Abstract
The bacterium Listeria monocytogenes causes meningoencephalitis in humans. In rodents, listeriosis is associated with granulomatous lesions in the liver and the spleen, but not with meningoencephalitis. Here, infant rats were infected intracisternally to generate experimental listeric meningoencephalitis. Dose-dependent effects of intracisternal inoculation with L. monocytogenes on survival and activity were noted; 104 L. monocytogenes organisms induced a self-limiting brain infection. Bacteria invaded the basal meninges, chorioid plexus and ependyme, spread to subependymal tissue and hippocampus, and disappeared by day 7. This was paralleled by recruitment and subsequent disappearance of macrophages expressing inducible nitric oxide synthase (iNOS) and nitrotyrosine accumulation, an indication of nitric oxide (NO⋅) production. Treatment with the spin-trapping agent α-phenyl-tert-butyl nitrone (PBN) dramatically increased mortality and led to bacterial numbers in the brain 2 orders of magnitude higher than in control animals. Treatment with the selective iNOS inhibitor l-N6-(1-iminoethyl)-lysine (L-NIL) increased mortality to a similar extent and led to 1 order of magnitude higher bacterial counts in the brain, compared with controls. The numbers of bacteria that spread to the spleen and liver did not significantly differ among L-NIL-treated, PBN-treated, and control animals. Thus, the infant rat brain is able to mobilize powerful antilisterial mechanisms, and both reactive oxygen and NO⋅ contribute to Listeria growth control.
Listeria monocytogenes, a gram-positive facultative intracellular rod, is the fourth most frequent cause of community-acquired bacterial meningitis overall, and the second most common pathogen in patients over 60 years of age and in children younger than 1 month (46). Gastrointestinal symptoms, bacteremia with liver and spleen involvement, and central nervous system (CNS) infections are the most common clinical presentations in patients (2, 44). The CNS disease often includes an encephalitic component, with early mental status alterations, cranial nerve deficits, and the occurrence of seizures. Formation of abscesses in the brain stem leads to the clinical picture of listeric rhombencephalitis. Serious cases can also result in abortion in pregnant women (25, 27) (Centers for Disease Control and Prevention, Atlanta, Ga. Media relations: facts about listeriosis, http://www.cdc.gov/od/oc/media/fact/lister.htm). Despite antibiotic therapy, the mortality rate in infants and immunocompromised adults is as high as 30% (48). In domestic animals, local outbreaks of listeriosis due to intake of contaminated food are seen (19, 22, 42). Ruminants are of particular interest in this regard because the bacterium can enter the food chain via the milk or meat of apparently uninfected animals and present a hazard for humans (20, 21). L. monocytogenes has been extensively used as an experimental rodent model to study cell-mediated immunity (15, 32, 39). In these animals, L. monocytogenes causes bacteremia with inflammation of liver and spleen without CNS involvement.
Nitric oxide (NO⋅) is a pleiotropic mediator playing an important part in antimicrobial host defense against intracellular pathogens (8, 33). Macrophages (Mφ) synthesize NO⋅ by induction of inducible nitric oxide synthase (iNOS) which converts l-arginine and oxygen into l-citrulline and NO⋅. A broad variety of intracellular pathogens are inhibited in their growth by NO⋅ and its congeners. Although firmly established for laboratory rodents, the ability of Mφ to synthesize NO⋅ exhibits a considerable interspecies variation, as has been shown, e.g., for ruminant and human Mφ (12, 29, 45, 49), and the role of NO⋅ in human host defense against intracellular pathogens is controversial. Furthermore, the role of NO⋅ in antilisterial activity is controversial (4, 6, 9, 18, 41, 43). Although the cited studies are of interest, it would be desirable to have information as to whether NO⋅ has a role in antilisterial activity and/or damage in the CNS. To establish an infection with L. monocytogenes in the brain, an intracisternal infection model was developed in infant rats. In this model, the host-pathogen relationship can be manipulated by varying the numbers of bacteria used for inoculation. The model presented here was a subacute self-limiting model of infection, allowing to study mechanisms of innate host defense in the brain. Using this route of infection, the role of NO⋅ and other oxidants on the animals' survival and the bacterial numbers in the brain were assessed. The current study suggests that the brains of infant rats have powerful antilisterial mechanisms and that iNOS-derived NO⋅ is one among several antilisterial mediators in the brain.
MATERIALS AND METHODS
Infecting organisms.
Bacteria used in the present study belonged to a strain of L. monocytogenes (serotype 4b) originally isolated from the cerebrospinal fluid (CSF) of a patient with meningitis (34). Bacteria were grown on blood agar plates. For infection, one bacterial colony was cultured overnight in brain heart infusion, diluted in fresh medium, grown at 37°C for 3 h to logarithmic phase, pelleted, and resuspended in normal saline to the desired density. The accuracy of the inoculum dose was confirmed by quantitative bacteriology for each experiment.
Infection model.
An established model of bacterial meningitis in infant rats (31) was modified. The animal studies were approved by the Animal Care and Experimentation Committee of the Canton of Bern, Switzerland, and followed National Institutes of Health, Bethesda, Md., guidelines for the performance of animal experiments. For all experiments, 11-day-old Sprague-Dawley rats with their dam were purchased from RCC Biotechnology and Animal Breeding (Füllingsdorf, Switzerland). The animals weighed 26 ± 4 g (mean ± standard deviation) at the onset of the experiments and remained with their mother through the whole course of the experiment.
