SUMMARY
Background:
Impaired cilial signaling in the melanocortin-4 receptor (MC4R) pathway may contribute to obesity in Bardet-Biedl syndrome (BBS) or Alström syndrome, rare genetic diseases associated with hyperphagia and early-onset, severe obesity. We evaluated the MC4R agonist setmelanotide for reducing body weight and hunger in these patients.
Methods:
This multicenter Phase 3 trial (NCT03746522) enrolled patients ≥6 years old with obesity and BBS or Alström syndrome in North America and Europe. Obesity was defined as weight >97th percentile (6–15 years old) or body mass index ≥30 kg/m2 (≥16 years old). Patients were randomized 1:1 to receive up to 3·0 mg of daily subcutaneous setmelanotide or placebo in a 14-week double-blind period, followed by open-label setmelanotide for 52 weeks of setmelanotide treatment total. The primary endpoint was the proportion of patients ≥12 years old in the full analysis set achieving ≥10% weight reduction after 52 weeks of setmelanotide. Hunger and safety were also assessed. This trial was completed at the time of analysis.
Findings:
Between December 10, 2018, and November 25, 2019, 38 patients with BBS or Alström syndrome were enrolled. The primary endpoint was achieved by 32·3% (95% CI, 16·7%, 51·4%; p=0·0006) of patients ≥12 years old, all of whom were patients with BBS. After 14 weeks in the placebo-controlled period, mean (SD) change in body weight was −2·4% (4·8%) and −0·3% (2·3%) with setmelanotide and placebo, respectively (difference, −2·1%; 95% CI, −4·6%, 0·4%; p=0·052). Skin hyperpigmentation (n=23; 60·5%) was the most common adverse event (AE). Two patients experienced serious AEs, none of which were considered related to setmelanotide treatment.
Interpretation:
Setmelanotide resulted in significant weight and hunger reductions after 1 year of treatment among patients with BBS; results in patients with Alström syndrome were not significant. Setmelanotide is the first approved treatment for obesity in BBS.
Funding:
Rhythm Pharmaceuticals, Inc.
Keywords: obesity, hyperphagia, rare genetic disease, MC4R, weight loss, Bardet-Biedl syndrome
INTRODUCTION
The central hypothalamic melanocortin pathway is a key regulator of energy balance.1 Pathway disruption leading to impaired melanocortin-4 receptor (MC4R) signaling may result in hyperphagia (a pathological insatiable hunger) and decreased energy expenditure, resulting in early-onset, severe obesity.1 Bardet-Biedl syndrome (BBS) is a rare autosomal recessive pleiotropic and genetically heterogeneous disease that arises from impaired primary cilium function.2 It is associated with hyperphagia, early-onset severe obesity, and other clinical features, including polydactyly and retinal degeneration.2,3 Obesity is present in 72%−92% of patients with BBS with onset frequently during infancy.2,3 Obesity and hyperphagia are among the most distressing manifestations of BBS,4 with each being a significant burden to patients and their caregivers.5,6 Alström syndrome, a rare genetic disease also associated with cilium dysfunction, is characterized by early-onset obesity, hyperphagia, and multisystem dysfunction including visual and auditory impairments, renal dysfunction, and cardiomyopathy.7
Both BBS and Alström syndrome are associated with rare genetic variants. Variants in 27 genes have been identified to cause BBS, and the associated proteins assist in the formation or function of cilia.2,8–11 Alström syndrome is caused by variants in ALMS1.7 Impaired cilial signaling is hypothesized to cause dysfunction in the MC4R pathway resulting in leptin resistance, hyperphagia, and obesity in patients with BBS, although the molecular mechanisms are not fully understood.11–16 In Alström syndrome, evidence suggests ALMS1 expression in the hypothalamus is involved in neuronal function, and variants may disrupt hunger signaling. There is no specific cure for BBS or Alström syndrome, and treatments targeting the underlying pathophysiology of hyperphagia do not exist. Rather, hyperphagia and obesity are managed symptomatically (eg, mostly through lifestyle modification and sometimes surgical intervention).2,5
Setmelanotide, an MC4R agonist, can restore MC4R signaling by acting on MC4R downstream of the locus for ciliopathy-related impaired melanocortin signaling. In a recent Phase 2 trial, setmelanotide reduced body weight and hunger in patients with BBS.13 Hence, a Phase 3 trial was designed to evaluate the effect of setmelanotide on body weight in a combined population of patients with BBS or Alström syndrome with the rationale that the diseases have some overlapping clinical features (including hyperphagia and early-onset obesity) and share underlying ciliary dysfunction.7,17
Clinical trials in rare diseases must enroll enough patients to test the hypotheses and manage the inevitable heterogeneity of the patient population.18 By combining two diseases, a larger trial could be performed, although including patients with Alström syndrome was expected to introduce heterogeneity into the trial. The confounding effect of pooling the two diseases became evident after unblinding for the primary and secondary analyses. Data from the prespecified pooled analysis are reported here, as well as the prespecified exploratory analysis of the BBS-only population, which made up the majority of the patient population. Importantly, these data represent the largest clinical trial to date in patients with BBS.
METHODS
Study Design and Patients
This multicenter Phase 3 trial (ClinicalTrials.gov, NCT03746522), with a randomized, double-blind, placebo-controlled period followed by an open-label period (Appendix p17), was performed at 12 sites in the United States, Canada, the United Kingdom, France, and Spain. This study was conducted in accordance with guidelines from the Declaration of Helsinki, Council for International Organizations of Medical Sciences, and International Council for Harmonisation at sites with approval of the Independent Ethics Committee or institutional review board. An Independent Data Monitoring Committee monitored the safety and efficacy data during the study to ensure the highest ethical standards. Details regarding background and study design have been previously published,5 and a summary is provided herein. Because BBS and Alström syndrome share some overlapping phenotypes, have related pathophysiology, and are both associated with energy balance and hunger dysregulation through the MC4R pathway, populations with both syndromes were recruited.2,19
The trial included patients age ≥6 years with a BBS or Alström syndrome clinical diagnosis3,20 and obesity, defined as body mass index (BMI) >97th percentile for age and sex on growth charts for those aged 6–15 years and ≥30 kg/m2 for those aged ≥16 years. These ages were used because data indicate that most patients ≥16 years have minimal additional growth in height. Key exclusion criteria included >2% weight loss from diet and/or exercise program in the prior 2 months, >10% durable weight loss from gastric bypass surgery, use of obesity medication within prior 3 months, glomerular filtration rate <30 mL/min, and any prior exposure to setmelanotide (Appendix p2–3). Patients and/or their parent/guardian provided informed consent/assent.
The safety analysis set (SAS) was defined as all patients who received ≥1 dose of placebo or setmelanotide. The full analysis set (FAS) included patients who received ≥1 dose of setmelanotide and had baseline data. The placebo-controlled analysis set (PCAS) was defined as patients who received ≥1 dose of placebo or setmelanotide and had baseline data, specifically defined for the double-blind, placebo-controlled period. A protocol amendment allowed for the enrollment of a supplemental cohort (Appendix p8–9). Given the rarity of the disease and trial design, data from the double-blind, placebo-controlled period of both pivotal and supplemental patients with BBS (described in Appendix p6–7) were also analyzed in a prespecified exploratory population of patients with BBS only.
