Cardiovascular disease (CVD) continues to be a leading cause of morbidity and mortality in women who have been underdiagnosed, undertreated, and under researched concerning prevention, diagnosis, and treatment of cardiac conditions that predominantly affect them.1 Notably, declines in CVD death rates have stalled in midlife women compared with those in men,1 suggesting sex-specific approaches might be needed. Ischemia with no obstructive coronary artery disease (INOCA) might affect up to 62% of women who undergo coronary angiography for suspected angina, with a higher prevalence in midlife women aged 45–65 years.2 The underlying pathophysiology includes coronary microvascular dysfunction (CMD), coronary endothelial dysfunction, and/or epicardial vasospasm.2 Myocardial infarction (MI) with no obstructive coronary artery disease (MINOCA), defined as evidence of clinical MI and absence of obstructive coronary artery disease (CAD; < 50% lesion), accounts for 6% of MI3 and is more frequently diagnosed in women. Common underlying causes of MINOCA include underlying plaque disruption, epicardial coronary vasospasm, occult microthrombi, or spontaneous coronary artery dissection.2 Although historically overlooked, INOCA and MINOCA are associated with adverse outcomes,2 and recent literature has contributed to increasing recognition,4 consensus on nomenclature,5,6 as well as potentially improving diagnostic workup2 and therapy.2
Because women represent most of the patients diagnosed with INOCA and MINOCA, clinical expertise in North America regarding diagnosis, treatment, and follow-up of these clinical entities has been centred in specialized women’s heart centres (WHCs), with the concept to improve patient outcomes, as well address knowledge gaps. In this issue of the Canadian Journal of Cardiology, Parvand et al. report on 1-year outcomes, including chest pain frequency, quality of life (QoL), depression and anxiety symptoms, and cardiovascular outcomes at the first such centre in Canada, established in Vancouver, British Columbia.7 A total of 154 women with no obstructive CAD on coronary angiography (n = 112 with INOCA and 42 with MINOCA) were enrolled. Overall, 71.4% of women with INOCA received a new probable or definite diagnosis after entry into the WHC, with the most common being probable CMD (60.7%), and 8.0% with definitive CMD. In the MINOCA cohort, 59.9% of women had a new diagnosis, with the most common being probable coronary vasospasm (54.8%) and 4.8% with definite vasospasm. Diagnostic testing included cardiac magnetic resonance imaging in 21.4% of INOCA and 52.4% of MINOCA patients, and invasive coronary reactivity testing in 13% of INOCA and 7% of MINOCA patients. Evaluation at the WHC also led to therapy modification2 resulting in improved cardiovascular risk factor control including blood pressure, total cholesterol, and low-density lipoprotein levels at 1 year compared with baseline. Importantly, the group experienced significantly improved mean angina frequency scores, QoL, and mental health.
We congratulate Dr Parvand and colleagues on this important study as one of the first in Canada to showcase the improvement in outcomes for women with INOCA or MINOCA through a specialized WHC. To date, there are now dedicated women’s heart programs and centres including the Barbra Streisand WHC at the Smidt Heart Institute, Cedars-Sinai Medical Center, the Emory WHC in Atlanta, Georgia, and notably four WHCs in Canada (Vancouver, Ottawa, Montreal, and Halifax).8 These WHCs and programs continue to increase awareness, improve education for patients and health care providers, and facilitate accessibility to specific diagnosis, risk stratification, and management. Furthermore, as illustrated by the authors in their study, the multidisciplinary foundation of WHCs foster collaborations across the various disciplines that provide care for women, bridging the continuity between advocacy, research, and clinical expertise.8 With the growing momentum from the past 3 decades, specialized WHCs are increasingly recognized as a long-term, sustainable model to address ongoing disparities facing women with cardiovascular risk factors and disease.2
Although encountered in the clinical setting for decades, the growing recognition of INOCA and MINOCA has only recently accelerated within the past 5–10 years when formalized names and diagnostic criteria for these heterogenous conditions emerged. A formal, updated definition for the broadly labelled term MINOCA has recently been published9 and from our search there are estimated nearly 400 publications related to this field. INOCA was first coined in 2017 by Bairey Merz et al. and has since increased to > 100 related publications in the past 6 years.2 However, there remains ongoing confusion with respect to uniform terminology to describe these patients, and a standardized diagnostic criteria for INOCA has not been unequivocally established. From a pragmatic perspective, symptom-related and anatomical terminology such as “ANOCA” (angina with nonobstructive CAD), “NOCAD” (nonobstructive CAD), INOCA, or functionally defined terminologies such as CMD, and coronary vasomotor disorders, which includes coronary vasospasm, likely generally all refer to the similar entities in the clinical setting.
