Abstract
Severe forms of pulmonary arterial hypertension (PAH) are most frequently the consequence of a lumen-obliterating angiopathy. One pathobiological model is that the initial pulmonary vascular endothelial cell injury and apoptosis is followed by the evolution of phenotypically altered, apoptosis-resistant, proliferating cells and an inflammatory vascular immune response. Although there may be a vasoconstrictive disease component, the increased pulmonary vascular shear stress in established PAH is caused largely by the vascular wall pathology. In this review, we revisit the “quasi-malignancy concept” of severe PAH and examine to what extent the hallmarks of PAH can be compared with the hallmarks of cancer. The cancer model of severe PAH, based on the growth of abnormal vascular and bone marrow-derived cells, may enable the emergence of novel cell-based PAH treatment strategies.
Keywords: cancer paradigm, pulmonary hypertension
INTRODUCTION
One day, we imagine that cancer biology and treatment—at present a patchwork quilt of cell biology, genetics, histopathology, biochemistry, immunology and pharmacology—will become a science with the conceptual structure and logical coherence that rivals that of chemistry and physics.
—D. Hanahan and R. A. Weinberg, 2000
The formal statement of the “quasi-malignancy paradigm of severe pulmonary hypertension” was published in 2008 (138); it had been encouraged by the seminal article, published in 2000 by Hanahan and Weinberg (65) in Cell (“The hallmarks of cancer”).
For the purpose of the following discussion, we consider as forms of “severe pulmonary arterial hypertension” those diseases that are characterized by an obliterative angiopathy that frequently leads despite therapy to right heart failure and death.
The 2008 publication (138) intended to provide, borrowing heavily from Hanahan and Weinberg (65), not a unifying hypothesis but an overarching pathobiological concept of severe pulmonary arterial hypertension (PAH). The initial question was: “Is there enough evidence and data cohesiveness to adopt the idea that PAH and cancers share important pathogenic mechanisms?”
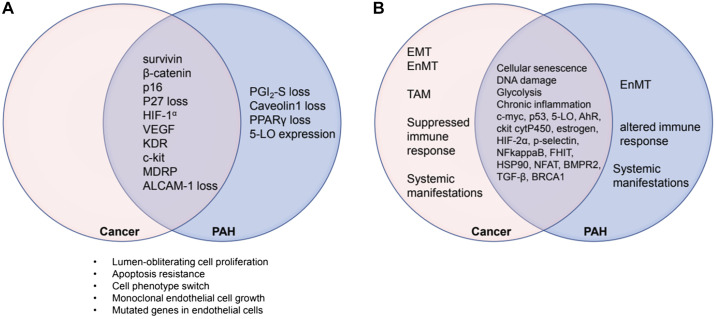
Shared between cancer and PAH are three of the hallmarks published in 2000: apoptosis-resistant cell growth, angiogenesis, and evasion of growth suppressors; whether replicative immortality and sustained proliferative signaling are shared is uncertain, as in-depth investigations of cells harvested and cultured from human PAH lung tissues are presently lacking. The hallmarks “invasion” and “metastasis” are not shared (65). Shared are the hallmarks, added in 2011: genome instability and mutations, inflammation, deregulation of cellular energetics, and avoidance of immune destruction (66). Figure 1 illustrates the similarities in the pattern of expressed genes and proteins as recognized in 2008 and the knowledge gained by 2019. “Quasi-malignancy” simply refers to the observation that there are several hallmarks shared between cancer and severe PAH (31, 174, 182). The reader is also referred to a recent publication that reflects a Pro-Con debate that took place in 2018 during the annual meeting of the American Thoracic Society (159).
Fig. 1.
Venn diagrams showing the state of knowledge in 2008 (A) and in 2019 (B). The overlapping areas represent proteins, which are expressed both in cancers and in pulmonary arterial hypertension (PAH) lung tissue. ALCAM-1, activated leukocyte cell adhesion molecule; EMT, epithelial mesenchymal transition; EnMT, endothelial mesenchymal transition; HIF-1α, hypoxia-inducible factor-1α; KDR, VEGF receptor 2; 5-LO, 5-lipoxygenase; MDRP, multiple drug resistance protein; PGI2, prostacyclin; PPARγ, peroxisome proliferator-activated receptor-γ; TAM, tumor-associated macrophages. The overlap area in B represents protein expression data and mechanistic aspects of the “quasi-malignancy” concept like “glycolysis” and “DNA damage” that have been added to the literature since 2008. For more details, see Table 1.
Is it reasonable to compare solid tumors caused by somatic mutations due to repetitive carcinogenic stimulation (inflammation, cigarette smoke, toxins) to a nonmetastatic, primarily vascular condition? Even when admitting that cigarette smoking can cause lung diseases associated with pulmonary hypertension, cigarette smoking has been related only rarely to the development of a plexogenic pulmonary arteriopathy (24). The comparison appears to be justified, as the theoretical framework proposed here to explain the development of the angioobliterative microvasculopathy is entirely compatible with the concept of cancer as defined by Harold Dvorak: “Cancer is the wound that never heals.” (42). It is likely that the concept of “wound healing gone awry” does also apply to PAH (182). Chronic endothelial injury (due to autoantibodies, infection, or high shear stress) and increased endothelial vulnerability to apoptosis (due to BMPR2 and other gene mutations; see Ref. 44) are central in the development of PAH.
Here, we critically review to what extent recent discoveries in the pulmonary vascular research field continue to support the concept of the cancer paradigm of PAH. This approach also allows for the identification of knowledge gaps and future research goals. It is not possible to discuss each of the PAH hallmarks in detail (excellent reviews have been published recently; 18, 60, 77, 134, 167). Instead, we chose to highlight the areas of endothelial cell mesenchymal transition (EnMT) (87), DNA damage, and mutations.
Lastly, why does this conversation about the cancer model of PAH matter? PAH patients continue to die because we still have no disease-modifying treatments for severe PAH.
Tumor, Hyperplasia Apoptosis Resistance, and Cancer
Most investigators have subscribed to Bert Vogelstein's definition that cancer “is caused by a sequential series of alterations in well-defined genes that alter the function of a limited number of pathways” and “that a set of driver genes and pathways is responsible for the most common forms of cancers. These alterations directly or indirectly increase the ratio of cell birth to cell death” (185). In contrast, a “tumor” is an abnormal or malignant new growth of tissue that possesses no physiological function and arises from uncontrolled, usually rapid cell proliferation (Merriam-Webster), while “hyperplasia” is the increase in the number of normal cells due to proliferation, which ceases after the triggering stimulus has been removed.
In severe PAH, the disease-defining cells within the vascular wall are abnormal, resulting in apoptosis-resistant cell growth (149, 182), which increases the ratio of cell birth to cell death. The concept of apoptosis-resistant cell growth—as PAH disease is defined—has found wide acceptance during the past decade (50, 167). Yet, it is important to emphasize that increased cellular proliferation in itself will lead to genetic alterations that may result in a vicious feed-forward loop, thereby raising the age-old chicken-egg question (3, 29). In Table 1, we compare some of the cellular and molecular hallmarks that are shared, or not, between cancer and PAH. In Table 2, we provide a list of the features and conditions, which do not support a cancer hypothesis.
Table 1.
Comparison of the cancer hallmarks with PAH hallmarks
| Hallmarks of Cancer (65) | Hallmarks of PAH |
|---|---|
| General aspects | General aspects |
| “Evolution gone awry” (66)* | “Wound healing gone awry” (42)** |
| Systemic disease*** | Small lung vessels, but PAH can be a manifestation of systemic diseases or heart diseases and there are systemic disease manifestations (115, 159) |
| Sustained proliferative signaling | |
| Growth factors, survival factors | Endothelial cell and vascular smooth muscle cell growth (31, 134, 138, 174) |
| Hormones, increased levels of receptor proteins, anti-apoptotic mechanisms (including survivin expression) | Increased sensitivity to growth factors (76, 78, 175) |
| Akt/PKB, Ras, c-MYC, c-KIT, p53, HIF-1α, HIF-2α, p27Kip1, FHIT (80), HSP90 (16), NFAT, β-catenin, FGF, MDRP (144), MRP4 (85), PDGF, 5-LO (89) | Increased expression of HIF-1α, HIF-2α, ARNT, VEGF, KDR (175), NFAT (15), 5-LO (190), β-catenin (138), MDRP (138), MRP4 (67) |
| Survivin (103, 138), loss of p27, Kip1 (31), HSP90 (19), c-KIT (38, 45, 46, 60, 108), FHIT (41) | |
| Genome instability, mutations | |
| Mutations (including BMPR2, BMP9, SOX2, KDR):“corruption of the TGF-β pathway,” PARP (65), many other gene mutations (KRAS, HRAS, BRAF, BRCA, PTEN, p53) (86, 157), KDR (170) | Mutations: BMPR2 (4), BMP9, SOX2 (60), aquaporin 1, CAV1 (177), SMAD9, ACVRL1, ENG, EIF2, AK4, KCN5, KCN3, PARP (103), KDR (44) |
| Chromosomal abnormalities | Chromosomal abnormalities (2) |
| Insensitivity to growth suppressors | |
| Cyclin-dependent kinases | Cyclin-dependent kinases (187) |
| Prostacyclin induces VSMC apoptosis via ERK1/2 (97), decreased BMPR2 signaling (4, 43) | |
| Resisting cell death | |
| Bcl-2 family, Bax, Bim, Puma, survivin | Decreased BMPR2 signaling (4, 43) Increased survivin expression (103, 138) |
| Loss of p53 function? Bax mutation (193) | |
| Enabling replicative immortality | |
| Telomerase (23) | Telomerase expression in PAH (111) supports hyper-proliferation of endothelial cells |
| Involvement in proliferation via the β-catenin/LEF transcriptional complex? (138) | |
| Sustained angiogenesis | |
| Upregulated by hypoxia and oncogenes; endogenous angiogenesis inhibitors Bone marrow-derived vascular progenitors |
VEGF, KDR (175) upregulated by hypoxia and oncogenes VEGF produced by inflammatory cells Angiogenic/anti-angiogenic imbalance Endostatin (39, 72), sFLT (148) and others |
| IL-32 (195) | IL-32 (119) |
| Phenotypic instability | |
| Epithelial mesenchymal transition | Endothelial mesenchymal transition (73, 87, 139) |
| 5-Lipoxygenase expression (107) | 5-Lipoxygenase expression (190), loss of TGF-β receptor 2 (193) |
| Microenvironment and inflammation providing growth signals and contributing to clonal expansion (33, 34, 156) | |
| Smoldering inflammation, presence of cells that provide growth and survival factors (181, 182) | |
| Il-1α, IL-1β (90) | IL-1α, IL-1β (180) |
| Stem cell niche? (171) Obesity (151), leptin (151) Red blood cell distribution width |
Stem cells (7, 48, 196) Obesity (23), leptin (75), dyslipidemia (70) Red blood cell distribution width |
| Reprogramming energy metabolism | |
| Glycolytic switch (Warburg effect) (191), glutaminolysis | Hypoxia upregulates HIF-1α and HIF-2α, which upregulate glycolysis (Warburg effect) (5, 19, 43, 196), upregulation of GLUT1 |
| Cancer stem cells (171) | |
| Bone marrow-derived stem cells (7), vessel wall stem cells (48, 155), circulating endothelial cell precursors (7, 22) | |
| Evasion of immune destruction | |
| Deficiency in development or function of cytotoxic CD8+ CD4+ Th1 helper cells Secretion of immunosuppressive TGF-β (66) |
Immunosuppressive T regs? (163) |
ACVRL1, activin A receptor like type 1; AK4, adenylate kinase 4; BMP9, bone morphogenetic protein 9; BMPR2, bone morphogenic protein receptor type II; CAV1, caveolin 1; EIF2, eukaryotic initiation factor 2; ENG, endoglin; GLUT1, glucose transporter 1; HIF-1α, hypoxia-inducible factor-1α; HIF-2α, hypoxia-inducible factor-2α; HSP90, heat shock protein 90; KDR, VEGF receptor 2; MDRP, multiple drug resistance protein; PAH, pulmonary arterial hypertension; PARP, poly (ADP-ribose) polymerase; PTEN, phosphatase and tensin homolog; sFlt, soluble VEGF receptor 1; SMAD9, SMAD family member 9; TGF, transforming growth factor.
This concept relates to a process “formally analogous to a Darwinian evolution, in which a succession of genetic changes, each conferring one or another growth advantage, leads to progressive conversion of normal human cells” (65).
“Wound healing gone awry” (42); injury to the endothelium is not repaired with a return to a normal endothelial cell monolayer; instead, exuberant endothelial cell growth (174) occurs, leading to lumen obliteration and fibrosis; inflammatory and immune cells participate in this process (174).
