ABSTRACT
Background
Parkinson's disease (PD) affects males more than females. The reasons for the gender differences in PD prevalence remain unclear.
Objective
The objective of this systematic review and meta‐analysis was to update the overall male/female prevalence ratios (OPR).
Methods
We updated previous work by searching MEDLINE, SCOPUS, and OVID for articles reporting PD prevalence for both genders between 2011 and 2021. We calculated OPRs and investigated heterogeneity in effect estimates.
Results
We included 19 new articles and 13 articles from a previously published meta‐analysis. The OPR was 1.18, 95% CI, [1.03, 1.36]. The OPR was lowest in Asia and appeared to be decreasing over time. Study design, national wealth, and participant age did not explain OPR heterogeneity.
Conclusion
Gender differences in PD prevalence may not be as stark as previously thought. Studies are needed to understand the role of other determinants of gender differences in PD prevalence.
Keywords: Parkinson's disease, meta‐analysis, systematic review, prevalence, gender differences.
The burden of Parkinson's disease (PD) appears to be increasing and is estimated to reach 13 million PD cases by 2040. 1 In observational studies, PD tends to affect males more frequently than females; however, the reasons are unclear. 2 , 3 , 4 , 5 In some parts of the world (eg, South Korea, Japan, and Bolivia), a male predominance of PD is less apparent. 6 , 7 , 8 Variation in prevalence may be explained by case ascertainment (specialist access), social determinants (health beliefs, insurance), or risk factors (ie, genetic, environmental factors, or their interaction). 9 , 10 , 11 , 12 A study by Pringsheim et al 3 demonstrated that the male/female ratio for prevalence increased with advancing age and varied by continent (lowest in Asian countries).
In this systematic review and meta‐analysis, we updated our knowledge about gender differences in PD prevalence, building on previous literature. 3 We hypothesized that study design, economic profile of the country, age at inclusion, or life expectancy could explain gender differences in PD prevalence. Furthermore, as the average age of the global population increased, we expected that there may be differences in the prevalence ratios reported in observational studies.
Methods
Data Selection
In this project, we updated a meta‐analysis conducted by Pringsheim et al 3 between 1985 and 2010. We searched for studies published from 2011 to 2021 with the terms: “Parkinson” and “prevalence”/“epidemiology” on MEDLINE, SCOPUS, and OVID (Table S1). Articles were included if they were population‐based (door‐to‐door surveys and predictive healthcare registry studies using PD diagnostic codes/medication), reported prevalence rates for males and females separately, and data were stratified by age groups. We excluded cohort studies because their design typically captures incidence and case–control studies because of potential selection bias. 13 Other exclusion criteria were lack of gender/age subgroup analysis, inclusion of secondary parkinsonism, and unavailable full‐text.
Two independent reviewers (A.Z. and S.C.R.) conducted the search and screened abstracts for eligibility. When differentially included, a third reviewer (A.J.N.) was involved. The list of selected prevalence studies was then combined with the studies from Pringsheim et al. 3 Because of low quality (30), lack of availability of full‐text articles in English (1), and lack of gender subgroups (3) from the list generated by Pringsheim et al, 3 we could only include 13 of the 47 studies that they included.
Data Extraction
Extraction of prevalence data was performed by each reviewer including study reference, study design, target population, male/female prevalence, case numbers, total population by gender, and age groups. Age‐standardized or crude prevalence rates were used and converted to cases per 100,000 persons (Supplementary material—Raw OPR data.pdf).
Additional data were extracted from World Health Organization life expectancy (LE) tables—defined as sex‐ and age‐specific death rates at birth for a specific year and region. Therefore, we used LE from the year 2000 for studies published before this year and from the year 2010 for later studies. 14 The LE gap was calculated as the difference between genders (female LE − male LE). Age at inclusion was extracted from articles either as reported (median) or was calculated from frequency tables (as the rank of the median). 15 World Bank data was used for the classification of high‐income countries (HIC) and low/middle‐income countries (L/MIC). 16
Data Analysis
Prevalence from each study was converted into a male/female ratio: overall male/female prevalence ratio (OPR) with log transformation for normalization. A random‐effects model was used because of high heterogeneity calculated by Cochrane Q statistic and I2. Subgroup meta‐analysis and single meta‐regression were used to determine the significance of the different variables: study design (survey vs. predictive), economic country profile, median age at study inclusion, LE gap, and study origin. For study origin, Europe was used as the reference continent. STATA v.19 was used for statistical analysis and figures (Supplementary File—Prevalence MA code).
Results
Our search identified 17,335 abstracts (Fig. 1A). Titles and abstracts were screened for relevance and to exclude case–control and cohort studies. From the first round, 350 articles were deemed relevant, with 25 articles included for a full‐text review after further exclusions. An in‐depth review of the articles resulted in the exclusion of another six articles because of insufficient data or no cases of PD identified for one gender (prevalence of 0). The final 19 articles from our search were added to the 13 articles from Pringsheim et al 3 (Fig. 1B, Table 1, Supplementary File—Supplementary References).