Infant rats were infected by direct intracisternal injection of 10-μl suspension of various doses of L. monocytogenes in saline ranging from 106 to 103 CFU by using a 32-gauge needle. After infection, rats were returned to their mother. Body weight and severity of the clinical symptoms were measured daily. Clinical severity was scored according to an activity scale graded from 5 to 0. A score of 5 indicated that the animals showed normal activity, 4 indicated minimal disease (ability to turn to an upright position within 5 s), 3 indicated moderate disease (unable to turn upright within 5 s), 2 indicated severe disease (lethargic, no ambulation), 1 indicated coma, and 0 indicated death. Animals which developed a score of 2 or lower or became terminally ill (cyanotic, exhibiting difficulty breathing or having seizures) were euthanatized for ethical reasons, using intraperitoneal injections of pentobarbital (200 mg/kg of body weight). These animals were henceforth scored as 0. The progress of the disease was such that this did not significantly distort the results.
Inhibitor studies.
Two different inhibitors were used as tools to delineate the role of NO⋅ in the antilisteric mechanisms of the CNS. First, the selective iNOS inhibitor l-N6-(1-iminoethyl)-lysine · 2HCl (L-NIL; ALEXIS Biochemicals, San Diego, Calif.) was used. Animals were injected subcutaneously with 5 mg of L-NIL per kg of body weight in 0.3 ml of sterile saline 3 h before infection and then once a day through the whole course of the experiment. Second, the spin-trapping agent α-phenyl-tert-butyl nitrone (PBN) (Calbiochem, Merck Eurolab, Darmstadt, Germany) was used. PBN acts by scavenging of oxygen- or nitrogen-derived radicals or suppression of their production (23, 36). PBN, which shows high concentrations in the cerebrospinal fluid due to its lipophilicity (10) was also administered subcutaneously 3 h before infection and then twice daily at a concentration of 150 mg/kg of body weight in saline. Animals of the control groups received 0.3 ml of sterile saline alone at the same time.
Quantitative bacteriology.
Immediately after being sacrificed, animals were perfused with phosphate-buffered saline (PBS) via the left cardiac ventricle. The spleens, cerebellums, and parts of the left liver lobes were removed aseptically, weighed, and homogenized in a 10-fold amount (wt/vol) of sterile saline. Bacterial counts were determined by plating 10-fold serial dilutions on blood agar plates after 24 h of culture at 37°C. A minimum of one colony count represented 1,000 CFU per g of organ tissue. For sterile cultures, after 24 h at 37°C the value of the detection limit (1,000 CFU) was used for statistical analysis.
Immunohistochemistry.
Animals were perfused either with 4% paraformaldehyde in PBS or with PBS alone if quantitative bacteriology was performed at the same time. The brains were harvested and immersion fixed in 4% paraformaldehyde in PBS. Slices of coronar cross sections of the thalamus region were embedded in paraffin, cut in 4-μm sections on a microtome, and mounted on positively charged glass slides.
Slides were deparaffinized shortly prior to immunohistochemical staining. Endogenous peroxidase was inactivated by treatment with H2O2 (1% in PBS for 15 min at room temperature). For L. monocytogenes and nitrotyrosine (NT) staining, antigens first had to be retrieved by treatment with trypsin (2 mg of trypsin 250 [Difco Laboratories, Detroit, Mich.] per ml in 50 mM Tris buffer, pH 7.5) for 15 min at 37°C on a shaker. Fc receptors were blocked by treatment with human immunoglobulin G (IgG) (10 mg of Globuman Berna [Swiss Serum and Vaccine Institute, Berne, Switzerland] per ml of PBS). Slides were covered with the specific antibodies diluted in dilution buffer (20 mM Tris, 0.25 M NaCl, 0.1% Tween 20, pH 7.5) at the concentrations given below for 1 h at 37°C, followed by an appropriate second antibody diluted in dilution buffer, and incubated for 45 min at 37°C. The Vectastain ABC Elite kit for peroxidase reaction or the Vectastain ABC-AP kit for alkaline phosphatase reaction, both from Vector (Geneva, Switzerland), or the Histostain-Plus broad spectrum kit (AEC) from Zymed (San Francisco, Calif.) was used as a detection system. With the Vectastain kits, either diaminobenzidine (DAB tablets; Sigma, St. Louis, Mo.) or fast red (1 mg of fast red [Chroma Gesellschaft, Schmid GmbH, Köngen, Germany] per ml plus 0.5 mg of Naphthol SX-MX per ml in 100 mM Tris-HCl, pH 8) was used as a substrate. With the Histostain-Plus kit, AEC served as a substrate and was included in the test kit. Slides were then embedded using Glycergel (DAKO, Glostrup, Denmark).
The primary antibodies used were as follows. For iNOS staining, a polyclonal rabbit anti-iNOS antibody (catalog no. 573, Upstate Biotechnology Inc., Lake Placid, N.Y.) was used at a dilution of 1:200; for L. monocytogenes staining, a polyclonal rabbit anti-L. monocytogenes antibody (serotypes 1 and 4, Difco Laboratories) at 1:200; and for detection of NT either a polyclonal NT-specific antibody (Upstate Biotechnology Inc.) at 1:200 or a monoclonal anti-NT antibody (kindly provided by M. Shigenaga, University of California—Berkeley) at 1:25 was used. The properties of the last antibody will be published in detail elsewhere (I. Girault et al., unpublished data). A validation of the NT staining is described elsewhere (H. Pfister et al., submitted for publication). Staining for the rat pan-Mφ marker ED1 was performed with a monoclonal antibody from Serotec (Oxford, United Kingdom) at 1:100. ED1 is expressed on blood-derived Mφ and activated microglia. As activated microglia cells and Mφ could not be differentiated in our model, the term Mφ was used to encompass both blood-derived Mφ and activated microglia cells.
