Abstract
The Cardiovascular Disease in Women Committee of the American College of Cardiology convened a working group to develop a consensus regarding the continuing rise of mortality rates in young women aged 35 to 54 years. Heart disease mortality rates in young women continue to increase. Young women have increased mortality secondary to ischemic heart disease (IHD) compared with comparably aged men and similar mortality to that observed among older women. The authors reviewed the published evidence, including observational and mechanistic/translational data, and identified knowledge gaps pertaining to young women. This paper provides clinicians with pragmatic, evidence-based management strategies for young women at risk for IHD. Next-step research opportunities are outlined. This report presents highlights of the working group review and a summary of suggested research directions to advance the IHD field in the next decade.
Keywords: disease, heart, ischemic, mortality, women
Over the past 4 decades, there has been a decrease in heart disease (HD) mortality across the general population, likely related to increases in implementation of national guideline recommendations for mitigating risk.1 Despite improving trends globally, among the general U.S. population, HD mortality rates in women aged 35 to 54 years continue to increase, largely driven by ischemic heart disease (IHD).2 The burden of cardiovascular disease (CVD) risk factors is disproportionately higher in women of ethnic and racial minorities.3 Black women have the highest rates of obesity of any racial group in the United States, recently exceeding 50%, as well as a higher prevalence of modifiable CVD risk factors resulting in higher rates of diabetes.1 Black women also have a persistent, 2-fold increased rate of chronic hypertension during pregnancy compared with White women.3 Compounding the problem, pregnancy-related CVD mortality in the United States is rising compared with other developed nations.4
This document reviews epidemiologic and pathophysiological contributions related to HD in young women. The definition of “young” when considering HD varies across reports; for this document, we refer to women <55 years of age as young. We review the recent evidence, including observational, mechanistic/translational, and randomized controlled trials in order to provide clinicians with pragmatic, evidence-based suggestions on how to increase awareness of HD risk factors and management options for young women with IHD.
ACUTE CORONARY SYNDROMES
The prevalence of acute coronary syndromes is lower among young women than other age groups, but there are concerning trends that merit attention.5 Younger patients hospitalized with acute myocardial infarction (AMI) are increasingly prevalent; the proportion attributable to young patients (aged 35 to 54 years) has increased from 27% to 32% over the past 2 decades, with the greatest increase in young women (21% to 31%).6 Table 1 summarizes ACS and AMI data in young women and men.
TABLE 1.
Acute Coronary Syndromes/AMI in Young Women and Men
| First Author, Year | Study Setting | Prevalence of the Different Etiologies of AMI |
|---|---|---|
|
| ||
| Safdar et al,64 2018 | U.S. VIRGO study AMI patients aged between 18 and 55 y from 103 geographically diverse hospitals in the United States, 24 in Spain, and 3 in Australia |
1,810 women MI-CAD 1,541 (85.1%) MINOCA 201 (11.1%) Coronary artery spasm 10 (0.6%) Coronary dissection 56 (3.1%) MI due to embolization 2 (0.1%) 863 men MI-CAD 833 (96.5%) MINOCA 23 (2.7%) Coronary artery spasm 1 (0.1%) Coronary dissection 5 (0.6%) MI due to embolization 1 (0.1%) |
| Raparelli et al,65 2018 | GENESIS-PRAXY study AMI patients aged between 18 and 55 y from 24 hospitals in Canada, 1 in the United States, and 1 in Switzerland |
327 women MI-CAD 293 (89.6%) MINOCA 34 (10.4%) 671 men MI-CAD 623 (92.8%) MINOCA 48 (7.2%) |
| Smilowitz et al,25 2017 | ACC-NCDR Chest Pain-MI Registry Retrospective inpatient data on consecutive patients with MI at >750 participating hospitals in the United States, patients aged 50–59 y |
38,281 women Age <50 y MI-CAD 13,080 (86%) MINOCA 2,052 (14%) Age 50–59 y MI-CAD 20,498 (89%) MINOCA 2,651 11%) 101,668 men Age <50 y MI-CAD 40,015 (94%) MINOCA 2,732 (6%) Age 50–59 y MI-CAD 61,653 (97%) MINOCA 1,942 (3%) |
Data are from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study, GENESIS-PRAXY (Gender and Sex Determinants of Cardiovascular Disease: From Bench to Beyond Premature Acute Coronary Syndrome) study, and the ACC-NCDR Chest Pain-MI Registry (American College of Cardiology National Cardiovascular Data Chest Pain-Myocardial Infarction Registry). This table focuses on the types of myocardial infarction (MI) and differences in prevalence between men and women.
AMI = acute myocardial infarction; CAD = coronary artery disease; MINOCA = myocardial infarction with nonobstructive coronary arteries.