Although metastatic spread turns cancer into a systemic disease, some cancers can remain localized.
Although stem cells in PAH are not “cancer” stem cells, they may nevertheless participate in the formation of complex vascular lesions.
Table 2.
Characteristics and conditions that do not support a cancer hypothesis of PAH
| Characteristics and Conditions |
|---|
| In PAH, there are no metastases to the liver, bone, brain, or adrenals. |
| Cells harvested from human PAH lungs do not exhibit unlimited growth.* |
| Tumor vessels are leaky; vessels obliterated by proliferating cells are not.** |
| Driver mutations of KRAS, HRAS, BRAF, and BRCA are not implicated in PAH. |
| There are long-term survivors of severe PAH. |
PAH, pulmonary arterial hypertension.
The cells harvested and cultured may not be the relevant vascular lesion cells; see text for more details.
The initial phase of the PAH lung vessel disease is unknown; whether in early PAH vessels exhibit an increase in permeability is unknown.
In the following, we take the Hanahan and Weinberg (65) cancer hallmarks as the blueprint for our discussion. It can be appreciated that the list of PAH-associated cellular and molecular abnormalities (Table 1) is most likely a work in progress. Following the publication of the second Hanahan and Weinberg (66) paper, other authors have added additional mechanistic aspects to the original “hallmarks” and emphasized telomeres, matrix-cell interactions, and other unifying aspects (22, 101, 104, 131, 156, 157, 171). Within the limits of available published reports in the pulmonary hypertension (PH) literature, these additional aspects are also considered in Table 1 (137, 139, 143, 152, 155, 175, 178, 180, 190, 193, 196).
The 2008 publication that introduced the concept of “quasi-malignancy” of severe, angioobliterative forms of PAH (138) has resonated with many investigators. A recent “Perspective” by Pullamsetti et al. (134) outlines proliferative and pro-survival signaling pathways and potential drug targets that are of interest in cancer and PAH, emphasizing mTOR and tyrosine kinase inhibitors that target signaling hubs. Boucherat et al. (18) published a comprehensive review entitled “The cancer theory of pulmonary arterial hypertension,” D’Alessandro et al. (38) recently reviewed “The Hallmarks of Pulmonary Hypertension,” and Guignabert et al. (60) discussed in their “Pathogenesis of pulmonary arterial hypertension” “the lessons learned from cancer”. A Canadian group of investigators report the aberrant expression of several genes in the lung specimens from PAH patients and appreciate the phenomenon of cell phenotype shifts as a Darwinian (evolutionary) “cancer cell identity crisis” (20, 147). Recently, Napoli et al. (113), emphasizing “the multifactorial nature of PAH”, focused on “epigenetic factors that can bridge genetic [e.g., BMPR2 (4, 43)] and environmental diseases,” favoring a concept of “dysregulation of a network-based molecular architecture”. Such an epigenomic hypothesis of PAH encourages the discovery of triggers or “hits” that are apparently required to turn BMPR2 mutation carriers into PAH patients; epigenomics can also begin to explain molecular mechanisms underlying drug-induced forms of PAH (123). For details concerning DNA methylation and histone modifications as part of the repertoire of mechanisms underlying epigenetic modifications, the reader is referred to this publication (113) and to the reviews by Boucherat et al. (18) and D’Alessandro et al. (38). A more recent paper adds to the list of epigenetic factors the aryl hydrocarbon receptor-cytochrome P450 axis (91).
Shared Expression of Proteins in Cancer and PAH: Examples
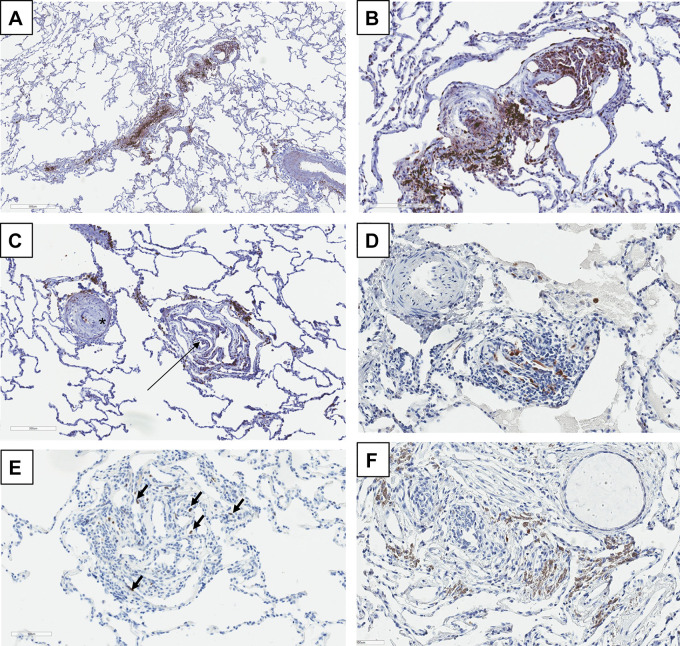
In PAH, the cellular senescence marker p16 is expressed in many of the plexiform lesions (Fig. 2) (138), and cellular senescence (142) can now be interpreted according to the Hallmarks 2 paper (66). “Cells with morphological features of senescence, including enlarged cytoplasm and the expression of the senescence-induced β-galactosidase enzyme are abundant in the tissues engineered to over-express certain oncogenes. These ostensibly paradoxical responses seem to reflect intrinsic cellular defense mechanisms designed to eliminate cells experiencing excessive levels of certain types of signaling.” Cellular senescence is an irreversible arrest of cell growth. It can be triggered by DNA damage (105), oncogene activation, and oxidative stress (176). These triggers are present in PAH. Noureddine et al. (120) reported p16 expression in the pulmonary artery smooth muscle cells from patients with COPD and pulmonary hypertension; conditioned medium from these p16+ cells stimulated the growth of nonsenescent smooth muscle cells via released paracrine factors. The role of telomerase in PH has been discussed by Mouraret et al. (111).
Fig. 2.
Immunohistochemical analysis of plexiform lesions. A: immunohistochemical stain for cMyc (brown color). Low-power view of a plexiform lesion showing near total obliteration of the lumen along the long axis of the small pulmonary artery. B: same vessel at higher power, demonstrating the nuclear staining of the endothelial cells lining the irregular lumina of the plexiform lesion. cMyc is a transcription factor regulating sets of genes involved in cell proliferation; it is an early growth response gene that is rapidly induced when quiescent cells receive a signal to divide. C: cMyc staining of a concentric obliterative and plexiform lesion from a different patient with pulmonary arterial hypertension (PAH). The concentric obliterative lesion on the left (asterisk) shows fewer positive cells than does the plexiform lesion on the right (arrow). D: immunohistochemical staining for the senescent cell marker p16 in a plexiform lesion. Note the strong nuclear endothelial cell staining. E: immunohistochemical stain for p53 (the antibody recognizes both normal and mutated p53 proteins). There are scattered positive cells demonstrating nuclear staining (arrows). There is a lack of p53 staining in the remainder of the lung parenchyma; only endothelial cells of the plexiform lesion are positive. F: a plexiform lesion stained for the HIF-1α protein (brown). There is a predominantly cytoplasmic staining pattern of the endothelial cells. Inset shows this staining pattern more clearly. A role for hypoxia-inducible factor-2α (HIF-2α) in pulmonary arterial cell proliferation has recently been described (165).
The presence of other proteins expressed in cancer cells has been documented in the vascular lesions from PAH patients (138), for example, β-catenin and the multiple drug resistance protein (MDRP). β-Catenin functions as an adherence junction protein for cell-cell adhesion and as a cancer driver via the Wnt/β-catenin pathway; β-catenin also plays a role in the T cell infiltration of tumors (54, 188). Indeed, the β-catenin/cancer literature is too voluminous to be reviewed here. The question of whether the high expression of β-catenin in plexiform lesions (138) is indicative of endothelial cells acquiring mesenchymal or stem cell properties has not been examined. However, β-catenin is required for EnMT during heart cushion development, and we postulate that high expression of β-catenin is likely part of the pulmonary vascular EnMT program (87). The multidrug resistance protein MDR1, also known as p-glycoprotein or ATP-binding cassette subfamily B member 1 (ABCB1), is expressed in macrophages and cardiac cells and highly expressed in a number of malignancies. This protein can efflux a number of cancer drugs, and its expression in cancer cells has been shown to correlate with a poor chemotherapy response and poor survival (35, 49, 64, 86). A large number of proteins expressed in cancer and PAH, including cMyc, also known as the “oncogene from hell” (189), the nuclear factor of activated T cells (NFAT) (15), STAT3, FOXO1 (153), YAP/TAZ (11), multiple drug resistance-associated protein 4 (MRP4) (67, 85, 152), and others have been added (see Fig. 1 and Table 1).
HALLMARKS OF CANCER AND PAH: THE LAST 10 YEARS
Cell-Cell Interactions
Central to the cancer hallmarks theory (66) and likewise central to the PAH hallmarks are the multiple cell-cell interactions.
Hanahan and Weinberg (65) have emphasized the importance of the tumor environment and of stromal cells, cancer stem cells, and cells of the immune system. As monocellular in vitro cancer studies are of a limited scope, it is doubtful that the investigation of cultured endothelial cells obtained from the lungs of PAH patients, which demonstrate a limited cell growth, can elucidate the cancer paradigm of PAH. While the normal wall of the small pulmonary vessels is complex, with the syncytial relationship between endothelial cells and VSMC, the “sick lung vessels” with their phenotypically changed cells and infiltration by multiple inflammatory cell types is infinitely more complex (174, 182). There are at least two reasons why cells cultured from PAH vessels do not exhibit unlimited growth. First, it is unclear whether the relevant vascular lesion cells have indeed been harvested and cultured; presently we lack important information regarding the phenotypical characterization of these cells. For example, we do not know whether the cultured endothelial cells (EC) express 5-LO, FLAP (190), and survivin (138) or lack expression of the PGI2 synthase and of caveolin and peroxisome proliferator-activated receptor-γ (PPARγ) (181). Second, precursor and stem cells (62, 63) and factors or cofactors that drive cell proliferation in situ may be missing in a monocellular culture environment. Complex multicellular lesions have been found at the sites of small vessel bifurcations believed to be sites of high shear stress and perhaps turbulent flow (31). Another explanation regarding the localization of the complex lesions is that the EC at these sites are phenotypically distinct or that the cell proliferation is largely driven by stem cells (48, 59, 172).
Infiltration of immune and inflammatory cells is a hallmark of cancer and plays a critical role in tumorigenesis by creating an inflammatory microenvironment that promotes tumor growth (150). Analogously, PAH is characterized by the perivascular infiltration of innate and adaptive immune cells, including mast cells, macrophages, and B and T cells (21, 88). Evidence from recent preclinical and epidemiological studies suggests a critical contribution of immunity and its regulation by inflammatory cytokines [in particular IL-6 (136)], leukotrienes [LTB4 and LTC4 (89, 169, 190)], and damage-associated molecular patterns [DAMPs; e. g. high-mobility group box 1 (HMGB1) (194)] in the pathogenesis and/or progression of PAH. These inflammatory mediators directly (and potentially, synergistically) stimulate proliferation of lung vascular cells via paracrine and endocrine pathways (88, 152). They may also drive the formation of perivascular ectopic tertiary lymphoid tissues consisting of B cells, T cells, and high-endothelial venules (130) as germinal centers for the generation of auto-antibodies (30), which in turn promote endothelial cell proliferation and PH development (99, 130). Endothelial cells of PAH patients show increased expression levels for a variety of adhesion molecules and increased interaction with peripheral blood mononuclear cells (94). Notably, expression of the adhesion molecule P-selectin has recently been shown for pulmonary artery smooth muscle cells of PAH patients, yet not in healthy controls, so that immune cells infiltrating the vascular media may potentially stimulate vascular remodeling via direct cell-cell interaction (121). While one finds detailed mechanistic studies of tumor-associated macrophages (162) and the role of neutrophiles in tumor metastasis (1), such detailed studies are still missing in PAH research.