FIG. 1.
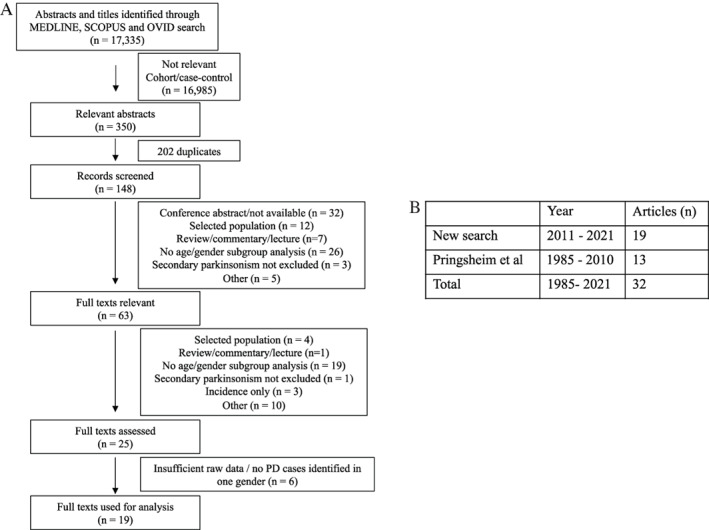
PRISMA flow diagram of article selection and final count of articles selected. (A) Results from search strategy. (B) Final results from the search strategy included 19 articles from 2011 to 2021. These were added to the list of articles from Pringsheim et al, 3 totaling 32 articles from the years 1985 to 2021
TABLE 1.
List of included prevalence studies with study characteristics
| Author, Year | OPR | Lower CI | Upper CI | Age* | M LE | F LE | F−M survival difference | Age group | Study type | Continent |
|---|---|---|---|---|---|---|---|---|---|---|
| Orozco, 2020 17 | 1.23 | 1.15 | 1.32 | 73 | 74 | 80.15 | 6.15 | 30+ | P | South America |
| Eusebi, 2019 18 | 0.99 | 0.94 | 1.05 | 82 | 79.47 | 84.22 | 4.75 | 40+ | P | Europe |
| Han, 2019 19 | 0.98 | 0.96 | 0.99 | 72 | 77.12 | 83.78 | 6.66 | 0+ | P | Asia |
| Park, 2019 6 | 0.63 | 0.63 | 0.64 | 85 | 77.12 | 83.78 | 6.66 | 0+ | P | Asia |
| Fleury, 2018 20 | 1.45 | 1.44 | 1.46 | 75 | 80.03 | 84.18 | 4.15 | 0+ | P | Europe |
| Kadastik‐Erme, 2018 21 | 1.21 | 0.99 | 1.47 | 77 | 70.86 | 80.45 | 9.59 | 0+ | P | Europe |
| Marras, 2018 22 | 1.37 | 1.34 | 1.39 | 80 | 78.56 | 80.8 | 2.24 | 45+ | P | North America |
| Valent, 2018 23 | 1.28 | 1.21 | 1.35 | 77 | 79.47 | 84.22 | 4.75 | 0+ | P | Europe |
| Nerius, 2017 24 | 1.05 | 0.99 | 1.11 | 77 | 77.78 | 82.54 | 4.76 | 0+ | P | Europe |
| Khan, 2016 25 | 1.77 | 0.47 | 6.64 | 72 | 61.53 | 63.9 | 2.37 | 50+ | S | Asia |
| Blin, 2015 26 | 0.97 | 0.97 | 0.98 | 80 | 77.96 | 84.34 | 6.38 | 18+ | P | Europe |
| Khedr, 2015 27 | 1.39 | 0.71 | 2.72 | 67 | 67.82 | 72.69 | 4.87 | 0+ | S | Africa |
| Yang, 2015 28 | 1.31 | 0.86 | 2 | 70 | 72.26 | 77.94 | 5.68 | 0+ | S | Asia |
| Zou, 2014 29 | 1.67 | 0.75 | 3.74 | 85 | 72.26 | 77.94 | 5.68 | 70+ | P | Asia |
| El‐Tallawy, 2013 62 | 1.32 | 0.66 | 2.66 | 65 | 67.82 | 72.69 | 4.87 | 40+ | S | Africa |
| Gordon, 2012 30 | 1.45 | 1.34 | 1.57 | 70 | 78.56 | 80.8 | 2.24 | 40+ | P | North America |
| Lökk, 2012 31 | 1.19 | 1.11 | 1.26 | 74 | 79.41 | 83.15 | 3.74 | 0+ | P | Europe |
| Seijo‐Martinez, 2011 32 | 2.09 | 0.75 | 5.81 | 80 | 78.76 | 84.58 | 5.82 | 65+ | S | Europe |
| Osaki, 2011 7 | 0.68 | 0.47 | 0.99 | 75 | 79.46 | 85.77 | 6.31 | 0+ | P | Asia |
| Das, 2008 33 | 0.48 | 0.18 | 1.28 | 80 | 65.69 | 68.94 | 3.25 | 60+ | S | India |
| Barbosa, 2006 34 | 1.27 | 0.68 | 2.36 | 82 | 70.57 | 77.98 | 7.41 | 65+ | S | South America |
| Zhang, 2005 35 | 1.17 | 0.92 | 1.48 | 80 | 72.26 | 77.94 | 5.68 | 55+ | S | Asia |