AMI presentation varies by age and sex. Young women are 50% more likely than similar aged men to present without chest pain when they have ST-segment elevation myocardial infarction (STEMI), with 1 in 5 women perceiving their symptoms as being related to anxiety or stress7 as opposed to AMI. However, it is important to stress that although AMI presentation without chest pain is more frequent in women than men, most women, and particularly most young women, do experience chest pain with AMI. Young women admitted with AMI often have more comorbidities compared with similarly aged men, with a higher prevalence of diabetes, hypertension, and/or chronic kidney disease.6,8 Some risk factors are more potent for young women; for example, smoking is more strongly associated with STEMI incidence among women aged 18 to 49 years vs similarly aged men and older women.9 Young women and men presenting with AMI have different psychosocial profiles, with significantly greater prevalence of depression and stress, poorer physical and mental health status, and lower quality of life among young women.10
Medical and invasive treatment of acute coronary syndromes are underutilized overall in women, but young women with AMI are much less likely to receive guideline-recommended therapies vs young men or even older women. This includes lipid-lowering medications, dual antiplatelet therapy, beta-blockers, and angiotensin-converting enzyme inhibitor/angiotensin receptor blockers.11–13 Women who receive an invasive strategy are less likely to achieve a door-to-balloon time of <90 minutes,14 a quality metric associated with survival in STEMI; although this may be in part due to delays in presentation.15 True sex differences may be even larger than reported, considering that young women are less likely to be diagnosed with and treated for AMI even when cardiac troponin is abnormal in the emergency department.16 Women hospitalized with AMI have consistently been reported to have greater in-hospital mortality rates than men,8,17 with these differences being particularly pronounced in the youngest age groups (eg, an ~2-fold greater odds of mortality in women with STEMI aged <45 years vs only ~30% in those aged >45 years).12
SPONTANEOUS CORONARY ARTERY DISSECTION
Spontaneous coronary artery dissection (SCAD) is an increasingly recognized cause of AMI and sudden cardiac death in young and middle-aged women who are often otherwise healthy and with few or no conventional HD risk factors.18 More than 90% of SCAD events occur in women, and although SCAD has been reported in individuals from late teens to the eighth decade, it is the number 1 cause of myocardial infarction (MI) in women <50 years old and the most frequent cause of pregnancy-associated MI.19 AMI secondary to SCAD complicates 1:16,000 pregnancies in the United States. Pregnancy-associated SCAD tends to be more severe, involving more proximal dissections, and resulting in larger infarctions, worse outcomes, and a high rate of maternal and fetal mortality.18
Early and accurate diagnosis of acute SCAD is critical. Undiagnosed AMI can be deadly, and many young women with SCAD often do not “look the part,” leading to delayed or missed diagnoses. A SCAD etiology is confirmed by coronary angiography showing either an intimal flap or, more frequently, abrupt smooth tapering of the vessel due to intramural hematoma. Intravascular imaging with ultrasound or optical coherence tomography is occasionally necessary to confirm the diagnosis. The etiologies of SCAD are largely unknown, although some may be attributed to systemic arteriopathies such as fibromuscular dysplasia and extracoronary aneurysms or dissections. SCAD patients should receive screening for these conditions by imaging the arteries from brain to pelvis with contrast computed tomography or magnetic resonance angiography. Recurrent SCAD is not uncommon, occurring in up to 30% of patients over 10 years.
Coronary interventions are much more challenging and less successful in the presence of SCAD,18 even in experienced hands. Conservative management now prevails as the recommended approach for patients who are stable. Patients with high-risk anatomy or ongoing ischemia may require revascularization either with percutaneous coronary intervention or coronary artery bypass grafting. Standard post-MI therapy, such as statins, is not effective in preventing subsequent SCAD and therefore are not recommended routinely.18 Hypertension has been associated with both initial and recurrent SCAD; blood pressure control and consideration of beta-blockers as agents of choice are routinely recommended and have been shown to reduce recurrent SCAD.