Parallels between the roles of inflammation and immunity in cancer and PAH have recently been highlighted by the discovery of extensive vascular remodeling in lung cancer tumor tissues and signs of elevated pulmonary artery systolic pressure and pulmonary artery enlargement in 45–50% of patients with lung cancer (135). Importantly, these morphological and hemodynamic changes are suppressed by genetic inhibition of NF-κB-dependent cytokine production in lung tumor cells in mice (141). Hence, the pro-inflammatory tumor micro-milieu favors cancer growth and lung vascular remodeling in a similar fashion. Conversely, in both cancer and PAH, the body’s natural immune defense against the hyperproliferating cells is suppressed. Cancers use inhibitory checkpoints such as CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) or PD1 (programed cell death) to suppress immune responses targeting cancer cells. In the normal lung, natural killer (NK) cells prevent vascular remodeling and the development of PH as demonstrated by the development of spontaneous PH in mice deficient in NK cells (141); importantly, NK cells are reduced in numbers and impaired in function in PH animal models and PAH patients (125). A similar inverse relationship exists for regulatory T cells and PH (163). As such, both cancers and PAH seem to actively suppress mechanisms of immune defenses. This notion may explain why, despite the important role of the inflammatory microenvironment outlined above, untargeted immunosuppressive therapies, e.g., corticosteroids, do not present promising therapeutic options in either disease.
Autoimmunity, Chronic Inflammation, and Cancer
Autoimmunity and cancer have a reciprocal relationship that may be germane to our general understanding of PAH triggers and progression. Like cancer, autoimmune disease can emerge in a single organ and cause chronic inflammation because of immune dysregulation. Cancer arises in part as a failure of the organism’s immune system to detect and destroy abnormal cells and grows because of a maladaptive form of immune tolerance (145). The basis of checkpoint inhibitors for cancer is to unfetter and re-engage immune surveillance and directed destruction. Not surprisingly, autoimmunity occurs after treatment with those therapeutics as collateral damage, arising as a consequence of excessive immune activation and loss of self-tolerance. A preclinical model of immune dysregulation-associated PH shows that blocking the key PD-1/PDL-1 tolerizing signal abrogates Treg-mediated protection against PH and, in this manner, shows how autoimmunity can induce PAH in a cancer-relevant context (164).
Autoimmunity may also be a stimulus for chronic inflammation, which can drive oncogenic processes (33, 57). Chronic inflammation causes DNA damage, which can lead to cancer. Malignancy arises at sites of chronic inflammation where the microenvironment fosters cellular proliferation, survival, and migration. Neoplastic cells in turn can co-opt signaling molecules of the innate immune system, including chemokines and selectins to facilitate abnormal patterns of cellular migration and invasion. In PAH, abnormal cell proliferation, survival, and migration are highly implicated in the processes, which lead to intimal obliteration. For these pulmonary vascular syndromes, the source of chronic inflammation, which drives cancer-like changes, may evolve as a consequence of the poor immune regulation of autoimmunity.
Vascular injury can transform a singular inflammatory event into a chronic inflammatory condition manifested within the pulmonary vascular lesion itself, which becomes a nidus for immunity. A notable illustration of this idea is the acquisition of intimal 5-lipoxygenase (5-LO) expression with the evolution of PAH (190, 197). This neo-expression of 5-LO may occur as a consequence of a single inflammatory vascular injury in the setting of poor immune regulation (169). As the enzyme responsible for leukotriene biosynthesis, neointimal 5-LO expression can drive the recruitment of inflammatory cells and create a self-sustained chronic inflammation that drives tissue changes reminiscent of malignant transformation (109). As we have mentioned, apoptosis-resistance, as documented by absence of protein signals in the complex vascular PAH lesions, which identify cells undergoing apoptosis, has been associated with the development of autoantibodies (6).
Limitations and Knowledge Gaps
As pointed out, autoantibodies have been associated with several forms of severe PAH (30), and mechanistic concepts are largely derived from animal models. We are uncertain whether infections trigger a lung vascular immune response and whether endothelial cell injury exposes epitopes that attract antibodies in the context of molecular mimicry.
Apoptosis, Phenotype Switch, DNA damage, Autophagy, and Anoikis
Tumor cells evolve a variety of strategies to limit or circumvent apoptosis. Most common is the loss of p53 tumor suppressor function (Figs. 2 and 3). Alternatively, tumors may achieve similar ends by increasing the expression of antiapoptotic regulators (Bcl-2, Bcl-xL) or of survival signals or by downregulating proapoptotic factors (Bax, Bim, Puma). Recent research has revealed intersections between the regulatory circuits governing autophagy, apoptosis, and cellular homeostasis (66). In the PAH vascular wall, cells undergo a phenotypic switch that generates apoptosis resistance, and endothelial cell mesenchymal transition (EnMT) is just one manifestation of the spectrum of pulmonary vascular endothelial cell phenotypes that spans from the quiescent monolayer to endothelial cell activation and inflamed endothelial cells to angiosarcomatous cells that have been detected in thromboendarterectomy samples (9).
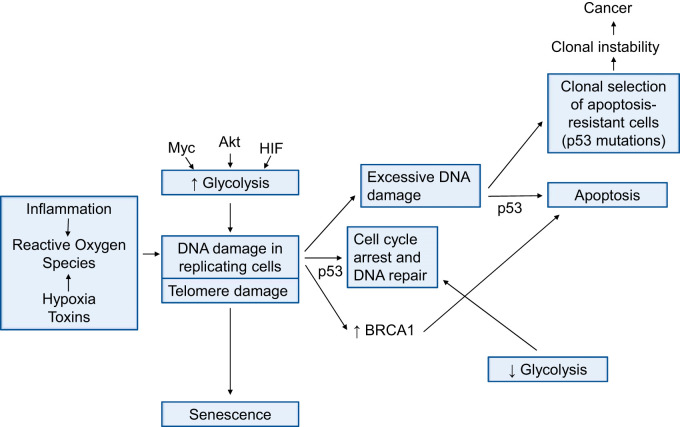
Fig. 3.
This diagram illustrates an example of how connecting dots between inflammation and hypoxia, DNA damage and repair, glycolysis control, oncogenes, p53, apoptosis, and emergence of apoptosis-resistant proliferating cells can generate specific hypotheses. The general hypothesis underlying this concept is that the initial pulmonary vascular endothelial cell damage is the critical disease-initiating event in genetically susceptible individuals. The diagram highlights the importance of endothelial cell DNA damage.
Phenotype Switch and Endothelial Cell Mesenchymal Transition
Altered cellular energy metabolism, in particular aerobic glycolysis (the Warburg effect), is a hallmark of cancer but also of severe angioproliferative PAH (5, 16, 18, 43, 134, 196). Glycolysis is controlled, among others, by p53, the oncogene cMyc and HIF (Fig. 3) (36, 68). Hypoxia-inducible factor-1α (HIF)-1α and HIF-2α play roles in cancer and PAH (36, 37); by binding to the hypoxia response elements (HRE), these proteins control the transcription of a large number of genes (the expression of HIF-1α in PAH arterioles is shown in Fig. 2). Because the mitochondrial and metabolic abnormalities in PAH cells have been extensively reviewed (5, 196), we restrict our discussion to examine why and how the Warburg effect supports cell proliferation in PAH. Briefly, when Otto Warburg in 1924 discovered that cancer cells “fermented” glucose to lactate, neither oncogenes nor growth factor signaling pathways had been discovered. Nowadays, it is appreciated that the metabolic requirements for cell proliferation are met through several signaling pathways that regulate both cell proliferation and metabolic pathways and that oncogenic mutations enable cancer cells to metabolize nutrients for proliferation, rather than efficient ATP production [see Vander Heiden et al. (177) for more details]. This redirection of energy metabolism, the “metabolic switch,” is one characteristic underlying the phenotype switch. Figure 3 illustrates an exercise in “connecting dots” to arrive at a hypothesis that needs to be tested in the context of PAH. The possible and perhaps likely interactions between glycolysis and cell growth and the influence of p53 and cMyc can be dissected when phenotypically relevant cells have become available.
EnMT is one example of the cell phenotype switch and may be the PAH counterpart of epithelial cell transition (EMT) in cancer, although EnMT does also occur in cancers (132). EnMT is the process whereby endothelial cells lose their typical endothelial cell markers and, importantly, their functions and adopt a mesenchymal-like cell type. A comprehensive recent review article by Kovacic et al. (87) dicusses EnMT in the context of cardiovascular diseases. Partial (pEnMT) and complete EnMT (cEnMT) cells have been described; cEnMT cells exert paracrine effects, stimulating mesenchymal cell migration and endothelial angiogenic capacity (161). One driver of EnMT is TGF signaling (87, 127), and EnMT can also be stimulated by extracellular vesicles. Inflammation via downregulation of BMPR2 expression promotes EnMT (73, 152), and so does galectin 3 (99). Mechanical factors and metabolic changes and HIF-2α (165) are also triggers (191). Finally, Tsutsumi et al. (173) reported that nintedanib reduced EnMT in the Sugen/hypoxia rat model; however, this was not confirmed by the study by Rol et al. (146). An important unanswered question is whether EnMT in the “sick lung circulation” (182) is central to pulmonary vascular remodeling and amenable to treatment.
DNA Damage, Causes, and Consequences: Apoptosis and Cancer
DNA damage (DNA breaks and other chromosomal abnormalities) triggers apoptosis but also cellular senescence and the selection of apoptosis-resistant cells (Fig. 3). Indeed, DNA damage and chromosomal abnormalities in PAH have been reported (2, 26, 96, 105, 140). Federici et al. (47) examined DNA damage in lung and blood cells from patients with severe forms of PAH and also found an increased sensitivity to DNA damage in relatives of PAH patients when exposing peripheral blood monocytes to DNA-damaging agents.
DNA damage results in an abnormal chemical structure of DNA. It is caused by inflammation- and hypoxia-related reactive oxygen species (186) and by (environmental) toxins. DNA damage triggers the activation of various damage response pathways and changes the activation of a number of cell fate controlling genes, including p53, p21, NF-κB, and BRCA1 (the reader is referred to several excellent reviews of the topic of DNA damage and repair; see Refs. 10, 53, 71, 128). Most cancers are associated with p53 mutations. One of the many functions of p53 is the suppression of glycolysis (81). Figure 2 shows the expression of p53 in a plexiform lesion; because the antibody used for this immunohistochemistry (IHC) stains mutated p53 as well as wild-type p53, we thus identify a knowledge gap and the need to examine whether there are p53 mutations in PAH. Whether there are p53 polymorphisms in PAH patients is also unknown. Figure 3 is a simplified diagram that shows how oncogenes, DNA damage, glycolysis, apoptosis, and cancer are connected. This diagram may serve as an aid to further investigate how DNA damage to pulmonary vascular cells results in a phenotype switch [see also (128)]. As an aside, there is evidence that endothelin 1 promotes anti-apoptosis in keratinocytes (81, 97). It would be of interest to find out whether endothelin 1 also contributes to the apoptosis resistance of endothelial cells in PAH.
It is still unclear to what extent the Sugen 5416-based models of severe PAH (116) mimic the various forms of severe human PAH, yet the Sugen/chronic hypoxia model data suggest that the profound pulmonary arteriolar obliterations are initiated by endothelial cell apoptosis. Sugen 5416 inhibits VEGF receptor 2 (KDR) (166), and a recent report describes KDR mutations, resulting in impaired VEGF signaling, in patients with familial PAH (44). In line with this notion of pathobiologically important endothelial cell apoptosis, induction of endothelial apoptosis suffices to induce obliterative vascular lesions in mice (58). Translating these findings to the human condition lets us arrive at a model of human PAH; initial endothelial cell damage and apoptosis is followed by the evolution of apoptosis-resistant cell types. Ngo et al. (114) found obliterated lung vessels in Ku 70-knockout mice. Ku 70 (Lupus Ku antigen p70) is essential for DNA double-stranded repair; Ku 70 is anti-apoptotic by suppressing intrinsic cell death signaling via Bax. Absence of Ku 70 leads to Bax hyperactivation. This finding is likely of importance because of the loss of the Ku 70 gene expression detected by gene microarray expression analysis of lungs from patients with idiopathic PAH (56).
Autophagy (51, 79) normally operates at low basal levels but can be strongly induced by cell stress. The autophagy marker LC3B was detected in the lungs from PAH patients (93); the combination of two cell stressors caused autophagy in macaque lungs (122), and Kato et al. (83) showed that autophagy can slow PAH progression. “Paradoxically, autophagy can be protective in cancer cells; severely stressed cancer cells have been shown to shrink via autophagy to a state of reversible dormancy. This survival response may enable the persistence and eventual regrowth of some latent -stage tumors following treatment with anticancer drugs” (66).
Anoikis (Greek: “homeless”) is defined as cell death following the detachment of cells from their matrix. Anoikis resistance is part of the survival skills of cancers. Interestingly, anoikis resistance can be induced by shear stress (98), STAT3 (100), and β-catenin (95, 124). We speculate that, in analogy to cancer, the phenotype switch of the cells and also an “integrin switch” (32) in the lumen-obliterating cell aggregates also affect anoikis-resistance. In the Sugen/hypoxia model of severe, angio-obliterative PAH, induction of anoikis markers has been reported, as shown by immunohistochemistry and protein analysis (14).
