exudates) was more strongly associated with the incidence of CLI and PAD than that of coronary heart disease or stroke (Figure 3).139 Of importance, the association of retinopathy with PAD and CLI was independent of the duration of diabetes and hemoglobin A1c levels. Similar results have been shown in US veterans.140
Figure 3. Hazard ratio (HR) of major atherosclerotic subtypes according to the presence versus absence of any retinopathy.139.
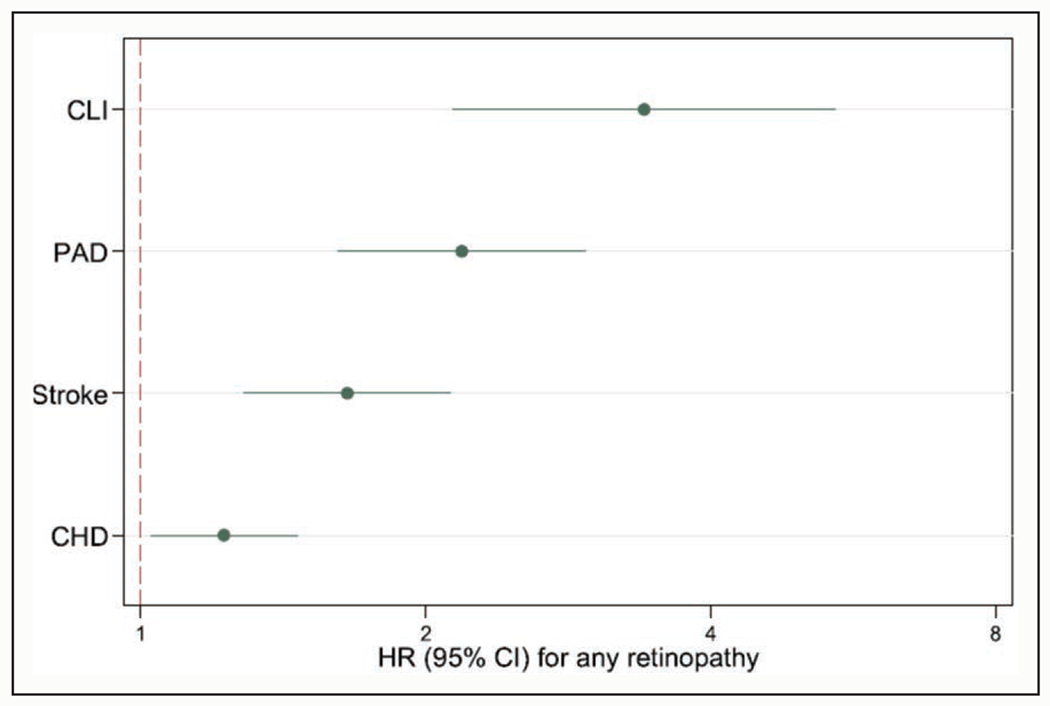
CHD indicates coronary heart disease; CLI, critical limb ischemia; and PAD, peripheral artery disease.
These observations have important diagnostic and therapeutic implications. For example, the ABI, which reflects stenosis in relatively large arteries, may not be helpful to classify the risk of CLI or leg amputation in some patients. A small case series has reported wide distribution of ABI (ranging from 0.7 to 1.1) in patients with diabetes and CLI.141 Of note, this study has demonstrated that all patients had TBI <0.7. Also, the current therapeutic options for patients with PAD (eg, statins and antiplatelets) are mainly based on evidence to prevent large artery disease or macrovascular disease (ie, coronary heart disease and stroke). Thus, future investigations on any therapeutic options targeting microvascular disease would be warranted.
COMPLICATIONS/COMORBIDITIES
Leg Symptoms, Physical Function, and Quality of Life
The magnitude and significance of functional impairment in PAD is underappreciated. Despite difficulty walking long distances, individuals with PAD frequently have atypical leg symptoms that can be mistaken for comorbidities such as hip or knee arthritis or spinal stenosis.142 Some clinicians may attribute difficulty walking to normal aging. Some people with PAD report no exertional leg symptoms (ie, are asymptomatic) either because they have restricted their physical activity or slowed their walking speed to avoid ischemic leg symptoms.142–144 Therefore, it is important for clinicians to suspect the possibility of PAD in people who report difficulty walking because of discomfort, weakness, cramping, or other symptoms in the hips, lower extremities, or feet. This is particularly the case if the symptoms resolve with rest and do not begin with rest and if the patient is >55 years of age with cardiovascular risk factors or a history of other cardiovascular disease. Cilostazol is the sole medication that the AHA/ACC PAD guideline recommends for ameliorating leg symptoms and improving walking distance in patients with PAD.1
The gradual but progressive nature of functional decline in PAD is also difficult for clinicians to detect without objective testing. Furthermore, patients with PAD who restrict their activity to avoid leg symptoms may not appreciate that their walking endurance has declined and may report stabilization of leg symptoms even as their 6-minute walk distance has declined.145 A 6-minute walk test can be used to measure objective change in walking ability. Greater declines in 6-minute walk distance over time are associated with adverse outcomes, including mortality and mobility loss.146
Atherosclerotic obstructions in lower extremity arteries prevent delivery of oxygenated blood to lower extremity skeletal muscle during walking activity, and many people with PAD cannot walk >2 to 3 blocks without stopping to rest because of ischemic leg symptoms such as cramping, weakness, or pain. It is important for health care providers to acknowledge patterns of atypical symptoms in patients with PAD. For example, hip, buttock, and lower back pain that occur with walking and resolve with rest are common in people with PAD and are likely attributable to atherosclerotic disease in locations proximal to the femoral arteries.
Consistent with the phenomenon of walking-induced ischemia, people with PAD have lower physical activity levels, poorer walking endurance, slower walking velocity, and poorer balance than people without PAD.142–144,147,148 More severe PAD is associated with lower physical activity levels and greater functional impairment.147 In the Walking and Leg Circulation Study cohort of 460 participants with PAD and 240 without PAD, lower ABI was progressively associated with a higher odds ratio of stopping to rest during a 6-minute walk test (eg, 11.7 [95% CI, 4.9–27.7] in ABI <0.50 and 6.6 [95% CI, 3.1–14.1] in ABI 0.50 to <0.70 compared with participants with ABI 0.9–1.5).
People with asymptomatic PAD also have significantly poorer functional performance than those without PAD.142,143,149 In 2 large observational studies of older community-dwelling men and women, ≈65% of those with an ABI <0.90 consistent with PAD were asymptomatic (ie, reported no exertional leg symptoms).143,149 Yet these individuals with asymptomatic PAD still had significantly slower walking velocity, lower physical activity, and poorer walking endurance than people without PAD who also report no exertional leg symptoms.143,149 Of note, borderline low ABI 0.9 to 1.0 has also been independently associated with reduced physical function.143,148
In addition to poorer performance on objective assessments of functional performance, people with PAD report poorer quality of life than those without PAD. In the ARIC Study with 5115 older adults, lower ABI was independently associated with lower quality of life.150 The association was more evident for physical domains than mental domains of quality of life. This pattern was consistently observed in other studies.151,152 Nonetheless, in a study of 957 patients with PAD presenting to 16 specialty clinics in the United States, Netherlands, and Australia, 336 (35%) had significant mental health concerns consisting of depressive symptoms, anxiety, and stress.153
Despite the significant functional impairment and impaired quality of life, people with PAD have traditionally been considered to have a benign natural history with regard to lower extremity outcomes.154–157 This is because relatively few people with PAD will develop CLI or require amputation.154–157 The gradual decline in walking performance may be less perceptible to patients and to clinicians than acute events such as ALI, creating a false perception of a benign natural history of lower extremity PAD.
Cognitive Function
Vascular dementia is a leading cause of dementia in older adults.158 Moreover, a growing body of evidence demonstrates that vascular dysfunction and its risk factors contribute to the pathophysiology of Alzheimer disease as well.159 Thus, it is not surprising that several studies documented associations of PAD and its severity with cognitive impairment and dementia.160–164 Cognitive deficits in individuals with PAD reveal patterns of neuropsychological impairment similar to individuals with a history of transient ischemic attack. In particular, 25% of patients with PAD scored in the bottom 5% of control groups on tests assessing attention and frontal lobe function.165 Although cognitive impairment is usually not considered as a complication of PAD, future studies are needed to evaluate the impact of cognitive function on the clinical management and prognosis among patients with PAD.
Leg Outcomes (CLI/ALI, Leg Amputations)
Lower extremity major amputations (typically defined at the level of the ankle or above) and ALI are often considered major adverse limb events. Amputation is not simply a complication but an important treatment option to save lives and proximal limbs. The association of PAD with mortality and other cardiovascular outcomes like myocardial infarction and stroke has been extensively evaluated. However, few studies have quantified the association of PAD (versus no PAD) with severe leg outcomes, although several clinical studies are exploring those outcomes only among PAD patients. There are no validated models to identify patients with PAD who are likely to develop CLI or need amputation. To the best of our knowledge, whether ABI is associated with future CLI or leg amputation in the general population has yet to be reported.