| Zhang, 2005 35 | 1.34 | 0.88 | 2.04 | 63 | 72.26 | 77.94 | 5.68 | 50+ | S | Asia |
| Bergareche, 2004 36 | 0.81 | 0.31 | 2.15 | 80 | 78.76 | 84.58 | 5.82 | 65+ | S | Europe |
| Tan, 2004 37 | 1.55 | 0.87 | 2.77 | 75 | 79.36 | 84.01 | 4.65 | 50+ | S | Asia |
| Benito‐Leon, 2003 63 | 1.58 | 1.03 | 2.44 | 77 | 78.76 | 84.58 | 5.82 | 65+ | S | Europe |
| Nicoletti, 2003 8 | 0.77 | 0.13 | 4.58 | 55 | 79.47 | 84.22 | 4.75 | 40+ | S | South America |
| Zhang, 2003 38 | 1.09 | 0.67 | 1.78 | 80 | 72.26 | 77.94 | 5.68 | 55+ | S | Asia |
| Kis, 2002 39 | 2.86 | 0.93 | 8.78 | 80 | 79.47 | 84.22 | 4.75 | 60+ | S | Europe |
| Wang, 1996 40 | 1.08 | 0.48 | 2.45 | 71.3 | 69.34 | 74.15 | 4.81 | 50+ | S | Asia |
| Trenkwalder, 1995 41 | 3.74 | 0.73 | 19.16 | 78 | 75.04 | 80.92 | 5.88 | 65+ | S | Europe |
| Wang, 1994 42 | 5.66 | 0.67 | 48.12 | 76.5 | 69.34 | 74.15 | 4.81 | 50+ | S | Asia |
Abbreviations: OPR, overall male to female prevalence ratio; CI, confidence interval; LE, life expectancy; F, female; M, male; P, predictive; S, survey; WHO, World Health Organization
*Age of inclusion is recorded as median. LE values for each gender were extracted from WHO LE at birth from the year 2000 for studies published before this year and from the year 2010 for later studies.
From the pooling effect estimates from the 32 articles in a random‐effects model, we calculated an OPR of 1.18, 95% confidence interval (CI), [1.03, 1.36] with a high degree of heterogeneity (I2 = 99.8%) (Figure S1A). There was no evidence of small study bias (P = 0.562) (Figure S1B).
We explored the high heterogeneity observed in the data by considering study design (door‐to‐door survey vs. health records analysis), national economic wealth (HIC vs. L/MIC), and continent. Meta‐regression for study design did not provide evidence that this was a source of heterogeneity (P = 0.141). However, studies from L/MIC had a higher OPR of 1.28, 95% CI [1.16, 1.4] compared to studies from HIC of 1.14, 95% CI [0.95, 1.35]. When we looked at OPR between continents, Asia was found to have the smallest OPR, 1.04, 95% CI [0.83, 1.29] (Fig. 2). Meta‐regression for categorical data—economic performance and continent, did not provide evidence that either were sources of heterogeneity (P = 0.362 and P = 0.100 for Asian compared to European studies, respectively). Similarly, no significant difference was seen between studies from our post‐2011 search and the previous studies included in the analysis (P = 0.362).
FIG. 2.
