Abstract
Left bundle branch area pacing has emerged as a safe and feasible alternative to conventional pacing. Acute septal injury, septal perforation, and arteriovenous fistula are potential risks of deep septal implants. Contrast drainage through the lesser cardiac veins and subsequent filling of major epicardial vessels may be benign observations noted during forceful hand injection. (Level of Difficulty: Advanced.)
Key Words: cardiac pacemaker, coronary circulation, left ventricle, x-ray fluoroscopy
Abbreviations and Acronyms: CS, coronary sinus; HTN, hypertension; LBBAP, left bundle branch area pacing; LBBP, left bundle branch pacing; LV, left ventricular; LVEF, left ventricular ejection fraction; NS, nonselective; pLVAT, peak left ventricular activation time; TTE, transthoracic echocardiography
Central Illustration

Left bundle branch area pacing (LBBAP) has emerged in recent years as a safe and feasible method of achieving conduction system pacing.1 Large observational studies have demonstrated its utility as an alternative to or bailout for the coronary sinus (CS) lead in patients requiring cardiac resynchronization.2,3 The technique involves deployment of the pacing lead deep within the interventricular septum below the left ventricular (LV) subendocardium to capture the left bundle or its branches.4 The reported complication rate ranges from 1.63% to 14% in different studies.4,5 Intraoperative contrast injection through the delivery sheath is frequently used to verify the depth of implantation. Acute septal myocardial injury, perforation into the LV cavity, atrioventricular block, and injury to septal perforator coronary arteries and veins are potential concerns as the operator embeds the lead deep into the septum.4,6 Most leads need not be repositioned even if there is evidence of septal staining provided the electrical parameters remain stable. We present 2 cases demonstrating drainage of contrast material into the coronary venous system that represents normal physiology in the absence of fistula formation.
Learning Objectives
-
•
To demonstrate contrast drainage through the lesser and greater cardiac venous system as a delineation of normal anatomy in the absence of septal perforation and/or coronary fistula formation.
-
•
To recognize the importance of avoiding forceful hand injection during a diagnostic angiogram or ventriculogram to limit myocardial staining, dissection, or perforation.
Case 1
A 92-year-old woman with a medical history significant for hypertension (HTN), diastolic dysfunction, chronic kidney disease, and paroxysmal atrial fibrillation treated with beta-blockers and amiodarone presented with altered mental status. The patient was afebrile on admission, with a heart rate of 30 beats/min and blood pressure of 118/78 mm Hg. Physical examination was noteworthy for mental confusion and bilateral pedal edema. The electrocardiogram showed sinus rhythm and complete heart block with a junctional escape rhythm. Preliminary laboratory data indicated acute kidney injury (serum creatinine, 1.9 mg/dL from a baseline value of 1.1-1.2 mg/dL) and an elevated N-terminal pro–B-type natriuretic peptide value of 4,586 pg/mL. Transthoracic echocardiography (TTE) showed a normal left ventricular ejection fraction (LVEF) of 50% to 55%, moderate biatrial enlargement, and moderate mitral regurgitation. Interventricular septal thickness was 0.78 cm.
A decision was made to proceed with dual-chamber pacemaker implantation using conduction system pacing targeted by His or LBBAP, which is the preferred practice at our institution (Geisinger Heart Institute, Geisinger Commonwealth School of Medicine, Wilkes-Barre, Pennsylvania, USA). Infra-Hisian block was noted during the procedure, and a stylet-driven left bundle branch pacing (LBBP) lead was implanted. Nonselective left bundle branch pacing (NS LBBP) capture was obtained with a threshold of 0.9 V at 0.4 milliseconds and a stimulation to peak left ventricular activation time (pLVAT) of 60 milliseconds. Unipolar pacing impedance was 721 ohms. Approximately 2 mL of contrast material was injected through the sheath to gauge the depth of implantation. A small area of septal staining was noted next to the lead tip along with flow of contrast material through the coronary venous branches into the body of the CS (Figure 1, Video 1). No residual contrast material was noted on fluoroscopy after sheath removal. Follow-up TTE immediately post-procedure and 3 months after implantation showed an appropriate lead position without evidence of septal perforation or fistula formation. Electrical parameters remained stable on subsequent device checks.