ALI is a vascular emergency requiring immediate treatment for limb salvage and has recently attracted attention as an important complication of PAD. ALI usually represents a rapid or sudden (eg, <2 weeks) decrease of leg perfusion causing pain, pulseless, pallor, sensory loss, or paralysis.1 However, to efficiently establish evidence on ALI, the field needs to develop a standardized definition of ALI. Nonetheless, a secondary analysis of the EUCLID trial (Examining Use of Ticagrelor in Peripheral Artery Disease) has reported that a history of leg revascularization was the most potent predictor of ALI (hazard ratio, 4.7 [95% CI, 3.3–6.8]), whereas the second strongest predictor, atrial fibrillation, had a hazard ratio of 1.8 (95% CI, 1.1–3.2).166
Mortality and Cardiovascular Outcomes
The ABI Collaboration reported a robust association of a low (≤0.90) and high (>1.40) ABI with all-cause and cardiovascular mortality from a meta-analysis of 16 population-based cohort studies.167 In persons with an ABI between 0.81 and 0.90, total mortality was doubled and in those with an ABI ≤0.70 it was quadrupled. In this study, borderline low ABI also demonstrated significantly elevated mortality. Multiple studies in diverse populations have demonstrated that persons with PAD have higher risk of other CVDs such as coronary heart disease, stroke, and abdominal aortic aneurysm.1,135 Another study adds heart failure to these outcomes.168 The elevated CVD risk has been shown to be only partially attributable to shared CVD risk factors, such that at any given level of CVD risk factors, PAD is independently related to future CVD events and mortality.169 PAD has also been shown to be predictive of future CVD events even when adjusted for other markers of subclinical atherosclerosis.170 As a result, the AHA/ACC 2018 lipid guideline lists low ABI ≤0.9 as a risk enhancer, to be considered for guiding lipid-lowering therapy on top of the predicted risk of atherosclerotic CVD based on the Pooled Cohort Equation.22
PAD recently gained attention in the context of polyvascular disease. This refers to a subset of patients with atherosclerotic involvement of multiple vascular beds, including PAD. In several trials assessing new lipid-lowering or antithrombotic therapies in the field of cardiovascular prevention such as the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Patients With Elevated Risk) and the COMPASS trial (Cardiovascular Outcomes for People Using Anticoagulation Strategies), patients with polyvascular disease demonstrated higher risk than those without, which was translated into higher absolute risk reduction with these new treatments.171,172 For example, in the FOURIER trial,171 as anticipated, PAD plus myocardial infarction/stroke had the highest risk of major adverse cardiovascular events (CVD mortality, myocardial infarction, and stroke), with 2.5-year risk of 14.9% (Figure 4). It is notable that PAD without myocardial infarction/stroke had a higher risk of major adverse cardiovascular events (10.3%) than myocardial infarction/stroke without PAD (7.6%).
Figure 4. Cumulative incidence of major adverse cardiovascular events in the placebo group according to CVD status at baseline.171.
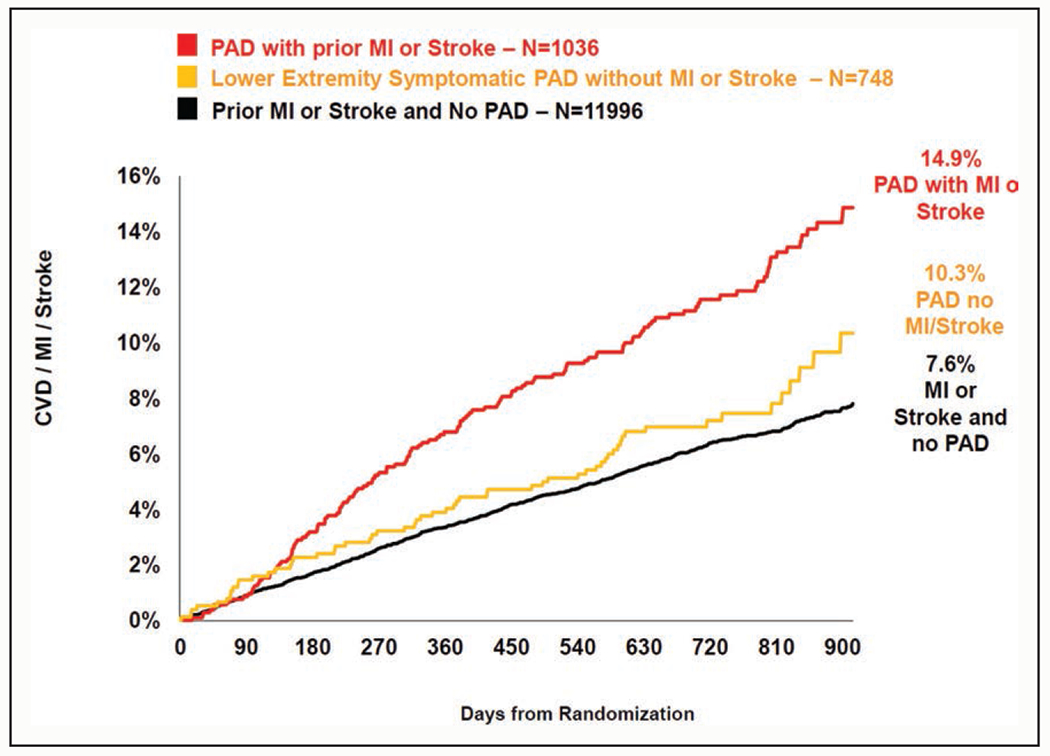
CVD indicates cardiovascular disease; MI, myocardial infarction; and PAD, peripheral artery disease.
GAPS AND CHALLENGES IN PAD MANAGEMENT
Underutilization of Evidence-Based Preventive Therapy
The most recent AHA/ACC PAD guideline was developed in 20161 and lists antiplatelet therapy, statins, antihypertensive agents, glycemic control, and smoking cessation as the Class I (strong) and IIa (moderate) recommendations. Despite these evidence-based guideline recommendations, patients with PAD remain undertreated (Table 1). In an analysis of persons with PAD (defined by ABI ≤0.9) from the National Health and Nutrition Examination Survey, the use of aspirin, statins, and renin-angiotensin system inhibitors was only 35.8%, 30.5%, and 24.9%, respectively.173 A more contemporary study of patients undergoing peripheral revascularization, a subgroup at heightened risk for cardiovascular and limb ischemic outcomes, reported use of aspirin, P2Y12 inhibitor, and renin-angiotensin system inhibitors in 67.3%, 57.7%, and 47.6% of patients, respectively, at discharge.174 In the latter analysis, only 61.7% of patients were discharged on a statin. Provider efforts to help patients with smoking cessation were examined among 1272 patients with PAD cared for in vascular specialty clinics followed in the PORTRAIT Registry (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories).176 In this study, 37.3% (n=474) were smoking actively at baseline. Of these, only 16% were referred to smoking cessation counseling, and 11% were prescribed pharmacological treatment. At 12 months, 72% of all individuals who smoked at baseline continued to smoke. The illustrated underutilization of preventive therapies may reflect the lack of clarity regarding prevention goals in PAD, because many trials have included PAD as a minority subgroup of broader atherosclerotic CVDs such as coronary heart disease and stroke. Nonetheless, these data clearly highlight the need for efforts to improve the use of evidence-based therapies in patients with PAD.
Table 1.
American Heart Association/American College of Cardiology 2016 PAD Guideline1 Class I (Evidence Level A) Recommendations and Adherence in Patients With PAD
| Class of recommendation | Level of recommendation | Recommendation | Adherence reported in literature |
|---|---|---|---|
| I | A | Aspirin or clopidogrel alone to reduce risk of myocardial infarction, stroke, and vascular death in patients with symptomatic PAD | 57.7%–67.3%173,174 |
| I | A | Statin therapy for all patients with PAD | 30.5% (asymptomatic)173 61.7% (symptomatic)174 |
| I | A | Antihypertensive therapy for all patients with hypertension and PAD to reduce risk of myocardial infarction, stroke, heart failure, and cardiovascular death | 48%–60% use of angiotensin-converting enzyme inhibitor in symptomatic PAD174,175 |
| I | A | Patients with PAD who smoke cigarettes or use other forms of tobacco should be advised at every visit to quit. Patients with PAD who smoke cigarettes should be assisted in developing a plan for quitting that includes pharmacotherapy (ie, varenicline, bupropion, and nicotine replacement therapy) or referral to a smoking cessation program | 16% were referred to smoking cessation counseling. 11% received pharmacological treatment176 |
PAD indicates peripheral artery disease.
Underutilization of Supervised Exercise Therapy
Supervised exercise is first-line therapy to improve walking impairment in people with PAD.1 Supervised treadmill exercise is the most thoroughly studied exercise therapy for people with PAD. More than 30 randomized clinical trials of supervised treadmill exercise in people with PAD involving >1400 participants have been completed. In 1 meta-analysis, mean improvement in treadmill walking distance was 180 meters and mean improvement in pain-free walking distance was 128 meters, compared with a nonexercise control group.177 Supervised exercise also significantly and meaningfully improves 6-minute walk distance and health-related quality of life in people with PAD.178–180 Several randomized trials have also demonstrated that arm and leg ergometry exercise, respectively, each significantly improve walking distance in people with PAD.181
In 2017, the Center for Medicare & Medicaid Services announced the coverage of supervised exercise for symptomatic patients with PAD.181 The Center for Medicare & Medicaid Services covers 12 weeks of supervised exercise, conducted 3 times weekly at a physician’s office.182 However, recent evidence showed that most people with PAD do not participate in supervised exercise programs.182 Of 135 vascular physicians across the United States, 54% reported that a facility for supervised exercise was not available to them and 49% reported that they had never referred a patient with PAD for supervised exercise. Barriers to supervised exercise participation include lack of medical center-based exercise facilities, costs, and the burden of repeated medical center visits.183
Structured home-based walking exercise interventions receive Class IIa recommendations in the AHA/ACC 2016 PAD guideline and have the potential to overcome some barriers of supervised exercise programs. However, home-based walking exercise interventions have had mixed benefits for improving walking ability in people with PAD.179,184–188,188a Three randomized trials of home-based walking exercise significantly improved walking ability, measured by 6-minute walk distance and treadmill walking performance, compared with a control group that did not exercise. These effective interventions have required periodic visits to the medical center for in-person coaching and feedback. A 6-month home-based exercise intervention that included weekly on-site visits to the medical center while helping patients with PAD adhere to walking exercise at home improved the 6-minute walk distance by 52 meters relative to a control group.186 In contrast, a 9-month randomized trial of home-based exercise that primarily relied on telephone calls, tapering to once per month did not show significant benefit compared with usual care.185 Although home-based exercise interventions can significantly and meaningfully improve 6-minute walk distance, it is important to keep in mind that the most effective interventions have incorporated regular visits to the medical center. A recent randomized clinical trial of home-based exercise in 305 participants with PAD demonstrated that exercise at an intensity that induced ischemic leg symptoms, but not exercise conducted at a comfortable pace without ischemic leg symptoms, significantly improved walking performance.188a
Gaps and Challenges in Revascularization
Revascularization for Intermittent Claudication
Guidelines from the AHA/ACC1 and the Society for Vascular Surgery61 recommend best medical treatment as the first-line treatment for claudication, with revascularization reserved for only refractory cases. These recommendations are based on data showing that there is a relatively low likelihood of limb loss associated with mild PAD189 and that long-term improvements in symptomatology may be limited.190 For example, recent data from the Invasive Revascularization or Not in Intermittent Claudication trial demonstrated that, after 5 years of follow-up, revascularization for claudication lost any early benefit and did not result in long-term health-related quality of life compared with best medical therapy.190 Despite guidelines recommending medical management as the first-line therapy for claudication, recent registry data from the Vascular Quality Initiative demonstrate that 27% of all open bypass procedures and even a higher percentage of endovascular interventions are performed for claudication.191 It is possible that many of the patients undergoing revascularization for claudication experienced severe claudication symptoms and that conservative management failed. For instance, in the CLEVER study (Claudication: Exercise Versus Endoluminal Revascularization),192 the revascularization group and the supervised exercise therapy group had better 18-month outcomes than optimal medical care alone. Quality improvement initiatives aimed at reducing unnecessary procedures are emerging to address outlier behavior in the overuse of invasive interventions for mild disease.177,193 Higher-quality data about the benefits of revascularization for severe claudication symptoms are needed.