Angiogenesis: Anti-angiogenesis
In severe forms of PAH, we find the pruning of the pulmonary vascular tree and angioobliteration due to a process of “misguided angiogenesis” (175). These side-by-side manifestations of vascular changes in PAH lungs have been a pathobiological puzzle. One possible explanation for the coexistence of pruning and obliteration is that both proangiogenic and anti-angiogenic factors are involved in the pathogenesis of PAH (39, 72, 148). However, if the root cause of vascular obliteration is endothelial cell apoptosis, as in the Sugen/hypoxia rat model (166), and, if indeed these findings that describe early and likely subsequent cell proliferation-initiating EC apoptosis in the animal model reflect early disease stages in human PAH, then the vascular homeostatic imbalance is initially tilted toward anti-angiogenesis. Yet functionally, the pruning of the vascular tree is not sufficient to fully explain the development of severe PAH, because many patients with end-stage emphysema and dramatic loss of lung vessels do not manifest severe PAH at rest.
VEGF is a cardinal angiogenesis and endothelial cell maintenance factor. Experimentally, inhibition of VEGF signaling causes emphysema associated with lung vessel loss (82). During the last decade, circulating anti-angiogenic proteins have been documented in PAH patients, and there are angiogenic and anti-angiogenic splice variants of the VEGF ligand (183). In this context, severe angioproliferative PAH may be a disease of anti-angiogenic loss of small lung vessels and a parallel or subsequent activation of an angioproliferative program. Anti-endothelial cell antibodies and molecular mimicry may play a role together with the anti-angiogenic circulating sFlt (soluble VEGF receptor 1) and endostatin (39, 92). While it is appreciated that angiogenesis is defined as the growth of new vessels from existing vessels in PAH, the growth of new vessels is within the vascular lumen (Fig. 2). Similarly as in tumor angiogenesis, the lumen-filling endothelial cells that form pseudo-lumina are abnormal and phenotypically switched (see above).
Histological analysis of premalignant, noninvasive lesions, including dysplasias and in situ carcinomas, has revealed the early tripping of the angiogenic switch. Some tumors, including such highly aggressive types as pancreatic ductal adenocarcinomas, are hypovascularized and replete with stromal “deserts” that are largely avascular and indeed may be actively antiangiogenic. The switching mechanism can vary in its form, even though the net result is a common inductive signal (e.g., VEGF). In some tumors, dominant oncogenes such as Ras and Myc (Fig. 2) can upregulate expression of angiogenic factors. Interestingly, wound healing is impaired by elevated expression of anti-angiogenic factor genes (66).
We conclude this discussion of the angiogenesis hallmark of PAH with a word of caution; well-intended anti-angiogenic treatment strategies designed to induce cell death of the pulmonary vascular lesion-obliterating cells may cause as collateral damage vessel loss in other organs, in particular in the failing right ventricle.
To summarize, while EnMT in PAH had previously been declared a hallmark that when present would strengthen the quasi-malignant concept of PAH (107), EnMT has now been documented (87). The inclusion of the angiogenesis/anti-angiogenesis part of the cancer-like disease model of PAH is justified, although the data in support of this concept are presently associations and correlations, and interventional studies in human PAH are lacking. Further studies, which would support a participation of autophagy and anoikis, need to be conducted using human PAH tissues.
Bone Marrow, Stem Cells, and Metastases
There is convincing evidence for a bone marrow-lung circulation axis (7, 8). Clinicians have long appreciated that patients with myelodysplastic disorders can develop severe PAH (55). On the other hand, there have been reports of myelofibrosis in patients with idiopathic PAH (133). There are also reports on patients developing severe PH following bone marrow transplantation (40). In addition, megakaryocytes (Cool CD, unpublished observations) and dendritic cells of bone marrow origin are found in and around plexiform lesions, and it remains unresolved whether some of the mast cells that are found in the plexiform lesions arrived from the bone marrow.
It has been pointed out that one of the hallmarks of cancer, “metastases,” is missing in PAH. “In addition to local signaling within the primary tumor environment, tumors also signal over long distances to sites of future metastases to promote the formation of a hospitable pre-metastatic niche that will foster growth of disseminated tumor cells upon their arrival” (129). We appreciate that avenues connecting the bone marrow and the lung vessels are the systemic bronchial vessels and the vasa vasorum (118). In severe PAH, the “sick lung circulation” (181) inflamed endothelial cells, macrophages, mast cells, and others, producing factors (for example, VEGF) that activate the bone marrow to release cells, including precursor cells. Abnormal endothelial cells are likely being sheared off and enter the systemic bronchial circulation, although the proof of such a “metastatic” lung vascular cell release is still pending. Such sheared-off cells could seed at the site of bronchial-pulmonary arterial anastomoses and form plexiform lesions (52). Perhaps liquid biopsies and single-cell RNA sequence analysis can test such a hypothesis. While there is no evidence of local vascular invasion, hematogenous and lymphatic spread of “sick lung cells” is possible.
Presently, a complete understanding of bone marrow-derived precursor cells, circulating and pulmonary resident stem cells, is lacking. Animal experiments have yielded data that demonstrate that bone marrow cells contribute to the pulmonary vascular disease but also that hematopoietic stem cells protect against PH (8, 60, 192).
Systemic Disease Components of PAH
Recent studies have drawn attention to extrapulmonary disease manifestations in patients with severe PAH. Coronary artery disease, renal disease, and skeletal muscle capillary rarefaction have been reported; a comprehensive review of these manifestations has recently been published (115), and the reader is also referred to a recently published pro and con debate (159). These systemic manifestations do not necessarily mean that severe idiopathic forms of PAH are systemic diseases. The more likely mechanistic interpretation is that the “sick lung circulation” produces “bad humors” that can cause damage in the periphery, for example, circulating anti-angiogenic proteins and lipids (115). Pathobiological contributions, either trigger factors or disease amplifiers, that emanate from the adipose tissue, as well as hormonal influences, need to be considered. Because there is an association between obesity and cancers (168), there is also an association between obesity and severe forms of PAH. Data from the REVEAL registry of pulmonary hypertension show that 30% of the PAH patients in the United States have a BMI > 35 (23). Adipose tissue is inflamed and undergoing angiogenic capillary sprouting and is a source of cytokines and growth factors (69, 74), and estrogen is produced by adiposites (102). These factors either directly or indirectly can affect injured lung vascular endothelial cells. It is also of interest that obesity has been associated with telomere shortening (112) and that adipose tissue undergoes EnMT (69).
The role of estrogen in breast and uterine cancers is well established, and in recent years the role of estrogen in the pathogenesis of severe PAH has received wide attention (27, 179). Estrogen receptor-related-α activates VEGF gene expression (160), perhaps one bridge between cancer and PAH. Estrogen metabolism is under the control of cytochrome P450 enzymes, another bridge between PAH and cancer (91).
SUMMARY AND OUTLOOK
Here, we have reexamined the quasi-malignancy concept of severe PAH. Ever since the publication of the “Cancer paradigm of PAH” article (138) in 2008, many publications provide data that support this concept (Fig. 1), including the recently pronounced “epigenomics” hypothesis (113). To summarize, there are robust hallmarks, which are shared between PAH and cancer: the phenotypic switch, the angiogenic switch, and the glycolytic switch. On the cellular level, the issue is one of cell survival and cell fate. In PAH, too many cells in the vascular wall are surviving, and too many cells are abnormal.
The past decade has evolved a more nuanced concept of the cancer hallmarks (13, 20, 25, 29, 34, 101, 156, 157, 171), and likewise, investigators in the PH research field continue to refine the hallmarks of PAH (4, 18, 38, 60, 70, 77, 106, 134). It is likely that advances in genotyping and phenotyping (61, 63) will also occur by putting to use liquid biopsies (12, 28) and analysis of circulating stem cells. For example, a recent whole genome-sequencing analysis of a large number of PAH patients discovered new rare mutations in the BMP9, aquaporin 1, and SOX17 genes (61, 62, 110), all of which have been associated with cancer and metastases. The last decade of PAH research has enlarged our knowledge of the pathobiology of severe forms of PAH by adding information relating to vascular inflammation and metabolic abnormalities of vascular cells; EnMT and DNA damage have been documented, and new gene mutations (44) have been discovered.
Are we there yet? We are not, but we are getting closer, and we are seeing the light at the end of the tunnel (184). What would novel, successful treatments for severe PAH look like? They would be disease modifying. In our opinion, there are two desirable treatment outcomes: 1) reversal of the disease in incident cases and 2) halting disease progression and stabilization of cardiac performance. For the second outcome goal, there is an example. It has not been universally appreciated that many of the aminorex-induced IPAH patients who had been diagnosed in the 1970s survived without treatment for many years. Their PAH did not disappear, but their disease did not progress.
If indeed many forms of angioobliterative PAH are quasi-malignant, as we suggest, then we can hope, and perhaps expect, that during the coming years new PAH treatment strategies will emerge that have been informed by cancer research, yet not to the exclusion of the concept of a vasoconstrictor/vasodilator imbalance. For instance, one can postulate that the disease of incident patients may be modifiable and that, as in cancer, there will be treatment responders and non-responders (184). Perhaps, informed by the hypothesis of a quasi-malignant pathobiology of severe PAH, clinical trials have begun to examine the effects of non-vasodilator drugs; examples are studies that have tested imatinib (45), bardoxolone (126), aromatase inhibition (84), and tacrolimus (158). Studies testing the treatment effect of rituximab in patients with scleroderma-associated PAH (117) and with 6-mercaptopurine in PAH patients (17) have recently been completed; cyclin-dependent kinases are also being considered as PAH treatment targets (187).
Visions of future PAH treatment strategies are to induce anoikis in anoikis-resistant lung vascular cells [see Bogaard et al. (14); this may require a detailed knowledge of integrin-matrix interactions that we are presently lacking), to correct the multilayered immunological deficiencies, and to reverse EnMT and steer stem cells toward differentiation.
Likely, in our opinion, future treatment strategies will target central disease components, like apoptosis-resistance and EnMT and immune mechanisms, rather than one particular enzyme or receptor. When strategies have been developed that reopen obliterated lung arterioles and venules, such treatments would reduce the pulmonary vascular resistance and unload the stressed right ventricle. We predict that strategies with the potential to reopen occluded pulmonary arterioles or halt disease progression will in all likelihood be informed by cancer research data and concepts.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.D.C., W.M.K., H.J.B., E.S., M.R.N., and N.F.V. prepared figures; C.D.C., W.M.K., H.J.B., E.S., M.R.N., and N.F.V. drafted manuscript; C.D.C., W.M.K., H.J.B., E.S., M.R.N., and N.F.V. edited and revised manuscript; C.D.C., W.M.K., H.J.B., E.S., M.R.N., and N.F.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors express appreciation for the skillfully generated immunohistochemistry provided by Jane Parr and to Felicitas Kern for editorial assistance.