Randomization and Masking
Investigators identified, screened, and enrolled patients, who then were stratified by age (≥12 years or <12 years) and clinical syndrome and randomized 1:1 to receive daily (administered in the morning) subcutaneous setmelanotide or placebo during the 14-week double-blind, placebo-controlled period. Randomization was assigned based on a numerical randomization code generated before study initiation (Appendix p4).
Procedures
At the beginning of the 14-week double-blind, placebo-controlled period, patients entered a 2-week dose titration phase, with doses titrated up to 3·0 mg on the basis of age (Appendix p10). Patients continued 3·0-mg setmelanotide or placebo through Week 14. After all patients repeated dose escalation in Weeks 15 and 16 to maintain the study blind, they continued open-label setmelanotide at 3·0 mg until Week 66, unless the investigator reduced the dose because of a favorable response (eg, marked weight loss). Study visits occurred approximately every 4 weeks during the double-blind, placebo-controlled period and approximately every 6 weeks during the open-label period. Body weight measures, height, waist circumference, and safety were assessed at every visit. Hunger scores were self-reported daily for those able to do so. Fasting lipids were assessed at Weeks 1, 15, 29, 53, and 66. Body composition was assessed using methods that were available at the site (eg, dual-energy x-ray absorptiometry scan, bioelectrical impedance) at Weeks 1, 53, and 66. Analyses were performed after all patients completed their last study visit. Adult and adolescent patients did not receive specific guidance on lifestyle modifications related to dietary intake or physical activity during the trial, although patients may have been familiar with, or had previously attempted, lifestyle modifications to control body weight gain. Nutritional counseling and monitoring was provided for pediatric patients to ensure adequate nutritional intake for proper growth and development.
Outcomes
The predefined primary and key secondary endpoints were in the combined BBS and Alström syndrome population among those ≥12 years old. The primary endpoint was the proportion of patients ≥12 years old who achieved ≥10% reduction in body weight from baseline after 52 weeks of setmelanotide treatment. Because weight loss assessment is confounded in children who are still growing, age ≥12 years was selected as the primary analysis age cutoff to allow maximum patient enrollment while minimizing the effect of growth on weight outcomes. Key secondary endpoints included the mean percent change from baseline in body weight, the mean percent change from baseline in weekly average of the daily hunger score, and the proportion of patients who achieved ≥25% reduction in weekly average of the daily hunger score from baseline, all after 52 weeks of setmelanotide treatment. Hunger was assessed in patients ≥12 years old without cognitive impairment (n=18) using 3 daily questions (Appendix p4) with a primary focus on the daily maximal hunger score. Other secondary efficacy endpoints were the mean percent change from baseline in body weight and weekly average of the daily hunger score at Week 14 compared with placebo. Frequency and severity of adverse events (AEs), laboratory evaluations, and vital signs were also assessed.
In addition to the predefined primary and secondary endpoints, a prespecified exploratory analysis of the subgroup of patients with BBS was performed in adult (≥18 years) and pediatric (<18 years) cohorts to eliminate the confounding effects of growth. The proportions of adult patients who achieved ≥5% and ≥10% body weight reduction and pediatric patients who achieved ≥0·2- and ≥0·3-point BMI Z score reduction were evaluated, based on suitable thresholds for a clinically important change in weight.21,22 A previous psychometric evaluation estimated the meaningful within-patient change (MWPC) thresholds of the daily maximal hunger score to be a reduction of 1 to 2 points (unpublished data on file, Rhythm Pharmaceuticals, Inc.). The proportion of patients who achieved this MWPC was also calculated in addition to the proportion of patients who achieved ≥25% reduction in weekly average of the daily hunger score from baseline.
Sample Size Determination
The sample size was determined on the basis of feasibility of the primary hypothesis, although the rarity of the population was also considered. A historical reference response rate was estimated from the Clinical Registry Investigating Bardet-Biedl Syndrome (CRIBBS; NCT02329210) longitudinal natural history registry for better understanding the clinical features of BBS and the impact of standard of care in these patients. Assuming a 66% response rate (ie, 66% of participants experiencing ≥10% reduction in body weight at 52 weeks), seven patients were required for 91% power to provide a statistically significant difference compared with a historical control rate of 10% at one-sided alpha level of 0·025. The null hypothesis is that the proportion of patients treated for ~52 weeks achieving ≥10% body weight reduction is less than or equal to a historical control rate of 10%; the alternative hypothesis is that the proportion is greater than the historical control rate. To have sufficient data for between-group analysis of the placebo-controlled, double-blind period and gain further insight into these rare diseases, planned enrollment was ~30 patients for the pivotal cohort, including six patients with Alström syndrome.
Statistical Analysis
Statistical analyses were assessed using SAS version 9·4. Efficacy analyses after 14 and 52 weeks of treatment were performed per protocol in the PCAS and FAS populations, respectively. Efficacy analyses at Week 14 (the placebo-controlled, double-blind period) reported here include a separate analysis of patients with BBS from both the pivotal and supplemental cohorts, and analyses at 52 weeks of active setmelanotide treatment include only pivotal patients because none of the supplemental patients with BBS had completed 52 weeks of setmelanotide at the end of the study. Safety analyses were conducted in the SAS population.
For patients who received ≥52 weeks of setmelanotide treatment by end of study, the analysis was performed at the time the last patient in the pivotal cohort completed 52 weeks of setmelanotide treatment. Generally, no substitutions were made to accommodate for missing data points. Due to the nature of the study design, a multiple imputation model was used to impute data for patients who received <52 weeks of setmelanotide treatment at the time of analysis (ie, a small percentage of patients in the original 14-week placebo arm). For analyses after 52 weeks of setmelanotide, baseline (named “active treatment baseline [ATB]”) is considered the last available measurement before the first dose of setmelanotide. For analyses after 14 weeks of treatment with either setmelanotide or placebo during the placebo-controlled period, baseline (named “placebo-controlled period baseline” [PCPB]) is considered the last available measurement before randomization to setmelanotide or placebo.
The primary hypothesis was tested using binomial proportions calculated for each of the 100 multiple imputed data sets, which were combined using Rubin’s rule. The null hypothesis was tested at a one-sided alpha level of 0·025, and the corresponding two-sided 95% confidence intervals (CIs) were provided. No adjustments for multiplicity were made for primary or secondary endpoints because of the small number of patients enrolled. Additional statistical analysis methods are described in the Appendix (p5–6).
Role of the Funding Source
Funding for this study was provided by Rhythm Pharmaceuticals. The funder assisted in study design, analysis and interpretation of data, and review of the manuscript.