Percutaneous Revascularization
The impact of percutaneous intervention in CLI is a subject of emergent research and the focus of active investigation. In a large observational study, percutaneous intervention compared with surgical therapy was associated with reduced in-hospital mortality (2.34% versus 2.73%, P<0.001), length of stay (8.7 days versus 10.7 days, P<0.001), and cost of hospitalization ($31 679 versus $32 485, P<0.001) despite similar rates of major amputation (6.5% versus 5.7%, P=0.75).20 Also, the increase in percutaneous leg revascularization has been related to a decline in leg amputation in the United States.20 Although many observational studies have suggested the benefit of percutaneous intervention in decreased amputation rates and mortality, to date, only one trial has compared percutaneous intervention with medical or surgical therapy in patients with CLI (see the Surgical revascularization section).
Furthermore, most studies to date have failed to account for anatomic factors that may influence patient selection toward percutaneous versus surgical intervention. The Society for Vascular Surgery has developed 2 limb-staging classification schemes to allow for more objective comparison of revascularization outcomes. The Wound, Ischemia, and foot Infection (WIfI) stage194 and the Global Anatomic Staging System (GLASS)16 are 2 classification systems intended to permit more meaningful analysis of outcomes for various forms of therapy in heterogeneous populations with CLI and should be reported whenever possible in major comparative studies moving forward.
With the increased use of percutaneous intervention in PAD, restenosis has been a continual obstacle. A growing proportion of patients are undergoing lower extremity bypass for a prior failed percutaneous intervention, and these secondary revascularization procedures have been associated with inferior 1-year outcomes.195 Although many devices lack comparative proof to support their use as a definite approach, multiple randomized studies of drug-eluting stent or drug-coated balloon show promising results for decreasing restenosis rates in the femoral-popliteal segment.196–202 Among the current therapeutic options, the paclitaxel-eluting or paclitaxel-coated devices consistently show a significantly higher primary patency rate, better target lesion revascularization rate, and cost effectiveness.203,204 Although a metaanalysis has reported an increase of mortality in patients receiving paclitaxel drug-coated balloon/drug-eluting stent DES compared with controls, there is some recent evidence against this finding.205 Nonetheless, the continued use of these devices should be individualized, carefully balancing the risks and benefits.170
Surgical Revascularization
The majority of open surgery for lower extremity revascularization is performed for CLI.206 Although lower extremity revascularization for PAD is becoming increasingly common in the United States, the rate of open surgery is stable or declining.207–209 Approximately 40% of all lower extremity revascularization procedures performed in the United States are open bypass surgery (versus 60% endovascular) because of the lower morbidity associated with endovascular procedures.210
However, there is still substantial debate about the efficacy of open surgery versus endovascular interventions for the treatment of PAD. In the BASIL trial (Bypass versus Angioplasty in Severe Ischaemia of the Leg), which is the only randomized controlled trial on the topic to date, a bypass-first strategy had overall outcomes similar to an angioplasty-first strategy.211 However, there was a significant overall survival benefit and a trend toward a benefit for amputation-free survival associated with open surgery among patients who survived >2 years.212 Since that trial concluded >15 years ago, there have been major advances in endovascular technology that are associated with better long-term outcomes at higher costs.213,214 As a result, the efficacy of endovascular versus open surgery revascularization for PAD remains unknown. The BEST-CLI trial (Best Endovascular vs Best Surgical Therapy for Patients with Critical Limb Ischemia), which just completed enrollment, will hopefully clarify optimal therapies for CLI.214 As noted earlier, the application of objective anatomic staging systems such as WIfI194 or GLASS16 are necessary to equalize clinical and anatomic factors in addition to baseline patient risk factors in clinical trials and observational studies moving forward.
SUMMARY
Lower extremity PAD is a global public health issue that has been systematically understudied and underappreciated. Table 2 summarizes major gaps in research, clinical practice, and implementation related to PAD that were covered in this scientific statement and should be filled. Health care professionals, researchers, expert organizations, health care organizations, government agencies, industry, and the community should collaborate to increase the awareness and understanding of PAD and improve the quality of PAD diagnosis, management, and prognosis.
Table 2.
Summary of Gaps Related to PAD in Research, Clinical Practice, and Implementation
| Research/clinical gaps |
| Contemporary data on the prevalence of PAD in the United States and globally |
| Larger studies with toe-brachial index (diagnostic accuracy and prognosis) |
| New and noninvasive techniques to visualize peripheral perfusion |
| Nonconventional risk factors and microvascular disease as potential preventive and therapeutic targets of PAD |
| Research to identify characteristics of effective home-based exercise interventions that are acceptable and accessible to patients with PAD |
| Behavioral methods to help patients with PAD adhere to home-based exercise long term |
| Community-based studies with severe leg outcomes |
| Randomized clinical trials comparing medical therapy, percutaneous revascularization, and surgical revascularization (with their latest evolutions) by indication and clinical staging |
| Medications or other oral therapies that significantly improve walking performance in PAD |
| Prediction models for developing critical limb ischemia and requiring lower extremity amputation |
| All PAD-related studies should include racially/ethnically diverse populations |
| Implementation gaps |
| Awareness of PAD among health care providers and patients |
| Screening of PAD with ankle-brachial index in high-risk populations |
| Broader use of toe-brachial index beyond ankle-brachial index>1.4, especially among patients with diabetes or chronic kidney disease |
| Adherence to evidence-based therapies in patients with PAD (medical therapies, supervised exercise therapy, and home-based exercise) |
| Avoiding unnecessary revascularization |
| All these implementation gaps should be filled across racially/ethnically diverse populations |
PAD indicates peripheral artery disease.
Acknowledgments
The authors appreciate A. Paskiewicz for her editorial help.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on June 4, 2021, and the American Heart Association Executive Committee on June 21, 2021. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com.
Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Michael H. Criqui | University of California, San Diego | None | None | None | None | None | None | None |
| Kunihiro Matsushita | Johns Hopkins Bloomberg School of Public Health | Kyowa Kirin† | None | None | None | None | Kyowa Kirin*; Akebia†; Fukuda Denshi* | None |
| Victor Aboyans | Dupuytren University Hospital, and Inserm 1094 & IRD, Limoges University (France) | None | None | Amgen*; Novartis*; Pfizer* | None | None | Bayer Healthcare*; NovoNordisk*; Sanofi*; AstraZeneca*; Boehringer Ingelheim*; BMS* | None |
| Connie N. Hess | University of Colorado School of Medicine | Amgen (research grant to CPC clinical research-study PI)†; Bayer (research grant to CPC clinical research)†; Janssen† (research grant to CPC clinical research)† | None | None | None | None | None | None |
| Caitlin W. Hicks | Johns Hopkins University School of Medicine | NIDDK (K23 award)* | None | None | None | None | None | None |
| Tak W. Kwan | Icahn School of Medicine at Mount Sinai | None | None | None | None | None | None | None |
| Mary M. McDermott | Northwestern University, Feinberg School of Medicine | Art Assist†; Chromadex*; Helixmith†; Mars*; Regeneron†; ReserveAge* | None | None | None | None | None | None |
| Sanjay Misra | Mayo Clinic | None | None | None | None | None | None | None |
| Francisco Ujueta | Mount Sinai Medical Center | None | None | None | None | None | None | None |
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Joshua A. Beckman | Vanderbilt University Medical Center | Novartis (DSMB)* | Bristol Myers Squibb (Investigator Initiated research on PE)† | None | None | None | Amgen*; Glaxo Smith Kline*; Sanofi†; Janone*; Janssen*; Bayer* | None |
| Michael S. Conte | University of California, San Francisco | NIDDK (Diabetic foot consortium)†; Profusa† | None | None | None | None | Abbott Vascular* | None |
| F. Gerry R. Fowkes | University of Edinburgh (United Kingdom) | None | None | None | None | None | None | None |
| J. Antonio T. Gutierrez | Duke University Medical Center | US Department of Veterans Affairs (Awarded Career Development Award via Health Services R&D branch of the Veterans Affairs. This award is for 5 years and provides full research funding starting July 2021.)† | None | None | None | None | Amgen*; Janssen Pharmaceuticals* | None |
REFERENCES
- 1.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317 [DOI] [PubMed] [Google Scholar]
- 3.Pradhan AD, Aday AW, Beckman JA. The Big MAC attack on peripheral artery disease. Circulation. 2020;141:1211–1213. doi: 10.1161/CIRCULATIONAHA.120.045627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creager MA, Belkin M, Bluth EI, Casey DE Jr, Chaturvedi S, Dake MD, Fleg JL, Hirsch AT, Jaff MR, Kern JA, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American College of Cardiology Foundation /American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease). Circulation. 2012;125:395–467 doi: 10.1161/CIR.0b013e31823299a1 [DOI] [PubMed] [Google Scholar]
- 5.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/ Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526 [DOI] [PubMed] [Google Scholar]
- 6.Hiatt WR, Goldstone J, Smith SC Jr, McDermott M, Moneta G, Oka R, Newman AB, Pearce WH; American Heart Association Writing Group 1. Atherosclerotic peripheral vascular disease symposium II: nomenclature for vascular diseases. Circulation. 2008;118:2826–2829. doi: 10.1161/CIRCULATIONAHA.108.191171 [DOI] [PubMed] [Google Scholar]
- 7.Bridgwood BM, Nickinson AT, Houghton JS, Pepper CJ, Sayers RD. Knowledge of peripheral artery disease: what do the public, healthcare practitioners, and trainees know? Vasc Med. 2020;25:263–273. doi: 10.1177/1358863X19893003 [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res. 2015;116:1540–1550. doi: 10.1161/CIRCRESAHA.114.303517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deleted in proof.