REFERENCES
- 1.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361: eaao4227, 2018. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldred MA, Comhair SA, Varella-Garcia M, Asosingh K, Xu W, Noon GP, Thistlethwaite PA, Tuder RM, Erzurum SC, Geraci MW, Coldren CD. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 182: 1153–1160, 2010. doi: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames BN, Gold LS. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science 249: 970–971, 1990. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- 4.Andruska A, Spiekerkoetter E. Consequences of BMPR2 deficiency in the pulmonary vasculature and beyond: contributions to pulmonary arterial hypertension. Int J Mol Sci 19: E2499, 2018. doi: 10.3390/ijms19092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer SL. Pyruvate kinase and Warburg metabolism in pulmonary arterial hypertension: uncoupled glycolysis and the cancer-like phenotype of pulmonary arterial hypertension. Circulation 136: 2486–2490, 2017. doi: 10.1161/CIRCULATIONAHA.117.031655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arneth B. Systemic lupus erythematosus and DNA degradation and elimination defects. Front Immunol 10: 1697, 2019. doi: 10.3389/fimmu.2019.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, Anand-Apte B, Yoder MC, Tuder RM, Erzurum SC. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 172: 615–627, 2008. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asosingh K, Farha S, Lichtin A, Graham B, George D, Aldred M, Hazen SL, Loyd J, Tuder R, Erzurum SC. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension. Blood 120: 1218–1227, 2012. doi: 10.1182/blood-2012-03-419275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandyopadhyay D, Panchabhai TS, Bajaj NS, Patil PD, Bunte MC. Primary pulmonary artery sarcoma: a close associate of pulmonary embolism-20-year observational analysis. J Thorac Dis 8: 2592–2601, 2016. doi: 10.21037/jtd.2016.08.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bautista-Niño PK, Portilla-Fernandez E, Vaughan DE, Danser AH, Roks AJ. DNA damage: a main determinant of vascular aging. Int J Mol Sci 17: E748, 2016. doi: 10.3390/ijms17050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J III, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Best MG, Sol N, In ’t Veld SGJG, Vancura A, Muller M, Niemeijer AN, Fejes AV, Tjon Kon Fat LA, Huis In ’t Veld AE, Leurs C, Le Large TY, Meijer LL, Kooi IE, Rustenburg F, Schellen P, Verschueren H, Post E, Wedekind LE, Bracht J, Esenkbrink M, Wils L, Favaro F, Schoonhoven JD, Tannous J, Meijers-Heijboer H, Kazemier G, Giovannetti E, Reijneveld JC, Idema S, Killestein J, Heger M, de Jager SC, Urbanus RT, Hoefer IE, Pasterkamp G, Mannhalter C, Gomez-Arroyo J, Bogaard HJ, Noske DP, Vandertop WP, van den Broek D, Ylstra B, Nilsson RJA, Wesseling P, Karachaliou N, Rosell R, Lee-Lewandrowski E, Lewandrowski KB, Tannous BA, de Langen AJ, Smit EF, van den Heuvel MM, Wurdinger T. Swarm intelligence-enhanced detection of non-small-cell lung cancer using tumor-educated platelets. Cancer Cell 32: 238–252.e9, 2017. doi: 10.1016/j.ccell.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blandin AF, Renner G, Lehmann M, Lelong-Rebel I, Martin S, Dontenwill M. β1 Integrins as therapeutic targets to disrupt hallmarks of cancer. Front Pharmacol 6: 279, 2015. doi: 10.3389/fphar.2015.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogaard HJ, Mizuno S, Guignabert C, Al Hussaini AA, Farkas D, Ruiter G, Kraskauskas D, Fadel E, Allegood JC, Humbert M, Vonk Noordegraaf A, Spiegel S, Farkas L, Voelkel NF. Copper dependence of angioproliferation in pulmonary arterial hypertension in rats and humans. Am J Respir Cell Mol Biol 46: 582–591, 2012. doi: 10.1165/rcmb.2011-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA 104: 11418–11423, 2007. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boroumand N, Saghi H, Avan A, Bahreyni A, Ryzhikov M, Khazaei M, Hassanian SM. Therapeutic potency of heat-shock protein-90 pharmacological inhibitors in the treatment of gastrointestinal cancer, current status and perspectives. J Pharm Pharmacol 70: 151–158, 2018. doi: 10.1111/jphp.12824. [DOI] [PubMed] [Google Scholar]
- 17.Botros L,Szulcek R,Jansen S,Smits J, Phelp P, Kurakula K, Goumans MJ, Nossent E, Boonstra A, Vonk-Noordegraaf A, Handoko ML, de Man F, Aman J, Bogaard HJ. Antiproliferative therapy with 6-mercaptopurine improves hemodynamics and BMPR2 expression in pulmonary arterial hypertension (Abstract no. A9386). ATS 2020. Philadelphia, PA, May 15-20, 2020. [Google Scholar]
- 18.Boucherat O, Vitry G, Trinh I, Paulin R, Provencher S, Bonnet S. The cancer theory of pulmonary arterial hypertension. Pulm Circ 7: 285–299, 2017. doi: 10.1177/2045893217701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boucherat O, Peterlini T, Bourgeois A, Nadeau V, Breuils-Bonnet S, Boilet-Molez S, Potus F, Meloche J, Chabot S, Lambert C, Tremblay E, Chae YC, Altieri DC, Sutendra G, Michelakis ED, Paulin R, Provencher S, Bonnet S. Mitochondrial HSP90 accumulation promotes vascular remodeling in pulmonary arterial hypertension. Am J Respir Crit Care Med 198: 90–103, 2018. doi: 10.1164/rccm.201708-1751OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgeois A, Omura J, Habbout K, Bonnet S, Boucherat O. Pulmonary arterial hypertension: New pathophysiological insights and emerging therapeutic targets. Int J Biochem Cell Biol 104: 9–13, 2018. doi: 10.1016/j.biocel.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Breitling S, Hui Z, Zabini D, Hu Y, Hoffmann J, Goldenberg NM, Tabuchi A, Buelow R, Dos Santos C, Kuebler WM. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 312: L710–L721, 2017. doi: 10.1152/ajplung.00311.2016. [DOI] [PubMed] [Google Scholar]
- 22.Bull TM, Golpon H, Hebbel RP, Solovey A, Cool CD, Tuder RM, Geraci MW, Voelkel NF. Circulating endothelial cells in pulmonary hypertension. Thromb Haemost 90: 698–703, 2003. doi: 10.1160/TH03-04-0251. [DOI] [PubMed] [Google Scholar]
- 23.Burger CD, Foreman AJ, Miller DP, Safford RE, McGoon MD, Badesch DB. Comparison of body habitus in patients with pulmonary arterial hypertension enrolled in the Registry to Evaluate Early and Long-term PAH Disease Management with normative values from the National Health and Nutrition Examination Survey. Mayo Clin Proc 86: 105–112, 2011. doi: 10.4065/mcp.2010.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlsen J, Hasseriis Andersen K, Boesgaard S, Iversen M, Steinbrüchel D, Bøgelund Andersen C. Pulmonary arterial lesions in explanted lungs after transplantation correlate with severity of pulmonary hypertension in chronic obstructive pulmonary disease. J Heart Lung Transplant 32: 347–354, 2013. doi: 10.1016/j.healun.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother 60: 319–326, 2011. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen PI, Cao A, Miyagawa K, Tojais NF, Hennigs JK, Li CG, Sweeney NM, Inglis AS, Wang L, Li D, Ye M, Feldman BJ, Rabinovitch M. Amphetamines promote mitochondrial dysfunction and DNA damage in pulmonary hypertension. JCI Insight 2: e90427, 2017. doi: 10.1172/jci.insight.90427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, Shay S, Trammell A, Phillips JA, Hamid R, Cogan JD, Dawson EP, Womble KE, Hedges LK, Martinez EG, Wheeler LA, Loyd JE, Majka SJ, West J, Austin ED. Estrogen Metabolite 16α-hydroxyestrone exacerbates bone morphogenetic protein receptor Type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation 133: 82–97, 2016. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, Schmidt CM, Yip-Schneider MT, Allen PJ, Schattner M, Brand RE, Singhi AD, Petersen GM, Hong SM, Kim SC, Falconi M, Doglioni C, Weiss MJ, Ahuja N, He J, Makary MA, Maitra A, Hanash SM, Dal Molin M, Wang Y, Li L, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Goggins MG, Hruban RH, Wolfgang CL, Klein AP, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Lennon AM. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci USA 114: 10202–10207, 2017. doi: 10.1073/pnas.1704961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SM, Ellwein LB. Cell proliferation in carcinogenesis. Science 249: 1007–1011, 1990. doi: 10.1126/science.2204108. [DOI] [PubMed] [Google Scholar]
- 30.Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, Yeager ME. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med 188: 1126–1136, 2013. doi: 10.1164/rccm.201302-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper J, Giancotti FG. Integrin Signaling in Cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 35: 347–367, 2019. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coussens LM, Werb Z. Inflammation and cancer. Nature 420: 860–867, 2002. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 12: 584–596, 2015. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 35.Cumber PM, Jacobs A, Hoy T, Fisher J, Whittaker JA, Tsuruo T, Padua RA. Expression of the multiple drug resistance gene (mdr-1) and epitope masking in chronic lymphatic leukaemia. Br J Haematol 76: 226–230, 1990. doi: 10.1111/j.1365-2141.1990.tb07876.x. [DOI] [PubMed] [Google Scholar]
- 36.Dabral S, Muecke C, Valasarajan C, Schmoranzer M, Wietelmann A, Semenza GL, Meister M, Muley T, Seeger-Nukpezah T, Samakovlis C, Weissmann N, Grimminger F, Seeger W, Savai R, Pullamsetti SS. A RASSF1A-HIF1α loop drives Warburg effect in cancer and pulmonary hypertension. Nat Commun 10: 2130, 2019. doi: 10.1038/s41467-019-10044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2α. Circulation 133: 2447–2458, 2016. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, Ježek P, Li M, Zhang H, Gupte SA, Stenmark KR. Hallmarks of pulmonary hypertension: mesenchymal and inflammatory cell metabolic reprogramming. Antioxid Redox Signal 28: 230–250, 2018. doi: 10.1089/ars.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damico R, Kolb TM, Valera L, Wang L, Housten T, Tedford RJ, Kass DA, Rafaels N, Gao L, Barnes KC, Benza RL, Rand JL, Hamid R, Loyd JE, Robbins IM, Hemnes AR, Chung WK, Austin ED, Drummond MB, Mathai SC, Hassoun PM. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 191: 208–218, 2015. doi: 10.1164/rccm.201409-1742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dandoy CE, Hirsch R, Chima R, Davies SM, Jodele S. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 19: 1546–1556, 2013. doi: 10.1016/j.bbmt.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Dannewitz Prosseda S, Tian X, Kuramoto K, Boehm M, Sudheendra D, Miyagawa K, Zhang F, Solow-Cordero D, Saldivar JC, Austin ED, Loyd JE, Wheeler L, Andruska A, Donato M, Wang L, Huebner K, Metzger RJ, Khatri P, Spiekerkoetter E. FHIT, a novel modifier gene in pulmonary arterial hypertension. Am J Respir Crit Care Med 199: 83–98, 2019. doi: 10.1164/rccm.201712-2553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dvorak HF. Tumors: wounds that do not heal—redux. Cancer Immunol Res 3: 1–11, 2015. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egnatchik RA, Brittain EL, Shah AT, Fares WH, Ford HJ, Monahan K, Kang CJ, Kocurek EG, Zhu S, Luong T, Nguyen TT, Hysinger E, Austin ED, Skala MC, Young JD, Roberts LJ II, Hemnes AR, West J, Fessel JP. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm Circ 7: 186–199, 2017. doi: 10.1086/690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eyries M, Montani D, Girerd B, Favrolt N, Riou M, Faivre L, Manaud G, Perros F, Gräf S, Morrell NW, Humbert M, Soubrier F. Familial pulmonary arterial hypertension by KDR heterozygous loss of function. Eur Respir J. In press. doi: 10.1183/13993003.02165-2019. [DOI] [PubMed] [Google Scholar]
- 45.Farkas D, Kraskauskas D, Drake JI, Alhussaini AA, Kraskauskiene V, Bogaard HJ, Cool CD, Voelkel NF, Farkas L. CXCR4 inhibition ameliorates severe obliterative pulmonary hypertension and accumulation of C-kit+ cells in rats. PLoS One 9: e89810, 2014. doi: 10.1371/journal.pone.0089810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farha S, Dweik R, Rahaghi F, Benza R, Hassoun P, Frantz R, Torres F, Quinn DA, Comhair S, Erzurum S, Asosingh K. Imatinib in pulmonary arterial hypertension: c-Kit inhibition. Pulm Circ 4: 452–455, 2014. doi: 10.1086/677359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Federici C, Drake KM, Rigelsky CM, McNelly LN, Meade SL, Comhair SA, Erzurum SC, Aldred MA. Increased mutagen sensitivity and DNA damage in pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 219–228, 2015. doi: 10.1164/rccm.201411-2128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felty Q, Sakao S, Voelkel NF. Pulmonary hypertension: a stem cell hypothesis. In: Lung Stem Cells in the Epithelium and Vasculature, edited by Firth A, Yuan JX. New York: Springer International Publishing, 2015, p. 289–306. [Google Scholar]