The magnitude of risk associated with pregnancy after SCAD is uncertain, and pregnancy is generally not advised. Post-SCAD pregnancies should be managed by a multidisciplinary cardio-obstetrics team. Similarly, although there is no clear link to increased SCAD risk with combined hormonal-based contraception and postmenopausal therapy, alternative agents are preferred. For contraception and the menorrhagia that often accompanies the use of dual antiplatelet therapy, levonorgestrel-releasing intrauterine devices may be a good option.18 The high burden of psychological stress and frequent post-SCAD chest pain confer a strong recommendation for cardiac rehabilitation in this population.18
IMPLICATIONS OF IHD IN PREGNANCY
As maternal age continues to increase in the United States with an associated rise in cardiovascular (CV) comorbidities, the need for prepregnancy CV assessment becomes very important. Recent guidelines and consensus statements provide insight into assessment of women with pre-existing CVD who are considering pregnancy. The CARPREG II (Cardiac Disease in Pregnancy Study) risk prediction score20 includes coronary artery disease (CAD) as a risk factor for maternal complications. European guidelines for management of CVD in pregnancy indicate maternal and fetal risks for women with pre-existing CAD, but pregnancy may be considered in the absence of residual ischemia and clinical signs of left ventricular dysfunction.21 Medications for the treatment of IHD in pregnancy are similar to non-pregnancy.22 Beta-blockers and low-dose aspirin can be continued throughout pregnancy. P2Y12 inhibitors need to be held 7 days before regional anesthesia. Statins may be considered for the highest-risk patients with known CAD because the U.S. Food and Drug Administration has recently recommended removing the contraindication of statins during pregnancy.22 In the ROPAC (Registry of Pregnancy and Cardiac Disease) registry, only 1.6% of women had IHD diagnosis, demonstrating the need for future studies in this population.23 In addition, the development of adverse pregnancy outcomes, such as preeclampsia, are associated with increased risk of IHD later in life.24
MYOCARDIAL INFARCTION WITH NO OBSTRUCTIVE CORONARY ARTERIES
The clinical definition of myocardial infarction with nonobstructive coronary arteries (MINOCA) requires the presence of universal AMI criteria in the setting of nonobstructive CAD (defined as ≤50% stenosis), with no overt cause such as pulmonary embolism.6,25 About 5% to 15% of AMI patients have MINOCA.26 The degree of coronary atherosclerosis may range from none to mild–moderate.6 MINOCA is more prevalent in women (40%–60%) than MI with obstructive CAD (MI-CAD, ~25%) and occurs at younger ages than MI-CAD. Approximately one-half of MINOCA patients are aged <60 years at the time of MI, and ~25% present at <50 years.1 Outcomes among MINOCA patients are better than in MI-CAD,27 but MINOCA may present as a fatal event.28 Recurrent MI occurs in ~7% of MINOCA patients over 4 years, but only about one-half of recurrent MIs present as MINOCA.29 MINOCA patients are less likely to receive secondary prevention medication prescriptions at hospital discharge vs MI-CAD patients, with marked variability in prescribing across hospitals.27,30 Interestingly, post-MI angina occurs in ~25% at 12 months, as high as patients with MI-CAD. Furthermore, there is an ~40% increase in all-cause mortality vs patients with stable angina who have nonobstructive CAD.31 MINOCA patients are not revascularization candidates, so new strategies are needed to improve their quality of life and clinical outcomes.32
MINOCA has multiple causes, including mild-to-moderate atherosclerosis with positive remodeling of the coronary artery, coronary artery spasm, plaque microrupture and/or erosion, coronary embolism, and microthrombosis, among others26 (Central Illustration). Intracoronary imaging and cardiac magnetic resonance imaging are recommended for etiologic diagnosis. However, until randomized trial evidence becomes available, the SWEDEHEART (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) registry data33 indicate that women with MINOCA are likely to benefit from statins and angiotensin-converting enzyme inhibitor/angiotensin receptor blockers, but not dual antiplatelet therapy.
CENTRAL ILLUSTRATION. Clinical Presentations of Underlying Etiologies Contributing to Myocardial Infarction With Nonobstructive Coronary Arteries.