- 10.Hishida M, Menez S, Matsushita K. Peripheral artery disease in CKD: anatomically peripheral but clinically central. Am J Kidney Dis. 2020;75:687–689. doi: 10.1053/j.ajkd.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 11.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 12.Song P Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, Fowkes FGR, Fowkes FJI, Rudan I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8:e721–e729. doi: 10.1016/S2214-109X(20)30117-0 [DOI] [PubMed] [Google Scholar]
- 13.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP et al. ; GBD-NHL-BI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010 [DOI] [PubMed] [Google Scholar]
- 15.Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: An analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab. 2018;20:667–671. doi: 10.1111/dom.13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH; GVG Writing Group. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69(6S):3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE, Driver VR, Jaff MR. Critical limb ischemia: a threat to life and limb. Endovasc Today. 2019;18:80–82. [Google Scholar]
- 18.Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, Conte MS, Murad MH. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642–1651.e3. doi: 10.1016/j.jvs.2015.07065 [DOI] [PubMed] [Google Scholar]
- 19.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695.e2. doi: 10.1016/j.jvs.2014.03.290 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003–2011. J Am Coll Cardiol. 2016;67:1901–1913. doi: 10.1016/j.jacc.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 21.Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care. 2019;42:50–54. doi: 10.2337/dc18-1380 [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushita K, Sang Y, Ning H, Ballew SH, Chow EK, Grams ME, Selvin E, Allison M, Criqui M, Coresh J, et al. Lifetime risk of lower-extremity peripheral artery disease defined by ankle-brachial index in the United States. J Am Heart Assoc. 2019;8:e012177. doi: 10.1161/JAHA.119.012177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns Hopkins University. Lifetime risk and prevalence of lower extremity peripheral artery disease (PAD). 2019;2020. Accessed July 1, 2021. http://ckdpcrisk.org/padrisk/
- 25.Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, et al. ; ESC Scientific Document Group. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 26.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, et al. ; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb [DOI] [PubMed] [Google Scholar]
- 27.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398. doi: 10.1016/0301-5629(96)00036-1 [DOI] [PubMed] [Google Scholar]
- 28.Stivalet O, Paisant A, Belabbas D, Omarjee L, Le Faucheur A, Landreau P, Garlantezec R, Jaquinandi V, Liedl DA, Wennberg PW, et al. Exercise testing criteria to diagnose lower extremity peripheral artery disease assessed by computed-tomography angiography. PLoS One. 2019;14:e0219082. doi: 10.1371/journal.pone.0219082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herraiz-Adillo Á, Cavero-Redondo I, Álvarez-Bueno C, Pozuelo-Carrascosa DP Solera-Martínez M. The accuracy of toe brachial index and ankle brachial index in the diagnosis of lower limb peripheral arterial disease: a systematic review and meta-analysis. Atherosclerosis. 2020;315:81–92. doi: 10.1016/j.atherosclerosis.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 30.Allison MA, Cushman M, Solomon C, Aboyans V, McDermott MM, Goff DC Jr, Criqui MH. Ethnicity and risk factors for change in the ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis. J Vasc Surg. 2009;50:1049–1056. doi: 10.1016/j.jvs.2009.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapoor R, Ayers C, Visotcky A, Mason P, Kulinski J. Association of sex and height with a lower ankle brachial index in the general population. Vasc Med. 2018;23:534–540. doi: 10.1177/1358863X18774845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC Jr, Manolio TA. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA). J Vasc Surg. 2007;45:319–327. doi: 10.1016/j.jvs.2006.10.032 [DOI] [PubMed] [Google Scholar]
- 33.Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41:110–116. doi: 10.1016/j.ejvs.2010.09.020 [DOI] [PubMed] [Google Scholar]
- 34.AbuRahma AF, Adams E, AbuRahma J, Mata LA, Dean LS, Caron C, Sloan J. Critical analysis and limitations of resting ankle-brachial index in the diagnosis of symptomatic peripheral arterial disease patients and the role of diabetes mellitus and chronic kidney disease. J Vasc Surg. 2020;71:937–945. doi: 10.1016/j.jvs.2019.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tehan PE, Santos D, Chuter VH. A systematic review of the sensitivity and specificity of the toe-brachial index for detecting peripheral artery disease. Vasc Med. 2016;21:382–389. doi: 10.1177/1358863X16645854 [DOI] [PubMed] [Google Scholar]
- 36.Chisalita SI, Wijkman M, Davidson LT, Spångeus A, Nyström F, Östgren CJ. Toe brachial index predicts major acute cardiovascular events in patients with type 2 diabetes independently of arterial stiffness. Diabetes Res Clin Pract. 2020;161:108040. doi: 10.1016/j.diabres.2020.108040 [DOI] [PubMed] [Google Scholar]
- 37.Wickström JE, Laivuori M, Aro E, Sund RT, Hautero O, Venermo M, Jalkanen J, Hakovirta H. Toe pressure and toe brachial index are predictive of cardiovascular mortality, overall mortality, and amputation free survival in patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2017;53:696–703. doi: 10.1016/j.ejvs.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 38.Hyun S, Forbang NI, Allison MA, Denenberg JO, Criqui MH, Ix JH. Ankle-brachial index, toe-brachial index, and cardiovascular mortality in persons with and without diabetes mellitus. J Vasc Surg. 2014;60:390–395. doi: 10.1016/j.jvs.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahe G, Pollak AW, Liedl DA, Cohoon KP, Mc Carter C, Rooke TW, Wennberg PW. Discordant diagnosis of lower extremity peripheral artery disease using American Heart Association postexercise guidelines. Medicine (Baltimore). 2015;94:e1277. doi: 10.1097/MD.0000000000001277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, Gamst A, Bundens WP, Fronek A. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507 [DOI] [PubMed] [Google Scholar]
- 41.Aboyans V, Lacroix P, Tran MH, Salamagne C, Galinat S, Archambeaud F, Criqui MH, Laskar M. The prognosis of diabetic patients with high ankle-brachial index depends on the coexistence of occlusive peripheral artery disease. J Vasc Surg. 2011;53:984–991. doi: 10.1016/j.jvs.2010.10.054 [DOI] [PubMed] [Google Scholar]
- 42.Misra S, Shishehbor MH, Takahashi EA, Aronow HD, Brewster LP, Bunte MC, Kim ESH, Lindner JR, Rich K; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; and Council on Cardiovascular and Stroke Nursing. Perfusion assessment in critical limb ischemia: principles for understanding and the development of evidence and evaluation of devices: a scientific statement from the American Heart Association. Circulation. 2019;140:e657–e672. doi: 10.1161/CIR.0000000000000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumamaru KK, Hoppel BE, Mather RT, Rybicki FJ. CT angiography: current technology and clinical use. Radiol Clin North Am. 2010;48:213–235, vii. doi: 10.1016/j.rcl.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–2045. doi: 10.1161/CIR.0b013e31822e80c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catalano C, Fraioli F, Laghi A, Napoli A, Bezzi M, Pediconi F, Danti M, Nofroni I, Passariello R. Infrarenal aortic and lower-extremity arterial disease: diagnostic performance of multi-detector row CT angiography. Radiology. 2004;231:555–563. doi: 10.1148/radiol.2312020920 [DOI] [PubMed] [Google Scholar]
- 46.Buls N, de Brucker Y, Aerden D, Devos H, Van Gompel G, Boonen PT, Nieboer K, Leiner T, de Mey J. Improving the diagnosis of peripheral arterial disease in below-the-knee arteries by adding time-resolved CT scan series to conventional run-off CT angiography. First experience with a 256-slice CT scanner. Eur J Radiol. 2019;110:136–141. doi: 10.1016/j.ejrad.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 47.Hur S, Jae HJ, Jang Y, Min SK, Min SI, Lee DY, Seo SG, Kim HC, Chung JW, Kim KG, et al. Quantitative assessment of foot blood flow by using dynamic volume perfusion CT technique: a feasibility study. Radiology. 2016;279:195–206. doi: 10.1148/radiol.2015150560 [DOI] [PubMed] [Google Scholar]
- 48.Sah BR, Veit-Haibach P, Strobel K, Banyai M, Huellner MW. CT-perfusion in peripheral arterial disease – correlation with angiographic and hemodynamic parameters. PLoS One. 2019;14:e0223066. doi: 10.1371/journal.pone.0223066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stacy MR, Yu DY Maxfield MW, Jaba IM, Jozwik BP, Zhuang ZW, Lin BA, Hawley CL, Caracciolo CM, Pal P, et al. Multimodality imaging approach for serial assessment of regional changes in lower extremity arteriogenesis and tissue perfusion in a porcine model of peripheral arterial disease. Circ Cardiovasc Imaging. 2014;7:92–99. doi: 10.1161/CIRCIMAGING.113.000884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chou TH, Atway SA, Bobbey AJ, Sarac TP, Go MR, Stacy MR. SPECT/CT imaging: a noninvasive approach for evaluating serial changes in angiosome foot perfusion in critical limb ischemia. Adv Wound Care (New Rochelle). 2020;9:103–110. doi: 10.1089/wound.2018.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi EA, Kinsman KA, Neidert NB, Young PM. Guiding peripheral arterial disease management with magnetic resonance imaging. Vasa. 2019;48:217–222. doi: 10.1024/0301-1526/a000742 [DOI] [PubMed] [Google Scholar]
