Abstract
The neurologic complications associated with the coronavirus disease 2019 (COVID-19) is becoming more prevalent in children after the appearance of the Omicron strain. An association between COVID-19 and posterior reversible encephalopathy (PRES) has been consistently reported in adults, but little information is available in the pediatric age group. There are only few case reports of COVID-19-related PRES in children, and all of these patients were either on some type of immunomodulatory medications or whose general condition was severe. The present case, a 9-year-old Japanese boy, who had no fever but vomited several times from days 1–4 of a COVID-19 infection had an afebrile seizure on the 8th day of his illness. The patient had no history of hypertension, and had not previously been administered any immunosuppressive drugs before or during the period of his COVID-19 infection. On admission, his physical findings were unremarkable, except for a high blood pressure. The results obtained by brain computed tomography and magnetic resonance imaging were consistent with PRES. The patient recovered with no sequelae after treatment with antihypertensive drugs. Further investigations did not suggest any underlying disease that could have caused the transient hypertension. Although PRES is relatively rare in children, pediatricians should keep in mind that this syndrome can be complicated, even in children with mild COVID-19 infections.
Keywords: COVID-19, Posterior reversible encephalopathy, Children, SARS-COV-2, Hypertension, Neuroimaging
Introduction
The novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome associated with the coronavirus 2 (SARS-COV-2) became more prevalent in children in 2022. Although most of the affected children presented asymptomatic or mild infections, severe complications could include respiratory failure, the multisystem inflammatory syndrome in children (MIS-C) and neurological manifestations. Neurologic complications such as febrile seizures, non-febrile seizures, encephalopathy, aseptic meningitis, encephalitis, brain abscesses and cerebral infarction have been reported in hospitalized children with COVID-19 [1].
The posterior reversible encephalopathy syndrome (PRES) is a clinical syndrome that usually arises as a part of some underlying diseases such as renal failure, autoimmune diseases and acute hypertension [2]. An association of PRES with COVID-19 has been consistently reported in adults [3], but there are only a few reports of this in children [4], [5], [6]. It appears that COVID-19-induced hyperinflammation or background immunomodulatory medications may have contributed to PRES in the previous cases. However, we present herein a pediatric case of mild COVID-19-related PRES without any preexisting risk factors for the syndrome.
Case
A 9-year-old boy was admitted to our hospital on the 8th day after the onset of a COVID-19 infection during the epidemic associated with the appearance of the Omicron strain. He had no fever and no respiratory symptoms but vomited frequently from days 1 through 4 of the illness, and received intravenous hydration at home by his parents who were medical doctors. In the morning of his hospitalization, he suddenly developed a generalized seizure that lasted several minutes.
Until he was about 5 years old, he had frequent episodes of vomiting, especially when he had a cold. He had a history of cryptorchidopexy and was diagnosed as having mild left renal pelvis dilation without vesicoureteral reflux. Mild psychomotor developmental delay was also recognized. A previous evaluation of brain magnetic resonance imaging (MRI), which was performed when he was around 1 year old, showed no abnormal findings. His blood pressure, that was measured 3 weeks before his hospitalization, was 96/52 mmHg, which was within the normal range for his age. He had not been vaccinated against SARS-COV-2.