- 49.Flens MJ, Zaman GJ, van der Valk P, Izquierdo MA, Schroeijers AB, Scheffer GL, van der Groep P, de Haas M, Meijer CJ, Scheper RJ. Tissue distribution of the multidrug resistance protein. Am J Pathol 148: 1237–1247, 1996. [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrer E, Dunmore BJ, Hassan D, Ormiston ML, Moore S, Deighton J, Long L, Yang XD, Stewart DJ, Morrell NW. A potential role for exosomal translationally controlled tumor protein export in vascular remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 59: 467–478, 2018. doi: 10.1165/rcmb.2017-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraidenburg DR, Yuan JX. Hungry for more: autophagy in the pathogenesis of pulmonary arterial hypertension. Circ Res 112: 1091–1093, 2013. doi: 10.1161/CIRCRESAHA.113.301247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2α oncogenic axis. Proc Natl Acad Sci USA 106: 21306–21311, 2009. doi: 10.1073/pnas.0906432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galambos C, Sims-Lucas S, Abman SH, Cool CD. Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 193: 574–576, 2016. doi: 10.1164/rccm.201507-1508LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganesh S, Shui X, Craig KP, Park J, Wang W, Brown BD, Abrams MT. RNAi-mediated β-catenin inhibition promotes T Cell infiltration and antitumor activity in combination with immune checkpoint blockade. Mol Ther 26: 2567–2579, 2018. doi: 10.1016/j.ymthe.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garypidou V, Vakalopoulou S, Dimitriadis D, Tziomalos K, Sfikas G, Perifanis V. Incidence of pulmonary hypertension in patients with chronic myeloproliferative disorders. Haematologica 89: 245–246, 2004. [PubMed] [Google Scholar]
- 56.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 88: 555–562, 2001. doi: 10.1161/01.RES.88.6.555. [DOI] [PubMed] [Google Scholar]
- 57.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev 16: 1049–1057, 2017. doi: 10.1016/j.autrev.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 58.Goldthorpe H, Jiang JY, Taha M, Deng Y, Sinclair T, Ge CX, Jurasz P, Turksen K, Mei SH, Stewart DJ. Occlusive lung arterial lesions in endothelial-targeted, fas-induced apoptosis transgenic mice. Am J Respir Cell Mol Biol 53: 712–718, 2015. doi: 10.1165/rcmb.2014-0311OC. [DOI] [PubMed] [Google Scholar]
- 59.Guerra G, Perrotta F, Testa G. Circulating endothelial progenitor cells biology and regenerative medicine in pulmonary vascular diseases. Curr Pharm Biotechnol 19: 700–707, 2018. doi: 10.2174/1389201019666181017161752. [DOI] [PubMed] [Google Scholar]
- 60.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev 22: 543–551, 2013. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gräf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, Hodgson J, Liu B, Salmon RM, Southwood M, Machado RD, Martin JM, Treacy CM, Yates K, Daugherty LC, Shamardina O, Whitehorn D, Holden S, Aldred M, Bogaard HJ, Church C, Coghlan G, Condliffe R, Corris PA, Danesino C, Eyries M, Gall H, Ghio S, Ghofrani HA, Gibbs JSR, Girerd B, Houweling AC, Howard L, Humbert M, Kiely DG, Kovacs G, MacKenzie Ross RV, Moledina S, Montani D, Newnham M, Olschewski A, Olschewski H, Peacock AJ, Pepke-Zaba J, Prokopenko I, Rhodes CJ, Scelsi L, Seeger W, Soubrier F, Stein DF, Suntharalingam J, Swietlik EM, Toshner MR, van Heel DA, Vonk Noordegraaf A, Waisfisz Q, Wharton J, Wort SJ, Ouwehand WH, Soranzo N, Lawrie A, Upton PD, Wilkins MR, Trembath RC, Morrell NW. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 9: 1416, 2018. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimm D, Bauer J, Wise P, Krüger M, Simonsen U, Wehland M, Infanger M, Corydon TJ. The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol. In press. doi: 10.1016/j.semcancer.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Hadinnapola C, Bleda M, Haimel M, Screaton N, Swift A, Dorfmüller P, Preston SD, Southwood M, Hernandez-Sanchez J, Martin J, Treacy C, Yates K, Bogaard H, Church C, Coghlan G, Condliffe R, Corris PA, Gibbs S, Girerd B, Holden S, Humbert M, Kiely DG, Lawrie A, Machado R, MacKenzie Ross R, Moledina S, Montani D, Newnham M, Peacock A, Pepke-Zaba J, Rayner-Matthews P, Shamardina O, Soubrier F, Southgate L, Suntharalingam J, Toshner M, Trembath R, Vonk Noordegraaf A, Wilkins MR, Wort SJ, Wharton J, Gräf S, Morrell NW; NIHR BioResource–Rare Diseases Consortium; UK National Cohort Study of Idiopathic and Heritable PAH . Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 136: 2022–2033, 2017. doi: 10.1161/CIRCULATIONAHA.117.028351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall MD, Handley MD, Gottesman MM. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol Sci 30: 546–556, 2009. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 66.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Hara Y, Sassi Y, Guibert C, Gambaryan N, Dorfmüller P, Eddahibi S, Lompré AM, Humbert M, Hulot JS. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Invest 121: 2888–2897, 2011. doi: 10.1172/JCI45023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashimoto N, Nagano H, Tanaka T. The role of tumor suppressor p53 in metabolism and energy regulation, and its implication in cancer and lifestyle-related diseases. Endocr J 66: 485–496, 2019. doi: 10.1507/endocrj.EJ18-0565. [DOI] [PubMed] [Google Scholar]
- 69.Haynes BA, Yang LF, Huyck RW, Lehrer EJ, Turner JM, Barabutis N, Correll VL, Mathiesen A, McPheat W, Semmes OJ, Dobrian AD. Endothelial-to-mesenchymal transition in human adipose tissue vasculature alters the particulate secretome and induces endothelial dysfunction. Arterioscler Thromb Vasc Biol 39: 2168–2191, 2019. doi: 10.1161/ATVBAHA.119.312826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hemnes AR, Luther JM, Rhodes CJ, Burgess JP, Carlson J, Fan R, Fessel JP, Fortune N, Gerszten RE, Halliday SJ, Hekmat R, Howard L, Newman JH, Niswender KD, Pugh ME, Robbins IM, Sheng Q, Shibao CA, Shyr Y, Sumner S, Talati M, Wharton J, Wilkins MR, Ye F, Yu C, West J, Brittain EL. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight 4: e123611, 2019. doi: 10.1172/jci.insight.123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374, 2001. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann J, Marsh LM, Pieper M, Stacher E, Ghanim B, Kovacs G, König P, Wilkens H, Haitchi HM, Hoefler G, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. Am J Physiol Lung Cell Mol Physiol 308: L1002–L1013, 2015. doi: 10.1152/ajplung.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, Rabinovitch M. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation 133: 1783–1794, 2016. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 75.Huertas A, Tu L, Thuillet R, Le Hiress M, Phan C, Ricard N, Nadaud S, Fadel E, Humbert M, Guignabert C. Leptin signalling system as a target for pulmonary arterial hypertension therapy. Eur Respir J 45: 1066–1080, 2015. doi: 10.1183/09031936.00193014. [DOI] [PubMed] [Google Scholar]
- 76.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, Capron F, Galanaud P, Duroux P, Simonneau G, Emilie D. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J 11: 554–559, 1998. [PubMed] [Google Scholar]
- 77.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 53: 1801887, 2019. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, Dartevelle P, Simonneau G, Adnot S, Maitre B, Raffestin B, Eddahibi S. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest 119: 512–523, 2009. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin Y, Choi AM. Cross talk between autophagy and apoptosis in pulmonary hypertension. Pulm Circ 2: 407–414, 2012. doi: 10.4103/2045-8932.105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joannes A, Bonnomet A, Bindels S, Polette M, Gilles C, Burlet H, Cutrona J, Zahm JM, Birembaut P, Nawrocki-Raby B. Fhit regulates invasion of lung tumor cells. Oncogene 29: 1203–1213, 2010. doi: 10.1038/onc.2009.418. [DOI] [PubMed] [Google Scholar]
- 81.Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA. α-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res 65: 4292–4299, 2005. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 82.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319, 2000. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kato F, Sakao S, Takeuchi T, Suzuki T, Nishimura R, Yasuda T, Tanabe N, Tatsumi K. Endothelial cell-related autophagic pathways in Sugen/hypoxia-exposed pulmonary arterial hypertensive rats. Am J Physiol Lung Cell Mol Physiol 313: L899–L915, 2017. doi: 10.1152/ajplung.00527.2016. [DOI] [PubMed] [Google Scholar]
- 84.Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, Palevsky HI, Palmisciano AJ, Patel M, Pinder D, Propert KJ, Smith KA, Stanczyk F, Tracy R, Vaidya A, Whittenhall ME, Ventetuolo CE. Anastrozole in pulmonary arterial hypertension. A randomized double-blind, placebo-controlled trial. Am J Respir Crit Care Med 195: 360–368, 2017. doi: 10.1164/rccm.201605-1024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kochel TJ, Reader JC, Ma X, Kundu N, Fulton AM. Multiple drug resistance-associated protein (MRP4) exports prostaglandin E2 (PGE2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget 8: 6540–6554, 2017. doi: 10.18632/oncotarget.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korkut A, Zaidi S, Kanchi RS, Rao S, Gough NR, Schultz A, Li X, Lorenzi PL, Berger AC, Robertson G, Kwong LN, Datto M, Roszik J, Ling S, Ravikumar V, Manyam G, Rao A, Shelley S, Liu Y, Ju Z, Hansel D, de Velasco G, Pennathur A, Andersen JB, O’Rourke CJ, Ohshiro K, Jogunoori W, Nguyen BN, Li S, Osmanbeyoglu HU, Ajani JA, Mani SA, Houseman A, Wiznerowicz M, Chen J, Gu S, et al.; Cancer Genome Atlas Research Network . A pan-cancer analysis reveals high-frequency genetic alterations in mediators of signaling by the TGF-β superfamily. Cell Syst 7: 422–437.e7, 2018. doi: 10.1016/j.cels.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G, Baker AH. Endothelial to mesenchymal transition in cardiovascular disease. J Am Coll Cardiol 73: 190–209, 2019. doi: 10.1016/j.jacc.2018.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuebler WM, Bonnet S, Tabuchi A. Inflammation and autoimmunity in pulmonary hypertension: is there a role for endothelial adhesion molecules? (2017 Grover Conference Series). Pulm Circ 8: 2045893218757596, 2018. doi: 10.1177/2045893218757596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 1851: 308–330, 2015. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurzrock R, Hickish T, Wyrwicz L, Saunders M, Wu Q, Stecher M, Mohanty P, Dinarello CA, Simard J. Interleukin-1 receptor antagonist levels predict favorable outcome after bermekimab, a first-in-class true human interleukin-1α antibody, in a phase III randomized study of advanced colorectal cancer. OncoImmunology 8: 1551651, 2019. doi: 10.1080/2162402X.2018.1551651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwapiszewska G, Johansen AKZ, Gomez-Arroyo J, Voelkel NF. Role of the aryl hydrocarbon receptor/ARNT/cytochrome p450 system in pulmonary vascular diseases. Circ Res 125: 356–366, 2019. doi: 10.1161/CIRCRESAHA.119.315054. [DOI] [PubMed] [Google Scholar]
- 92.Lassus P, Turanlahti M, Heikkilä P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164: 1981–1987, 2001. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 93.Lee SJ, Smith A, Guo L, Alastalo TP, Li M, Sawada H, Liu X, Chen ZH, Ifedigbo E, Jin Y, Feghali-Bostwick C, Ryter SW, Kim HP, Rabinovitch M, Choi AM. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med 183: 649–658, 2011. doi: 10.1164/rccm.201005-0746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le Hiress M, Tu L, Ricard N, Phan C, Thuillet R, Fadel E, Dorfmüller P, Montani D, de Man F, Humbert M, Huertas A, Guignabert C. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension. Role of the macrophage migration inhibitory factor/CD74 complex. Am J Respir Crit Care Med 192: 983–997, 2015. doi: 10.1164/rccm.201402-0322OC. [DOI] [PubMed] [Google Scholar]
- 95.Li H, Ray G, Yoo BH, Erdogan M, Rosen KV. Down-regulation of death-associated protein kinase-2 is required for β-catenin-induced anoikis resistance of malignant epithelial cells. J Biol Chem 284: 2012–2022, 2009. doi: 10.1074/jbc.M805612200. [DOI] [PubMed] [Google Scholar]