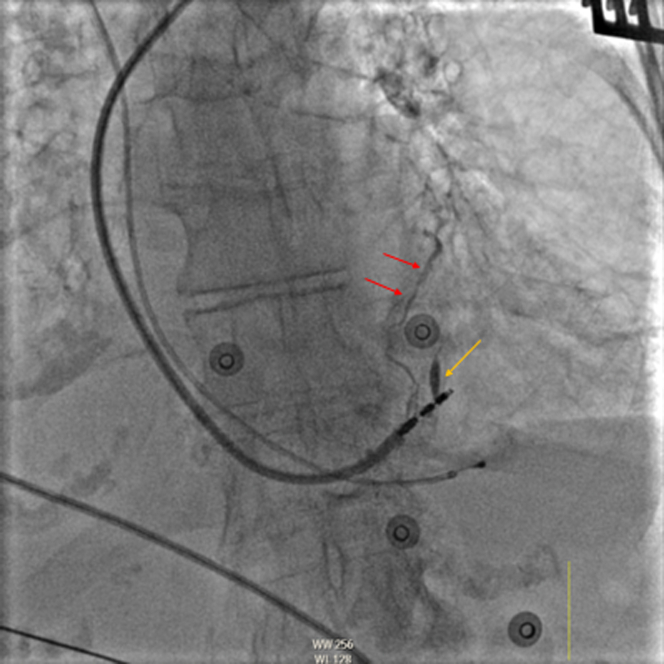
Figure 1.
Case 1: Fluoroscopy of a Stylet-Driven Left Bundle Branch Pacing Lead in the Left Anterior Oblique Projection
Contrast material filling a high anterolateral venous branch through the thebesian veins (red arrows) and tract created by the initial lead position (yellow arrow) is visible.
Case 2
A 75-year-old man presented to the emergency department with worsening shortness of breath. His past medical history was significant for HTN, type 2 diabetes, hyperlipidemia, benign prostatic hypertrophy, a left-sided dual-chamber pacemaker implanted for tachy-brady syndrome that was extracted because of Enterococcus faecalis bacteremia, and endocarditis treated with intravenous antibiotics. Physical examination was unremarkable except for tachycardia. Telemetry showed paroxysmal atrial tachycardia with an average heart rate of 110 to 120 beats/min correlating with symptoms. Antiarrhythmic therapy was limited by bradycardia; therefore, a decision was made to proceed with reimplantation of the pacemaker after reported blood culture results were negative.
A right-sided pacemaker was implanted with LBBAP achieved using a Medtronic SelectSecure lead. The pLVAT was 78 milliseconds, with an NS LBBP capture threshold of 0.8 V at 0.4 milliseconds and impedance of 570 ohms. During contrast material injection to assess lead depth, small anterolateral veins were visualized with subsequent drainage into the CS body (Figure 2, Video 2). There was no residual contrast material retention. Postprocedure TTE showed mild concentric LV hypertrophy, LVEF of 55% to 60%, mild tricuspid regurgitation, and an appropriately placed deep septal lead with no evidence of septal perforation, hematoma formation, or pericardial effusion. The echocardiographic findings, as well as device electrical parameters, remained unchanged at 3-month follow-up.
Figure 2.
Case 2: Fluoroscopic Image in the Left Anterior Oblique Projection After Deep Septal Lead Implantation
Contrast material injection with retrograde filling of the lesser cardiac veins (red arrows) and an epicardial high anterolateral branch vein (yellow arrow) draining subsequently into the body of the coronary sinus (green dashed lines) are shown.