For the secondary antibodies, either the biotinylated anti-rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, Pa.) specific for the polyclonal antibodies or a biotinylated goat anti-mouse IgG (human and rat serum absorbed, Kirkegaard & Perry Laboratories, Inc., Gaithesburg, Md.) specific for the monoclonal antibodies was applied.
Evaluation of immunohistochemistry results.
In order to objectively score the staining intensity, slides were randomized and scored in a blinded fashion from 0 to 4. Individual scores for the meninges and plexus and periventricular regions were calculated as averages of three and four microscopic fields, respectively. A score of 0 indicated that there was no positive staining on the slide, 1 referred to an average of less than 2 positive cells, 2 indicated 2 to 20 positive cells, 3 indicated between 21 and 200 positive cells, and 4 indicated more than 200 positive cells. The scores were then averaged for each individual animal.
Statistical analysis.
For determining statistical significance, data from identical experimental groups run in several experiments were compiled. Results were expressed as averages ± standard deviations unless otherwise indicated. Multiple group comparisons were performed by nonparametric one-way analysis of variance (Kruskal-Wallis test). For group comparisons, the Mann-Whitney test was used. Survival curves were analyzed by the Kaplan-Meier test.
RESULTS
Dose response and time course of intracisternal inoculation with L. monocytogenes.
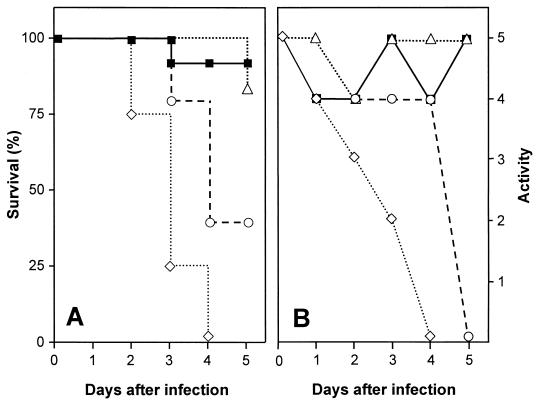
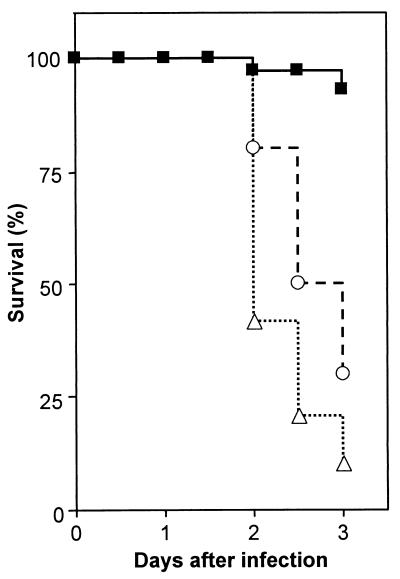
Infant rats of 11 days of age were inoculated with several doses of L. monocytogenes, ranging from 103 to 106 CFU, by the intracisternal route (Table 1). Rats were monitored over time with regard to mortality and morbidity (Fig. 1). Infant rats inoculated with 106 CFU or more of L. monocytogenes showed marked decreases in their activity scores and succumbed to the infection within the first 3 days. Animals inoculated with 103 to 105 CFU showed transient decreases in their activity scores around day 2 of infection. Mortality in the group inoculated with 105 CFU was 60% and was much lower in the groups inoculated with 103 and 104 CFU, with animals succumbing to the infection only rarely. Additional observations suggested that animals which survived until day 5 recovered from the infection. As an appropriate dose, 104 CFU of L. monocytogenes was chosen for subsequent experiments in which the course of the infection was followed for up to 1 week. Days 3, 5, and 7 were selected as time points to sacrifice the animals for immunohistochemistry, while days 2, 4, and 7 were used to sacrifice the animals for quantitative bacteriology, due to the high mortality rate of inhibitor-treated animals after day 2 (see below).
TABLE 1.
Dose-dependent effects of intracisternal inoculation of rats with L. monocytogenes
| Days postinfection | Median activity score (range) with indicated no. of intracisternally injected CFU
|
|||
|---|---|---|---|---|
| 5 × 103 | 5 × 104 | 5 × 105 | 5 × 106 | |
| 0 | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) |
| 1 | 4 (4–5) | 5 (4–5) | 4 (4–5) | 4 (4–4) |
| 2 | 4 (4–5) | 4 (4–5) | 4 (3–5) | 3 (0–5) |
| 3 | 5 (3–5) | 5 (4–5) | 4 (0–5) | 2 (2–4) |
| 4 | 4 (0–5) | 5 (3–5) | 4 (0–5) | 0 (0–2) |
| 5 | 5 (5–5) | 5 (5–5) | 0 (0–5) | NAa |
NA, not applicable.
FIG. 1.
Survival (A) and activity (B) profiles of rats inoculated intracisternally with various doses of L. monocytogenes. Data were compiled from three experiments; each dose group contained four or more animals. Symbols refer to doses of L. monocytogenes as follows: closed squares, 103 CFU; open triangles, 104 CFU; open circles, 105 CFU; open diamonds, 106 CFU.
Kinetics of L. monocytogenes growth.