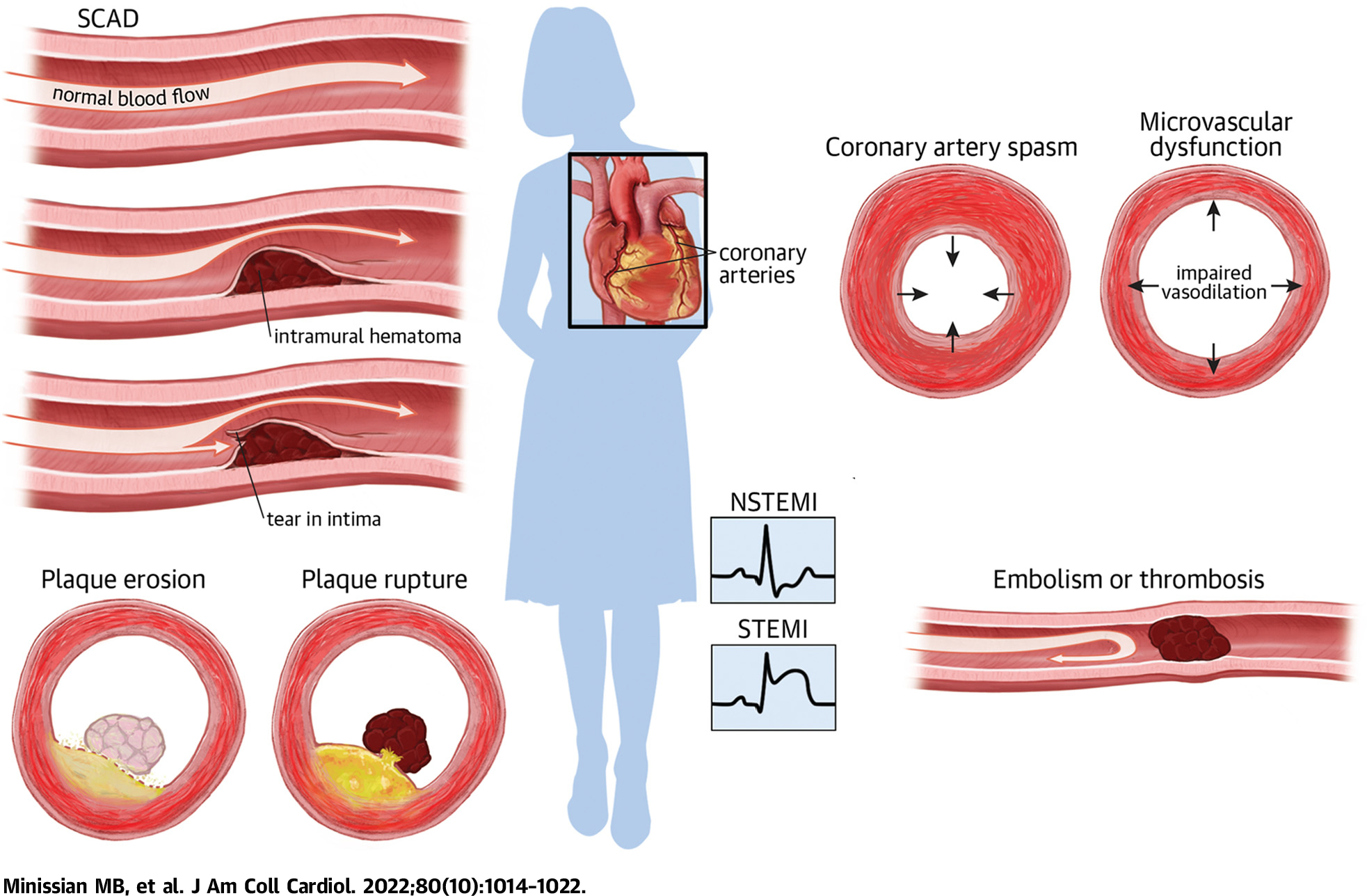
A summary of visuals to highlight etiologies of ischemic heart disease. NSTEMI = non–ST-segment elevation myocardial infarction; SCAD = spontaneous coronary artery dissection; STEMI = ST-segment elevation myocardial infarction.
CORONARY ARTERY SPASM
Coronary spasm is a cause of MI in young patients (both women and men) but is often challenging to diagnose because it may not be apparent at initial angiography and may present as MINOCA. Patients with coronary spasm tend to be younger and male, and have more antecedent angina than patients with obstructive CAD, which may be atypical in character.34,35 It typically presents as rest angina occurring at night and early morning hours. Risk factors include smoking, cocaine, and methamphetamine use,36,37 as well as certain chemotherapy agents such as 5-fluorouracil and paclitaxel.38,39 Young women (<50 years) with coronary spasm have worse long-term prognosis than older women, which may reflect the higher smoking prevalence in young women.40 Mechanisms of coronary spasm include vascular smooth muscle cell hyperreactivity and endothelial dysfunction, with superimposed vasoconstrictor stimuli due to sympathetic activity, allergic reactions, hyperventilation-induced alkalosis, or platelet activation.41
Diagnostic criteria for vasospastic angina include: 1) nitrate-responsive angina, with either 2) transient ischemic electrocardiographic changes or 3) evidence of coronary artery spasm as defined as a transient >90% coronary artery constriction with symptoms and ischemic electrocardiographic changes either occurring spontaneously or in response to a provocative stimulus.42 One-quarter to two-thirds of patients undergoing coronary angiography for MINOCA had evidence of vasospasm during intracoronary acetylcholine provocation,35,43 and the presence of provoked coronary spasm predicts major adverse cardiac events (MACE) following AMI.44 Coronary microvascular spasm is another potential cause of MI.45 Severe myocardial ischemia caused by coronary spasm can lead to life-threatening arrhythmias in 5% to 10% of patients, with sudden cardiac death resulting from either bradyarrhythmias or ventricular tachyarrhythmias.46 Prognosis of coronary artery spasm is not benign, because coronary MACE-free survival rates in patients with vasospastic angina are approximate 80% at 5 years for Caucasian patients and 90% for Japanese patients.1 The JCSA (Japanese Coronary Spasm Association) Risk Score can be used to predict the risk of MACE.38 Effective vasodilator therapy (calcium-channel blockers, nitrates) and avoidance of vasoconstrictor stimuli should be initiated promptly to avert the occurrence of MACE in these patients.47
CORONARY MICROVASCULAR DYSFUNCTION
Coronary microvascular dysfunction (CMD) (defined as impaired coronary flow reserve [CFR] and/or high index of microvascular resistance) and/or endothelial dysfunction is seen in at least 50% of women with signs and symptoms of ischemia with no obstructive coronary arteries.48,49 CFR may be measured invasively in the catheterization laboratory48 or by using noninvasive modalities such as cardiac magnetic resonance imaging, positron emission tomography, or transthoracic echo Doppler,50 or more recently, by coronary computed tomographic angiography.51 Reduced CFR has been linked to CV events, and therefore, the clinician must guard against inappropriate reassurance of women without obstructive CAD on angiography.52,53
An invasive diagnostic approach followed by medical therapy for CMD, which included risk factor modification, angina relief with beta-blockers, as well as aspirin and high-dose statins for concomitant nonobstructive CAD, has been shown to be safe and to improve angina symptoms in a small, randomized trial.54 Quinapril reduced angina frequency and improved CFR in a small, randomized trial.55 Although smaller (n = 20–58, mostly women) trials showed that ranolazine might improve angina in patients with microvascular dysfunction,56,57 a larger randomized trial did not replicate this benefit.58