RESULTS
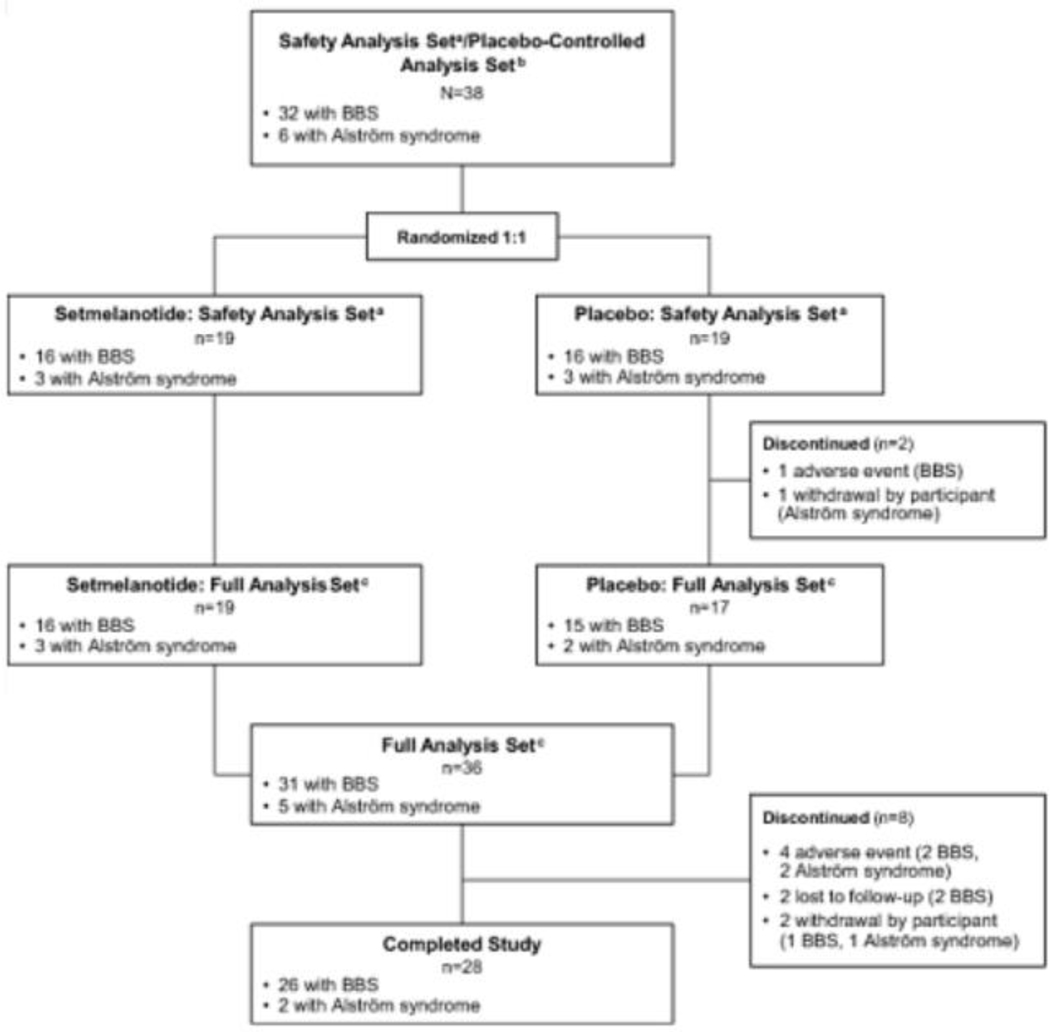
Between December 10, 2018, and November 25, 2019, 38 patients were enrolled and randomized (BBS, n=32; Alström syndrome, n=6) (Figure 1). Two patients (BBS, n=1; Alström syndrome, n=1) discontinued study drug after receiving placebo but before receiving setmelanotide. Baseline demographics are reported in Table 1. Three reported type 2 diabetes mellitus. Among those with BBS in either the pivotal or supplemental cohorts, the most common genetic variants identified were in BBS1 (12/44; 27·3%) and BBS10 (11/44; 25·0%) (Appendix p11). During the study, 5 patients had dose reductions (setmelanotide, n=2; placebo, n=3); all but 1 patient resumed the full 3·0 mg dosage following a brief reduction.
Figure 1.
Disposition of pivotal and supplemental patients with BBS and Alström syndrome. BBS, Bardet-Biedl syndrome. aSafety analysis set includes patients who received ≥1 dose of study drug (setmelanotide or placebo). bPlacebo-controlled analysis set includes randomized patients who received ≥1 dose of placebo or setmelanotide and had baseline data. cFull analysis set includes randomized patients who received ≥1 dose of setmelanotide and had baseline data.
Table 1.
Baseline Characteristics of Patients
| Pivotal patients with BBS and Alstrӧm syndrome (SAS) (N=38) | Pivotal patients ≥12 years old with BBS and Alstrӧm syndrome (FAS) (n=31) | Pivotal patients with BBS (n=32) | |
|---|---|---|---|
| Age, yearsa | 19·8 (10·2) [16·5; 12·0–24·0] | 21·6 (10·3) [19·0; 13·0–28·0] | 20·2 (10·2) [17·5; 12·0–25·5] |
| Female, n (%) | 23 (60·5) | 17 (54·8) | 17 (53·1) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 1 (2·6) | 1 (3·2) | 1 (3·1) |
| Not Hispanic or Latino | 37 (97·4) | 30 (96·8) | 31 (96·9) |
| Race, n (%) | |||
| White | 31 (81·6) | 26 (83·9) | 28 (87·5) |
| Black or African American | 3 (7·9) | 2 (6·5) | 1 (3·1) |
| Asian | 1 (2·6) | 0 | 0 |
| Other | 3 (7·9) | 3 (9·7) | 3 (9·4) |
| Cognitive impairment, n (%) | 19 (50·0) | 15 (48·4) | 17 (53·1) |
| Weight, kga | 111·7 (30·4) [112·3; 92·0–129·8] | 117·2 (29·2) [113·8; 98·7–130·2] | 112·3 (27·9) [113·4; 94·6–129·9] |
| BMI, kg/m2a | 42·3 (11·0) [41·0; 34·9–47·1] | 43·5 (11·4) [41·5; 36·9–47·4] | 41·6 (90) [41·3; 35·4–46·7] |
| Maximal hunger scoreb | — | 7·3 (2·0) | 6·8 (1·8) |
Data are mean (SD) [median; interquartile range] unless otherwise specified. BBS, Bardet-Biedl syndrome; BMI, body mass index; FAS, full analysis set; SAS, safety analysis set; SD, standard deviation.
At placebo-controlled period baseline.
At active treatment baseline in patients ≥12 years old without cognitive impairment; self-reported (FAS: n=16; pivotal patients with BBS: n=18).
The primary endpoint of the study was achieved, with 32·3% (95% CI, 16·7%, 51·4%; p=0·0006) of patients ≥12 years (in the FAS achieving ≥10% reduction in body weight after 52 weeks of setmelanotide (all of whom had BBS), with mean (standard deviation [SD]) percent change in body weight of −5·2% (7·9%; 95% CI, −8·1%, −2·3%; p=0·0005) from ATB (Table 2).
Table 2.