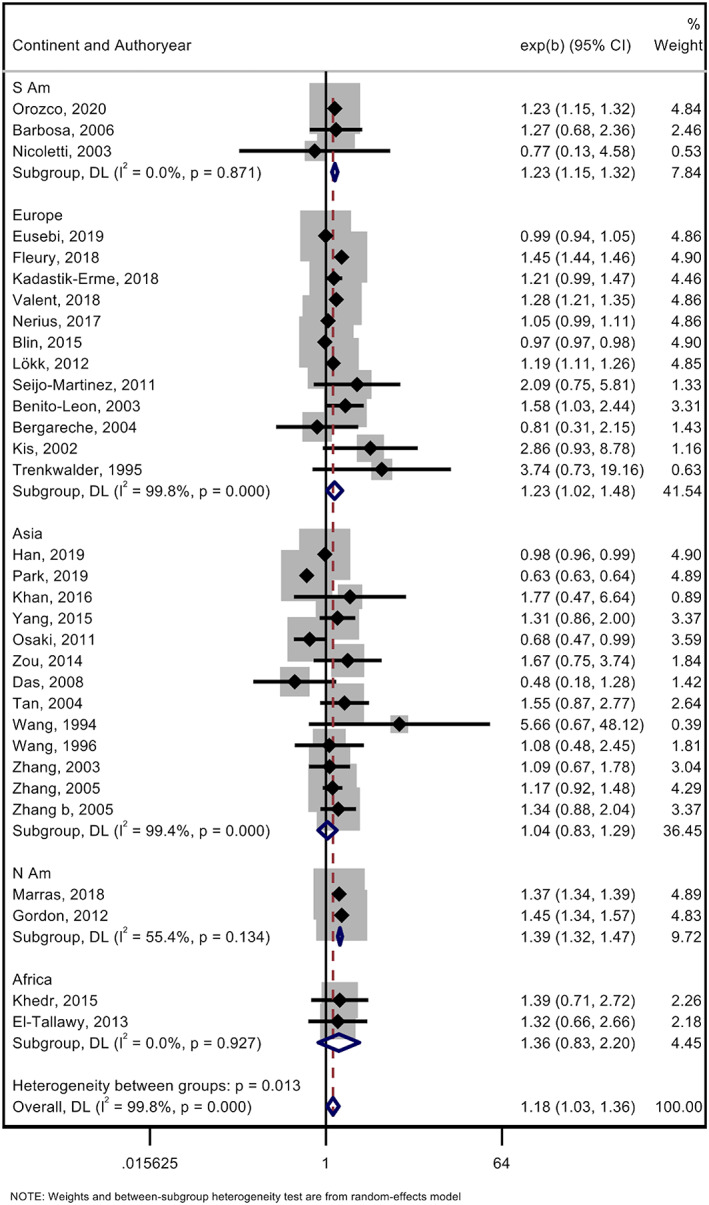
Subgroup meta‐analysis using random effects model for each study continent in the male/female (M/F) prevalence ratio. Random effects meta‐analysis with Cochrane Q statistic of the overall M/F prevalence ratio of each study categorized by its respective continent. The pooled prevalence ratio for all the studies was 1.18, 95% CI, [1.03, 1.36], but heterogeneity was high between groups (I 2 = 99.8%, P = 0.013).
Next, we investigated whether age at inclusion or LE gap could explain the heterogeneity. The median age for PD patients from the 32 studies was 75.4 (range, 55–85). Meta‐regression using median age did not suggest that this was a cleasource of observed heterogeneity (P = 0.059). The LE gap also did not account for observed heterogeneity (P = 0.080).
Last, we investigated whether the OPR varied with publication year. A subgroup analysis showed a decreasing trend in the last 30 years: 1990 to 2000 OPR, 2.06; 95% CI [0.72, 5.87], 2000 to 2010 OPR, 1.24; 95% CI [1.06, 1.45] and 2010‐present OPR, 1.15; 95% CI [0.98, 1.35], P = 0.019 (Figure S2). However, regression by year of publication was not significant (continuous P = 0.158; categorical P = 0.311). Multiple regression by publication year and study design was also not significant (P = 0.390).
Discussion
This systematic review and meta‐analysis suggested that the male/female prevalence ratio for PD is lower than has previously been reported by other studies. 2 The lowest male/female ratio was seen in studies from Asia. We also observed that the OPR may be decreasing over time and may be higher in L/MIC compared with HIC. Such findings may start to shift the paradigm of PD being a “male” dominant disorder, at least in some regions.
Large population‐based studies are best suited for determining the prevalence of diseases like PD. 13 We included data from healthcare registries and door‐to‐door cross‐sectional studies. Healthcare registries may only cover 50% to 100% of a particular population, 6 , 24 meaning that for population‐based prevalence estimates, door‐to‐door studies are considered the gold standard. PD diagnostic criteria vary across regions and institutions, causing potential differences in prevalence estimates for door‐to‐door and healthcare registries. 24 , 43 Our analysis did not identify study design as a source of heterogeneity in prevalence ratios, which could be because of our strict inclusion criteria for study design.
There was little evidence to suggest that age at inclusion might explain differences in the gender ratio. Previous studies have suggested that in patients with younger age at onset (AAO), the ratio is closer to 1. 2 One might expect that genetic factors may have a greater contribution to a lower AAO, although genome‐wide association studies to date have not identified different genetic risk factors for PD between sexes. 12 Many of these studies over‐represented participants of European ancestry and had lower female inclusion rates. 9 Efforts to create large trans‐ethnic genomic datasets will help us to better understand PD genetics in under‐represented populations. 44
A source of heterogeneity that we were unable to explore in‐depth was the role of environmental factors. Rural living and occupational pesticide exposure are associated with an increase in PD risk. 45 , 46 Males have traditionally held agricultural occupations, which could explain the higher male PD prevalence. 47 Increasing urbanization and decreasing organophosphate use in some regions may explain variability in OPRs between continents. Other putative risks/protective factors, including the Mediterranean diet, type 2 diabetes, smoking, and alcohol consumption, 48 , 49 , 50 , 51 , 52 , 53 might contribute to the male predominance of PD.