STRATEGIES TO IMPROVE AWARENESS AND OUTCOMES OF IHD IN YOUNG WOMEN
A recent nationwide survey demonstrated a marked decline in awareness of HD as the leading cause of death among women, with the largest declines among young women and women of racial and ethnic minorities.59 Significant socioeconomic disparities in IHD risk factors and outcomes exist among women.1 Major contributing factors include reduced access to care, low-income level and social support, language and cultural barriers, and lack of guidance from current research. Policies are needed to provide affordable health insurance and consistent access to care and primary prevention across a woman’s life-span. The use of new technology and digital medicine in raising awareness, prevention, and treatment is critical in this younger population.60,61 Social media and digital tools utilizing remote monitoring62 can be used to promote health knowledge and improve heart health behaviors. Telemedicine can be used to engage women in health care who are often juggling multiple responsibilities.60
Strategies to improve IHD outcomes include creating education curriculums for health care providers on evidence-based guidelines for primary and secondary prevention and management of IHD in young women. In addition, bias training for providers to address structural racism and implementation of standardized processes of care and reporting of clinical and institutional data by sex and race/ethnicity to assess adherence to evidence-based guidelines and appropriateness of care. Lastly, continued efforts to diversify the cardiology workforce would provide benefit to all women.
KNOWLEDGE GAPS AND RESEARCH OPPORTUNITIES
There are many important knowledge gaps in the optimal strategies for IHD prevention and treatment in young adults, particularly women, given that they are largely underrepresented in most CVD trials. These knowledge gaps are further widened by the social and technological transformations differentiating contemporary women from prior research cohorts of young adults.60 Research is needed to understand the effect of lifestyle changes that promote healthy habits including differences that may exist among racial/ethnic minorities. Implementation science is needed to determine the best tools to increase engagement of young women in the health care system and how technology and digital medicine are best integrated. Clinical trials also need to focus on the inclusion of young women in both behavioral and pharmacologic interventions. Further research is also needed to understand IHD that are more common in women, such as SCAD, ischemia with no obstructive coronary arteries, MINOCA, CMD, that lack clinical guidelines due to a dearth of research.
CONCLUSIONS
Despite improving trends globally among the general U.S. population, IHD mortality rates in women aged 35 to 54 years continue to stagnate or increase,1 a trend that is particularly prominent among African American women.63 Differences in AMI mortality rates between sexes remain, with women receiving less guideline-recommended pharmacotherapy and invasive coronary management. AMI mortality rates in women are potentially modifiable through improved concordance with guideline-indicated care. Although many women will experience obstructive CAD as the etiology for their IHD, women have higher rates of MINOCA, SCAD, coronary vasospasm, and CMD compared with men. Women with pre-existing CAD considering pregnancy need to be appropriately assessed and advised regarding both maternal and neonatal risk before conception.