Changes in Anthropometric and Metabolic Parameters Compared With Baseline After 52 Weeks of Setmelanotide Treatment: Pivotal Patients With BBS and Alström Syndrome (FAS; n=36)
| ≥12 years old (n=31) | ||||
|---|---|---|---|---|
|
| ||||
| ATB | Week 52 | Change from ATB | % change from ATB | |
| Body weight, kg | 117·0 (29·3) | 111·1 (31·0) | −5·9 (9·3) [−9·3, −25] p=0·0007 | −5·2 (7·9) [−8·1, −2·3] p=0·0005 |
| Achieved >10% lossa, % | — | 32·3 [16·7, 51·4] p=0·0006 | — | — |
| Maximal hunger scoreb | 7·3 (2·0) | 5·0 (2·4) | −2·3 (2·0) [−3·3, −1·2] p=0·0002 | −30·9 (24·7) [−44·1, −17·7] p<0·0001 |
| Achieved >25% reduction, %a,b | — | 62·5 [35·4, 84·8] p<0·0001 | — | — |
|
| ||||
| All ages, ≥6 years old (n=36) | ||||
|
| ||||
| ATB | Week 52 | Change from ATB | % change from ATB | |
|
| ||||
| Waist circumference, cmc | 118·1 (18·1) | 113·2 (24·8) | −5·1 (10·9) [−9·3, −0·8] | −4·7 (9·2) [−8·3, −1·2] |
| Body fat, kgd | 51·9 (21·0) | 43·3 (16·2) | −4·6 (12·0) [−10·2, 1·3] | −8·9 (26·1) [−21·5, 3·6] |
| Lean muscle, kgd | 57·8 (14·4) | 56·2 (12·6) | −0·7 (4·0) [−2·7, 1·2] | −1·0 (7·0) [−4·4, 2·4] |
| Lipids, mmol/Le | ||||
| Total cholesterol | 4·4 (1·0) | 3·9 (0·9) | −0·3 (0·4) | −7·0 (10·2) [−11·1, −2·8] |
| HDL cholesterol | 1·1 (0·2) | 1·1 (0·2) | 0·1 (0·1) | 4·3 (11·6) [−0·4, 9·0] |
| LDL cholesterol | 3·0 (1·0) | 2·3 (0·9) | −0·2 (0·4) | −8·8 (16·2) [−15·3, −2·3] |
| Triglycerides | 2·0 (1·4) | 1·4 (0·8) | −0·2 (0·6) | −10·7 (32·0) [−23·6, 2·2] |
Data are the mean (SD), mean [95% CI], or mean (SD) [95% CI] unless otherwise specified. ATB, active treatment baseline (defined as last measurement before the first dose of setmelanotide; ie, Week 0 for setmelanotide group and Week 14 for placebo group); BBS, Bardet-Biedl syndrome; CI, confidence interval; FAS, full analysis set; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Multiple imputation model used to impute measurements for patients with <52 weeks of treatment.
Estimated proportion on the basis of qualifying patients in the FAS (ie, ≥12 years old without cognitive impairment; n=16).
Patients with measurements at ATB (n=36) and Week 52 (n=28).
Patients with measurements at ATB (n=33), Week 52 (n=20), and both (n=19).
Patients with measurements at ATB (n=36) and Week 52 (n=26).
Among those ≥12 years old with BBS or Alström syndrome and no cognitive impairment, 62·5% (95% CI, 35·4%, 84·8%; p<0·0001) of patients achieved ≥25% reduction in the weekly average of the daily maximal hunger score after 52 weeks of setmelanotide, with mean (SD) percent change in maximal hunger of −30·9% (24·7%; 95% CI, −44·1%, −17·7%; p<0·0001) from ATB.
Outcomes at Week 14 following the double-blind, placebo-controlled period were prespecified secondary endpoints. Baseline characteristics were similar among those randomized to each arm (Appendix p12). After 14 weeks in the double-blind, placebo-controlled period, patients ≥12 years receiving setmelanotide and placebo lost a mean (SD) of −2·4% (4·8%) and −0·3% (2·3%) body weight, respectively, representing a −2·1% further reduction in body weight compared with those receiving placebo (95% CI, −4·6%, 0·4%; p=0·052) (Appendix p14). A significantly greater proportion of patients achieved ≥25% reduction in maximal hunger score at Week 14 with setmelanotide (71·4%) versus placebo (20·2%; difference, 51·2% [95% CI, 9·4%, 93·0%]; p=0·0081), with a mean (SD) change in maximal hunger score of −26·7% (19·0%) versus −14·8% (14·6%) from baseline for setmelanotide and placebo, respectively. Results in the BBS-only population are included in the Appendix (p6–7).
All patients in the FAS achieving the primary endpoint of ≥10% reduction in body weight were patients with BBS, which strongly indicated a separate analysis of these patients was necessary. In addition, of the 32 patients with BBS in the pivotal cohort who received ≥1 dose of the study drug, 11 grew >2 cm from baseline to the primary endpoint time point or last available time point, necessitating additional analyses of body weight measures such as BMI Z score and BMI percent of the 95th percentile. Thus, additional analyses were performed in patients with BBS based on an age cutoff of 18 years.
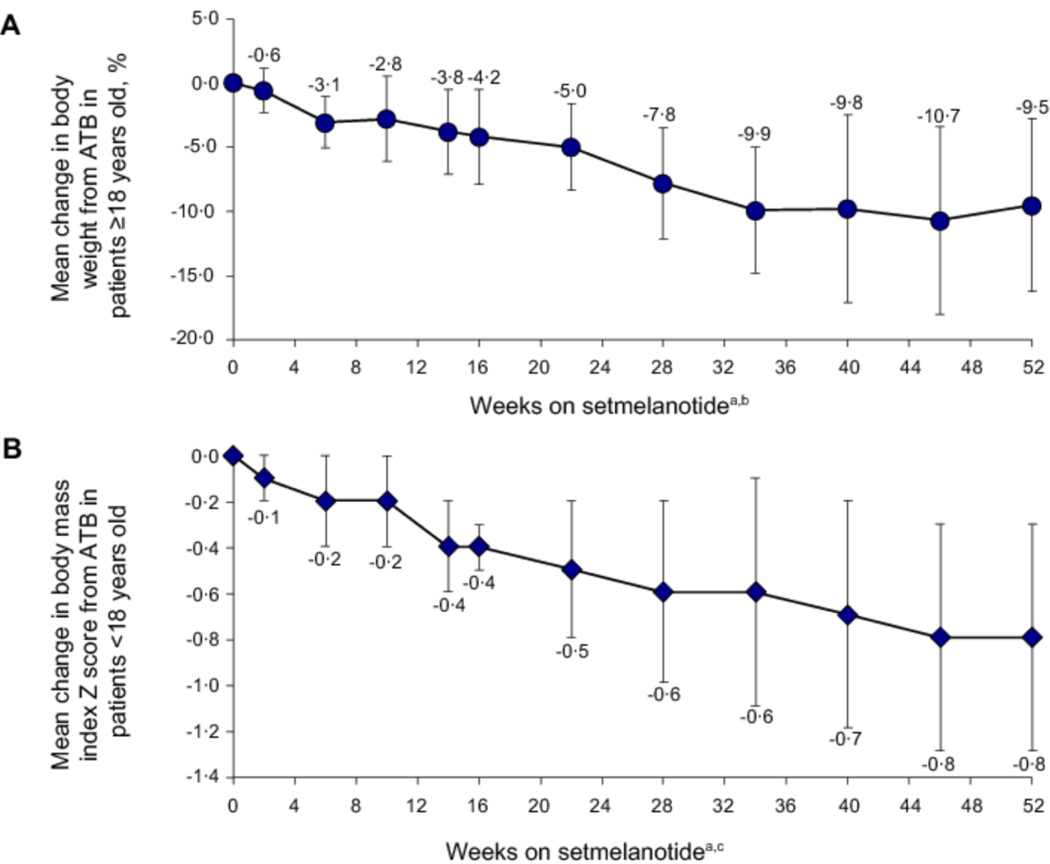
Among patients with BBS ≥18 years (n=15), seven (46·7%) achieved ≥10% and nine (60·0%) achieved ≥5% reduction in body weight after 52 weeks of setmelanotide. Mean (SD) change in BMI was −4·2 (3·3; 95% CI, −6·3, −2·1) kg/m2 and mean (SD) percent change was −9·1% (6·8%; 95% CI, −13·4%, −4·8%) from ATB (Table 3). Mean percent change in body weight by study week is shown in Figure 2A.