- 96.Li M, Vattulainen S, Aho J, Orcholski M, Rojas V, Yuan K, Helenius M, Taimen P, Myllykangas S, De Jesus Perez V, Koskenvuo JW, Alastalo TP. Loss of bone morphogenetic protein receptor 2 is associated with abnormal DNA repair in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 50: 1118–1128, 2014. doi: 10.1165/rcmb.2013-0349OC. [DOI] [PubMed] [Google Scholar]
- 97.Li RC, Cindrova-Davies T, Skepper JN, Sellers LA. Prostacyclin induces apoptosis of vascular smooth muscle cells by a cAMP-mediated inhibition of extracellular signal-regulated kinase activity and can counteract the mitogenic activity of endothelin-1 or basic fibroblast growth factor. Circ Res 94: 759–767, 2004. doi: 10.1161/01.RES.0000121568.40692.97. [DOI] [PubMed] [Google Scholar]
- 98.Li S, Chen Y, Zhang Y, Jiang X, Jiang Y, Qin X, Yang H, Wu C, Liu Y. Shear stress promotes anoikis resistance of cancer cells via caveolin-1-dependent extrinsic and intrinsic apoptotic pathways. J Cell Physiol 234: 3730–3743, 2019. doi: 10.1002/jcp.27149. [DOI] [PubMed] [Google Scholar]
- 99.Li T, Zha L, Luo H, Li S, Zhao L, He J, Li X, Qi Q, Liu Y, Yu Z. Galectin-3 mediates endothelial-to-mesenchymal transition in pulmonary arterial hypertension. Aging Dis 10: 731–745, 2019. doi: 10.14336/AD.2018.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin CH, Chiang MC, Chen YJ. STAT3 mediates resistance to anoikis and promotes invasiveness of nasopharyngeal cancer cells. Int J Mol Med 40: 1549–1556, 2017. doi: 10.3892/ijmm.2017.3151. [DOI] [PubMed] [Google Scholar]
- 101.Low KC, Tergaonkar V. Telomerase: central regulator of all of the hallmarks of cancer. Trends Biochem Sci 38: 426–434, 2013. doi: 10.1016/j.tibs.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 102.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115: 1479–1491, 2005. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meehan K, Vella LJ. The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci 53: 121–131, 2016. doi: 10.3109/10408363.2015.1092496. [DOI] [PubMed] [Google Scholar]
- 105.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, Couture C, Michelakis ED, Provencher S, Bonnet S. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation 129: 786–797, 2014. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 106.Meloche J, Le Guen M, Potus F, Vinck J, Ranchoux B, Johnson I, Antigny F, Tremblay E, Breuils-Bonnet S, Perros F, Provencher S, Bonnet S. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol 309: C363–C372, 2015. doi: 10.1152/ajpcell.00149.2015. [DOI] [PubMed] [Google Scholar]
- 107.Mertelsmann R, Waesch R. On the pathobiology and treatment of cancer and pulmonary arterialhypertension. In: Pulmonary Hypertension: The Present and Future, edited by Voelkel NF. Shelton, CT: People’s Medical Publishing House-USA, 2011, p. 317–340. [Google Scholar]
- 108.Montani D, Perros F, Gambaryan N, Girerd B, Dorfmuller P, Price LC, Huertas A, Hammad H, Lambrecht B, Simonneau G, Launay JM, Cohen-Kaminsky S, Humbert M. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 116–123, 2011. doi: 10.1164/rccm.201006-0905OC. [DOI] [PubMed] [Google Scholar]
- 109.Moore GY, Pidgeon GP. Cross-talk between cancer cells and the tumour microenvironment: the role of the 5-lipoxygenase pathway. Int J Mol Sci 18: 236, 2017. doi: 10.3390/ijms18020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moosavi MS, Elham Y. Aquaporins 1, 3 and 5 in different tumors, their expression, prognosis value and role as new therapeutic targets. Pathol Oncol Res. In press. doi: 10.1007/s12253-019-00646-9. [DOI] [PubMed] [Google Scholar]
- 111.Mouraret N, Houssaïni A, Abid S, Quarck R, Marcos E, Parpaleix A, Gary-Bobo G, Dubois-Randé JL, Derumeaux G, Boczkowski J, Delcroix M, Blasco MA, Lipskaia L, Amsellem V, Adnot S. Role for telomerase in pulmonary hypertension. Circulation 131: 742–755, 2015. doi: 10.1161/CIRCULATIONAHA.114.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mundstock E, Sarria EE, Zatti H, Mattos Louzada F, Kich Grun L, Herbert Jones M, Guma FT, Mazzola In Memoriam J, Epifanio M, Stein RT, Barbé-Tuana FM, Mattiello R. Effect of obesity on telomere length: systematic review and meta-analysis. Obesity (Silver Spring) 23: 2165–2174, 2015. doi: 10.1002/oby.21183. [DOI] [PubMed] [Google Scholar]
- 113.Napoli C, Benincasa G, Loscalzo J. Epigenetic inheritance underlying pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 39: 653–664, 2019. doi: 10.1161/ATVBAHA.118.312262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ngo J, Matsuyama M, Kim C, Poventud-Fuentes I, Bates A, Siedlak SL, Lee HG, Doughman YQ, Watanabe M, Liner A, Hoit B, Voelkel N, Gerson S, Hasty P, Matsuyama S. Bax deficiency extends the survival of Ku70 knockout mice that develop lung and heart diseases. Cell Death Dis 6: e1706, 2015. doi: 10.1038/cddis.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nickel NP, Yuan K, Dorfmuller P, Provencher S, Lai YC, Bonnet S, Austin ED, Koch CD, Morris A, Perros F, Montani D, Zamanian RT, de Jesus Perez VA. Beyond the lungs: systemic manifestations of pulmonary arterial hypertension. Am J Respir Crit Care Med 201: 148–157, 2020. doi: 10.1164/rccm.201903-0656CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nicolls MR, Mizuno S, Taraseviciene-Stewart L, Farkas L, Drake JI, Al Husseini A, Gomez-Arroyo JG, Voelkel NF, Bogaard HJ. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm Circ 2: 434–442, 2012. doi: 10.4103/2045-8932.105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nicolls M, Badesch D, Chung L, Domsic R, Medsger T, Pinckney A, Keyes-Elstein L, D’Aveta C, Spychala M, White RJ, Hassoun P, Torres F, Molitor J, Khanna D, Maeker H, Welch B, Goldmuntz E, Zamanian R. Safety and efficacy of B-cell depletion with rituximab for the treatment of systemic sclerosis-associated pulmonary arterial hypertension in a multi-center NIH clinical trial (Abstract No. 867). 2019 ACR/ARP Annual Meeting. Atlanta, GA, November 8–13, 2019. [Google Scholar]
- 118.Nijmeh H, Balasubramaniam V, Burns N, Ahmad A, Stenmark KR, Gerasimovskaya EV. High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization. Am J Physiol Lung Cell Mol Physiol 306: L661–L671, 2014. doi: 10.1152/ajplung.00244.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nold-Petry CA, Rudloff I, Baumer Y, Ruvo M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, Dinkel H, Palmer BE, Boisvert WA, Cool CD, Taraseviciene-Stewart L, Heinhuis B, Joosten LA, Dinarello CA, Voelkel NF, Nold MF. IL-32 promotes angiogenesis. J Immunol 192: 589–602, 2014. doi: 10.4049/jimmunol.1202802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noureddine H, Gary-Bobo G, Alifano M, Marcos E, Saker M, Vienney N, Amsellem V, Maitre B, Chaouat A, Chouaid C, Dubois-Rande JL, Damotte D, Adnot S. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res 109: 543–553, 2011. doi: 10.1161/CIRCRESAHA.111.241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Novoyatleva T, Kojonazarov B, Owczarek A, Veeroju S, Rai N, Henneke I, Böhm M, Grimminger F, Ghofrani HA, Seeger W, Weissmann N, Schermuly RT. Evidence for fucoidan-P-selectin axis as a therapeutic target on hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med 199: 1407–1420, 2019. doi: 10.1164/rccm.201806-1170OC. [DOI] [PubMed] [Google Scholar]
- 122.Dalvi P, Sharma H, Chinnappan M, Sanderson M, Allen J, Zeng R, Choi A, O’Brien-Ladner A, Dhillon NK. Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: role in HIV-related pulmonary arterial hypertension. Autophagy 12: 2420–2438, 2016. doi: 10.1080/15548627.2016.1238551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orcholski ME, Yuan K, Rajasingh C, Tsai H, Shamskhou EA, Dhillon NK, Voelkel NF, Zamanian RT, de Jesus Perez VA. Drug-induced pulmonary arterial hypertension: a primer for clinicians and scientists. Am J Physiol Lung Cell Mol Physiol 314: L967–L983, 2018. doi: 10.1152/ajplung.00553.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Orford K, Orford CC, Byers SW. Exogenous expression of β-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol 146: 855–868, 1999. doi: 10.1083/jcb.146.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ormiston ML, Chang C, Long LL, Soon E, Jones D, Machado R, Treacy C, Toshner MR, Campbell K, Riding A, Southwood M, Pepke-Zaba J, Exley A, Trembath RC, Colucci F, Wills M, Trowsdale J, Morrell NW. Impaired natural killer cell phenotype and function in idiopathic and heritable pulmonary arterial hypertension. Circulation 126: 1099–1109, 2012. doi: 10.1161/CIRCULATIONAHA.112.110619. [DOI] [PubMed] [Google Scholar]
- 126.Oudiz R, Meyer C, Chin M, Feldman J, Goldsberry A, Mc Connell J, McCullough P, O’Grady M, Tapson V, Torres F, Waxman A, White R. Initial data report from “LARIAT”: A phase 2 study of bardoxolone methyl in PAH patients on stable background therapy (Abstract). Chest 148: 639A, 2015. doi: 10.1378/chest.2345856. [DOI] [Google Scholar]
- 127.Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci 18: 2157, 2017. doi: 10.3390/ijms18102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pastukh V, Roberts JT, Clark DW, Bardwell GC, Patel M, Al-Mehdi AB, Borchert GM, Gillespie MN. An oxidative DNA “damage” and repair mechanism localized in the VEGF promoter is important for hypoxia-induced VEGF mRNA expression. Am J Physiol Lung Cell Mol Physiol 309: L1367–L1375, 2015. doi: 10.1152/ajplung.00236.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17: 302–317, 2017. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 130.Perros F, Dorfmüller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 185: 311–321, 2012. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 131.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15: 1243–1253, 2014. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Platel V, Faure S, Corre I, Clere N. Endothelial-to-mesenchymal transition (EndoMT): roles in tumorigenesis, metastatic extravasation and therapy resistance. J Oncol 2019: 1–13, 2019. doi: 10.1155/2019/8361945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Popat U, Frost A, Liu E, May R, Bag R, Reddy V, Prchal JT. New onset of myelofibrosis in association with pulmonary arterial hypertension. Ann Intern Med 143: 466–467, 2005. doi: 10.7326/0003-4819-143-6-200509200-00017. [DOI] [PubMed] [Google Scholar]
- 134.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med 195: 425–437, 2017. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pullamsetti SS, Kojonazarov B, Storn S, Gall H, Salazar Y, Wolf J, Weigert A, El-Nikhely N, Ghofrani HA, Krombach GA, Fink L, Gattenlöhner S, Rapp UR, Schermuly RT, Grimminger F, Seeger W, Savai R. Lung cancer-associated pulmonary hypertension: Role of microenvironmental inflammation based on tumor cell-immune cell cross-talk. Sci Transl Med 9: eaai9048, 2017. doi: 10.1126/scitranslmed.aai9048. [DOI] [PubMed] [Google Scholar]
- 136.Pullamsetti SS, Seeger W, Savai R. Classical IL-6 signaling: a promising therapeutic target for pulmonary arterial hypertension. J Clin Invest 128: 1720–1723, 2018. doi: 10.1172/JCI120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qian Z, Li Y, Yang H, Chen J, Li X, Gou D. PDGFBB promotes proliferation and migration via regulating miR-1181/STAT3 axis in human pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 315: L965–L976, 2018. doi: 10.1152/ajplung.00224.2018. [DOI] [PubMed] [Google Scholar]
- 138.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 558–564, 2008. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131: 1006–1018, 2015. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 140.Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA Damage and pulmonary hypertension. Int J Mol Sci 17: 990, 2016. doi: 10.3390/ijms17060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rätsep MT, Moore SD, Jafri S, Mitchell M, Brady HJM, Mandelboim O, Southwood M, Morrell NW, Colucci F, Ormiston ML. Spontaneous pulmonary hypertension in genetic mouse models of natural killer cell deficiency. Am J Physiol Lung Cell Mol Physiol 315: L977–L990, 2018. doi: 10.1152/ajplung.00477.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer 130: 1715–1725, 2012. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren W, Liu Y, Wan S, Fei C, Wang W, Chen Y, Zhang Z, Wang T, Wang J, Zhou L, Weng Y, He T, Zhang Y. BMP9 inhibits proliferation and metastasis of HER2-positive SK-BR-3 breast cancer cells through ERK1/2 and PI3K/AKT pathways. PLoS One 9: e96816, 2014. doi: 10.1371/journal.pone.0096816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18: 452–464, 2018. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rogovskii VS. The linkage between inflammation and immune tolerance: interfering with inflammation in cancer. Curr Cancer Drug Targets 17: 325–332, 2017. doi: 10.2174/1568009617666170109110816. [DOI] [PubMed] [Google Scholar]