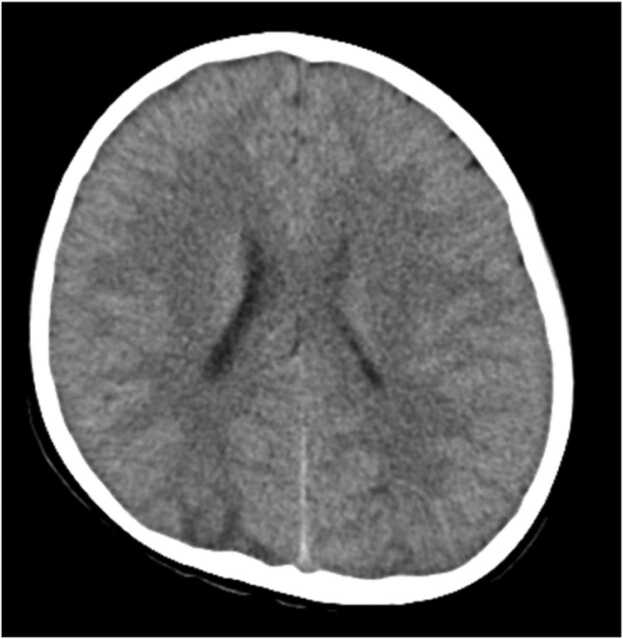
He was conscious on admission and physical findings including a neurological examination were unremarkable. His temperature, 37.0 °C; heart rate, 88 bpm; percutaneous oxygen saturation, 99%; and blood pressure was 143/92 mmHg. A blood examination revealed the following results: white blood cell counts, 10.3 × 103/μL; c-reactive protein, 1.03 mg/dL; sodium, 133 mEq/L; urinary nitrogen, 14.8 mg/dL; creatinine, 0.45 mg/dL; ferritin, 202 ng/mL; N-terminal prohormone of the brain natriuretic peptide, 90 pg/mL. His chest x-ray finding was unremarkable. Brain computed tomography (CT) showed low density areas that predominantly affected the posterior bilateral regions (Fig. 1). Cranial MRI revealed multiple subcortical-cortical high intensity signals on T2 weighted and fluid-attenuated inversion recovery (FLAIR) sequences in the bilateral posterior and parietal regions (Fig. 2). Cranial magnetic resonance angiography showed no abnormal findings. These results and physical findings did not meet the criteria for MIS-C but were consistent with PRES. We started to treat him with continuous intravenous infusion of nicardipine hydrochloride, which as 0.9 μg/kg of body weight/minute was needed to control his blood pressure. Oral amlodipine besilate was administered one week later, followed by reducing the intravenous administration of nicardipine hydrochloride. Antihypertensive treatment was finally discontinued on the 14th day of his stay in the hospital, but his blood pressure remained within the normal range. Follow-up brain MRI, performed on the 15th day of his hospital stay, indicated that the subcortical high intensity signals on T2 weighted and FLAIR images were dramatically resolved and he was discharged without any neurological sequelae.
Fig. 1.
Brain CT on the day of hospitalization showed low density areas on the occipital lobes.
Fig. 2.
Brain MRI, performed on 3rd hospital day, revealed multiple subcortical-cortical high intensity signals on FLAIR sequence in the left posterior-temporal lobe (A) and bilateral occipital lobes (B).
We sought to re-assess him for any underlying diseases that could trigger the development of PRES with transient hypertension. The levels of glucose, thyroid functions, serum cortisol, adrenocorticotropic hormones, epinephrine and norepinephrine levels, serum immunoglobulins and complement factors were all within the normal range. Anti-nuclear antibody, anti-glomerular basement membrane antibody and anti-neutrophil cytoplasmic antibodies were all negative. Plasma renin activity and aldosterone levels, measured on the 4th day of his hospitalization, were increased to 115 pg/mL and 499.3 pg/mL, respectively, but returned to normal after a week. Not only the cranial but also the renal MR angiography did not suggest arterial stenosis. Mild left renal enlargement was recognized on abdominal ultrasonography, but renal excretory function was not impaired, as assessed by diuretic renogram. A cerebrospinal fluid examination, performed on the 16th day of his hospitalization revealed normal results. A cardiac evaluation by electrocardiography and ultrasonography confirmed a normal heart. The electroencephalogram, which was performed after 2 weeks from the onset of PRES, showed no unusual findings. The results of an ophthalmic examination were normal. There were no indications of a specific metabolic disease, based on tandem mass screening and a urine metabolomic analysis.
Discussion
PRES is characterized by an impairment in brain blood vessel autoregulation after severe hypertension, or as a consequence of a severe infection, inflammation or vasotoxicity [7]. A pathophysiological mechanism of developing PRES is the disruption of the blood-brain barrier secondary to endothelial dysfunction and a failure of autoregulation at high blood pressures and subsequent hyperperfusion followed by cerebral edema, predominantly in the posterior brain [8], [9]. A preexisting autoimmune condition and renal failure are significant risk factors for developing PRES.