Table 3.
Changes in Anthropometric and Metabolic Parameters Compared With Baseline After 52 Weeks of Setmelanotide Treatment: Pivotal Patients With BBS (n=32)
| ≥12 years old (n=28) | ||||
|---|---|---|---|---|
|
| ||||
| ATB | Week 52 | Change from ATB | % change from ATB | |
| Body weight, kg | 115·9 (26·7) | 108·5 (27·0) | −7·4 (8·2) [−10·6, −4·2] p<0·0001 | −6·5 (7·0) [−9·2, −3·8] p<0 p<0·001 |
| Maximal hunger scorea | 7·0 (1·9) | 4·9 (2·5) | −21 (2·0) [−3·3, −0·9] p=0·0010 | −30·5 (26 ·5) [−45·7, −15·2] p=0 ·0004 |
| Achieved ≥25% reduction in | — | 571c [28·9, 82·3] | — | — |
| body weightb, % | p<0·0001 | |||
| ≥1-point reduction, % | — | 71·4 | — | — |
| ≥2-point reduction, % | — | 42·9 | — | — |
|
| ||||
| All ages, ≥6 years old (n=31) | ||||
|
| ||||
| ATB | Week 52 | Change from ATB | % change from ATB | |
|
| ||||
| Waist circumference, cmd | 117·9 (18·0) | 110·3 (21·0) | −7·2 (7·4) [−10·2, −4·1] | −6·3 (7·4) [−9·4, −3·3] |
| Body fat, kge | 51·1 (18·9) | 43·1(16·3) | −5·6 (12·0) [−11·8, 0·5] | −11·3 (26·3) [−24·9, 2·2] |
| Lean muscle, kge | 58·9 (14·1) | 57·6 (12·4) | −12 (3·9) [−3·2, 0·8] | −2·0 (6·5) [−5·4, 1·3] |
| Lipids, mmol/Lf | ||||
| Total cholesterol | 4·4 (1·0) | 3·9 (0·9) | −0·3 (0·4) | −6·1 (10·6) [−22·6, 19·2] |
| HDL cholesterol | 1·1 (0·2) | 1·1 (0·2) | 0·1 (0·1) | 5·3 (11·6) [−14·3, |
| LDL cholesterol | 3·0 (10) | 2·6 (0·9) | −0·2 (0·4) | 30·8] −7·8 (168) [−33·3, |
| Triglycerides | 1·9 (0·9) | 1·4 (0·8) | −0·2 (0·6) | 33·3] −9·6 (32·5) [−69·9,67·2] |
|
| ||||
| ≥18 years old (n=15) | ||||
|
| ||||
| Body weight, kg | 128·4 (16·6) | 119·0 (20·6) | −9·4 (9·4) [−14·6, −4·2] p=0·0008 | −7·6 (−7·1) [−11·5, −36] p=0·0005 |
| BMI, kg/m2g | 46·4 (5·9) | 43·3 (7·2) | −4·2 (3·3) [−6·3, −21] | −9·1(6·8) [−13·4, −4·8] |
|
| ||||
| <18 years old (n=16) | ||||
|
| ||||
| BMI,h kg/m2 | 37·4 (9·4) | 34·2 (10·1) | −3·4 (2·1) [−4·6, −2·2] | −95 (6·4) [−13·2, −5·8] |
| BMI Z scoreh | 3·7 (1·3) | 3·0 (1·5) | −0·8 (0·5) [−1·0, −0·5] | — |
| % 95th BMI percentile | 144·5 (35·8) | 126·8 (37·1) | −17·3 (7·7) [−21·7, −12·9] | — |
Data are the mean (SD), mean [95% CI], or mean (SD) [95% CI] unless otherwise specified. ATB, active treatment baseline; BBS; Bardet-Biedl syndrome; BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Patients ≥12 years old without cognitive impairment and with hunger scores at ATB and Week 52 (n=14).
Multiple imputation model used to impute measurements for patients with <52 weeks of treatment.
Estimated proportion based on qualifying patients in the full analysis set (ie, ≥12 years old without cognitive impairment; n=16).
Patients with measurements at ATB (n=31) and Week 52 (n=25).
Patients with measurements at ATB (n=29), Week 52 (n=18), and both (n=17).
Patients with measurements at ATB (n=31) and Week 52 (n=23).
Patients with measurements at ATB (n=15) and Week 52 (n=12).
Patients with measurements at ATB (n=16) and Week 52 (n=14).
Figure 2.
(A) Body weight percent change in the exploratory cohort of patients with BBS ≥18 years old (n=15). (B) Body mass index Z score absolute change in pivotal patients with BBS <18 years old (n=16). ATB, active treatment baseline (defined as last measurement before the first dose of setmelanotide; ie, Week 0 for setmelanotide group and Week 14 for placebo group). aData shown by study visit do not include data imputed for patients who received <52 weeks of setmelanotide at time of analysis. bPopulation sizes ranged from 7 to 15, with n=12 at 52 weeks on active treatment. cPopulation sizes ranged from 8 to 16, with n=14 at 52 weeks on active treatment. Error bars are the standard deviation.
Among patients with BBS <18 years (n=14), ten (71·4%) achieved ≥0·3-point reduction in BMI Z score and 12 (85·7%) achieved ≥0·2-point reduction after 52 weeks of setmelanotide. Mean (SD) change in BMI Z score was −0·8 (0·5; 95% CI, −1·0, −0·5) and mean (SD) change in BMI percent of the 95th percentile was −17·3 (7·7; 95% CI, −21·7, −12·9) (Figure 2B; Table 3).
Among patients with BBS ≥12 years old who reported hunger scores (n=14), 57·1% (95% CI, 28·9%, 82·3%; p<0·0001) of patients achieved ≥25% reduction in the weekly average of the daily maximal and morning hunger score after 52 weeks of setmelanotide with a mean (SD) percent change in maximal hunger score of −30·5% (26·5%; 95% CI, −45·7%, −15·2%; p=0·0004) (n=14). In addition, ten (71·4%) and six (42·9%) of 14 patients achieved the MWPC reduction thresholds of the daily maximal hunger score of ≥1 and ≥2 points, respectively. Decreases in total cholesterol, low-density lipoprotein cholesterol, triglycerides, body fat, and waist circumference were also observed (Table 3). Among 17 of 29 patients with body composition measurements at baseline and Week 52, mean (SD) fat mass decreased by 11·3% (26·3%), while lean body mass decreased by 2·0% (6·5%).