- 52.Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med. 2020;180:223–230. doi: 10.1001/jamainternmed.2019.5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavallo AU, Koktzoglou I, Edelman RR, Gilkeson R, Mihai G, Shin T, Rajagopalan S. Noncontrast magnetic resonance angiography for the diagnosis of peripheral vascular disease. Circ Cardiovasc Imaging. 2019;12: e008844. doi: 10.1161/CIRCIMAGING.118.008844 [DOI] [PubMed] [Google Scholar]
- 54.Bajwa A, Wesolowski R, Patel A, Saha P, Ludwinski F, Ikram M, Albayati M, Smith A, Nagel E, Modarai B. Blood oxygenation level-dependent CMR-derived measures in critical limb ischemia and changes with revascularization. J Am Coll Cardiol. 2016;67:420–431. doi: 10.1016/j.jacc.2015.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajwa A, Wesolowski R, Patel A, Saha P, Ludwinski F, Smith A, Nagel E, Modarai B. Assessment of tissue perfusion in the lower limb: current methods and techniques under development. Circ Cardiovasc Imaging. 2014;7:836–843. doi: 10.1161/CIRCIMAGING.114.002123 [DOI] [PubMed] [Google Scholar]
- 56.Allard L, Cloutier G, Durand LG, Roederer GO, Langlois YE. Limitations of ultrasonic duplex scanning for diagnosing lower limb arterial stenoses in the presence of adjacent segment disease. J Vasc Surg. 1994;19:650–657 doi: 10.1016/s0741-5214(94)70038-9 [DOI] [PubMed] [Google Scholar]
- 57.Mohler ER 3rd, Gornik HL, Gerhard-Herman M, Misra S, Olin JW, Zierler RE. ACCF/ACR/AIUM/ASE/ASN/ICAVL/SCAI/SCCT/SIR/SVM/SVS 2012 appropriate use criteria for peripheral vascular ultrasound and physiological testing part I: arterial ultrasound and physiological testing: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American College of Radiology, American Institute of Ultrasound in Medicine, American Society of Echocardiography, American Society of Nephrology, Intersocietal Commission for the Accreditation of Vascular Laboratories, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery [published correction appears in J Am Coll Cardiol. 2013;62:1540]. J Am Coll Cardiol. 2012;60:242–276. doi: 10.1016/j.jacc.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou XX, Chu GH, Yu Y. Prospects of contrast-enhanced ultrasonography for the diagnosis of peripheral arterial disease: a meta-analysis. J Ultrasound Med. 2018;37:1081–1090. doi: 10.1002/jum.14451 [DOI] [PubMed] [Google Scholar]
- 59.Serhal A, Koktzoglou I, Edelman RR. Feasibility of image fusion for concurrent MRI evaluation of vessel lumen and vascular calcifications in peripheral arterial disease. AJR Am J Roentgenol. 2019;212:914–918. doi: 10.2214/AJR.18.20000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.US Preventive Services Task Force: Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, et al. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:177–183. doi: 10.1001/jama.2018.8357 [DOI] [PubMed] [Google Scholar]
- 61.Conte MS, Pomposelli FB. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities management of asymptomatic disease and claudication. Introduction. J Vasc Surg. 2015;61(3 suppl):1S. doi: 10.1016/j.jvs.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 62.Lindholt JS, Søgaard R. Population screening and intervention for vascular disease in Danish men (VIVA): a randomised controlled trial. Lancet. 2017;390:2256–2265. doi: 10.1016/S0140-6736(17)32250-X [DOI] [PubMed] [Google Scholar]
- 63.American College of Cardiology, American College of Radiology, American Heart Association, Society for Vascular Medicine, Society of Interventional Radiology. Joint comments to USPSTF on PAD ABI screening. February 12, 2018. https://www.heart.org/-/media/files/get-involved/advocacy/joint-comments-to-uspstf-on-pad-abi-screening-021218.pdf?la=en
- 64.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515. doi: 10.1161/01.cir.71.3.510 [DOI] [PubMed] [Google Scholar]
- 65.Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–1191. doi: 10.1016/j.jvs.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 66.Moussa ID, Jaff MR, Mehran R, Gray W, Dangas G, Lazic Z, Moses JW. Prevalence and prediction of previously unrecognized peripheral arterial disease in patients with coronary artery disease: the Peripheral Arterial Disease in Interventional Patients Study. Catheter Cardiovasc Interv. 2009;73:719–724. doi: 10.1002/ccd.21969 [DOI] [PubMed] [Google Scholar]
- 67.Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, Pittrow D, von Stritzky B, Tepohl G, Trampisch HJ. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1 [DOI] [PubMed] [Google Scholar]
- 68.Kalbaugh CA, Kucharska-Newton A, Wruck L, Lund JL, Selvin E, Matsushita K, Bengtson LGS, Heiss G, Loehr L. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among Medicare fee-for-service beneficiaries in the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2017;6:e003796. doi: 10.1161/JAHA.116.003796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ortmann J, Nüesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. 2012;55:98–104. doi: 10.1016/j.jvs.2011.07.074 [DOI] [PubMed] [Google Scholar]
- 70.Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, Henke PK, Gurm HS, Grossman PM. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardioi. 2014;63:2525–2530. doi: 10.1016/j.jacc.2014.03.036 [DOI] [PubMed] [Google Scholar]
- 71.Kullo IJ, Bailey KR, Kardia SL, Mosley TH Jr, Boerwinkle E, Turner ST. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc Med. 2003;8:237–242. doi: [DOI] [PubMed] [Google Scholar]
- 72.Soden PA, Zettervall SL, Deery SE, Hughes K, Stoner MC, Goodney PP Vouyouka AG, Schermerhorn ML; Society for Vascular Surgery Vascular Quality Initiative. Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J Vasc Surg. 2018;67:549–556.e3. doi: 10.1016/j.jvs.2017.06.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arya S, Binney Z, Khakharia A, Brewster LP, Goodney P, Patzer R, Hockenberry J, Wilson PWF. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc. 2018;7:e007425. doi: 10.1161/JAHA.117.007425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–539. doi: 10.1161/CIRC0UTC0MES.113.000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vart P, Coresh J, Kwak L, Ballew SH, Heiss G, Matsushita K. Socioeconomic status and incidence of hospitalization with lower-extremity peripheral artery disease: Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2017;6:e004995. doi: 10.1161/JAHA.116.004995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Subherwal S, Patel MR, Tang F, Smolderen KG, Jones WS, Tsai TT, Ting HH, Bhatt DL, Spertus JA, Chan PS. Socioeconomic disparities in the use of cardioprotective medications among patients with peripheral artery disease: an analysis of the American College of Cardiology’s NCDR PINNACLE Registry. J Am Coll Cardiol. 2013;62:51–57. doi: 10.1016/j.jacc.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srivaratharajah K, Abramson BL. Women and peripheral arterial disease: a review of sex differences in epidemiology, clinical manifestations, and outcomes. Can J Cardiol. 2018;34:356–361. doi: 10.1016/j.cjca.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 78.Nguyen LL, Henry AJ. Disparities in vascular surgery: is it biology or environment? J Vasc Surg. 2010;51 (4 suppl):36S–41S. doi: 10.1016/j.jvs.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mustapha JA, Fisher BT Sr, Rizzo JA, Chen J, Martinsen BJ, Kotlarz H, Ryan M, Gunnarsson C. Explaining racial disparities in amputation rates for the treatment of peripheral artery disease (PAD) using decomposition methods. J Racial Ethn Health Disparities. 2017;4:784–795. doi: 10.1007/s40615-016-0261-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rowe VL, Weaver FA, Lane JS, Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998–2006. J Vasc Surg. 2010;51(4 suppl):21s–26s. doi: 10.1016/j.jvs.2009.09.066 [DOI] [PubMed] [Google Scholar]
- 81.Hicks CW, Wang P, Bruhn WE, Abularrage CJ, Lum YW, Perler BA, Black JH 3rd, Makary MA. Race and socioeconomic differences associated with endovascular peripheral vascular interventions for newly diagnosed claudication. J Vasc Surg. 2020;72:611–621.e5. doi: 10.1016/j.jvs.2019.10.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Regenbogen SE, Gawande AA, Lipsitz SR, Greenberg CC, Jha AK. Do differences in hospital and surgeon quality explain racial disparities in lower-extremity vascular amputations? Ann Surg. 2009;250:424–431. doi: 10.1097/SLA.0b013e3181b41d53 [DOI] [PubMed] [Google Scholar]
- 83.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. ; on behalf of the American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 84.Farah BQ, Ritti-Dias RM, Montgomery PS, Casanegra AI, Silva-Palacios F, Gardner AW. Sedentary behavior is associated with impaired biomarkers in claudicants. J Vasc Surg. 2016;63:657–663. doi: 10.1016/j.jvs.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Housley E, Leng GC, Donnan PT, Fowkes FG. Physical activity and risk of peripheral arterial disease in the general population: Edinburgh Artery Study. J Epidemiol Community Health. 1993;47:475–480. doi: 10.1136/jech.47.6.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141 :e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 87.Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, Blaha MJ, Allison M, Heiss G, Selvin E, et al. Cigarette smoking, smoking cessation, and long-term risk of 3 major atherosclerotic diseases. J Am Coll Cardiol. 2019;74:498–507 doi: 10.1016/j.jacc.2019.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. doi: 10.2337/diacare.26.12.3333 [DOI] [PubMed] [Google Scholar]
- 89.Brand FN, Abbott RD, Kannel WB. Diabetes, intermittent claudication, and risk of cardiovascular events. The Framingham Study. Diabetes. 1989;38:504–509. doi: 10.2337/diab.38.4.504 [DOI] [PubMed] [Google Scholar]
