Abstract
Infection of leadless pacemakers (LPM) is rare, even in patients at high risk for infections. Only 3 cases of LPM infection have been documented in the literature, all occurring within 1 month of device implantation. We report the first case, to our knowledge, of late-onset LPM infection, developing almost 2 years after implantation. (Level of Difficulty: Beginner.)
Key Words: cardiac implantable electronic device, infection, leadless pacemaker
Abbreviations and Acronyms: ECG, electrocardiogram; LPM, leadless pacemaker; RBB, right bundle branch
Central Illustration
History of Presentation
An 89-year-old woman presented to the emergency department with generalized weakness, chest pain, and shortness of breath for 6 hours. Two days prior, she had been diagnosed with cellulitis of her right forearm and treated with oral cephalexin. Physical examination findings were unremarkable except for the presence of an arteriovenous graft on her right forearm, with faint erythema not involving the graft.
Learning Objectives
-
•
To demonstrate that LPM infection can occur late after device implantation.
-
•
To show that agents that inhibit endothelization, such as leflunomide, may predispose LPM to late infections.
Past Medical History
The patient had a history of type 2 diabetes mellitus; heart failure with preserved ejection fraction; coronary artery disease; moderate aortic stenosis; end-stage renal disease, for which she was on hemodialysis via arteriovenous graft access; and seronegative rheumatoid arthritis, for which she was on daily prednisone and leflunomide. Approximately 1.5 years prior (558 days), the patient underwent atrioventricular node ablation and concomitant implantation of a leadless pacemaker (LPM) (Micra, Medtronic) because of episodes of junctional bradycardia and paroxysmal atrial fibrillation with rapid ventricular response refractory to maximally tolerated drug therapy. Despite a high risk of stroke (CHA2DS2-VASc score: 9), the patient was not anticoagulated because of a history of severe gastrointestinal bleeding.
Differential Diagnosis
The differential diagnosis included acute coronary syndrome, decompensated heart failure, pulmonary embolism, worsening aortic stenosis, pacemaker malfunction, and sepsis.
Investigations
The patient was normotensive (125/57 mm Hg) and afebrile (36.7 °C). Electrocardiogram (ECG) showed a ventricular paced rhythm at 60 beats/min. Troponin was mildly elevated (0.36 ng/mL), similar to prior values. Chest radiography findings were negative for pulmonary edema or infiltrates. Blood count revealed leukocytosis (23,380 leukocytes/μL). Empiric antibiotic therapy was initiated. Blood cultures grew methicillin-sensitive Staphylococcus aureus. Transthoracic echocardiogram showed no changes compared to a study done 1 year prior (left ventricular ejection fraction: 70%; aortic valve area: 1.28 cm2). The LPM was not well visualized on transthoracic echocardiogram because of poor acoustic windows in its region (Video 1). A transesophageal echocardiogram was requested because of the presence of a pacemaker and revealed a vegetation on the mid to distal part of the LPM (Figure 1, Video 2), a vegetation vs Lambl’s excrescence (fibrous strands at valvular coaptation sites) on the aortic valve, and a large thrombus within the left atrial appendage. During her inpatient course, the patient quickly deteriorated with lethargy and neurologic deficits. Brain computed tomography showed acute left cerebral infarcts and cerebritis, concerning for septic emboli.