The diagnostic challenge for INOCA and MINOCA is further reflected in the latest version of the International Classification of Diseases for Mortality and Morbidity Statistics, 11th Revision,10 which includes the following terms: BA85 (coronary vasospastic disease), BA86 (coronary microvascular disease), BA8Z (diseases of coronary artery, unspecified), BA41.Z (acute MI, unspecified), BA82 (coronary artery dissection), and BC43.5 (stress-induced cardiomyopathy), with notable lack of codes for MI due to plaque erosion. These codes underscore the important recognition that INOCA and MINOCA are working/broad diagnoses for more specific phenotype/endotype with potentially different diagnostic pathways. To facilitate future research and inform practice-changing health care policies in these areas, formal diagnostic International Classification of Diseases codes specific for INOCA and MINOCA should be considered for ease of recognition and to facilitate management.
Despite this progress, many important knowledge gaps remain. Although identification and treatment of cardiovascular risk factors is the cornerstone of INOCA management, whether intensive medical therapy alters outcomes in patients with INOCA remains to be seen. We await the results of the Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD (WARRIOR; NCT03417388), a large, multicentre clinical trial of 4422 participants randomized to optimal medical management strategy (high intensity statin, angiotensin converting enzyme inhibitor [ACEI]/angiotensin receptor blocker [ARB], and low-dose aspirin) compared with usual care, and the Randomized Evaluation of Beta Blocker and ACEI/ARB Treatment in MINOCA Patients (MINOCA-BAT) trial (NCT03686696) of 3500 patients randomized into 4 groups (eg, ACEI/ARB and β-blocker, β-blocker only, ACEI/ARB only, and neither ACEI/ARB nor β-blocker), and followed for a mean of 4 years. In patients with predominantly smooth muscle hyperactivity and vasospasm, calcium channel blockers and nitrates remain the standard treatment; however, in those with chest pain due to CMD (ie, low coronary flow reserve and/or high index of microcirculatory resistance), there is equipoise regarding firstline management.
Historical “cardiac syndrome x” literature that lacked endotype characterization suggested that β-blockers were superior to calcium channel blockers for the outcomes of exercise tolerance and angina. Newer generation β-blockers such as carvedilol and nebivolol appear to have CMD benefits,11 but these have not been compared with calcium channel blockers. Of note, the recent Efficacy of Diltiazem to Improve Coronary Microvascular Dysfunction (EDIT-CMD) trial, which evaluated diltiazem on the outcomes of coronary function testing, angina, and QoL, failed to show improvement these in patients with angiographically defined general CMD,12 although the small subgroup of patients with a vasospastic component improved, suggesting it is premature to completely rule out calcium channel blockers in angina management for INOCA.
Cardiometabolic diseases such as obesity and insulin resistance are associated with heart failure with preserved ejection fraction (HFpEF), and near-term follow-up of women with suspected INOCA showed that predominantly HFpEF hospitalization is the most frequent major adverse cardiac event.13 Thus it is logical to hypothesize that CMD might represent a trajectory to HFpEF,14 a condition also predominant in women, a hypothesis which is being actively investigated (NCT03876223). Epicardial adipose tissue, underlying vascular inflammation, and abnormal vasoregulation are targets for INOCA investigation. Novel methods using proteomic and metabolomic biomarker approaches to detect subgroups that are at higher major adverse cardiovascular events risk are needed. Because INOCA appears to be a heterogenous condition, mechanistically driven research on various INOCA endotypes and subgroups with appropriate controls is needed to advance therapeutics in this area.