- 90.Semple R Diabetes and peripheral arterial disease; a clinical study. Lancet. 1953;1:1064–1068. doi: 10.1016/s0140-6736(53)92200-4 [DOI] [PubMed] [Google Scholar]
- 91.Herzstein J, Weinroth LA. Arteriosclerotic peripheral vascular disease in diabetes. Arch Intern Med 1945;76:34–38. doi: 10.1001/archinte.1945.00210310042005 [DOI] [Google Scholar]
- 92.Ding N, Kwak L, Ballew SH, Jaar B, Hoogeveen RC, Ballantyne CM, Sharrett AR, Folsom AR, Heiss G, Salameh M, et al. Traditional and nontraditional glycemic markers and risk of peripheral artery disease: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2018;274:86–93. doi: 10.1016/j.atherosclerosis.2018.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433–1437 doi: 10.2337/diacare.24.8.1433 [DOI] [PubMed] [Google Scholar]
- 94.Pyörälä K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206 [DOI] [PubMed] [Google Scholar]
- 95.Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, Fujimoto WY, Imperatore G. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA. 2019;322:2389–2398. doi: 10.1001/jama.2019.19365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493–1498. doi: 10.1111/dme.13054 [DOI] [PubMed] [Google Scholar]
- 97.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37:651–658. doi: 10.2337/dc13-2176 [DOI] [PubMed] [Google Scholar]
- 98.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49:106–116. doi: 10.1080/07853890.2016.1231932 [DOI] [PubMed] [Google Scholar]
- 99.Hinchliffe RJ, Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J, et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(suppl 1):e3276. doi: 10.1002/dmrr.3276 [DOI] [PubMed] [Google Scholar]
- 100.Morbach S, Furchert H, Gröblinghoff U, Hoffmeier H, Kersten K, Klauke GT, Klemp U, Roden T, Icks A, Haastert B, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35:2021–2027 doi: 10.2337/dc12-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bevilacqua NJ, Rogers LC, Armstrong DG. Diabetic foot surgery: classifying patients to predict complications. Diabetes Metab Res Rev. 2008;24(suppl 1):S81–S83. doi: 10.1002/dmrr.858 [DOI] [PubMed] [Google Scholar]
- 102.Willigendael EM, Teijink JA, Bartelink ML, Kuiken BW, Boiten J, Moll FL, Büller HR, Prins MH. Influence of smoking on incidence and prevalence of peripheral arterial disease. J Vasc Surg. 2004;40:1158–1165. doi: 10.1016/j.jvs.2004.08.049 [DOI] [PubMed] [Google Scholar]
- 103.Leng GC, Lee AJ, Fowkes FG, Lowe GD, Housley E. The relationship between cigarette smoking and cardiovascular risk factors in peripheral arterial disease compared with ischaemic heart disease. The Edinburgh Artery Study. Eur Heart J. 1995;16:1542–1548. doi: 10.1093/oxfordjournals.eurheartj.a060775 [DOI] [PubMed] [Google Scholar]
- 104.Huxley RR, Yatsuya H, Lutsey PL, Woodward M, Alonso A, Folsom AR. Impact of age at smoking initiation, dosage, and time since quitting on cardiovascular disease in African Americans and Whites: the Atherosclerosis Risk in Communities study Am J Epidemiol. 2012;175:816–826. doi: 10.1093/aje/kwr391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Planas A, Clará A, Marrugat J, Pou JM, Gasol A, de Moner A, Contreras C, Vidal-Barraquer F. Age at onset of smoking is an independent risk factor in peripheral artery disease development. J Vasc Surg. 2002;35:506–509. doi: 10.1067/mva.2002.120030 [DOI] [PubMed] [Google Scholar]
- 106.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837 [DOI] [PubMed] [Google Scholar]
- 107.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44 [DOI] [PubMed] [Google Scholar]
- 108.Powell JT, Edwards RJ, Worrell PC, Franks PJ, Greenhalgh RM, Poulter NR. Risk factors associated with the development of peripheral arterial disease in smokers: a case-control study. Atherosclerosis. 1997;129:41–48. doi: 10.1016/s0021-9150(96)06012-1 [DOI] [PubMed] [Google Scholar]
- 109.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–362. doi: 10.1093/eurheartj/ehl441 [DOI] [PubMed] [Google Scholar]
- 110.Gurdasani D, Sjouke B, Tsimikas S, Hovingh GK, Luben RN, Wainwright NW, Pomilla C, Wareham NJ, Khaw KT, Boekholdt SM, et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2012;32:3058–3065. doi: 10.1161/ATVBAHA.112.255521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, et al. ; AKCEA-APO(a)-LRx Study Investigators. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239 [DOI] [PubMed] [Google Scholar]
- 112.Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation. 2018;138:2330–2341. doi: 10.1161/CIRCULATIONAHA.118.035432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, et al. ; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 114.Meijer WT, Grobbee DE, Hunink MG, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: the Rotterdam study. Arch Intern Med. 2000;160:2934–2938. doi: 10.1001/archinte.160.19.2934 [DOI] [PubMed] [Google Scholar]
- 115.Lu Y, Ballew SH, Tanaka H, Szklo M, Heiss G, Coresh J, Matsushita K. 2017 ACC/AHA blood pressure classification and incident peripheral artery disease: The Atherosclerosis Risk in Communities (ARIC) Study. Eur J Prev Cardiol. 2020;27:51–59. doi: 10.1177/2047487319865378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith FB, Lee AJ, Hau CM, Rumley A, Lowe GD, Fowkes FG. Plasma fibrinogen, haemostatic factors and prediction of peripheral arterial disease in the Edinburgh Artery Study. Blood Coagul Fibrinolysis. 2000;11:43–50. [PubMed] [Google Scholar]
- 117.Wattanakit K, Folsom AR, Selvin E, Weatherley BD, Pankow JS, Brancati FL, Hirsch AT. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2005;180:389–397. doi: 10.1016/j.atherosclerosis.2004.11.024 [DOI] [PubMed] [Google Scholar]
- 118.Beckman JA, Duncan MS, Alcorn CW, So-Armah K, Butt AA, Goetz MB, Tindle HA, Sico JJ, Tracy RP Justice AC, et al. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation. 2018;138:255–265. doi: 10.1161/CIRCULATIONAHA.117032647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cedarbaum E, Ma Y, Scherzer R, Price JC, Adimora AA, Bamman M, Cohen M, Fischl MA, Matsushita K, Ofotokun I, et al. Contributions of HIV, hepatitis C virus, and traditional vascular risk factors to peripheral artery disease in women. AIDS. 2019;33:2025–2033. doi: 10.1097/QAD.0000000000002319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018;3:e177–e184. doi: 10.1016/S2468-2667(18)30025-2 [DOI] [PubMed] [Google Scholar]
- 121.Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S, Khan H, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310. doi: 10.1136/bmj.k3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321 [DOI] [PubMed] [Google Scholar]
- 123.Møller L, Kristensen TS. Blood lead as a cardiovascular risk factor. Am J Epidemiol. 1992;136:1091–1100. doi: 10.1093/oxfordjournals.aje.a116574 [DOI] [PubMed] [Google Scholar]
- 124.Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–2449. doi: 10.1001/archinte.162.21.2443 [DOI] [PubMed] [Google Scholar]
- 125.Nawrot TS, Staessen JA. Low-level environmental exposure to lead unmasked as silent killer. Circulation. 2006;114:1347–1349. doi: 10.1161/CIRCULATIONAHA.106.650440 [DOI] [PubMed] [Google Scholar]
- 126.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2 [DOI] [PubMed] [Google Scholar]
- 127.Tellez-Plaza M, Guallar E, Fabsitz RR, Howard BV, Umans JG, Francesconi KA, Goessler W, Devereux RB, Navas-Acien A. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2013;6:626–633. doi: 10.1161/CIRCOUTCOMES.112.000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ujueta F, Arenas IA, Diaz D, Yates T, Beasley R, Navas-Acien A, Lamas GA. Cadmium level and severity of peripheral artery disease in patients with coronary artery disease. Eur J Prev Cardiol. 2019;26:1456–1458. doi: 10.1177/2047487318796585 [DOI] [PubMed] [Google Scholar]
- 129.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401 [DOI] [PubMed] [Google Scholar]
- 130.Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, Astor B, Keeler J. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:667–675. doi: 10.1093/aje/kwm359 [DOI] [PubMed] [Google Scholar]
- 131.Hoffmann B, Moebus S, Kröger K, Stang A, Möhlenkamp S, Dragano N, Schmermund A, Memmesheimer M, Erbel R, Jöckel KH. Residential exposure to urban air pollution, ankle-brachial index, and peripheral arterial disease. Epidemiology. 2009;20:280–288. doi: 10.1097/EDE.0b013e3181961ac2 [DOI] [PubMed] [Google Scholar]
- 132.An W, Xian L, Zhao L, Detrano R, Criqui MH, Wu Y. Distribution of the ankle-brachial index and peripheral arterial disease in middle-aged and elderly Chinese: a population-based study of 18,000 men and women. Circulation. 2010;122:e43. [Google Scholar]
- 133.Wattanakit K, Williams JE, Schreiner PJ, Hirsch AT, Folsom AR. Association of anger proneness, depression and low social support with peripheral arterial disease: the Atherosclerosis Risk in Communities Study. Vasc Med. 2005;10:199–206. doi: 10.1191/1358863x05vm622oa [DOI] [PubMed] [Google Scholar]