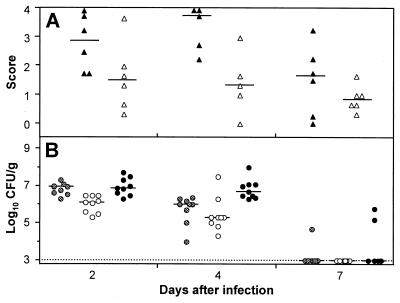
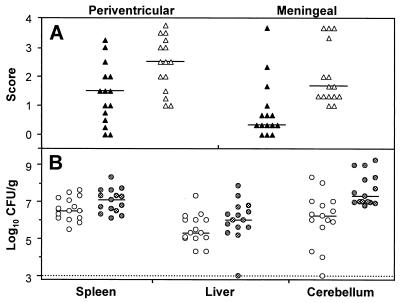
Immunohistochemical staining showed L. monocytogenes inside phagocytic cells of Mφ-like appearance and free in the tissue. The organisms were located in the basal meninges, the chorioid plexus, and the ependyme. Bacteria were abundant at day 3 of infection. At day 5 the bacteria had spread further into the subependymal tissue and in the hippocampus, causing fulminant local inflammation, but had already started to disappear from the meninges (Fig. 2A and 3A to D). At day 7, few bacteria were still present in the inflamed areas.
FIG. 2.
Growth kinetics of and tissue infiltration by L. monocytogenes in the brains of intracisternally infected rats. Tissues were analyzed at various times after infection with 104 CFU of L. monocytogenes. (A) Scores taken in a blind fashion in meninges (closed symbols) and in plexus and periventricular regions (open symbols) were averaged for individual animals as described in Materials and Methods. Each group consisted of a minimum of five rats. The horizontal lines indicate the medians of the groups (B) CFU of L. monocytogenes in spleen (hatched dots), liver (open dots), and cerebellum (black dots) tissues. For animals with no detectable bacteria, the threshold of detection was entered. The detection threshold is indicated in the graph by the dotted line.
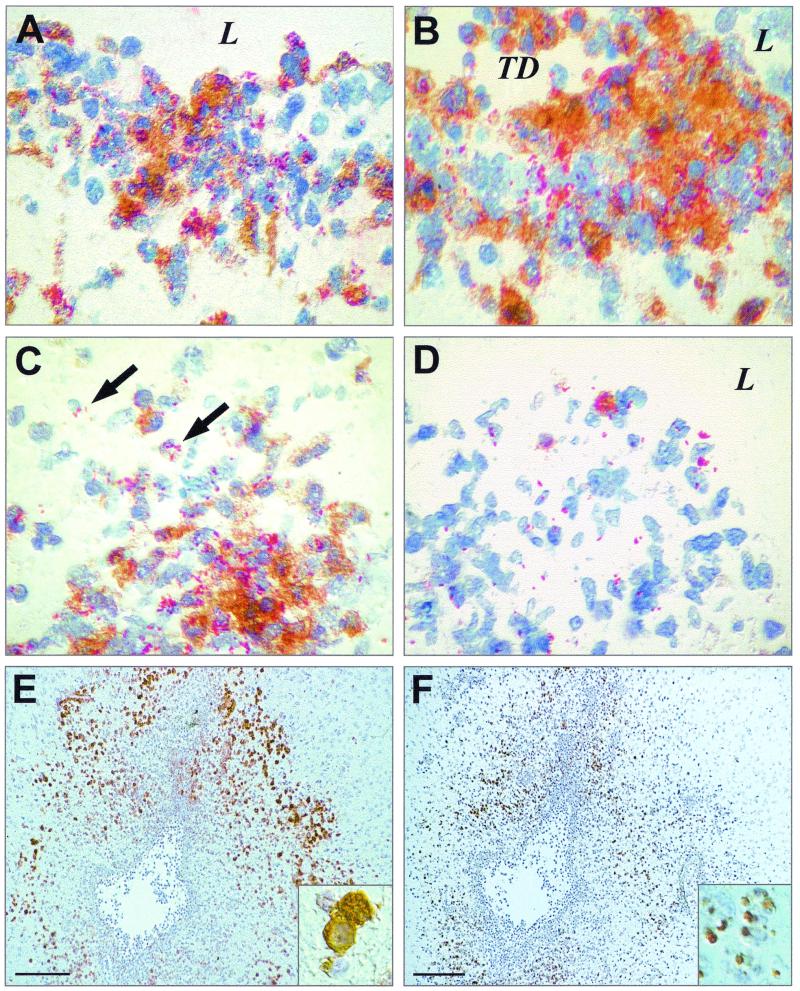
FIG. 3.
Rat brain sections showing areas of inflammation due to infection with L. monocytogenes around the third ventricle. They were stained for bacteria (red, panels A to D), iNOS (brown, panels A to E), or NT (brown, panel F). L indicates the lumen of the ventricle. TD indicates tissue destruction due to massive inflammation. Arrows point to intracellular bacteria. (A) Ependymal tissue at day 3. (B) Subependymal tissue at day 5. (C) Sequestration of bacteria at day 5. (D) Clearing of infection at day 7. (E) Overview of the third ventricle at day 3 after infection showing staining for iNOS; insert, iNOS-expressing Mφ. (F) Section adjacent to E stained for NT, insert, NT granules in Mφ. Magnification, ×400 (A to D), ×100 (E and F), and ×3,000 (inserts of E and F). The bars in E and F represent 1 mm.
L. monocytogenes injected intracisternally also spread to other organs. Thus, bacteria were detected in the liver and spleen around day 2 but already had declined at day 4 and dropped to subdetectable levels at day 7 (Fig. 2B).
Pathological findings.