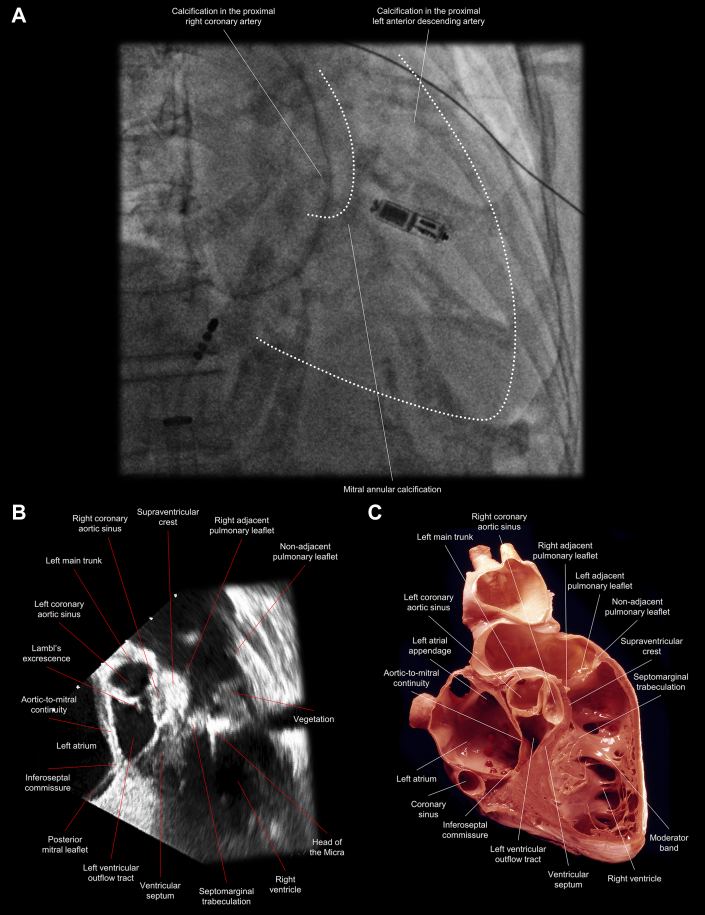
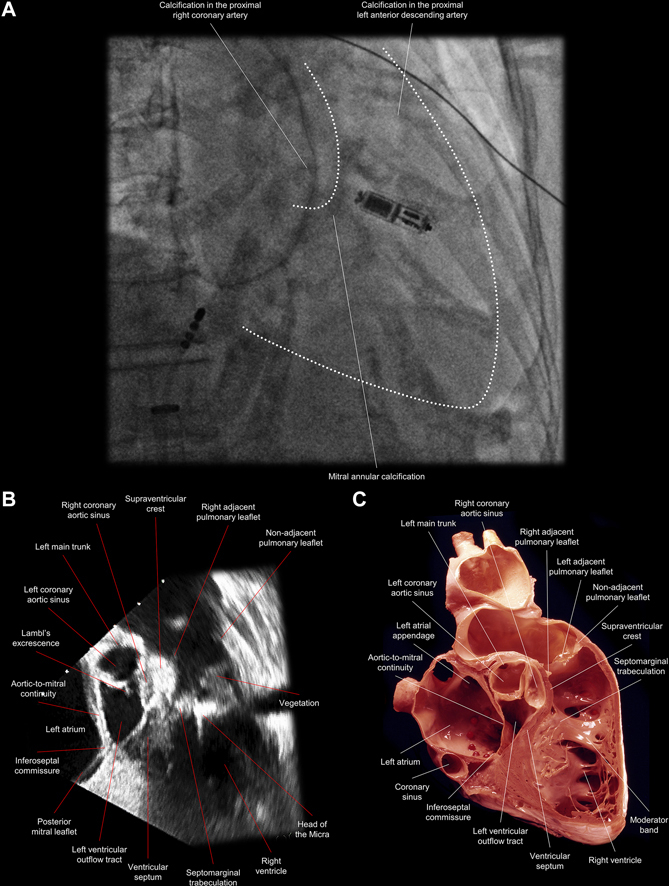
Figure 1.
Comparative Images Showing the Anatomic Location of the Leadless Pacemaker and a Large Vegetation Attached to It
(A) A right anterior oblique fluoroscopic view shows that the leadless pacemaker was implanted along the septomarginal trabeculation, at the base of the moderator band; white dots delineate the right ventricle and pulmonary root. (B) A transesophageal echocardiographic view of the corresponding sectional plane shows a large vegetation (1.4 × 0.8 cm) attached to the mid to distal part of the leadless pacemaker and extending toward the pulmonary valve. (C) An anatomic image shows the corresponding sagittal plane of the right ventricular outflow tract. (Illustration courtesy of UCLA Cardiac Arrhythmia Center, Wallace A. McAlpine MD Collection.)
Management
It was discussed with the patient’s family that the infection would not resolve unless the LPM was extracted. However, given the patient’s advanced age, comorbidities, cerebral infarcts, and extremely frail status, the family declined invasive procedures, so the device was not extracted, and the patient was transitioned to comfort care.
Discussion
We report a case of infection of an LPM 1.5 years after its implantation in an immunocompromised (on prednisone/leflunomide) patient on dialysis. The infection was likely caused by hematogenous bacterial seeding of the device in the setting of bacteremia following a forearm skin infection. To our knowledge, this is the first reported case of late-onset infection in an LPM.