Across all patients, the most commonly reported treatment-emergent AEs in the SAS were skin hyperpigmentation (n=23; 60·5%) and injection site erythema (n=18; 47·7%; Table 4). Nausea and vomiting occurred in 13 patients (34·2%) and 10 patients (26·3%), respectively. All cases of nausea and vomiting were mild or moderate in severity, none were serious, and were infrequent after the first month of setmelanotide treatment. After 52 weeks of treatment, no noteworthy increases in systolic blood pressure (mean [SD] change, −2·4 [16·1; 95% CI, −8·7, 3·8] mm Hg), diastolic blood pressure (−2·0 [9·4; 95% CI, −5·6, 1·7] mm Hg) or heart rate (0·4 [10·9; 95% CI, −3·8, 4·6] beats/min) were observed with setmelanotide treatment. During the placebo-controlled period, skin hyperpigmentation was the most notable AE and was reported only in patients who received setmelanotide (n=11; 57·9%) (Appendix p15).
Table 4.
Treatment-Emergent Adverse Events
| Pivotal patients with BBS and Alstrӧm syndrome (SAS) (N=38) | Pivotal patients with BBS (n=32) | |
|---|---|---|
| Overall treatment-emergent AEs | 38 (100) | 32 (100) |
| Treatment-emergent AEs occurring in ≥10% of patients | ||
| Skin hyperpigmentation | 23 (60·5) | 18 (56·3) |
| Injection site erythema | 18 (47·7) | 16 (50·0) |
| Injection site pruritus | 13 (34·2) | 11 (34·4) |
| Nausea | 13 (34·2) | 11 (34·4) |
| Injection site bruising | 11 (28·9) | 11 (34·4) |
| Injection site pain | 11 (28·9) | 10 (31·3) |
| Vomiting | 10 (26·3) | 9 (28·1) |
| Injection site induration | 9 (23·7) | 8 (25·0) |
| Diarrhea | 7 (18·4) | 7 (21·9) |
| Headache | 10 (26·3) | 9 (28·1) |
| Back pain | 4 (10·5) | 2 (6·3) |
| Cough | 4 (10·5) | 2 (6·3) |
| HDL cholesterol decrease | 4 (10·5) | 3 (9·4) |
| Injection site edema | 4 (10·5) | 4 (12·5) |
| Melanocytic nevus | 4 (10·5) | 4 (12·5) |
| Nasopharyngitis | 4 (10·5) | 3 (9·4) |
| Spontaneous penile erection | 4 (10·5) | 4 (12·5) |
| Abdominal pain | 4 (10·5) | 2 (6·3) |
| Treatment-related AEs | 37 (97·4) | 32 (100) |
| Serious AEs | 2 (5·3) | 2 (6·3) |
| Serious treatment-related AEs | 1 (2·6)a | 1 (3·1)a |
| AEs leading to study drug withdrawal | 5 (13·2) | 3 (9·4) |
| AEs leading to death | 0 | 0 |
Data are n (%) unless otherwise specified. AE, adverse event; BBS, Bardet-Biedl syndrome; HDL, high-density lipoprotein; SAS, safety analysis set.
One patient with BBS experienced an anaphylactic reaction to study drug (placebo).
Two patients experienced a total of four serious AEs (blindness, anaphylactic reaction, and suicidal ideation); none were considered related to setmelanotide treatment (Appendix p7). In five patients, treatment-emergent AEs led to study drug withdrawal: one patient with anaphylaxis (while on placebo); one with nausea, vomiting, eye redness, and skin darkening; one with worsening acne and hidradenitis suppurativa; one with hot flashes, nausea, headaches, vomiting, and abdominal pain; and one with nausea and vomiting. No treatment-emergent AEs led to death during the study. The AE profile in patients with BBS was highly similar to the aggregate population (Table 4). The majority of all patients (42/54; 77·8%) who had enrolled in this Phase 3 study or a previous Phase 213 study have opted to continue receiving setmelanotide in the long-term extension trial (NCT03651765).
DISCUSSION
To our knowledge, this is the largest Phase 3 interventional trial in patients with BBS and Alström syndrome, and the first showing statistically significant and clinically beneficial weight loss and hunger reduction in individuals with BBS. Administration of setmelanotide, an MC4R agonist, resulted in statistically significant improvements in weight-related measures and hunger, both at Week 14 relative to placebo, as well as after 1 year in the combined patient population despite the absence of specific dietary or exercise guidance. Body composition and metabolic measures were also improved after 1 year of setmelanotide. Setmelanotide was generally well tolerated. While results in Alström syndrome were inconclusive, the results in patients with BBS were striking and drove the benefit seen in the combined population. As a targeted therapy, setmelanotide may provide personalized, precision treatment to individuals with rare variants in the MC4R pathway, including BBS.
Despite disease rarity, the trial enrolled a sufficiently sized cohort of patients with BBS to allow for statistical comparison between setmelanotide and placebo, demonstrating the initial safety and efficacy of setmelanotide. Hunger reduction occurred within the first several weeks of setmelanotide and continued throughout the study. Patients who had lost weight during the first 14 weeks of the study continued to lose weight throughout the study. Statistically and clinically significant weight loss (and related measures) and hunger reduction were observed after 52 weeks of setmelanotide in patients with BBS. Most patients with BBS lost weight during the 52 weeks of setmelanotide, which is a much better outcome than the described natural history of BBS in which patients rapidly gain weight early in childhood that persists into adulthood.4 Importantly, weight loss in this study was predominantly related to loss in fat mass with relative preservation of lean body mass. The results of the current analysis are consistent with earlier setmelanotide trials in rare genetic diseases of obesity, including a Phase 2 trial in patients with BBS.13
Hyperphagia especially can be an overwhelming burden to patients and their caregivers.23 Patients with BBS have complications due to unremitting hunger and incessant food-seeking behaviors, including the inability to focus on tasks and negatively impacted social participation.24 A clinically significant change in weight or BMI Z score was achieved by most patients with BBS in the study; however, the observed benefits of setmelanotide go beyond weight loss and include parallel reductions in hunger and improvements in health-related quality of life.25 Improvements in health-related quality of life from setmelanotide treatment are highly meaningful to patients with BBS and their caregivers,24 with the full impact not always reflected in improvements in body weight or hunger as recorded on a numerical rating scale. Individual narratives may better capture the overall patient experience.
Of the six patients with Alström syndrome enrolled in the trial, none met the primary endpoint of ≥10% weight loss after 52 weeks of setmelanotide. In part due to the rarity of the disease, we were able to enroll only a small cohort of patients with Alström syndrome, limiting the power of the analysis. Future analyses further exploring the role of the MC4R pathway and clinical benefit of setmelanotide beyond weight in this patient population are warranted.
In addition to the impaired signaling through the MC4R, patients with BBS and Alström syndrome may have other complicating biologic and environmental factors that were not systematically controlled in the current study. These factors include concomitant medications and the onset of the COVID-19 pandemic during the trial, which may have impacted weight in some patients in ways that are difficult to assess, yet should not be underestimated. Large interpatient variation in weight change is a common observation in pharmacotherapy for obesity, and speaks to the challenge of treating general obesity, let alone obesity associated with rare genetic diseases such as BBS or Alström syndrome.26 Notably, the impact of setmelanotide on hunger in this population highlights the benefit of treatment regardless of interpatient variability, with 57·1% of patients with BBS achieving ≥25% reduction in worst reported hunger levels.