The inflammation was restricted to the basal meninges, the chorioid plexus, the ependyme, and subependymal tissue. At day 3, the animals showed moderate purulent meningitis, mild purulent ependymitis with many mononuclear cells, plexus chorioiditis, and inflammation of the hippocampus with infiltration of polymorphonuclear leukocytes and Mφ. At day 5, the inflammation had spread to the subependymal tissue, where strong abscess formation, especially around the third ventricle, could be observed. Mφ became the dominant cell type in the inflamed areas of the hippocampus and also in the subependyme as revealed by immunohistochemistry. At day 7, the number of inflammatory cells around the ventricles and in the hippocampus had declined. Few inflammatory cells were left in the meninges. As a remnant of the inflammation, dilatation of the lateral ventricles and destruction of the hippocampus could be observed.
Expression of iNOS.
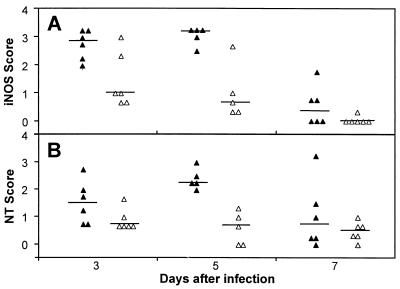
iNOS was expressed by cells in foci of infection (Fig. 3A to E). The majority of these iNOS-expressing cells were Mφ. Weak iNOS expression could also be observed in a subset of polymorphonuclear leukocytes, as indicated by nucleus morphology. At day 3, strong iNOS expression could be observed in the basal meninges, the chorioid plexus, and the hippocampus. While iNOS expression in the meninges already declined at day 5, it further intensified in the subependymal tissue around the third ventricle and the hippocampus, where it declined only at day 7 (Fig. 3D and 4A). The expression of iNOS was colocalized with bacteria in the tissue, and the presence of bacteria could also be demonstrated directly inside Mφ expressing iNOS (Fig. 3C). At day 7, when bacteria had almost disappeared, iNOS expression was limited to only a small number of Mφ in close proximity to bacteria.
FIG. 4.
Kinetics and tissue distribution of iNOS (A) and NT (B) in the brains of intracisternally infected rats. Tissues were analyzed at days 3, 5, and 7 postinfection with 104 CFU of L. monocytogenes. Scores were determined in meninges (filled triangles) and in the plexus and periventricular regions (open triangles) as described in Materials and Methods. The animals analyzed here were the same as those used for Fig. 2. The horizontal lines indicate the medians of the groups.
Expression of NT.
The expression of iNOS suggests, but does not prove, the local synthesis of NO⋅. One of the footprints of NO⋅ in tissues is NT (5, 7, 16, 28). Therefore, the appearance of NT in the tissue is a hallmark of NO⋅ formation. Immunohistochemical staining showed the onset of NT formation as early as day 3. Significant amounts of NT were seen in the form of massive granules of various sizes inside cells of Mφ type around day 5 (Fig. 3F and 4B). NT-containing cells were located in abscesses and inflamed areas with infiltration of Mφ. They were in close contact with iNOS-expressing cells, although the NT-containing cells themselves only weakly expressed iNOS, if at all. Strongly iNOS-expressing cells contained no or only single small NT granules. Toward the end of iNOS-expression, at day 7, the presence of NT could no longer be demonstrated.
Effect of treatment with L-NIL and PBN.
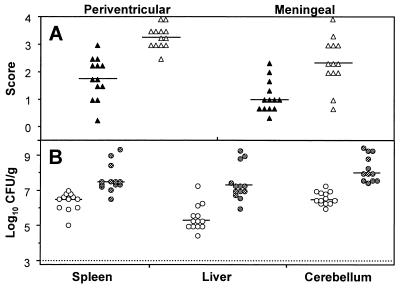
The observation that iNOS expression and NT accumulation precede the disappearance of L. monocytogenes is consistent with a role of NO⋅ in antilisterial host defense in the brain. To substantiate this, infant rats were treated with the competitive iNOS inhibitor L-NIL shortly before being infected with L. monocytogenes. At the chosen doses, L-NIL had no adverse effect on the survival and activity of healthy animals as determined in preliminary experiments (data not shown). In contrast, animals inoculated with 104 CFU of L. monocytogenes treated with L-NIL prior to infection died between days 2 and 4, whereas infected control animals succumbed to the infection only occasionally (Fig. 5). This was confirmed in experiments in which amino guanidine was used as an iNOS inhibitor (data not shown). Numbers of bacteria in the cerebellums, livers, and spleens were determined. The iNOS inhibitor L-NIL significantly increased the number of bacteria in the cerebellum; the increases seen in the liver and spleen were not significant. Immunohistochemistry of the thalamus region showed that L. monocytogenes scores in this part of the brain had increased accordingly (Fig. 6).
FIG. 5.
Survival of rats infected with L. monocytogenes and treated with either PBN (open circles) or L-NIL (open triangles) or left untreated (closed squares). The graph incorporates several experiments. Each group represents at least 13 animals.
FIG. 6.
L. monocytogenes growth in organs of animals treated with a selective iNOS inhibitor, L-NIL. (A) L. monocytogenes score at day 2 as determined in L-NIL-treated (open triangles) or mock-treated animals (closed triangles). (B) CFU in spleen, liver, and cerebellum tissues at day 2 after treatment with L-NIL (hatched dots) or mock treatment (open dots). The graph accounts for 15 animals per group, infected at different days. The difference between the control group and the L-NIL-treated group is significant (P < 0.01) for the cerebellum. The dotted line indicates the threshold of detection. Horizontal bars indicate the medians in each panel.
When using the spin-trapping agent PBN, animals succumbed to the infection between days 2 and 3 (Fig. 5). PBN significantly increased the bacterial counts in the cerebellums, livers, and spleens of the infected animals by about 2 orders of magnitude. Immunohistochemistry of the thalamus region revealed that elevated L. monocytogenes scores appeared concomitantly with an increase of the iNOS scores (Fig. 7 and data not shown).