A systematic review of the association of PRES with COVID-19 has been reported [3]. They analyzed 56 documented cases and reported that the mean age of the patients was 56.6 ± 15.3 years and approximately half of them were under mechanical ventilation for severe COVID-19 pneumonia. The most common comorbidities were hypertension and diabetes mellitus. Tocilizumab had been administered for 16.1% of the patients. Lallana et al. reported on 8 adults with COVID-19-related PRES; all of these patients had severe pneumonia and half had been treated with tocilizumab [10].
The mechanism by which PRES may be associated with COVID-19 has not been clarified, but numerous studies have proposed possible explanations. As summarized in a recent review article [3], the mechanism may include: (1) toxic damage to the endothelium of the blood brain barrier mediated by a cytokine storm in COVID-19, (2) endothelial damage induced by the binding of the spike protein of SARS-COV-2 to the angiotensin-converting enzyme 2 (ACE2) receptor on the endothelium, and (3) direct neuronal damage caused by the binding of SARS-COV-2 to the ACE2 receptor on the neurons and glial cells in the brain.
In the pediatric age group, neoplastic, renal disorders and hematopoietic stem cell transplantation represent the main disorders associated with PRES [11]. Chemotherapeutic drugs, immunosuppressants, and hypertension appear to be the main risk factors for the development of pediatric PRES [11]. A retrospective study has suggested no association between sepsis and PRES in children [12]. Three pediatric patients with PRES in association with COVID-19 but not MIS-C have recently been reported (Table 1) [4], [5], [6]. Al Haboob et al. reported on a child with the Miller Fischer syndrome (MFS) and a PRES post COVID-19 infection [4]. The child had been exposed to COVID-19 two months prior to his illness. During the treatment with intravenous immunoglobulins (IVIG) for MFS, he experienced fluctuations in his blood pressure, impaired consciousness and seizures. The second patient developed PRES while recovering from COVID-19 pneumonia that was managed with favipiravir, and dexamethasone [5]. The third patient, who was being treated with cyclosporine and dexamethasone for hemophagocytic lymphohistiocytosis, developed PRES following severe COVID-19 pneumoniae that had been treated with inodilator infusion, hydroxychloroquine and tocilizumab [6]. It has been suggested that PRES may be a complication of MFS with or without IVIG treatment [13], [14]. As shown in Table 1, it appears that all of the previous children either had severe COVID-19 complications or had some risk factors for PRES including immunosuppressive therapies.
Table 1.
Presentation and outcome of pediatric patients with COVID-19-related PRES.
| Patient [Reference] | Patient 1[4] | Patient 2[5] | Patient 3[6] | Present case |
|---|---|---|---|---|
| Age, sex | 11 year, Male | 10 year, Male | 5 year, Female | 9 year, Male |
| Comorbidity | None | None | HLH | Psychomotor delay |
| Time from COVID-19 | > 3 weeks | < 1 month | 7 days | 8 days |
| COVID-19 complication | Miller Fischer syndrome | Severe pneumonia | Severe pneumonia | None |
| Seizure due to PRES | Yes | Yes | Yes | Yes |
| Treatment just before or at the onset of seizure | IVIG | Favipiravir Antibiotics Dexamethasone |
Hydroxychloroquine Antibiotics Dexamethasone Cyclosporine Tocilizumab |
None |
| Treatment for PRES | Levetiracetam Hydralazine Antibiotics Remdesivir mPSL ASA |
Levetiracetam Phenytoin Amlodipine |
Levetiracetam Esmolol |
Nicardipine Amlodipine |
| Outcome | Recovered | Recovered | Recovered | Recovered |
HLH=hemophagocytic lymphohistiocytosis, IVIG=intravenous immunoglobulins, mPSL=methylprednisolone, ASA=acetyl salicylic acids
On the contrary, our case presented mild symptoms of COVID-19, and was free from any risk factors for PRES. Although the precise pathology remains to be elucidated, our case suggests that PRES can be a complication of even mild COVID-19 infections in children.