Healthcare inequalities could account for observed differences in male/female PD prevalence. In L/MIC, such as Benin, 60% of females reported socioeconomic and marital barriers to accessing healthcare. 54 , 55 Stigma related to PD symptoms and cultural beliefs about “normal” aging/PD awareness may explain the different levels of healthcare access globally. 56 Our data suggested that studies from L/MIC have a higher OPR, which may reflect unequal access to healthcare.
Another important consideration is the relationship between disease prevalence, incidence, and survival. Females have a higher AAO and a lower incidence of PD, but also have a longer LE than males. 57 Female longevity may influence the male/female prevalence imbalance, similar to other neurodegenerative diseases, like Alzheimer's disease. 58 Our analysis did not support this hypothesis, because LE did not explain heterogeneity in the OPR. However, the LE gap has changed over time, 59 and this may partly explain why the OPR is also seen to be decreasing over the last 30 years. The recent decrease in the LE gap may be related to environmental exposure, such as increased smoking rates among females 60 or the increasing integration of females into jobs traditionally held by males. Further studies are needed to understand the effect of LE and environmental/societal factors on PD prevalence.
A limitation of our study was not conducting the search before 2011. We included both door‐to‐door studies—similar to Pringsheim et al, 3 as well as studies with the newer methodology of predictive algorithms. However, no significant difference was detected between the newly identified studies and those from Pringsheim et al. 3 Another limitation in our study was the use of both crude and age‐standardized prevalence ratios. A third limitation was classifying the articles by geographic regions rather than individual countries, as genetics, environmental, and demographic factors may vary even between countries from the same region. 61 This may have impacted our results and further studies of differences within regions are needed.
In conclusion, this systematic review and meta‐analysis showed that the gender ratio for PD prevalence is lower in Asian populations and may have been decreasing in recent years. Further high‐quality epidemiological studies in diverse populations are needed to understand whether the decrease in OPR stems from environmental, societal, and/or genetic factors.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.Z.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B, 3C.
S.C.R: 1B, 1C, 2C, 3A, 3B.
J.B.: 2A, 2B, 2C, 3B.
R.R.: 3B.
C.M.: 3B.
C.B.: 3B.
I.F.M.: 1C, 3B.
A.J.N.: 1A, 1B, 1C, 2C, 3B.
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board and patient consent was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding sources and Conflict of Interest: The Preventive Neurology Unit is funded by the Barts Charity. This work was supported in part by the Intramural Research Program of the National Institute on Aging (NIA). This is present in the disclosures, no conflict but AJN has reported funding sources.
Financial Disclosures for the previous 12 months: A.J.N. reports grants from the Barts Charity, Parkinson's United Kingdom, Aligning Science Across Parkinson's, The Michael J. Fox Foundation, and the Virginia Keiley Benefaction. Personal fees/honoraria from Britannia, BIAL, AbbVie, Global Kinetics Corporation, Profile, Biogen, Roche, and UCB are outside of the submitted work. I.F.M. reports grants from Aligning Science Across Parkinson's and The Michael J. Fox Foundation, The Parkinson's Foundation, American Parkinson Disease Association, and National Institutes of Health.
Supporting information
Figure S1. Meta‐funnel plot analyses for reporting bias in prevalence studies. (A) Meta‐analysis using random effects and Cochrane Q statistic for male/female prevalence ratios with an overall prevalence ratio of 1.18, 95% CI, [1.03, 1.36]. (B) Meta‐funnel plot of included studies did not identify any evidence of small studying reporting bias (P = 0.562).
Figure S2. Subgroup meta‐analysis using random effects model for time‐trends in the M/F prevalence ratio. Random effects meta‐analysis with Cochrane Q statistic of the M/F prevalence ratio of each study categorized by the year of publication. This shows a decreasing trend from 2.06, 95% CI, [0.72, 5.87] in 1990–2000 to 1.24, 95% CI, [1.06, 1.45] in 2000–2010, and to 1.15, 95% CI, [0.98, 1.35] in 2010–2021.
Table S1. Search strategies for MEDLINE, SCOPUS, and OVID. Published PD prevalence articles were searched through key terms with the following filters: published between years 1/1/2011–12/30/2021, in English in MEDLINE, SCOPUS, and OVID, sorted by most recent. Abstracts and article information were collated into an excel file, which served as a basis for screening.
Prevalence MA code
Raw OPR data
Relevant disclosures and conflict of interest are listed at the end of this article.
Alexandra Zirra and Shilpa C. Rao are joint first authors.