FIG. 7.
L. monocytogenes growth in organs of animals treated with PBN. (A) L. monocytogenes scores at day 2 as determined in PBN-treated (open triangles) or mock-treated animals (closed triangles). (B) CFU in cerebellum, spleen, and liver tissues at day 2 after treatment with PBN (hatched dots) or mock treatment (open dots). The graph accounts for 13 animals per group, infected at different days. The differences in the results of control and PBN-treated tissues are significant (P < 0.001) for all groups. The dotted line indicates the threshold of detection. Horizontal bars represent medians of the groups in each panel.
DISCUSSION
An infection with L. monocytogenes causes meningoencephalitis in humans but granulomatous lesions in the liver and spleen in rodents. We have established an experimental model of listeric meningoencephalitis using an intracisternal route of infection. Infant rats infected intracisternally with a moderate dose of L. monocytogenes developed a self-limiting meningoencephalitis. Bacteria spread to other organs such as the liver and the spleen, but powerful antilisterial mechanisms led to a complete elimination of L. monocytogenes from the brain and other organs. Dose-response studies showed that survival and behavioral activity of the animals are dependent on the number of L. monocytogenes organisms used for inoculation. This model is therefore suited to study host defense mechanisms against L. monocytogenes in the brain.
The L. monocytogenes-induced inflammation was mainly restricted to the ventricles, chorioid plexus, and the neighboring tissue, which demonstrates that L. monocytogenes has a preference for the ependyme and subependymal tissue in the infant rat model. This histopathological picture closely resembles the situation in human meningoencephalitis, which is due to a hematogenous spread of L. monocytogenes to the brain.
Current infective encephalitis models are usually acute and fatal within 3 days. This is also true for models of murine listeric meningoencephalitis (24). An intracisternal inoculation has the advantage that the brain is minimally damaged by the injection, thereby causing minimal trauma and nonspecific inflammation. A listeriosis model using intracisternal infection of infant rats has recently been published (34). Whereas that model used high bacterial numbers, in the present model lower numbers were used. This allowed to address recovery, to monitor the infection kinetically, and to study which host defense elements contribute to survival and recovery.
One candidate mediator contributing to rapid elimination of L. monocytogenes from the brain is, at least in the rat, macrophage-derived NO⋅ or its congeners. Several lines of evidence support the notion that NO⋅ synthesized by iNOS does contribute to the growth control and/or elimination of the bacteria in the brain: (i) we show by immunohistochemistry that iNOS is strongly expressed in foci of L. monocytogenes infection but not in other sites of the brain; (ii) staining for NT suggests that the enzyme iNOS is active; (iii) abolishment of iNOS-mediated NO⋅ synthesis by a selective iNOS inhibitor significantly impairs survival and increases the number of bacteria in the brain, as shown by quantitative bacteriology of the cerebellum and by microscopic examination of the hypothalamus area. Earlier rodent studies indicate that the role of NO⋅ in antilisterial activity is controversial (4, 6, 9, 18, 41, 43). In one study, NO⋅ was shown to contribute to bacterial elimination in rodent but not in human Mφ (6). The latter were shown by many other groups not to contribute to iNOS-mediated NO⋅ synthesis to a large extent (12, 45, 49).
Examination of the sections showed a preferential expression of iNOS in Mφ; neutrophils were only occasionally stained, and if so, only weakly. This parallels our findings in ruminants, in which neutrophils were negative for iNOS in listeric encephalitis (30). It is noteworthy that others reported iNOS to be expressed by rat neutrophils in other systems (35, 50).
When NO⋅ was prevented from being synthesized, bacterial numbers were moderately increased (12-fold, on the average). In contrast, when both reactive oxygen and nitrogen species were downregulated with PBN (23, 36), bacterial numbers were increased by 2 orders of magnitude in all organs tested. A likely explanation for this discrepancy is that oxidants other than NO⋅ also contribute to the control of L. monocytogenes growth in the rat brain. This is consistent with in vitro studies showing that both reactive oxygen and nitrogen species contribute to antilisterial host defense (41). Genetic deficiency of a constituent of the NAD(P)H-dependent oxidase has a moderate effect on resistance to a listeria infection (13, 17), and deficiency in both the iNOS gene and the NAD(P)H-dependent oxidase gene renders the animals more susceptible to an L. monocytogenes infection than deficiency in a single gene only (47). In addition to an antilisterial effect of the single agents NO⋅ and O2−, a recent in vitro study suggested that the combined effect can be explained by a mechanism mediated by peroxynitrite, a direct reaction product of NO⋅ and O2− (37).
Tissue-associated NT may be regarded as a hallmark of local NO⋅ synthesis be it due to nitration by peroxynitrite or by the more recently described mechanism based on a reaction of nitrite and hypochlorous acid (16). In this study, it was manifested in the form of granules which were seen mainly within macrophages. Specificity for NT was demonstrated (i) by disappearance of staining in the presence of free NT, (ii) by lack of a color reaction upon pretreatment of the sections with dithionite, and (iii) by obtaining parallel results with four distinct NT-specific antibodies (H. Pfister et al., submitted for publication). Observations over time showed that NT disappeared shortly after the disappearance of iNOS. The exact nature of antibody-labeled nitrated proteins and the characteristics of their removal remain to be investigated.
The temporally and spatially limited NT expression is consistent with the view that either NO⋅ or its congener peroxynitrite is generated in the same area in which the bacteria are seen and in which iNOS is being expressed. The observation that a given cell preferentially expresses either iNOS or NT does not detract from this idea, since there could be a temporal difference between iNOS expression and NT accumulation.