- 134.Cherr GS, Zimmerman PM, Wang J, Dosluoglu HH. Patients with depression are at increased risk for secondary cardiovascular events after lower extremity revascularization. J Gen Intern Med. 2008;23:629–634. doi: 10.1007/s11606-008-0560-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grenon SM, Hiramoto J, Smolderen KG, Vittinghoff E, Whooley MA, Cohen BE. Association between depression and peripheral artery disease: insights from the Heart and Soul Study. J Am Heart Assoc. 2012;1:e002667 doi: 10.1161/JAHA.112.002667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McDermott MM, Guralnik JM, Tian L, Kibbe MR, Ferrucci L, Zhao L, Liu K, Liao Y, Gao Y, Criqui MH. Incidence and prognostic significance of depressive symptoms in peripheral artery disease. J Am Heart Assoc. 2016;5:e002959. doi: 10.1161/JAHA.115.002959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruo B, Liu K, Tian L, Tan J, Ferrucci L, Guralnik JM, McDermott MM. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosom Med. 2007;69:415–424. doi: 10.1097/PSY.0b013e318063ef5c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Matsushita K, Ballew SH, Coresh J, Arima H, Ärnlöv J, Cirillo M, Ebert N, Hiramoto JS, Kimm H, Shlipak MG, et al. ; Chronic Kidney Disease Prognosis Consortium. Measures of chronic kidney disease and risk of incident peripheral artery disease: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017;5:718–728. doi: 10.1016/S2213-8587(17)30183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang C, Kwak L, Ballew SH, Jaar BG, Deal JA, Folsom AR, Heiss G, Sharrett AR, Selvin E, Sabanayagam C, et al. Retinal microvascular findings and risk of incident peripheral artery disease: an analysis from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2020;294:62–71. doi: 10.1016/j.atherosclerosis.2019.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beckman JA, Duncan MS, Damrauer SM, Wells QS, Barnett JV, Wasserman DH, Bedimo RJ, Butt AA, Marconi VC, Sico JJ, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140:449–458. doi: 10.1161/CIRCULATIONAHA.119.040672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park SC, Choi CY Ha YI, Yang HE. Utility of toe-brachial index for diagnosis of peripheral artery disease. Arch Plast Surg. 2012;39:227–231. doi: 10.5999/aps.2012.39.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McDermott MM, Greenland P Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599 [DOI] [PubMed] [Google Scholar]
- 143.McDermott MM, Applegate WB, Bonds DE, Buford TW, Church T, Espeland MA, Gill TM, Guralnik JM, Haskell W, Lovato LC, et al. Ankle brachial index values, leg symptoms, and functional performance among community-dwelling older men and women in the Lifestyle Interventions and Independence for Elders study. J Am Heart Assoc. 2013;2:e000257 doi: 10.1161/JAHA.113.000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McDermott MM, Guralnik JM, Ferrucci L, Tian L, Liu K, Liao Y, Green D, Sufit R, Hoff F, Nishida T, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484–2491. doi: 10.1161/CIRCULATIONAHA.107.736108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:61–68. doi: 10.1161/CIRCULATI0NAHA.114.007002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morris DR, Rodriguez AJ, Moxon JV, Cunningham MA, McDermott MM, Myers J, Leeper NJ, Jones RE, Golledge J. Association of lower extremity performance with cardiovascular and all-cause mortality in patients with peripheral artery disease: a systematic review and meta-analysis. J Am Heart Assoc. 2014;3:e001105. doi: 10.1161/JAHA.114.001105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McDermott MM, Greenland P Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008 [DOI] [PubMed] [Google Scholar]
- 148.Matsushita K, Ballew SH, Sang Y, Kalbaugh C, Loehr LR, Hirsch AT, Tanaka H, Heiss G, Windham BG, Selvin E, et al. Ankle-brachial index and physical function in older individuals: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2017;257:208–215. doi: 10.1016/j.atherosclerosis.2016.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the Women’s Health and Aging Study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007 [DOI] [PubMed] [Google Scholar]
- 150.Wu A, Coresh J, Selvin E, Tanaka H, Heiss G, Hirsch AT, Jaar BG, Matsushita K. Lower extremity peripheral artery disease and quality of life among older individuals in the community. J Am Heart Assoc. 2017;6:e004519. doi: 10.1161/JAHA.116.004519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Korhonen PE, Seppälä T, Kautiainen H, Jäivenpää S, Aarnio PT, Kivelä SL. Ankle-brachial index and health-related quality of life. Eur J Prev Cardiol. 2012;19:901–907. doi: 10.1177/1741826711420346 [DOI] [PubMed] [Google Scholar]
- 152.Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, Hirsch AT. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13:15–24. doi: 10.1177/1358863X07084911 [DOI] [PubMed] [Google Scholar]
- 153.Thomas M, Patel KK, Gosch K, Labrosciano C, Mena-Hurtado C, Fitridge R, Spertus JA, Smolderen KG. Mental health concerns in patients with symptomatic peripheral artery disease: insights from the PORTRAIT registry. J Psychosom Res. 2020;131:109963. doi: 10.1016/j.jpsychores.2020.109963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Imparato AM, Kim GE, Davidson T, Crowley JG. Intermittent claudication: its natural course. Surgery. 1975;78:795–799. [PubMed] [Google Scholar]
- 155.McAllister FF. The fate of patients with intermittent claudication managed nonoperatively. Am J Surg. 1976;132:593–595. doi: 10.1016/0002-9610(76)90351-2 [DOI] [PubMed] [Google Scholar]
- 156.Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–1181. doi: 10.1093/ije/25.6.1172 [DOI] [PubMed] [Google Scholar]
- 157.Morley RL, Sharma A, Horsch AD, Hinchliffe RJ. Peripheral artery disease. BMJ. 2018;360:j5842. doi: 10.1136/bmj.j5842 [DOI] [PubMed] [Google Scholar]
- 158.Uwagbai O, Kalish VB. Vascular Dementia. StatPearls. Online book. StatPearls Publishing; Jan 26, 2021. Accessed July 1, 2021. https://www.ncbi.nlm.nih.gov/books/NBK430817 [Google Scholar]
- 159.Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging: JACC focus seminar. J Am Coll Cardiol. 2020;75:942–951. doi: 10.1016/j.jacc.2019.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Fowkes FG. Cardiovascular diseases and decline in cognitive function in an elderly community population: the Edinburgh Artery Study. Psychosom Med. 2007;69:425–434. doi: 10.1097/psy.0b013e318068fce4 [DOI] [PubMed] [Google Scholar]
- 161.Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: evidence from the Whitehall II Study. J Am Geriatr Soc. 2003;51:1445–1450. doi: 10.1046/j.1532-5415.2003.51464.x [DOI] [PubMed] [Google Scholar]
- 162.Laurin D, Masaki KH, White LR, Launer LJ. Ankle-to-brachial index and dementia: the Honolulu-Asia Aging Study. Circulation. 2007;116:2269–2274. doi: 10.1161/CIRCULATIONAHA.106.686477 [DOI] [PubMed] [Google Scholar]
- 163.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE ε4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40 [DOI] [PubMed] [Google Scholar]
- 164.Phillips NA, Mate-Kole CC. Cognitive deficits in peripheral vascular disease. A comparison of mild stroke patients and normal control subjects. Stroke. 1997;28:777–784. doi: 10.1161/01.str.28.4.777 [DOI] [PubMed] [Google Scholar]
- 165.Rao R, Jackson S, Howard R. Neuropsychological impairment in stroke, carotid stenosis, and peripheral vascular disease. A comparison with healthy community residents. Stroke. 1999;30:2167–2173. doi: 10.1161/01.str.30.10.2167 [DOI] [PubMed] [Google Scholar]
- 166.Hess CN, Huang Z, Patel MR, Baumgartner I, Berger JS, Blomster JI, Fowkes FGR, Held P Jones WS, Katona B, et al. Acute limb ischemia in peripheral artery disease. Circulation. 2019;140:556–565. doi: 10.1161/CIRCULATIONAHA.119.039773 [DOI] [PubMed] [Google Scholar]
- 167.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta DK, Skali H, Claggett B, Kasabov R, Cheng S, Shah AM, Loehr LR, Heiss G, Nambi V, Aguilar D, et al. Heart failure risk across the spectrum of ankle-brachial index: the ARIC study (Atherosclerosis Risk In Communities). JACC Heart Fail. 2014;2:447–454. doi: 10.1016/j.jchf.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605 [DOI] [PubMed] [Google Scholar]
- 170.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bonaca MP Nault P, Giugliano RP Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338–350. doi: 10.1161/CIRCULATIONAHA.117.032235 [DOI] [PubMed] [Google Scholar]
- 172.Steffel J, Eikelboom JW, Anand SS, Shestakovska O, Yusuf S, Fox KAA. The COMPASS Trial: net clinical benefit of low-dose rivaroxaban plus aspirin as compared with aspirin in patients with chronic vascular disease. Circulation. 2020;142:40–48. doi: 10.1161/CIRCULATIONAHA.120.046048 [DOI] [PubMed] [Google Scholar]
- 173.Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hess CN, Rogers RK, Wang TY, Fu R, Gundrum J, Allen LaPointe NM, Hiatt WR. Major adverse limb events and 1-year outcomes after peripheral artery revascularization. J Am Coll Cardiol. 2018;72:999–1011. doi: 10.1016/j.jacc.2018.06.041 [DOI] [PubMed] [Google Scholar]
- 175.Armstrong EJ, Chen DC, Westin GG, Singh S, McCoach CE, Bang H, Yeo KK, Anderson D, Amsterdam EA, Laird JR. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc. 2014;3:e000697. doi: 10.1161/JAHA.113.000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Patel KK, Jones PG, Ellerbeck EF, Buchanan DM, Chan PS, Pacheco CM, Moneta G, Spertus JA, Smolderen KG. Underutilization of evidence-based smoking cessation support strategies despite high smoking addiction burden in peripheral artery disease specialty care: insights from the International PORTRAIT Registry. J Am Heart Assoc. 2018;7:e010076. doi: 10.1161/JAHA.118.010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fakhry F, van de Luijtgaarden KM, Bax L, den Hoed PT, Hunink MG, Rouwet EV, Spronk S. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg. 2012;56:1132–1142. doi: 10.1016/j.jvs.2012.04.046 [DOI] [PubMed] [Google Scholar]