References
- 1. Dorsey ER, Bloem BR. The Parkinson pandemic‐a call to action. JAMA Neurol 2018;75:9–10. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Parkinson's Disease Collaborators . Global, regional, and national burden of Parkinson's disease, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol 2018;17:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pringsheim T, Jette N, Frolkis A, Steeves TDL. The prevalence of Parkinson's disease: A systematic review and meta‐analysis. Mov Disord 2014;29:1583–1590. [DOI] [PubMed] [Google Scholar]
- 4. Ma C‐L, Su L, Xie JJ, Long JX, Wu P, Gu L. The prevalence and incidence of Parkinson's disease in China: A systematic review and meta‐analysis. J Neural Transm 2014;121:123–134. [DOI] [PubMed] [Google Scholar]
- 5. Riccò M et al. Prevalence of Parkinson disease in Italy: A systematic review and meta‐analysis. Acta Biomed 2020;91:e2020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park J‐H, Kim DH, Kwon DY, et al. Trends in the incidence and prevalence of Parkinson's disease in Korea: A nationwide, population‐based study. BMC Geriatr 2019;19:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osaki Y, Morita Y, Kuwahara T, Miyano I, Doi Y. Prevalence of Parkinson's disease and atypical parkinsonian syndromes in a rural Japanese district. Acta Neurol Scand 2011;124:182–187. [DOI] [PubMed] [Google Scholar]
- 8. Nicoletti A, Sofia V, Bartoloni A, Bartalesi F, Gamboa Barahon H, Giuffrida S, Reggio A. Prevalence of Parkinson's disease: A door‐to‐door survey in rural Bolivia. Parkinsonism Relat Disord 2003;10:19–21. [DOI] [PubMed] [Google Scholar]
- 9. Nalls MA, Blauwendraat C, Vallerga CL, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: A meta‐analysis of genome‐wide association studies. Lancet Neurol 2019;18:1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: Risk factors and prevention. Lancet Neurol 2016;15:1257–1272. [DOI] [PubMed] [Google Scholar]
- 11. Elbaz A, Carcaillon L, Kab S, Moisan F. Epidemiology of Parkinson's disease. Rev Neurol 2016;172:14–26. [DOI] [PubMed] [Google Scholar]
- 12. Blauwendraat C, Heilbron K, Vallerga CL, et al. Parkinson's disease age at onset genome‐wide association study: Defining heritability, genetic loci, and α‐synuclein mechanisms. Mov Disord 2019;34:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belbasis L, Bellou V. Introduction to epidemiological studies. Methods Mol Biol 2018;1793:1–6. [DOI] [PubMed] [Google Scholar]
- 14. Life expectancy at birth (years) . https://www.who.int/data/gho/data/indicators/indicator-details/GHO/life-expectancy-at-birth-(years).
- 15. Ch 3. Numbers about Numbers/SWT . https://brownmath.com/swt/chap03.htm.
- 16. Data Catalog . https://datacatalog.worldbank.org.
- 17. Orozco, Jorge Luis et al. Parkinson's disease prevalence, age distribution and staging in Colombia. Neurology international vol. 12, 8401. 10 Jul. 2020, doi: 10.4081/ni.2020.8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eusebi P, Franchini D, De Giorgi M, et al. Incidence and prevalence of Parkinson's disease in the Italian region of Umbria: a population‐based study using healthcare administrative databases. Neurological Sciences 2019;40(8):1709–1712. 10.1007/s10072-019-03872-w. [DOI] [PubMed] [Google Scholar]
- 19. Han S, Kim S, Kim H, Shin H‐W, Na K‐S, Suh HS. Prevalence and incidence of Parkinson's disease and drug‐induced parkinsonism in Korea. BMC Public Health 2019;19(1). 10.1186/s12889-019-7664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fleury V, Brindel P, Nicastro N, Burkhard PR. Descriptive epidemiology of parkinsonism in the Canton of Geneva, Switzerland. Parkinsonism & Related Disorders 2018;54:30–39. 10.1016/j.parkreldis.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 21. Kadastik‐Eerme L, Taba N, Asser T, Taba P. The increasing prevalence of Parkinson's disease in Estonia. Acta Neurologica Scandinavica 2018;138(3):251–258. 10.1111/ane.12948. [DOI] [PubMed] [Google Scholar]
- 22. Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson's disease across North America. Npj Parkinson's Disease 2018;4(1). 10.1038/s41531-018-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valent F, Devigili G, Rinaldo S, Del Zotto S, Tullio A, Eleopra R. The epidemiology of Parkinson's disease in the Italian region Friuli Venezia Giulia: a population‐based study with administrative data. Neurological Sciences 2018;39(4):699–704. 10.1007/s10072-018-3273-x. [DOI] [PubMed] [Google Scholar]