The present study suggests that in the rat brain, early resistance to L. monocytogenes is mediated by mechanism(s) abolished by PBN. Several reasons argue for the involvement of innate mechanisms. These include the kinetics of bacterial spread, followed by growth control, the paucity of lymphocytes observed in lesions (K. A. Remer, unpublished observation), and the observation that heat-killed L. monocytogenes combined with gamma interferon is one of the strongest inducer of iNOS in cultured Mφ. Both neutrophils (11) and gamma interferon-producing cells other than T cells (1, 3, 14, 38, 40) have been reported to be essential for controlling an L. monocytogenes infection in the murine liver and spleen. Recovery from an infection at a later time point may be less dependent on these mediators. Older studies show that both innate and acquired immune mechanisms contribute to antilisterial host defense. There is evidence that acquired resistance, which rests on both activated CD4 and CD8 cells, is less dependent on NO⋅ than innate resistance (26, 43).
In conclusion, we developed a new model of infection with L. monocytogenes allowing experimental manipulation by varying the number of bacteria inoculated and by using specific inhibitors. We provide evidence that reactive nitrogen intermediates produced before an effective T-cell response could be established have a role in antilisterial defense in the brain. Future studies have to evaluate more precisely the role of nitric oxide versus other oxidants in cerebral antilisterial defense mechanisms and the contribution to host defense by cells other than macrophages.
ACKNOWLEDGMENTS
This work was supported by grants from the Swiss National Science Foundation (54041.98, 32-61654, and NRP 4038-52841) and the National Institutes of Health (NS-35902).
The technical assistance of Hedi Pfister, Andrea Grunder, Carmen Cardoso, and Oliver Schuetz is gratefully appreciated. We acknowledge the critical reading of the manuscript by and the helpful discussions with E. Peterhans, Institute of Veterinary Virology, and S. Christen, Institute of Infectious Diseases, University of Berne.
REFERENCES
- 1.Andersson A, Dai W J, Di Santo J P, Brombacher F. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998;161:5600–5606. [PubMed] [Google Scholar]
- 2.Armstrong R W, Fung P C. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes; case report and review Clin. Infect Dis. 1993;16:689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft G J, Schreiber R D, Unanue E R. T cell-independent macrophage activation in scid mice. Curr Top Microbiol Immunol. 1989;152:235–242. doi: 10.1007/978-3-642-74974-2_28. [DOI] [PubMed] [Google Scholar]
- 4.Beckerman K P, Rogers H W, Corbett J A, Schreiber R D, McDaniel M L, Unanue E R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–895. [PubMed] [Google Scholar]
- 5.Beckman J S, Koppenol W H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez L E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages. The role of nitric oxide. Clin Exp Immunol. 1993;91:277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betz Corradin S, Mauel J, Gallay P, Heumann D, Ulevitch R J, Tobias P S. Enhancement of murine macrophage binding of and response to bacterial lipopolysaccharide (LPS) by LPS-binding protein. J Leukoc Biol. 1992;52:363–368. doi: 10.1002/jlb.52.4.363. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan C, Röllinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 9.Boockvar K S, Granger D L, Poston R M, Maybodi M, Washington M K, Hibbs J B, Jr, Kurlander R L. Nitric oxide produced during murine listeriosis is protective. Infect Immun. 1994;62:1089–1100. doi: 10.1128/iai.62.3.1089-1100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Griffin M, Poyer J L, McCay P B. HPLC procedure for the pharmacokinetic study of the spin-trapping agent, alpha-phenyl-N-tert-butyl nitrone (PBN) Free Radic Biol Med. 1990;9:93–98. doi: 10.1016/0891-5849(90)90110-5. [DOI] [PubMed] [Google Scholar]
- 11.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 13.Dinauer M C, Deck M B, Unanue E R. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J Immunol. 1997;158:5581–5583. [PubMed] [Google Scholar]
- 14.Dunn P L, North R J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelson B T, Unanue E R. Immunity to listeria infection. Curr Opin Immunol. 2000;12:425–431. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 16.Eiserich J P, Hristova M, Cross C E, Jones A D, Freeman B A, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 17.Endres R, Luz A, Schulze H, Neubauer H, Futterer A, Holland S M, Wagner H, Pfeffer K. Listeriosis in p47 (phox−/−) and TRp55−/− mice protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7:419–432. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- 18.Fehr T, Schoedon G, Odermatt B, Holtschke T, Schneemann M, Bachmann M F, Mak T W, Horak I, Zinkernagel R M. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J Exp Med. 1997;185:921–931. doi: 10.1084/jem.185.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenlon D R. Wild birds and silage as reservoirs of Listeria in the agricultural environment. J Appl Bacteriol. 1985;59:537–543. doi: 10.1111/j.1365-2672.1985.tb03357.x. [DOI] [PubMed] [Google Scholar]
- 20.Fenlon D R, Stewart T, Donachie W. The incidence, numbers and types of Listeria monocytogenes isolated from farm bulk tank milks. Lett Appl Microbiol. 1995;20:57–60. doi: 10.1111/j.1472-765x.1995.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 21.Fenlon D R, Wilson J, Donachie W. The incidence and level of Listeria monocytogenes contamination of food sources at primary production and initial processing. J Appl Bacteriol. 1996;81:641–650. doi: 10.1111/j.1365-2672.1996.tb03559.x. [DOI] [PubMed] [Google Scholar]