Future directions (Table 2) should be focused on: 1) utilizing technology to engage and educate young women on optimal lifestyle strategies and the impact of these strategies on CV health; 2) using cutting-edge digital approaches to tailor effective CV management to reduce IHD; 3) educating health care providers to implement established evidence-based guidelines in young women for primary and secondary prevention of IHD and bias training; 4) standardizing processes of care to overcome biases; 5) reporting of clinical and institutional data by sex and race/ethnicity to assess adherence to evidence-based guidelines and appropriateness of care; and 6) increasing participation of young women in clinical research to create guidelines driven by sex-specific data, including research on IHD pathophysiology in women.
TABLE 2.
Actionable Future Directions
| 1 | Utilize technology to engage and educate young women on optimal lifestyle strategies and the impact of these strategies on CV health |
| 2 | Use of cutting-edge digital approaches to tailor effective management to reduce IHD |
| 3 | Educating health care providers to implement established evidence-based guidelines in young women for primary and secondary prevention of IHD and bias training |
| 4 | Standardizing processes of care to overcome biases |
| 5 | Reporting of clinical and institutional data by sex and race/ethnicity to assess adherence to evidenced-based guidelines and appropriateness of care |
| 6 | Increase participation of young women in clinical research to create guidelines driven by sex-specific data |
This table highlights future directions for clinician scientists to investigate in relation to gaps in knowledge around young women and cardiovascular disease.
CV = cardiovascular; IHD = ischemic heart disease.
HIGHLIGHTS.
Ischemic heart disease mortality rates for women age 35 to 54 years are not decreasing.
Although the most frequent cause of AMI in these young women is obstructive CAD, up to 15% have unobstructed coronary arteries.
Women with AMI less often receive guideline-recommended pharmacotherapy and invasive management than men, contributing to higher mortality.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This study was funded by National Institute for Nursing Research grants 1K99NR018679-01 and F31NR015725, UCLA Clinical and Translational Science Institutes grants UL1TR000124 and UL1TR001881-01, and the Preventive Cardiovascular Nurses Association grant #5362 through the American Nurses Foundation (Dr Minissian). Additional support was provided by the Cedars-Sinai Department of Obstetrics and Gynecology, the Cedars-Sinai Brawerman Nursing Institute, Department of Nursing Research, and the Cedars-Sinai Barbra Streisand Women’s Heart Center, Beta Chi Chapter, and Cedars-Sinai Precision Health Institute grant #42254 (Dr Minissian). Dr Wei is supported by National Institutes of Health (NIH) grants K23HL125941 and R01HL146158. Dr Bairey Merz is supported by NIH grants R01HL124649 and U54 AG065141, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center Los Angeles, the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California. Dr Reynolds is supported by American Heart Association grant SFRN 20-A0-00-1005066. Dr Quesada is supported by NIH grants T32HL116273 and K23HL151867. Dr Minissian has received honoraria from the National Lipid Association, North American Menopause Society, MJH Life Sciences, Vox Media, Medtelligence, Minneapolis Heart Institute, Primed, Good Samaritan Hospital Los Angeles, California, Cardiometabolic Health Congress, American Heart Association, Preventive Cardiovascular Nurses Association, and the American College of Cardiology; has been a consultant for and served on a medical advisory board for Amgen; and has served as a co-chair for CME for the North American Center for Continuing Medical Education honorarium. Dr Wei has received institutional fees for serving on an advisory board for Abbott Vascular. Dr Bairey Merz has received consulting fees from Abbott Diagnostics, Caladrius, and Sanofi Vascular; and receives consulting fees, is on the board of directors and owns stock in iRhythm. Dr Volgman has served as a consultant and on advisory boards for Merck, Bristol Myers Squibb Foundation, Novo Nordisk, and Janssen; has received research funding from Novartis; and hold stocks in Apple, Inc. Dr Elgendy has received research grants from Caladrius Biosciences, Inc, unrelated to this paper. Dr Mamas has been a speaker for Daiichi-Sankyo. Dr Reynolds has received in-kind donations for research from Abbott Vascular, Siemens, and BioTelemetry, Inc. Dr Piazza has received research grants from BMS/Pfizer, Janssen, Alexion, Bayer, Amgen, and BSC; and has an advisory role in BSC, Amgen, BCRI, PERC, Syntactx, BMS, and Janssen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AMI
acute myocardial infarction
- CAD
coronary artery disease
- CFR
coronary flow reserve
- CMD
coronary microvascular dysfunction
- CV
cardiovascular
- CVD
cardiovascular disease
- HD
heart disease
- IHD
ischemic heart disease
- MACE
major adverse cardiac events
- MI
myocardial infarction
- MINOCA
myocardial infarction with nonobstructive coronary arteries
- SCAD