- 24. Nerius M, Fink A, Doblhammer G. Parkinson's disease in Germany: Prevalence and incidence based on health claims data. Acta Neurol Scand 2017;136:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan S, Nabi G, Naeem M, Ali L, Silburn P, Mellick G. A door‐to‐door survey to estimate the prevalence of Parkinsonism in Pakistan. Neuropsychiatric Disease and Treatment 2016;1499. 10.2147/ndt.s86329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blin P, Dureau‐Pournin C, Foubert‐Samier A, et al. Parkinson's disease incidence and prevalence assessment in France using the national healthcare insurance database. European Journal of Neurology 2014;22(3):464–471. 10.1111/ene.12592. [DOI] [PubMed] [Google Scholar]
- 27. Khedr EM, Fawi G, Abbas MAA, Mohammed TA, El‐Fetoh NA, Attar GA, Zaki AF. Prevalence of Parkinsonism and Parkinson's disease in Qena governorate/Egypt: a cross‐sectional community‐based survey. Neurological Research 2015;37(7):607–618. 10.1179/1743132815y.0000000020. [DOI] [PubMed] [Google Scholar]
- 28. Yang XL, Luo Q, Song HX, Wang YL, Yao YN, Xia H. Related factors and prevalence of Parkinson's disease among Uygur residents in Hetian, Xinjiang Uygur Autonomous Region. Genetics and Molecular Research 2015;14(3):8539–8546. 10.4238/2015.july.31.1. [DOI] [PubMed] [Google Scholar]
- 29. Zou Y‐M et al. The prevalence of Parkinson's disease continues to rise after 80 years of age: a cross‐sectional study of Chinese veterans. European review for medical and pharmacological sciences vol. 2014;18(24):3908–3915. [PubMed] [Google Scholar]
- 30. Gordon PH, Mehal JM, Holman RC, Rowland AS, Cheek JE. Parkinson's disease among American Indians and Alaska natives: A nationwide prevalence study. Movement Disorders 2012;27(11):1456–1459. 10.1002/mds.25153. [DOI] [PubMed] [Google Scholar]
- 31. Lökk J, Borg S, Svensson J, Persson U, Ljunggren G. Drug and treatment costs in Parkinson's disease patients in Sweden. Acta Neurologica Scandinavica 2011;125(2):142–147. 10.1111/j.1600-0404.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 32. Seijo‐Martinez M, Castro del Rio M, Rodríguez Alvarez J, Suarez Prado R, Torres Salgado E, Paz Esquete J, Sobrido MJ. Prevalence of parkinsonism and Parkinson's disease in the Arosa Island (Spain): A community‐based door‐to‐door survey. Journal of the Neurological Sciences 2011;304(1–2):49–54. 10.1016/j.jns.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 33. Das SK et al. Prevalence of major neurological disorders among geriatric population in the metropolitan city of Kolkata. The Journal of the Association of Physicians of India 2008;56:175–181. [PubMed] [Google Scholar]
- 34. Barbosa MT, Caramelli P, Maia DP, Cunningham MCQ, Guerra HL, Lima‐Costa MF, Cardoso F. Parkinsonism and Parkinson's disease in the elderly: A community‐based survey in Brazil (the Bambuí study). Movement Disorders 2006;21(6):800–808. 10.1002/mds.20806. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Z, Roman G, Hong Z, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. The Lancet 2005;365(9459):595–597. 10.1016/s0140-6736(05)70801-1. [DOI] [PubMed] [Google Scholar]
- 36. Bergareche A, De La Puente E, de Munain AL, Sarasqueta C, de Arce A, Poza JJ, Martí‐Massó JF. Prevalence of Parkinson?s disease and other types of Parkinsonism. Journal of Neurology 2004;251(3):340–345. 10.1007/s00415-004-0333-3. [DOI] [PubMed] [Google Scholar]
- 37. Tan LCS, Venketasubramanian N, Hong CY, et al. Prevalence of Parkinson disease in Singapore: Chinese vs Malays vs Indians. Neurology 2004;62(11):1999–2004. 10.1212/01.wnl.0000128090.79756.10. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Z‐X, Anderson DW, Huang J‐B, et al. Prevalence of Parkinson's disease and related disorders in the elderly population of greater Beijing, China. Movement Disorders 2003;18(7):764–772. 10.1002/mds.10445. [DOI] [PubMed] [Google Scholar]
- 39. Kis B, Schrag A, Ben‐Shlomo Y, et al. Novel three‐stage ascertainment method. Neurology 2002;58(12):1820–1825. 10.1212/wnl.58.12.1820. [DOI] [PubMed] [Google Scholar]
- 40. Wang S‐J, Fuh J‐L, Teng EL, et al. A Door‐to‐Door Survey of Parkinson's Disease in a Chinese Population in Kinmen. Archives of Neurology 1996;53(1):66–71. 10.1001/archneur.1996.00550010084020. [DOI] [PubMed] [Google Scholar]
- 41. Trenkwalder C. Starnberg Trial on Epidemiology of Parkinsonism and Hypertension in the Elderly. Archives of Neurology 1995;52(10):1017. 10.1001/archneur.1995.00540340109020. [DOI] [PubMed] [Google Scholar]
- 42. Wang SJ, Fuh JL, Liu CY, et al. Parkinson's Disease in Kin‐Hu, Kinmen: A Community Survey by Neurologists. Neuroepidemiology 1994;13(1–2):69–74. 10.1159/000110361. [DOI] [PubMed] [Google Scholar]
- 43. de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MMB, Maraganore DM. A population perspective on diagnostic criteria for Parkinson's disease. Neurology 1997;48:1277–1281. [DOI] [PubMed] [Google Scholar]