Because CMD symptoms can present at rest and often due to emotional stress, the cardiac autonomic nervous system dysfunction might be a contributing factor in CMD-related ischemia. We and others have reported abnormal cardiac sympathetic activity detected using 123I-meta-iodobenzylguanidine imaging in patients with INOCA.15 It is unclear whether CMD-related ischemia triggers nociceptive pain pathways, or abnormal central processing of normal nociceptive input. Indeed, a portion of CMD patients have chest pain that is out of proportion to objective evidence of ischemia on stress testing, and neural correlates of angina have been reported in obstructive CAD; this idea requires further study in well phenotyped INOCA subgroups compared with matched controls. Investigation of strategies to modulate the cardiac autonomic nervous system to improve angina and QoL are needed.
In summary, the stagnation of the CVD mortality rate decline in midlife women and the increasingly recognized INOCA and MINOCA populations provide opportunity to improve outcomes for women (and men) using clinical practice, guidelines, clinical trials, and research concepts (Fig. 1). Evidence presented by Parvand et al.7 suggests that specific care offers promise to improve care quality and QoL outcomes. Diagnostic coding and improved phenotyping of INOCA and MINOCA using noninvasive and invasive strategies provides treatment targets for guideline-directed risk factor and angina management. Ongoing trials are testing the effect on major adverse cardiac events, as well as pathophysiology characterization for novel treatment targets including the autonomic nervous system.
Figure 1.
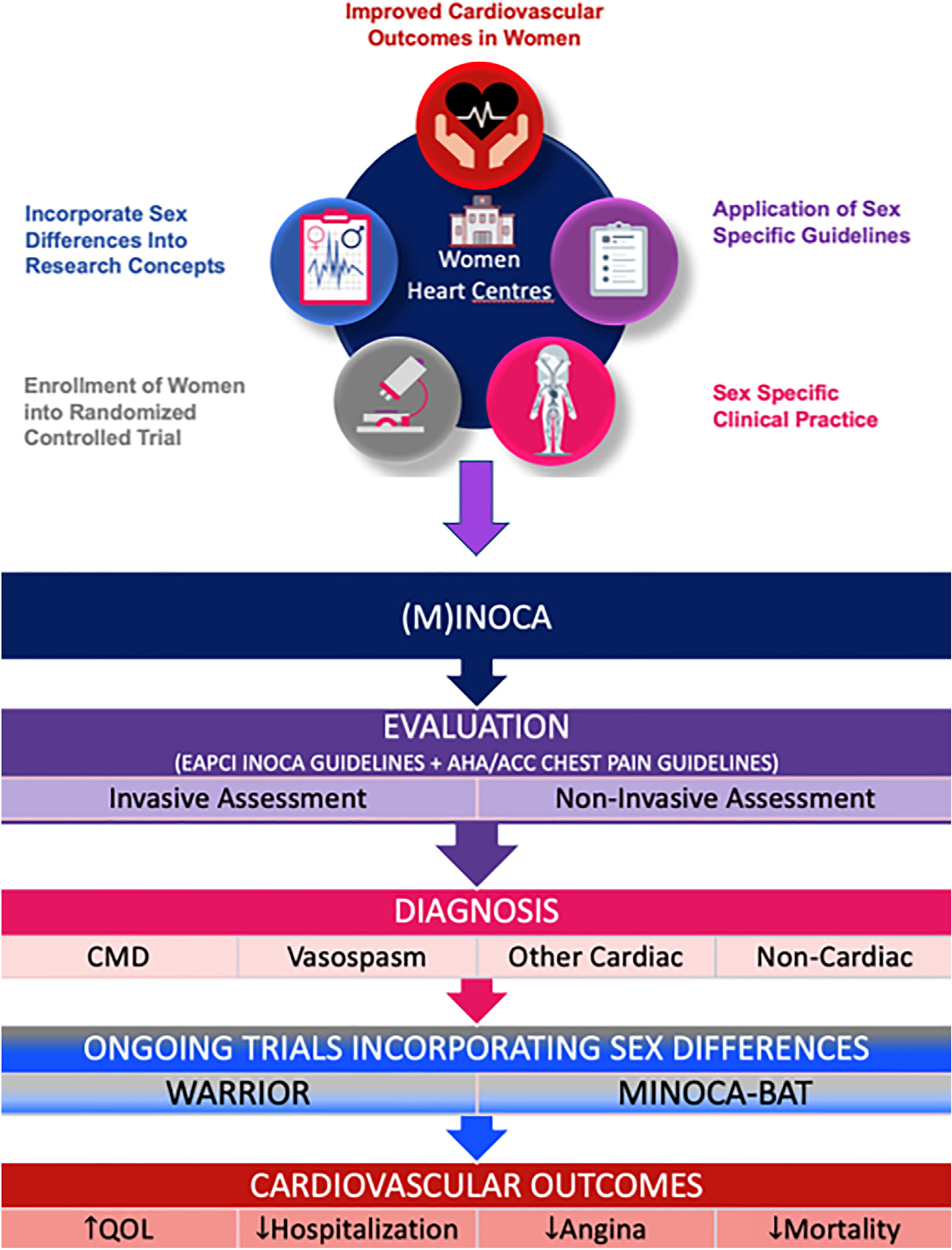
Given the stagnation of cardiovascular disease mortality rate decline in midlife women and the increasingly recognized ischemia with no obstructive coronary artery disease (INOCA) and myocardial infarction with no obstructive coronary artery disease (MINOCA) populations, the opportunity to improve outcomes for women (and men) is provided using clinical practice, guidelines, clinical trials, and research concepts. ACC, American College of Cardiology; AHA, American Heart Association; CMD, coronary microvascular dysfunction; EAPCI, European Association of Percutaneous Cardiovascular Interventions; MINOCA-BAT, Randomized Evaluation of Beta Blocker and ACE/ARB Treatment in MINOCA Patients; QOL, quality of life; WARRIOR, Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD.
Acknowledgements
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding Sources
This work was supported by the National Institutes of Health R01HL124649, 1R01HL157311, and U54 AG065141, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research, Washington, DC, the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California.
Footnotes
Disclosures