Discussion
Septal injury, perforation, and rarely coronary arteriovenous fistula formation are potential complications of deep septal lead implantation.4,6 Acute septal injury with contrast material retention during injection was reported in 0.65% of cases by Chen et al.5 There is also a published case report of septal coronary artery fistula with a stylet-driven LBBP lead.6 In both instances, the patients were managed conservatively, and there were no long-term sequelae. The representative images from our cases likely show venous drainage through the lesser cardiac venous system (thebesian veins or venae cordis minimae). This system is unique to the myocardium and provides 30% of its venous drainage directly into the chambers, as well as the large epicardial coronary vessels.7 Of the 340 patients retrospectively analyzed, we observed the phenomenon in 9 cases (2.6%). The implantation site was retained in all but 1 case. In Figure 1, contrast material filling of the tract created by the initial lead placement is also noted, which is not an uncommon finding if lead positioning requires multiple attempts. Care should be exercised nonetheless during forceful injection of contrast material to avoid myocardial staining and dissection that may cause ventricular arrhythmia or, rarely, perforation during diagnostic angiograms.8,9 This is the first report, to our knowledge, describing filling of coronary venous branches that are normal anatomical correlates during contrast material injection along the septum.
Conclusions
Visualization of lesser or greater cardiac veins with contrast injection after deep septal lead placement maybe a benign finding and not indicative of fistula formation.
Funding Support and Author Disclosures
Dr Batul has received honorarium from Medtronic. Dr Subzposh has received honorarium from Medtronic. Dr Vijayaraman has received fellowship support from Medtronic; has served as a speaker and researcher for Medtronic; has served as a consultant for Medtronic, Biotronik, and Abbott; and has received honorarium from Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this article.
Appendix
Case 1. Fluoroscopy contrast material injection through an 8.7-F delivery sheath in the left anterior oblique projection.
Case 2. Left anterior oblique projection of contrast material injection through a 7-F delivery catheter showing thebesian veins connecting directly with the right ventricular cavity and draining through the epicardial branches into the coronary sinus.
References
- 1.Huang W., Su L., Wu S., et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1731–1736. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P., Patel N.R., Ravi V., et al. Clinical outcomes of let bundle branch area pacing compared to right ventricular pacing: results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2022;19:3–11. doi: 10.1016/j.hrthm.2021.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Vijayaraman P., Ponnusamy S., Cano O., et al. Left bundle branch area pacing for cardiac resynchronization therapy. Results from the international LBBAP Collaborative Study Group. J Am Coll Cardiol EP. 2021;7:135–147. doi: 10.1016/j.jacep.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Ponnusamy S., Basil W., Vijayaraman P. Electrophysiological characteristics of septal perforation during left bundle branch pacing. Heart Rhythm. 2022;19(5):728–734. doi: 10.1016/j.hrthm.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Chen X., Wei L., Bai J., et al. Procedure-related complications of left bundle branch: a single-center experience. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.645947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pooter J.D., Calle S., Demulier L., Timmermans F., Van Heuverswyn H. Septal coronary artery fistula following left bundle branch area pacing. J Am Coll Cardiol EP. 2020;6(10):1337–1338. doi: 10.1016/j.jacep.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Nordick K., Weber C., Singh P. StatPearls Publishing; 2022. Anatomy, Thorax, Heart Thebesian Veins.http://www.ncbi.nlm.nih.gov/books/NBK541040/ [PubMed] [Google Scholar]
- 8.Mehmet F., Ozeke O., Duman I., Canplot U., Kisacik A.L. Massive myocardial staining and thebesian venous opacification during complicated coronary angiography. Int J Angiol. 2016;25:e19–e20. doi: 10.1055/s-0034-1396930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frizelli J.D., Alkouz M., Sheldone M.W. Left ventricular perforation during ventriculogram using an Optitorque Tiger catheter. J Am Coll Cardiol Intv. 2014;7(12):1456–1457. doi: 10.1016/j.jcin.2014.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case 1. Fluoroscopy contrast material injection through an 8.7-F delivery sheath in the left anterior oblique projection.
Case 2. Left anterior oblique projection of contrast material injection through a 7-F delivery catheter showing thebesian veins connecting directly with the right ventricular cavity and draining through the epicardial branches into the coronary sinus.