spontaneous coronary artery dissection
- STEMI
ST-segment elevation myocardial infarction
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan AS, Schieb L, Casper M. Historic and recent trends in county-level coronary heart disease death rates by race, gender, and age group, United States, 1979–2017. PLoS One. 2020;15:e0235839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananth CV, Duzyj CM, Yadava S, Schwebel M, Tita ATN, Joseph KS. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension. 2019;74:1089–1095. [DOI] [PubMed] [Google Scholar]
- 4.Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 6.Arora S, Stouffer GA, Kucharska-Newton AM, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction: the ARIC Community Surveillance Study. Circulation. 2019;139(8):1047–1056. 10.1161/CIRCULATIONAHA.118.037137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichtman JH, Leifheit EC, Safdar B, et al. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients). Circulation. 2018;137:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Wang Y, Spertus JA, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer J, Lloyd A, Steele L, et al. Differential risk of ST-segment elevation myocardial infarction in male and female smokers. J Am Coll Cardiol. 2019;73:3259–3266. [DOI] [PubMed] [Google Scholar]
- 10.Bucholz EM, Strait KM, Dreyer RP, et al. Editor’s choice-sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6:610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smolina K, Ball L, Humphries KH, Khan N, Morgan SG. Sex disparities in post-acute myocardial infarction pharmacologic treatment initiation and adherence: problem for young women. Circ Cardiovasc Qual Outcomes. 2015;8:586–592. [DOI] [PubMed] [Google Scholar]
- 12.Bangalore S, Fonarow GC, Peterson ED, et al. Age and gender differences in quality of care and outcomes for patients with ST-segment elevation myocardial infarction. Am J Med. 2012;125:1000–1009. [DOI] [PubMed] [Google Scholar]
- 13.Koopman C, Vaartjes I, Heintjes EM, et al. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur Heart J. 2013;34:3198–3205. [DOI] [PubMed] [Google Scholar]
- 14.Stehli J, Martin C, Brennan A, Dinh DT, Lefkovits J, Zaman S. Sex differences persist in time to presentation, revascularization, and mortality in myocardial infarction treated with percutaneous coronary intervention. J Am Heart Assoc. 2019;8:e012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugiardini R, Ricci B, Cenko E, et al. Delayed care and mortality among women and men with myocardial infarction. J Am Heart Assoc. 2017;6(8):e005968. 10.1161/JAHA.117.005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphries KH, Lee MK, Izadnegahdar M, et al. Sex differences in diagnoses, treatment, and outcomes for emergency department patients with chest pain and elevated cardiac troponin. Acad Emerg Med. 2018;25:413–424. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson C, Bebb O, Dondo TB, et al. Sex differences in quality indicator attainment for myocardial infarction: a nationwide cohort study. Heart. 2019;105:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes SN, Kim ESH, Saw J, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–1702. [DOI] [PubMed] [Google Scholar]
- 20.Silversides CK, Grewal J, Mason J, et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71:2419–2430. [DOI] [PubMed] [Google Scholar]
- 21.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 22.Park K, Bairey Merz CN, Bello NA, et al. Management of women with acquired cardiovascular disease from pre-conception through pregnancy and postpartum: JACC Focus Seminar 3/5. J Am Coll Cardiol. 2021;77:1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J. 2019;40:3848–3855. [DOI] [PubMed] [Google Scholar]
- 24.Sondergaard MM, Hlatky MA, Stefanick ML, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020;5:1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smilowitz NR, Mahajan AM, Roe MT, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes. 2017;10:e003443. [DOI] [PubMed] [Google Scholar]
- 26.Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 27.Smilowitz NR, Dubner R, Hellkamp AS, et al. Variability of discharge medical therapy for secondary prevention among patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) in the United States. PLoS One. 2021;16(8):e0255462. 10.1371/journal.pone.0255462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J. 2011;161:681–688. [DOI] [PubMed] [Google Scholar]
- 29.Nordenskjold AM, Lagerqvist B, Baron T, et al. Reinfarction in patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): coronary findings and prognosis. Am J Med. 2019;132:335–346. [DOI] [PubMed] [Google Scholar]
- 30.Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3:632–641. [DOI] [PubMed] [Google Scholar]
- 31.Kissel CK, Chen G, Southern DA, Galbraith PD, Anderson TJ. Impact of clinical presentation and presence of coronary sclerosis on long-term outcome of patients with non-obstructive coronary artery disease. BMC Cardiovasc Disord. 2018;18:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475–481. [DOI] [PubMed] [Google Scholar]
- 33.Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 34.Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999;33:1442–1452. [DOI] [PubMed] [Google Scholar]