- 178.McDermott MM, Ades P Guralnik JM, Dyer A, Ferrucci L, Liu K, Nelson M, Lloyd-Jones D, Van Horn L, Garside D, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3:e001107. doi: 10.1161/JAHA.114.001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McDermott MM, Ferrucci L, Tian L, Guralnik JM, Lloyd-Jones D, Kibbe MR, Polonsky TS, Domanchuk K, Stein JH, Zhao L, et al. Effect of granulocyte-macrophage colony-stimulating factor with or without supervised exercise on walking performance in patients with peripheral artery disease: the PROPEL randomized clinical trial. JAMA. 2017;318:2089–2098. doi: 10.1001/jama.2017.17437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Treat-Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, Gardner AW, Hiatt WR, Regensteiner JG, Rich K; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Council on Cardiovascular and Stroke Nursing. Optimal exercise programs for patients with peripheral artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e10–e33. doi: 10.1161/CIR.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 182.Treat-Jacobson D, McDermott MM, Beckman JA, Burt MA, Creager MA, Ehrman JK, Gardner AW, Mays RJ, Regensteiner JG, Salisbury DL, et al. ; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Lifestyle and Cardiometabolic Health. Implementation of supervised exercise therapy for patients with symptomatic peripheral artery disease: a science advisory from the American Heart Association. Circulation. 2019;140:e700–e710. doi: 10.1161/CIR.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 183.Dua A, Gologorsky R, Savage D, Rens N, Gandhi N, Brooke B, Corriere M, Jackson E, Aalami O. National assessment of availability, awareness, and utilization of supervised exercise therapy for peripheral artery disease patients with intermittent claudication. J Vasc Surg. 2020;71:1702–1707. doi: 10.1016/j.jvs.2019.08.238 [DOI] [PubMed] [Google Scholar]
- 184.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–498. doi: 10.1161/CIRCULATIONAHA.110.963066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McDermott MM, Spring B, Berger JS, Treat-Jacobson D, Conte MS, Creager MA, Criqui MH, Ferrucci L, Gornik HL, Guralnik JM, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319:1665–1676. doi: 10.1001/jama.2018.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, Domanchuk K, Ferrucci L, Lloyd-Jones D, Kibbe M, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:57–65. doi: 10.1001/jama.2013.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Collins TC, Lu L, Ahluwalia JS, Nollen NL, Sirard J, Marcotte R, Post S, Zackula R. Efficacy of community-based exercise therapy among African American patients with peripheral artery disease: a randomized clinical trial. JAMA Netw Open. 2019;2:e187959. doi: 10.1001/jamanetworkopen.2018.7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, Hodges JS. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011;34:2174–2179. doi: 10.2337/dc10-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188a.McDermott MM, Spring B, Tian L, Treat-Jacobson D, Ferrucci L, Lloyd-Jones D, Zhao L, Polonsky T, Kibbe MR, Bazzano L, et al. Effect of low-intensity vs high-intensity home-based walking exercise on walk distance in patients with peripheral artery disease: the LITE randomized clinical trial. JAMA. 2021;325:1266–1276. doi: 10.1001/jama.2021.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aquino R, Johnnides C, Makaroun M, Whittle JC, Muluk VS, Kelley ME, Muluk SC. Natural history of claudication: long-term serial followup study of 1244 claudicants. J Vasc Surg. 2001;34:962–970. doi: 10.1067/mva.2001.119749 [DOI] [PubMed] [Google Scholar]
- 190.Djerf H, Millinger J, Falkenberg M, Jivegård L, Svensson M, Nordanstig J. Absence of long-term benefit of revascularization in patients with intermittent claudication: five-year results from the IRONIC randomized controlled trial. Circ Cardiovasc Interv. 2020;13:e008450. doi: 10.1161/CIRCINTERVENTIONS.119.008450 [DOI] [PubMed] [Google Scholar]
- 191.Soden PA, Zettervall SL, Shean KE, Vouyouka AG, Goodney PP Mills JL, Hallett JW Jr, Schermerhorn ML; Society for Vascular Surgery Vascular Quality Initiative. Regional variation in outcomes for lower extremity vascular disease in the Vascular Quality Initiative. J Vasc Surg. 2017;66:810–818. doi: 10.1016/j.jvs.2017.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Murphy TP Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, et al. ; CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hicks CW, Holscher CM, Wang P Black JH 3rd, Abularrage CJ, Makary MA. Overuse of early peripheral vascular interventions for claudication. J Vasc Surg. 2020;71:121–130.e1. doi: 10.1016/j.jvs.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 194.Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G; Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220-234.e1-2. doi: 10.1016/j.jvs.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 195.Jones DW, Schanzer A, Zhao Y MacKenzie TA, Nolan BW, Conte MS, Goodney PP; Vascular Study Group of New England. Growing impact of restenosis on the surgical treatment of peripheral arterial disease. J Am Heart Assoc. 2013;2:e000345. doi: 10.1161/JAHA.113.000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zeller T, Dake MD, Tepe G, Brechtel K, Noory E, Beschorner U, Kultgen PL, Rastan A. Treatment of femoropopliteal in-stent restenosis with paclitaxel-eluting stents. JACC Cardiovasc Interv. 2013;6:274–281. doi: 10.1016/j.jcin.2012.12.118 [DOI] [PubMed] [Google Scholar]
- 197.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Machan LS, Snyder SA, O’Leary EE, Ragheb AO, et al. ; Zilver PTX Investigators. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016;133:1472–1483; discussion 1483. doi: 10.1161/CIRCULATIONAHA.115.016900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Giacoppo D, Cassese S, Harada Y, Colleran R, Michel J, Fusaro M, Kastrati A, Byrne RA. Drug-coated balloon versus plain balloon angioplasty for the treatment of femoropopliteal artery disease: an updated systematic review and meta-analysis of randomized clinical trials. JACC Cardiovasc Interv. 2016;9:1731–1742. doi: 10.1016/j.jcin.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 199.Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, et al. ; LEVANT 2 Investigators. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. doi: 10.1056/NEJMoa1406235 [DOI] [PubMed] [Google Scholar]
- 200.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, et al. ; IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schroeder H, Werner M, Meyer DR, Reimer P, Krüger K, Jaff MR, Brodmann M; ILLUMENATE EU RCT Investigators. Low-dose paclitaxel-coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one-year results of the ILLUMENATE European randomized clinical trial (randomized trial of a novel paclitaxel-coated percutaneous angioplasty balloon). Circulation. 2017;135:2227–2236. doi: 10.1161/CIRCULATIONAHA.116.026493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Krishnan P Faries P Niazi K, Jain A, Sachar R, Bachinsky WB, Cardenas J, Werner M, Brodmann M, Mustapha JA, et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136:1102–1113. doi: 10.1161/CIRCULATIONAHA.117028893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Salisbury AC, Li H, Vilain KR, Jaff MR, Schneider PA, Laird JR, Cohen DJ. Cost-effectiveness of endovascular femoropopliteal intervention using drug-coated balloons versus standard percutaneous transluminal angioplasty: results from the IN.PACT SFA II trial. JACC Cardiovasc Interv. 2016;9:2343–2352. doi: 10.1016/j.jcin.2016.08.036 [DOI] [PubMed] [Google Scholar]
- 204.Sridharan ND, Boitet A, Smith K, Noorbakhsh K, Avgerinos E, Eslami MH, Makaroun M, Chaer R. Cost-effectiveness analysis of drug-coated therapies in the superficial femoral artery. J Vasc Surg. 2018;67:343–352. doi: 10.1016/j.jvs.2017.06.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e011245. doi: 10.1161/JAHA.118.011245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lo RC, Bensley RP Dahlberg SE, Matyal R, Hamdan AD, Wyers M, Chaikof EL, Schermerhorn ML. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–418.e3. doi: 10.1016/j.jvs.2013.07.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Goodney PP Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035 [DOI] [PubMed] [Google Scholar]
- 208.Rowe VL, Lee W, Weaver FA, Etzioni D. Patterns of treatment for peripheral arterial disease in the United States: 1996-2005. J Vasc Surg. 2009;49:910–917. doi: 10.1016/j.jvs.2008.11.054 [DOI] [PubMed] [Google Scholar]
- 209.Simons JP Schanzer A, Flahive JM, Osborne NH, Mills JL Sr, Bradbury AW, Conte MS. Survival prediction in patients with chronic limb-threatening ischemia who undergo infrainguinal revascularization. J Vasc Surg. 2019;69(6S):137S–151S.e3. doi: 10.1016/j.jvs.2018.08.169 [DOI] [PubMed] [Google Scholar]
- 210.Egorova NN, Guillerme S, Gelijns A, Morrissey N, Dayal R, McKinsey JF, Nowygrod R. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51:878–885, 885.e1. doi: 10.1016/j.jvs.2009.10.102 [DOI] [PubMed] [Google Scholar]
- 211.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, et al. ; BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5 [DOI] [PubMed] [Google Scholar]
- 212.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, Ruckley CV, Raab GM. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010;51(5 suppl):5s–17s. doi: 10.1016/j.jvs.2010.01.073 [DOI] [PubMed] [Google Scholar]
- 213.Janas A, Buszman PP Milewski KP Wiernek S, Janas K, Pruski M, Wojakowski W, Błachut A, Picheta W, Buszman P et al. Long-term outcomes after percutaneous lower extremity arterial interventions with atherectomy vs. balloon angioplasty-propensity score-matched registry. Circ J. 2017;81:376–382. doi: 10.1253/circj.CJ-16-0856 [DOI] [PubMed] [Google Scholar]
- 214.Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54:1021–1031.e1. doi: 10.1016/j.jvs.2011.03.281 [DOI] [PubMed] [Google Scholar]
- 215.Menard MT, Farber A, Assmann SF, Choudhry NK, Conte MS, Creager MA, Dake MD, Jaff MR, Kaufman JA, Powell RJ, et al. Design and rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) trial. J Am Heart Assoc. 2016;5:e003219. doi: 10.1161/JAHA.116.003219 [DOI] [PMC free article] [PubMed] [Google Scholar]