The tolerability profile of setmelanotide was consistent with that in other studies of setmelanotide in patients with other rare genetic diseases of obesity, and no new safety concerns emerged.13,27 The onset of treatment-related AEs typically occurred during the first few weeks of setmelanotide. AEs were generally mild and transient and infrequently led to setmelanotide discontinuation. Hyperpigmentation was not transient but plateaued within the initial months of treatment and infrequently led to withdrawal. Hyperpigmentation due to setmelanotide is caused by activation of the melanocortin-1 receptor (MC1R) on the melanocyte with subsequent release of melanin.28 The only treatment-related serious AE occurred in a patient receiving blinded placebo at the time of the event. No changes in blood pressure and heart rate were identified across the treatment population. Most patients opted to continue setmelanotide in the long-term extension trial, suggesting strong patient satisfaction and clinical benefit with setmelanotide.
Until recently, available therapies for BBS were largely supportive and targeted other clinical characteristics of the disease.2 In 2022, setmelanotide was approved by the US Food and Drug Administration and EU European Medicines Agency for chronic weight management in patients with BBS ≥6 years old.29,30 It is possible that the earlier hyperphagia and obesity are treated, the greater the benefit for reducing associated comorbidities that might develop later in life. Given the similar response between adult and pediatric patients in this study, early intervention in pediatric patients may be a sound and useful option for this population.
The results of this trial were bolstered by the inclusion of a 14-week double-blind, placebo-controlled period, the results of which underscore those at the 52-week endpoints of the full study. Weight loss was observed during this period in patients who received setmelanotide but was negligible in those who received placebo. The absence of a clear placebo effect demonstrates the challenges patients with an impaired MC4R pathway have with obesity management. Hunger scores appeared to separate during the placebo-controlled period for patients who received setmelanotide compared with placebo, with those receiving placebo experiencing smaller reported reductions in hunger. When patients who initially received placebo began setmelanotide, they experienced a rapid reduction in hunger and soon matched the reduced hunger levels of those initially randomized to setmelanotide. The reduction in hunger is consistent with the mechanism of action of setmelanotide.
In addition to total body weight loss in adult patients, reductions in BMI (kg/m2), BMI Z score, and BMI percent of the 95th percentile were assessed in pediatric patients with BBS. This is relevant because these metrics more accurately reflect the true benefit of an intervention like setmelanotide in pediatric patients who are still growing. The failure to lose weight should not strictly be interpreted as nonresponse to treatment. Benefits of setmelanotide were consistently observed for patients <18 years old and for those ≥18 years old, when clinically relevant weightrelated measures were used.
A limitation of this study is the scientific challenge of combining 2 separate patient populations as well as the need to combine both children and adults in the analysis of weight reduction. While related, BBS and Alström syndrome are distinct diseases, and thus evaluating response to setmelanotide only in the combined population may mask the true benefit observed in this trial. For this reason, exploratory analyses of the BBS-only population were performed using the 18 years cutoff to specifically evaluate the effect of setmelanotide in both adult and pediatric populations. Another limitation of the study is that patients with only a subset of genetic variants known to cause BBS were enrolled, which is not surprising considering the rarity of the condition. In our small data set, there were no apparent differences in response as a function of underlying genotype.
Patients with moderate-to-severe renal dysfunction were not eligible for the trial because dosing in patients with renal insufficiency had not been studied at the time this trial was initiated. An estimated 53% to 82% of patients with BBS have renal disease, and approximately 8% develop kidney failure.2 A full 52-week double-blind study might have been superior to assess the effects of setmelanotide, but was not likely to be achievable in this young population with a rare disease. In light of the rarity of the disease, the enrolled trial population was small and heterogeneous. Nevertheless, this represents the largest interventional trial reported to date for patients with BBS.
BBS places a significant burden on both the patients and their families.2,5 Setmelanotide was associated with greater weight loss and hunger reduction compared with placebo after 14 weeks of treatment. Patients who were initially randomized to placebo experienced a reduction in hunger once they began setmelanotide treatment. Further, setmelanotide reduced weigh-trelated measures, as well as hunger, after 52 weeks of treatment. The effects of setmelanotide in Alström syndrome were inconclusive in this study and require further exploration. Setmelanotide was generally well tolerated, and the safety profile was aligned with that established in other rare genetic diseases of obesity. An ongoing extension study, which most patients from this trial entered, is evaluating the long-term effects of setmelanotide in patients with rare genetic diseases of obesity (NCT03651765). In conclusion, the positive results reported in this Phase 3 trial indicate that setmelanotide represents a new therapy for obesity and hyperphagia in patients with BBS.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed for English-language articles published before July 12, 2022, describing interventional clinical trials in patients with Bardet-Biedl syndrome (BBS) or Alström syndrome using the search string “(bardet-biedl OR “alstrom syndrome”) AND (“clinical trial” OR “clinical study” OR “intervention” OR “treatment”)” with the “Clinical Trials” filter off. Search results returned 18 relevant articles relating to BBS: 16 describing case reports or series with fewer than five patients; one describing the study design for this trial; and one describing an earlier Phase 2 open-label trial investigating setmelanotide in ten patients with BBS, in which setmelanotide treatment was associated with reduced body weight and hunger. Search results returned 12 relevant articles relating to Alström syndrome: 9 case reports or series with fewer than five patients; two clinical trial designs (one describing the study design for this trial); and one study of lifestyle interventions in two patients.
Added value of this study
Hyperphagia, a pathological insatiable hunger, and consequent obesity in patients with the genetic disease BBS and Alström syndrome start early in life, and currently no approved treatment modalities targeting the underlying pathophysiology exist. In this multicenter Phase 3 trial, setmelanotide was associated with greater weight loss and hunger reduction compared with placebo after 14 weeks of treatment, with further reductions in body weight and hunger after 52 weeks of setmelanotide in patients with BBS. This trial represents the largest interventional trial of patients with BBS, and results are consistent with the previous Phase 2 trial.
Implications of the available evidence
In line with previous reports, these results support the use of setmelanotide for the treatment of obesity and hyperphagia in patients with BBS and indicate that evaluation of setmelanotide in other genetic diseases with impaired signaling in the central hypothalamic melanocortin pathway and melanocortin-4 receptor is warranted.
ACKNOWLEDGMENTS
Funding for this study was provided by Rhythm Pharmaceuticals. We thank the participants and their families, trial coordinators, nurses, and members of the clinical investigation centers. Writing and editorial assistance was provided under the direction of the authors by Kristin French, PhD, Rhyomi Sellnow, PhD, CMPP, and David Boffa, ELS of MedThink SciCom, and funded by Rhythm Pharmaceuticals.