- 146.Rol N, de Raaf MA, Sun XQ, Kuiper VP, da Silva Gonçalves Bos D, Happé C, Kurakula K, Dickhoff C, Thuillet R, Tu L, Guignabert C, Schalij I, Lodder K, Pan X, Herrmann FE, van Nieuw Amerongen GP, Koolwijk P, Vonk-Noordegraaf A, de Man FS, Wollin L, Goumans MJ, Szulcek R, Bogaard HJ. Nintedanib improves cardiac fibrosis but leaves pulmonary vascular remodelling unaltered in experimental pulmonary hypertension. Cardiovasc Res 115: 432–439, 2019. doi: 10.1093/cvr/cvy186. [DOI] [PubMed] [Google Scholar]
- 147.Ruffenach G, Bonnet S, Rousseaux S, Khochbin S, Provencher S, Perros F. Identity crisis in pulmonary arterial hypertension. Pulm Circ 8: 2045893217746054, 2018. doi: 10.1177/2045893217746054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Säleby J, Bouzina H, Lundgren J, Rådegran G. Angiogenic and inflammatory biomarkers in the differentiation of pulmonary hypertension. Scand Cardiovasc J 51: 261–270, 2017. doi: 10.1080/14017431.2017.1359419. [DOI] [PubMed] [Google Scholar]
- 149.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180, 2005. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 150.Sánchez-Duffhues G, García de Vinuesa A, van de Pol V, Geerts ME, de Vries MR, Janson SG, van Dam H, Lindeman JH, Goumans MJ, Ten Dijke P. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J Pathol 247: 333–346, 2019. doi: 10.1002/path.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol 9: 596, 2019. doi: 10.3389/fonc.2019.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 897–908, 2012. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 153.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, Schermuly RT, Pullamsetti SS. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med 20: 1289–1300, 2014. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 155.Sekine A, Nishiwaki T, Nishimura R, Kawasaki T, Urushibara T, Suda R, Suzuki T, Takayanagi S, Terada J, Sakao S, Tada Y, Iwama A, Tatsumi K. Prominin-1/CD133 expression as potential tissue-resident vascular endothelial progenitor cells in the pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 310: L1130–L1142, 2016. doi: 10.1152/ajplung.00375.2014. [DOI] [PubMed] [Google Scholar]
- 156.Soave DF, Miguel MP, Tomé FD, de Menezes LB, Nagib PR, Celes MR. The fate of the tumor in the hands of microenvironment: role of TAMs and mTOR pathway. Mediators Inflamm 2016: 1–7, 2016. doi: 10.1155/2016/8910520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Solomon H, Madar S, Rotter V. Mutant p53 gain of function is interwoven into the hallmarks of cancer. J Pathol 225: 475–478, 2011. doi: 10.1002/path.2988. [DOI] [PubMed] [Google Scholar]
- 158.Spiekerkoetter E, Sung YK, Sudheendra D, Bill M, Aldred MA, van de Veerdonk MC, Vonk Noordegraaf A, Long-Boyle J, Dash R, Yang PC, Lawrie A, Swift AJ, Rabinovitch M, Zamanian RT. Low-dose FK506 (Tacrolimus) in end-stage pulmonary arterial hypertension. Am J Respir Crit Care Med 192: 254–257, 2015. doi: 10.1164/rccm.201411-2061LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spiekerkoetter E, Goncharova EA, Guignabert C, Stenmark K, Kwapiszewska G, Rabinovitch M, Voelkel N, Bogaard HJ, Graham B, Pullamsetti SS, Kuebler WM. Hot topics in the mechanisms of pulmonary arterial hypertension disease: cancer-like pathobiology, the role of the adventitia, systemic involvement, and right ventricular failure. Pulm Circ 9: 2045894019889775, 2019. doi: 10.1177/2045894019889775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stein RA, Gaillard S, McDonnell DP. Estrogen-related receptor alpha induces the expression of vascular endothelial growth factor in breast cancer cells. J Steroid Biochem Mol Biol 114: 106–112, 2009. doi: 10.1016/j.jsbmb.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Suzuki T, Carrier EJ, Talati MH, Rathinasabapathy A, Chen X, Nishimura R, Tada Y, Tatsumi K, West J. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 314: L118–L126, 2018. doi: 10.1152/ajplung.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, Gutiérrez-Vázquez C, Kenison J, Tjon EC, Barroso A, Vandeventer T, de Lima KA, Rothweiler S, Mayo L, Ghannam S, Zandee S, Healy L, Sherr D, Farez MF, Prat A, Antel J, Reardon DA, Zhang H, Robson SC, Getz G, Weiner HL, Quintana FJ. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci 22: 729–740, 2019. [Erratum in Nat Neurosci 22: 1533, 2019.] doi: 10.1038/s41593-019-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 109: 867–879, 2011. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tamosiuniene R, Manouvakhova O, Mesange P, Saito T, Qian J, Sanyal M, Lin YC, Nguyen LP, Luria A, Tu AB, Sante JM, Rabinovitch M, Fitzgerald DJ, Graham BB, Habtezion A, Voelkel NF, Aurelian L, Nicolls MR. Dominant role for regulatory T cells in protecting females against pulmonary hypertension. Circ Res 122: 1689–1702, 2018. doi: 10.1161/CIRCRESAHA.117.312058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang H, Babicheva A, McDermott KM, Gu Y, Ayon RJ, Song S, Wang Z, Gupta A, Zhou T, Sun X, Dash S, Wang Z, Balistrieri A, Zheng Q, Cordery AG, Desai AA, Rischard F, Khalpey Z, Wang J, Black SM, Garcia JGN, Makino A, Yuan JX. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol 314: L256–L275, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 167.Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 360: j5492, 2018. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Teslow EA, Mitrea C, Bao B, Mohammad RM, Polin LA, Dyson G, Purrington KS, Bollig-Fischer A. Obesity-induced MBD2_v2 expression promotes tumor-initiating triple-negative breast cancer stem cells. Mol Oncol 13: 894–908, 2019. doi: 10.1002/1878-0261.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 5: 200ra117, 2013. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Toledo RA, Garralda E, Mitsi M, Pons T, Monsech J, Vega E, Otero Á, Albarran MI, Baños N, Durán Y, Bonilla V, Sarno F, Camacho-Artacho M, Sanchez-Perez T, Perea S, Álvarez R, De Martino A, Lietha D, Blanco-Aparicio C, Cubillo A, Domínguez O, Martínez-Torrecuadrada JL, Hidalgo M. Exome sequencing of plasma DNA portrays the mutation landscape of colorectal cancer and discovers mutated VEGFR2 receptors as modulators of antiangiogenic therapies. Clin Cancer Res 24: 3550–3559, 2018. doi: 10.1158/1078-0432.CCR-18-0103. [DOI] [PubMed] [Google Scholar]
- 171.Tomasetti C, Durrett R, Kimmel M, Lambert A, Parmigiani G, Zauber A, Vogelstein B. Role of stem-cell divisions in cancer risk. Nature 548: E13–E14, 2017. doi: 10.1038/nature23302. [DOI] [PubMed] [Google Scholar]
- 172.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LSG, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, Stewart S, Hecker M, Zhu Z, Gehling U, Seeger W, Pepke-Zaba J, Morrell NW. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 180: 780–787, 2009. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsutsumi T, Nagaoka T, Yoshida T, Wang L, Kuriyama S, Suzuki Y, Nagata Y, Harada N, Kodama Y, Takahashi F, Morio Y, Takahashi K. Nintedanib ameliorates experimental pulmonary arterial hypertension via inhibition of endothelial mesenchymal transition and smooth muscle cell proliferation. PLoS One 14: e0214697, 2019. doi: 10.1371/journal.pone.0214697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994. [PMC free article] [PubMed] [Google Scholar]
- 175.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 176.van der Feen DE, Berger RMF, Bartelds B. Converging paths of pulmonary arterial hypertension and cellular senescence. Am J Respir Cell Mol Biol 61: 11–20, 2019. doi: 10.1165/rcmb.2018-0329TR. [DOI] [PubMed] [Google Scholar]
- 177.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vattulainen-Collanus S, Southwood M, Yang XD, Moore S, Ghatpande P, Morrell NW, Lagna G, Hata A. Bone morphogenetic protein signaling is required for RAD51-mediated maintenance of genome integrity in vascular endothelial cells. Commun Biol 1: 149, 2018. doi: 10.1038/s42003-018-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, Palmisciano AJ, Krishnan I, Pinder D, Preston IR, Roberts KE, Kawut SM. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med 193: 1168–1175, 2016. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol 11: 664–675, 1994. doi: 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- 181.Voelkel NF. The wide spectrum of pulmonary vascular diseases. The sick lung circulation. In: Pulmonary Hypertension: The Present and Future, edited by Voelkel NF. Shelton, CT: People’s Medical Publishing House-USA, 2011, p. 1–9. [Google Scholar]
- 182.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J 40: 1555–1565, 2012. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Voelkel NF, Gomez-Arroyo J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol 51: 474–484, 2014. doi: 10.1165/rcmb.2014-0045TR. [DOI] [PubMed] [Google Scholar]
- 184.Voelkel NF, Newman JH. The light at the end of the long pulmonary hypertension tunnel brightens. Am J Respir Crit Care Med 198: 818–820, 2018. doi: 10.1164/rccm.201802-0325LE. [DOI] [PubMed] [Google Scholar]
- 185.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science 339: 1546–1558, 2013. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wauchope OR, Mitchener MM, Beavers WN, Galligan JJ, Camarillo JM, Sanders WD, Kingsley PJ, Shim HN, Blackwell T, Luong T, deCaestecker M, Fessel JP, Marnett LJ. Oxidative stress increases M1dG, a major peroxidation-derived DNA adduct, in mitochondrial DNA. Nucleic Acids Res 46: 3458–3467, 2018. doi: 10.1093/nar/gky089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weiss A, Neubauer MC, Yerabolu D, Kojonazarov B, Schlueter BC, Neubert L, Jonigk D, Baal N, Ruppert C, Dorfmuller P, Pullamsetti SS, Weissmann N, Ghofrani HA, Grimminger F, Seeger W, Schermuly RT. Targeting cyclin-dependent kinases for the treatment of pulmonary arterial hypertension. Nat Commun 10: 2204, 2019. doi: 10.1038/s41467-019-10135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.White KA, Grillo-Hill BK, Esquivel M, Peralta J, Bui VN, Chire I, Barber DL. β-Catenin is a pH sensor with decreased stability at higher intracellular pH. J Cell Biol 217: 3965–3976, 2018. doi: 10.1083/jcb.201712041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Whitfield JR, Soucek L. Tumor microenvironment: becoming sick of Myc. Cell Mol Life Sci 69: 931–934, 2012. doi: 10.1007/s00018-011-0860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wright L, Tuder RM, Wang J, Cool CD, Lepley RA, Voelkel NF. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am J Respir Crit Care Med 157: 219–229, 1998. doi: 10.1164/ajrccm.157.1.9704003. [DOI] [PubMed] [Google Scholar]
- 191.Wu D, Birukov K. Endothelial cell mechano-metabolomic coupling to disease states in the lung microvasculature. Front Bioeng Biotechnol 7: 172, 2019. doi: 10.3389/fbioe.2019.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yan L, Chen X, Talati M, Nunley BW, Gladson S, Blackwell T, Cogan J, Austin E, Wheeler F, Loyd J, West J, Hamid R. Bone marrow-derived cells contribute to the pathogenesis of pulmonary arterial hypertension. Am J Respir Crit Care Med 193: 898–909, 2016. doi: 10.1164/rccm.201502-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 88: E2–E11, 2001. doi: 10.1161/01.RES.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 194.Zabini D, Crnkovic S, Xu H, Tscherner M, Ghanim B, Klepetko W, Olschewski A, Kwapiszewska G, Marsh LM. High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J Cell Mol Med 19: 1151–1161, 2015. doi: 10.1111/jcmm.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhai JM, An YH, Wang W, Fan YG, Yao GL. IL-32 expression indicates unfavorable prognosis in patients with colon cancer. Oncol Lett 17: 4655–4660, 2019. doi: 10.3892/ol.2019.10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Ježek P, Morrell NW, Hu CJ, Stenmark KR. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a MicroRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation 136: 2468–2485, 2017. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang YY, Walker JL, Huang A, Keaney JF Jr, Clish CB, Serhan CN, Loscalzo J. Expression of 5-lipoxygenase in pulmonary artery endothelial cells. Biochem J 361: 267–276, 2002. doi: 10.1042/bj3610267. [DOI] [PMC free article] [PubMed] [Google Scholar]