LPMs are known for their resistance to infection. Although transvenous pacemakers have an infection rate ranging from 0.7% to 2.1%, no cases of LPM infection were reported in clinical trials enrolling more than 3,000 patients.1 Studies including patients with high risk for cardiac device infection (eg, diabetes, cancer, end-stage renal disease, bacteremia, endocarditis) also did not report LPM infections.1,2
There are only 3 cases of documented LPM infection reported in the literature, all occurring within 30 days of device implantation. In 2 of these cases, the LPM had been implanted immediately following explantation of an infected transvenous device. In all 3 cases, the LPM was extracted, and infection was then successfully cleared.3, 4, 5
The mechanisms that explain the apparent resistance of LPMs to infection are still not fully understood, but encapsulation (endothelialization) of the LPM probably plays a pivotal role by reducing or eliminating the surface area of the device exposed to the bloodstream, thus preventing pathogens from accessing the device to seed infection.1 Presumably, LPMs are completely endothelialized around 4 months after implantation.6 All 3 previously documented cases of LPM infection occurred early, within 1 month of device implantation, at a time when full endothelialization of the device is not expected.
The infection in our case occurred 558 days after device implantation; therefore, our case is the first reported case of an LPM late infection (“late” defined here as infection more than 1 month after implantation). Our patient did not undergo autopsy, so we cannot confirm whether the device was fully endothelialized, but failure to endothelialize remains the most likely explanation in this case. A primary contributing factor was likely leflunomide use, a pyrimidine synthesis inhibitor agent, which has been shown to disrupt normal endothelial cell proliferation and migration.7
The lack of histologic diagnosis may raise the possibility that the mobile echodensity attached to the LPM was a thrombus (or a secondarily infected thrombus) rather than a vegetation. The ability of echocardiography to distinguish between vegetation and thrombi is limited; however, prior reports of LPM-attached thrombi were thin adherent structures representing various stages of fibrotic encapsulation and endothelization.8 A mass-like echodensity, as was seen in our patient, in the setting of Staphylococcus aureus bacteremia made a vegetation much more likely, also similar to descriptions of the previously reported LPM infections.3,4
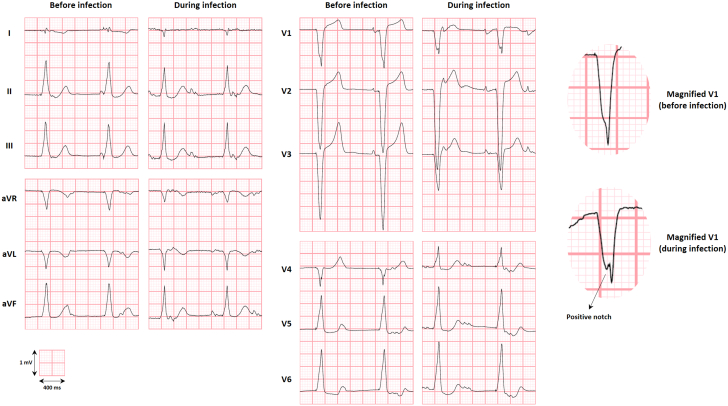
An interesting observation in our case is that, compared with previous ECGs, the patient’s ECG following device infection showed possible right bundle branch (RBB) injury (Figure 2). Considering that the LPM had been implanted adjacent to the moderator band (Figure 1, Videos 2 and 3), which contains the RBB, it is possible that the RBB was damaged because of contiguous infection of the ventricular tissue in contact with the device.
Figure 2.
The 12-Lead Electrocardiogram Before and After Leadless Pacemaker Infection, Suggesting Right Bundle Branch Injury
Although the change is minor, the appearance of a positive notch within the QRS complex of V1, indicative of a delay in right ventricular depolarization, may be caused by an infectious involvement of the right bundle branch in the setting of right ventricular septal pacing near the base of the moderator band. An alternative explanation for the notch would be that it represents a myocardial scar or anisotropic conduction in the setting of chronic or acute hemodynamic shifts, given that the V1 voltage dropped from 14 mV to 10 mV and the P-wave developed a deeper negative deflection suggestive of a P-mitrale pattern. The electrocardiograms were recorded 9 months apart and, for the low- and high-pass filters, were set at 150 Hz and 0.05 Hz, respectively. Of note, the shift in precordial transition (V5 preinfection vs V4 during infection) seems to reflect only changes in the position of the leads, as this shift was not consistent across serial electrocardiograms.