Ethical approval statement
The patient’s parents provided informed consent for all the established procedures.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Acknowledgement
We wish to thank Dr. Satoshi Terae for his helpful expert comments on diagnostic imaging.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Antoon J.W., Hall M., Howard L.M., Herndon A., Freundlich K.L., Grijalva C.G., et al. COVID-19 and acute neurologic complications in children. Pediatrics. 2022 doi: 10.1542/peds.2022-058167. (prepublication release) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto K., Motohashi K., Fujiwara H., Ishihara T., Ninomiya I., Onodera O., et al. PRES: posterior reversible encephalopathy syndrome. Brain Nerve (Tokyo) 2017;69:129–141. doi: 10.11477/mf.1416200653. (Japanese) [DOI] [PubMed] [Google Scholar]
- 3.Iftikhar S., Rehman A.U., Ameer M.Z., Nawaz A., Rehman M.A.U., Farooq H., et al. The association of posterior reversible encephalopathy syndrome with COVID-19: a systematic review. Ann Med Surg. 2021;72 doi: 10.1016/j.amsu.2021.103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Haboob A.A. Miller Fischer and posterior reversible encephalopathy syndromes post COVID-19 infection. Neurosciences. 2021;26(3):295–299. doi: 10.17712/nsj.2021.3.20210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korkmazer B., Ozogul M., Hikmat E., Kilic H., Aygun F., Arslan S., et al. Posterior reversible encephalopathy syndrome in a pediatric COVID-19 patient. Pedia Infect Dis J. 2021;40:e240–e242. doi: 10.1016/j.amsu.2021.103080. [DOI] [PubMed] [Google Scholar]
- 6.Arslan G., Besci T., Karaca O., Gelen S.A. Posterior reversible encephalopathy syndrome related COVID-19 in a child. Pedia Int. 2022;64 doi: 10.1111/ped.14908. [DOI] [PubMed] [Google Scholar]
- 7.Fugate J.E., Rabinstein A.A. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–925. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R.C., Patel V., Sheikh-Bahaei N., Lju C.S.J., Rajamohan A.G., Shiroishi M.S., et al. Posterior reversible encephalopathy syndrome (PRES): pathophysiology and neuro-imaging. Front Neurol. 2020;11:463. doi: 10.3389/fneur.2020.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartynski W.S. Posterior reversible encephalopathy syndrome, Part 2: controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lallana S., Chen A., Requena M., Rubiera M., Sanchez A., Siegler J.E., et al. Posterior reversible encephalopathy syndrome (PRES) associated with COVID-19. J Clin Neurosci. 2021;88:108–112. doi: 10.1016/j.jocn.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darwish A.M. Posterior reversible encephalopathy syndrome in children: a prospective follow-up study. L Clin Neurol. 2020;35:55–62. doi: 10.1177/0883073819876470. [DOI] [PubMed] [Google Scholar]
- 12.Fisler G., Monty M.A., Kohn N., Assaad P., Trope R., Kessel A. Characteristics and outcomes of critically ill pediatric patients with posterior reversible encephalopathy syndrome. Neurocrit Care. 2020;32:145–151. doi: 10.1007/s12028-019-00720-9. [DOI] [PubMed] [Google Scholar]
- 13.Stetefeld H.R., Lehmann H.C., Fink G.R., Burghaus L. Posterior reversible encephalopathy syndrome and stroke after intravenous immunoglobulin treatment in Miller-Fisher syndrome/Bickerstaff brain stem encephalitis overlap syndrome. J Stroke Cereb Dis. 2014;23(9):e423–e425. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Yokoi K., Ando T., Kawakami O. Case of posterior reversible encephalopathy syndrome caused by Fisher syndrome. Rinsho Shinkeigaku. 2018;58(1):45–48. doi: 10.5692/clinicalneurol.cn-001089. [DOI] [PubMed] [Google Scholar]