DECLARATION OF INTERESTS
RMH has received study drugs, grant support for clinical trials of setmelanotide in obesity, payments for lectures, and support for attending meetings from Rhythm Pharmaceuticals, Inc, as well as consulting fees from Rhythm Pharmaceuticals, Inc, and Axovia Therapeutics, LLC, and participated in the Data Safety Monitoring Board for setmelanotide clinical trials for Rhythm Pharmaceuticals, Inc. AMH has received grant funding from the Weston Family Microbiome Initiative and Canadian Institutes of Health Research, received payment as a speaker for Pfizer Canada, Inc, is a member of the BBS advisory board for Rhythm Pharmaceuticals, Inc, is a member of the 2021 Somatrogon advisory board for Pfizer, Inc, and heads the scientific advisory board for the Prader-Willi Syndrome Association USA (PWSA). WKC has received study funding, consulting fees, and payment for speaker bureaus from Rhythm Pharmaceuticals, Inc. HD has received consulting fees once from Rhythm Pharmaceuticals, Inc, and participated in the BBS advisory board for Rhythm Pharmaceuticals, Inc. GÁM-M has received payment for lectures from and participated in the BBS advisory board for Rhythm Pharmaceuticals, Inc. CP has received support for attending meetings from Rhythm Pharmaceuticals, Inc, and grant funding for clinical trials of setmelanotide in obesity from Rhythm Pharmaceuticals, Inc; for clinical trials in Prader-Willi syndrome from Millendo; and for clinical trials in obesity from Novo Nordisk. JAY has received grant support for clinical trials of setmelanotide in obesity from Rhythm Pharmaceuticals, Inc, received grant support for clinical trials of diazoxide choline controlled release in Prader-Willi syndrome from Soleno Therapeutics, Inc, and received study drugs for clinical trials in obesity from Hikma Pharmaceuticals. RSM is an employee of and stockholder in Rhythm Pharmaceuticals, Inc. GY is an employee of and stockholder in Rhythm Pharmaceuticals, Inc. EF has received consulting fees from Rhythm Pharmaceuticals, Inc, and participated in the BBS advisory board for Rhythm Pharmaceuticals, Inc and is a clinical investigator for clinical trials of setmelanotide in Bardet-Biedl syndrome for Rhythm Pharmaceuticals, Inc. KC has received grant funding from Ysopia, Integrative Phenomics, and Confo Therapeutics and is a clinical investigator for clinical trials of setmelanotide in Bardet-Biedl syndrome for Rhythm Pharmaceuticals, Inc. JA has received payment for lectures from and participated in the BBS advisory board for Rhythm Pharmaceuticals, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA SHARING STATEMENT
Anonymized individual patient data and study documents can be requested from Rhythm Pharmaceuticals for further research.
REFERENCES
- 1.Sadaf Farooqi I. Monogenic obesity syndromes provide insights into the hypothalamic regulation of appetite and associated behaviors. Biol Psychiatry 2022; 91: 856–9. [DOI] [PubMed] [Google Scholar]
- 2.Forsythe E, Kenny J, Bacchelli C, Beales PL. Managing Bardet-Biedl Syndrome—now and in the future. Front Pediatr 2018; 6: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet 1999; 36: 437–46. [PMC free article] [PubMed] [Google Scholar]
- 4.Pomeroy J, Krentz AD, Richardson JG, Berg RL, VanWormer JJ, Haws RM. Bardet-Biedl syndrome: weight patterns and genetics in a rare obesity syndrome. Pediatr Obes 2021; 16: e12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haws RM, Gordon G, Han JC, Yanovski JA, Yuan G, Stewart MW. The efficacy and safety of setmelanotide in individuals with Bardet-Biedl syndrome or Alström syndrome: phase 3 trial design. Contemp Clin Trials Commun 2021; 22: 100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA 2003; 289: 1813–9. [DOI] [PubMed] [Google Scholar]
- 7.Girard D, Petrovsky N. Alström syndrome: insights into the pathogenesis of metabolic disorders. Nat Rev Endocrinol 2011; 7: 77–88. [DOI] [PubMed] [Google Scholar]
- 8.Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Gene. 2019; 95: 23–40. [DOI] [PubMed] [Google Scholar]
- 9.Lindstrand A, Frangakis S, Carvalho CMB, et al. Copy-number variation contributes to the mutational load of Bardet-Biedl syndrome. Am J Hum Genet 2016; 99: 318–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamseldin HE, Shaheen R, Ewida N, et al. The morbid genome of ciliopathies: an update. Genet Med 2020; 22: 1051–60. [DOI] [PubMed] [Google Scholar]
- 11.Guo D-F, Lin Z, Wu Y, et al. The BBSome in POMC and AgRP neurons is necessary for body weight regulation and sorting of metabolic receptors. Diabetes 2019; 68: 1591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Bernard A, Comblain F, et al. Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J Clin Invest 2021; 131: e142064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haws R, Brady S, Davis E, et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab 2020; 22: 2133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuillan PP, Ng D, Han JC, et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J Clin Endocrinol Metab 2011; 96: E528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Liu Y, Stratigopoulos G, et al. Bardet-Biedl syndrome proteins regulate intracellular signaling and neuronal function in patient-specific iPSC-derived neurons. J Clin Invest 2021; 131: e146287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quarta C, Claret M, Zeltser LM, et al. POMC neuronal heterogeneity in energy balance and beyond: an integrated view. Nat Metab 2021; 3: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet 2013; 21: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augustine EF, Adams HR, Mink JW. Clinical trials in rare disease: challenges and opportunities. J Child Neurol 2013; 28: 1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davenport JR, Watts AJ, Roper VC, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol 2007; 17: 1586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall JD, Beck S, Maffei P, Naggert JK. Alström syndrome. Eur J Hum Genet 2007; 15: 1193–202. [DOI] [PubMed] [Google Scholar]
- 21.Knowler WC, Barrett-Connor E, Fowler SE, et al. , and the Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Preventive Services Task Force, Grossman DC, Bibbins-Domingo K, et al. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation Statement. JAMA 2017; 317: 2417–26. [DOI] [PubMed] [Google Scholar]
- 23.Heymsfield SB, Avena NM, Baier L, et al. Hyperphagia: current concepts and future directions proceedings of the 2nd international conference on hyperphagia. Obesity (Silver Spring) 2014; 22 (suppl 1): S1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ervin C, Norcross L, Mallya UG, et al. Patient- and caregiver-reported experiences of hyperphagia in Bardet-Biedl syndrome before and during setmelanotide treatment. Presented at: The Pediatric Endocrine Society Annual Meeting; April 28-May 1, 2022; Virtual. [Google Scholar]
- 25.Forsythe E, Haws R, Argente J, et al. Quality of life in patients with Bardet-Biedl syndrome in a setmelanotide phase 3 trial [poster 257 ]. Presented at: ObesityWeek®; November 1–5, 2021; Virtual. [Google Scholar]
- 26.Tchang BG, Aras M, Kumar RB, Aronne LJ. Pharmacologic Treatment of overweight and obesity in adults. Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000. [Google Scholar]
- 27.Clément K, van den Akker E, Argente J, et al. , for the Setmelanotide POMC and LEPR Phase 3 Trial Investigators. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol 2020; 8: 960–70. [DOI] [PubMed] [Google Scholar]
- 28.Clément K, Biebermann H, Farooqi IS, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med 2018; 24: 551–5. [DOI] [PubMed] [Google Scholar]
- 29.IMCIVREE® (setmelanotide) [prescribing information]. Boston, MA: Rhythm Pharmaceuticals, Inc.; 2022. [Google Scholar]
- 30.IMCIVREE® (setmelanotide) [summary of product characteristics]. Amsterdam, Netherlands: Rhythm Pharmaceuticals, Inc.; 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual patient data and study documents can be requested from Rhythm Pharmaceuticals for further research.