- 22.Fligger J M, Blum J W, Jungi T W. Induction of intracellular arginase activity does not diminish the capacity of macrophages to produce nitric oxide in vitro. Immunobiology. 1999;200:169–186. doi: 10.1016/s0171-2985(99)80068-0. [DOI] [PubMed] [Google Scholar]
- 23.Floyd R A, Hensley K. Nitrone inhibition of age-associated oxidative damage. Ann N Y Acad Sci. 2000;899:222–237. doi: 10.1111/j.1749-6632.2000.tb06189.x. [DOI] [PubMed] [Google Scholar]
- 24.Frei K, Nadal D, Pfister H W, Fontana A. Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin 10. J Exp Med. 1993;178:1255–1261. doi: 10.1084/jem.178.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulet V, Marchetti P. Listeriosis in 225 non-pregnant patients in 1992: clinical aspects and outcome in relation to predisposing conditions. Scand J Infect Dis. 1996;28:367–374. doi: 10.3109/00365549609037921. [DOI] [PubMed] [Google Scholar]
- 26.Harty J T, White D. A knockout approach to understanding CD8+ cell effector mechanisms in adaptive immunity to Listeria monocytogenes. Immunobiology. 1999;201:196–204. doi: 10.1016/S0171-2985(99)80059-X. [DOI] [PubMed] [Google Scholar]
- 27.Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. Clin Microbiol Rev. 1997;10:345–357. doi: 10.1128/cmr.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin J C, Smith C D, Beckman J S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 29.Jungi T W, Adler H, Adler B, Thöny M, Krampe M, Peterhans E. Inducible nitric oxide synthase of macrophages. Present knowledge and evidence for species-specific regulation. Vet Immunol Immunopathol. 1996;54:323–330. doi: 10.1016/s0165-2427(96)05690-5. [DOI] [PubMed] [Google Scholar]
- 30.Jungi T W, Pfister H, Sager H, Fatzer R, Vandevelde M, Zurbriggen A. Comparison of inducible nitric oxide synthase expression in the brains of Listeria monocytogenes-infected cattle, sheep, and goats and in macrophages stimulated in vitro. Infect Immun. 1997;65:5279–5288. doi: 10.1128/iai.65.12.5279-5288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leib S L, Kim Y S, Chow L L, Sheldon R A, Täuber M G. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Investig. 1996;98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 33.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 34.Michelet C, Leib S L, Bentue-Ferrer D, Täuber M G. Comparative efficacies of antibiotics in a rat model of meningoencephalitis due to Listeria monocytogenes. Antimicrob Agents Chemother. 1999;43:1651–1656. doi: 10.1128/aac.43.7.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles A M, Owens M W, Milligan S, Johnson G G, Fields J Z, Ing T S, Kottapalli V, Keshavarzian A, Grisham M B. Nitric oxide synthase in circulating vs. extravasated polymorphonuclear leukocytes. J Leukoc Biol. 1995;58:616–622. doi: 10.1002/jlb.58.5.616. [DOI] [PubMed] [Google Scholar]
- 36.Miyajima T, Kotake Y. Spin trapping agent, phenyl N-tert-butyl nitrone, inhibits induction of nitric oxide synthase in endotoxin-induced shock in mice. Biochem Biophys Res Commun. 1995;215:114–121. doi: 10.1006/bbrc.1995.2440. [DOI] [PubMed] [Google Scholar]
- 37.Muller M, Althaus R, Frohlich D, Frei K, Eugster H P. Reduced antilisterial activity of TNF-deficient bone marrow-derived macrophages is due to impaired superoxide production. Eur J Immunol. 1999;29:3089–3097. doi: 10.1002/(SICI)1521-4141(199910)29:10<3089::AID-IMMU3089>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.North R J, Dunn P L, Conlan J W. Murine listeriosis as a model of antimicrobial defense. Immunol Rev. 1997;158:27–36. doi: 10.1111/j.1600-065x.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 40.Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, Koyasu S. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J Exp Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohya S, Tanabe Y, Makino M, Nomura T, Xiong H, Arakawa M, Mitsuyama M. The contributions of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and postinfection. Infect Immun. 1998;66:4043–4049. doi: 10.1128/iai.66.9.4043-4049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebhun W C, deLahunta A. Diagnosis and treatment of bovine listeriosis. J Am Vet Med Assoc. 1982;180:395–398. [PubMed] [Google Scholar]
- 43.Samsom J N, Langermans J A, Groeneveld P H, van Furth R. Acquired resistance against a secondary infection with Listeria monocytogenes in mice is not dependent on reactive nitrogen intermediates. Infect Immun. 1996;64:1197–1202. doi: 10.1128/iai.64.4.1197-1202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlech W F I. Foodborne listeriosis. Clin Infect Dis. 2000;31:770–775. doi: 10.1086/314008. [DOI] [PubMed] [Google Scholar]
- 45.Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167:1358–1363. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- 46.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. Active surveillance team. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 47.Shiloh M U, MacMicking J D, Nicholson S, Brause J E, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 48.Skogberg K, Syrjanen J, Jahkola M, Renkonen O V, Paavonen J, Ahonen J, Kontiainen S, Ruutu P, Valtonen V. Clinical presentation and outcome of listeriosis in patients with and without immunosuppressive therapy. Clin Infect Dis. 1992;14:815–821. doi: 10.1093/clinids/14.4.815. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg J B, Misukonis M A, Shami P J, Mason S N, Sauls D L, Dittman W A, Wood E R, Smith G K, McDonald B, Bachus K E. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 50.Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Ohkawa S, Ohnishi K, Terao S, Kawai C. Calmodulin-independent nitric oxide synthase from rat polymorphonuclear neutrophils. J Biol Chem. 1991;266:3369–3371. [PubMed] [Google Scholar]