- 35.Choo EH, Chang K, Lee KY, et al. Prognosis and predictors of mortality in patients suffering myocardial infarction with non-obstructive coronary arteries. J Am Heart Assoc. 2019;8(14):e011990. 10.1161/JAHA.119.011990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havakuk O, Rezkalla SH, Kloner RA. The cardiovascular effects of cocaine. J Am Coll Cardiol. 2017;70:101–113. [DOI] [PubMed] [Google Scholar]
- 37.Paratz ED, Cunningham NJ, MacIsaac AI. The cardiac complications of methamphetamines. Heart Lung Circ. 2016;25:325–332. [DOI] [PubMed] [Google Scholar]
- 38.Beltrame JF, Crea F, Kaski JC, et al. The who, what, why, when, how and where of vasospastic angina. Circ J. 2016;80:289–298. [DOI] [PubMed] [Google Scholar]
- 39.Herrmann J, Yang EH, Iliescu CA, et al. Vascular toxicities of cancer therapies: the old and the new–an evolving avenue. Circulation. 2016;133:1272–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawana A, Takahashi J, Takagi Y, et al. Gender differences in the clinical characteristics and outcomes of patients with vasospastic angina–a report from the Japanese Coronary Spasm Association. Circ J. 2013;77:1267–1274. [DOI] [PubMed] [Google Scholar]
- 41.Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. [DOI] [PubMed] [Google Scholar]
- 42.Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 43.Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) study. J Am Coll Cardiol. 2008;52:523–527. [DOI] [PubMed] [Google Scholar]
- 44.Wakabayashi K, Suzuki H, Honda Y, et al. Provoked coronary spasm predicts adverse outcome in patients with acute myocardial infarction: a novel predictor of prognosis after acute myocardial infarction. J Am Coll Cardiol. 2008;52:518–522. [DOI] [PubMed] [Google Scholar]
- 45.Pirozzolo G, Seitz A, Athanasiadis A, Bekeredjian R, Sechtem U, Ong P. Microvascular spasm in non-ST-segment elevation myocardial infarction without culprit lesion (MINOCA). Clin Res Cardiol. 2020;109(2):246–254. 10.1007/s00392-019-01507-w [DOI] [PubMed] [Google Scholar]
- 46.Romagnoli E, Lanza GA. Acute myocardial infarction with normal coronary arteries: role of coronary artery spasm and arrhythmic complications. Int J Cardiol. 2007;117:3–5. [DOI] [PubMed] [Google Scholar]
- 47.Yasue H, Takizawa A, Nagao M, et al. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988;78:1–9. [DOI] [PubMed] [Google Scholar]
- 48.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol Intv. 2012;5:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford TJ, Yii E, Sidik N, et al. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv. 2019;12:e008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serruys PW, Hara H, Garg S, et al. Coronary computed tomographic angiography for complete assessment of coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:713–736. [DOI] [PubMed] [Google Scholar]
- 52.AlBadri A, Bairey Merz CN, Johnson BD, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 55.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rayner-Hartley E, Parvand M, Humphries KH, Starovoytov A, Park JE, Sedlak T. Ranolazine for symptomatic management of microvascular angina. Am J Ther. 2020;27:e151–e158. [DOI] [PubMed] [Google Scholar]
- 57.Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. J Am Coll Cardiol Img. 2011;4:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cushman M, Shay CM, Howard VJ, et al. Ten-year differences in women’s awareness related to coronary heart disease: results of the 2019 American Heart Association National Survey: a special report from the American Heart Association. Circulation. 2021;143:e239–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gooding HC, Gidding SS, Moran AE, et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: report from a National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc. 2020;9:e016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel J, Pallazola VA, Dudum R, et al. Assessment of coronary artery calcium scoring to guide statin therapy allocation according to risk-enhancing factors: the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol. 2021;6(10):1161–1170. 10.1001/jamacardio.2021.2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alshurafa N, Sideris C, Pourhomayoun M, Kalantarian H, Sarrafzadeh M, Eastwood JA. Remote health monitoring outcome success prediction using baseline and first month intervention data. IEEE J Biomed Health Inform. 2017;21:507–514. [DOI] [PubMed] [Google Scholar]
- 63.Smilowitz NR, Maduro GA Jr, Lobach IV, Chen Y, Reynolds HR. Adverse trends in ischemic heart disease mortality among young New Yorkers, particularly young black women. PLoS One. 2016;11:e0149015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Safdar B, Spatz ES, Dreyer RP, et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc. 2018;7(13):e009174. 10.1161/JAHA.118.009174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raparelli V, Elharram M, Shimony A, Eisenberg MJ, Cheema AN, Pilote L. Myocardial infarction with no obstructive coronary artery disease: angiographic and clinical insights in patients with premature presentation. Can J Cardiol. 2018;34:468–476. [DOI] [PubMed] [Google Scholar]