- 44. Global Parkinson's Genetics Program . GP2: The global Parkinson's genetics program. Mov Disord 2021;36:842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ritz BR, Paul KC, Bronstein JM. Of pesticides and men: A California story of genes and environment in Parkinson's disease. Curr Environ Health Rep 2016;3:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Narayan S, Liew Z, Bronstein JM, Ritz B. Occupational pesticide use and Parkinson's disease in the Parkinson environment gene (PEG) study. Environ Int 2017;107:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Semchuk KM, Love EJ, Lee RG. Parkinson's disease and exposure to agricultural work and pesticide chemicals. Neurology 1992;42:1328–1335. [DOI] [PubMed] [Google Scholar]
- 48. Maraki MI, Yannakoulia M, Stamelou M, et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson's disease. Mov Disord 2019;34:48–57. [DOI] [PubMed] [Google Scholar]
- 49. Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, Ascherio A. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr 2007;86:1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li X, Li W, Liu G, Shen X, Tang Y. Association between cigarette smoking and Parkinson's disease: A meta‐analysis. Arch Gerontol Geriatr 2015;61:510–516. [DOI] [PubMed] [Google Scholar]
- 51. Gallo V, Vineis P, Cancellieri M, et al. Exploring causality of the association between smoking and Parkinson's disease. Int J Epidemiol 2019;48:912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim R, Yoo D, Jung YJ, Han K, Lee J‐Y. Sex differences in smoking, alcohol consumption, and risk of Parkinson's disease: A nationwide cohort study. Parkinsonism Relat Disord 2020;71:60–65. [DOI] [PubMed] [Google Scholar]
- 53. Choi HK, Curhan G. Beer, liquor, and wine consumption and serum uric acid level: The third National Health and nutrition examination survey. Arthritis Rheum 2004;51:1023–1029. [DOI] [PubMed] [Google Scholar]
- 54. Zegeye B, el‐Khatib Z, Ameyaw EK, Seidu AA, Ahinkorah BO, Keetile M, Yaya S. Breaking barriers to healthcare access: A multilevel analysis of individual‐ and community‐level factors affecting Women's access to healthcare Services in Benin. Int J Environ Res Public Health 2021;18:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matin BK, Williamson HJ, Karyani AK, Rezaei S, Soofi M, Soltani S. Barriers in access to healthcare for women with disabilities: A systematic review in qualitative studies. BMC Womens Health 2021;21:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mshana G, Dotchin CL, Walker RW. ‘We call it the shaking illness’: Perceptions and experiences of Parkinson's disease in rural northern Tanzania. BMC Public Health 2011;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol 2016;160:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. GBD 2019 Demographics Collaborators . Global age‐sex‐specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the global burden of disease study 2019. Lancet 2020;396:1160–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Janssen F. Changing contribution of smoking to the sex differences in life expectancy in Europe, 1950‐2014. Eur J Epidemiol 2020;35:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lim S‐Y, Tan AH, Ahmad‐Annuar A, et al. Parkinson's disease in the Western Pacific region. Lancet Neurol 2019;18:865–879. [DOI] [PubMed] [Google Scholar]
- 62.El‐Tallawy, Hamdy N, et al. Prevalence of Parkinson's disease and other types of Parkinsonism in Al Kharga district, Egypt. Neuropsychiatr Dis Treat 2013;9:1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benito‐León J, Bermejo‐Pareja F, Rodríguez J, et al. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord 2003;18(3):267–274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Meta‐funnel plot analyses for reporting bias in prevalence studies. (A) Meta‐analysis using random effects and Cochrane Q statistic for male/female prevalence ratios with an overall prevalence ratio of 1.18, 95% CI, [1.03, 1.36]. (B) Meta‐funnel plot of included studies did not identify any evidence of small studying reporting bias (P = 0.562).
Figure S2. Subgroup meta‐analysis using random effects model for time‐trends in the M/F prevalence ratio. Random effects meta‐analysis with Cochrane Q statistic of the M/F prevalence ratio of each study categorized by the year of publication. This shows a decreasing trend from 2.06, 95% CI, [0.72, 5.87] in 1990–2000 to 1.24, 95% CI, [1.06, 1.45] in 2000–2010, and to 1.15, 95% CI, [0.98, 1.35] in 2010–2021.
Table S1. Search strategies for MEDLINE, SCOPUS, and OVID. Published PD prevalence articles were searched through key terms with the following filters: published between years 1/1/2011–12/30/2021, in English in MEDLINE, SCOPUS, and OVID, sorted by most recent. Abstracts and article information were collated into an excel file, which served as a basis for screening.
Prevalence MA code
Raw OPR data