Follow-Up
The patient died 3 days after the transition to palliative care.
Conclusions
Infection of LPMs is rare, and the only 3 documented cases occurred within 1 month of device implantation. We reported the first case of late-onset infection in an LPM, 1.5 years after its implantation.
Funding Support and Author Disclosures
Dr Do has received research funding and speaker fees from Medtronic, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transthoracic Echocardiography Obtained in Parasternal Long Axis With Slight Tilt to Better Visualize the Right Ventricular Outflow Tract and LPM. Acoustic shadowing from the patient’s ribs was present, limiting visualization of the LPM.
Transesophageal Echocardiography, With 82° Counterclockwise Rotation, Showing the Sagittal Plane of the Right Ventricular Outflow Tract. A large vegetation (1.4 × 0.8 cm) is seen attached to the LPM, extending toward the pulmonary valve during systole with the pulsatile blood flow.
Cine Fluoroscopy, Obtained in the Right Anterior Oblique Projection, Showing That the LPM Was Implanted Between the Right Ventricular Inflow and Outflow Tract Along the Septomarginal Trabeculation, at the Basal Part of the Moderator Band
References
- 1.El-Chami M.F., Bonner M., Holbrook R., et al. Leadless pacemakers reduce risk of device-related infection: review of the potential mechanisms. Heart Rhythm. 2020;17(8):1393–1397. doi: 10.1016/j.hrthm.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 2.El-Chami M.F., Soejima K., Piccini J.P., et al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: data from the Micra IDE study. Pacing Clin Electrophysiol. 2019;42(8):1105–1110. doi: 10.1111/pace.13752. [DOI] [PubMed] [Google Scholar]
- 3.Koay A., Khelae S., Wei K.K., Muhammad Z., Mohd Ali R., Omar R. Treating an infected transcatheter pacemaker system via percutaneous extraction. HeartRhythm Case Rep. 2016;2(4):360–362. doi: 10.1016/j.hrcr.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison K., Hesselson A., Ayoub K., Leung S., Gurley J. Retrieval of an infected leadless pacemaker. HeartRhythm Case Rep. 2020;6(11):863–866. doi: 10.1016/j.hrcr.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada A., Shoda M., Tabata H., et al. Simultaneous infection of abandoned leads and newly implanted leadless cardiac pacemaker: why did this occur? J Cardiol Cases. 2021;23(1):35–37. doi: 10.1016/j.jccase.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgquist R., Ljungström E., Koul B., Höijer C.J. Leadless Medtronic Micra pacemaker almost completely endothelialized already after 4 months: first clinical experience from an explanted heart. Eur Heart J. 2016;37(31):2503. doi: 10.1093/eurheartj/ehw137. [DOI] [PubMed] [Google Scholar]
- 7.Waldman W.J., Bickerstaff A., Gordillo G., Orosz K., Knight D.A., Orosz C.G. Inhibition of angiogenesis-related endothelial activity by the experimental immunosuppressive agent leflunomide. Transplantation. 2001;72(9):1578–1582. doi: 10.1097/00007890-200111150-00018. [DOI] [PubMed] [Google Scholar]
- 8.Breeman K.T.N., du Long R., Beurskens N.E.G., et al. Tissues attached to retrieved leadless pacemakers: histopathological evaluation of tissue composition in relation to implantation time and complications. Heart Rhythm. 2021;18(12):2101–2109. doi: 10.1016/j.hrthm.2021.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic Echocardiography Obtained in Parasternal Long Axis With Slight Tilt to Better Visualize the Right Ventricular Outflow Tract and LPM. Acoustic shadowing from the patient’s ribs was present, limiting visualization of the LPM.
Transesophageal Echocardiography, With 82° Counterclockwise Rotation, Showing the Sagittal Plane of the Right Ventricular Outflow Tract. A large vegetation (1.4 × 0.8 cm) is seen attached to the LPM, extending toward the pulmonary valve during systole with the pulsatile blood flow.
Cine Fluoroscopy, Obtained in the Right Anterior Oblique Projection, Showing That the LPM Was Implanted Between the Right Ventricular Inflow and Outflow Tract Along the Septomarginal Trabeculation, at the Basal Part of the Moderator Band