Dr C. Noel Bairey Merz serves as Board of Director for iRhythm, fees paid through Cedars Sinai Medical Center from Abbott Diagnostics and Sanofi. Dr Pacheco has received honoraria from KYE pharmaceuticals, Pfizer, and Novartis, and consulting fees from KYE pharmaceuticals. Dr Wei served on an advisory board for Abbott Vascular. The remaining authors have no conflicts of interest to disclose.
References
- 1.Vogel B, Acevedo M, Appelman Y, et al. The Lancet Women and Cardiovascular Disease Commission: reducing the global burden by 2030. Lancet 2021;397:2385–438. [DOI] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Pepine CJ, Walsh MN, et al. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacheco C, Mullen KA, Coutinho T, et al. The Canadian Women’s Heart Health Alliance Atlas on the epidemiology, diagnosis, and management of cardiovascular disease in women - chapter 5: sex- and gender-unique manifestations of cardiovascular disease. CJC Open 2022;4:243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunadian V, Chieffo A, Camici PG, et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020;41:3504–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 6.Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565–8. [DOI] [PubMed] [Google Scholar]
- 7.Parvand M, Cai L, Ghadiri S, et al. One-year prospective follow-up of women with INOCA and MINOCA at a Canadian women’s heart centre. Can J Cardiol 2022;38:1600–10. [DOI] [PubMed] [Google Scholar]
- 8.Gulati M, Hendry C, Parapid B, et al. Why we need specialised centres for women’s hearts: changing the face of cardiovascular care for women. Eur Cardiol 2021;16:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:e891–908. [DOI] [PubMed] [Google Scholar]
- 10.ICD-11 for Mortality and Morbidity Statistics (Version : 02/2022). Available at: https://icd.who.int/browse11/l-m/en. Accessed May 13, 2022.
- 11.Bairey Merz CN, Pepine CJ, Shimokawa H, et al. Treatment of coronary microvascular dysfunction. Cardiovasc Res 2020;116:856–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen TPJ, Konst RE, de Vos A, et al. Efficacy of diltiazem to improve coronary vasomotor dysfunction in ANOCA: results of the EDIT-CMD randomized clinical trial. JACC Cardiovasc Imaging 2022;15:1473–84. [DOI] [PubMed] [Google Scholar]
- 13.Bakir M, Nelson MD, Jones E, et al. Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the Women’s Ischemia Syndrome Evaluation study. Int J Cardiol 2016;223:936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson MD, Wei J, Bairey Merz CN. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J 2018;39:850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta PK, Thomson LEJ, Slomka PJ, et al. Cardiac sympathetic activity by (123)I-meta-iodobenzylguanidine imaging in women with coronary microvascular dysfunction: a pilot study. JACC Cardiovasc Imaging 2021;14:1873–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


