To the Editor: The continued evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the emergence of the B.1.1.529 (omicron) variant and numerous sublineages that often evade neutralizing-antibody responses induced by infection or vaccination.1 In response to this worrisome trend, the Food and Drug Administration granted emergency use authorizations to bivalent formulations of the messenger RNA (mRNA) vaccines mRNA-1273 (Moderna) and BNT162b2 (Pfizer–BioNTech) that target both the omicron BA.4–BA.5 spike and the ancestral wild-type (D614G) spike of SARS-CoV-2.2 Published data on antibody responses to the bivalent vaccines have been limited to studies in animals and in humans that have used another bivalent mRNA vaccine targeting the BA.1 spike in addition to the D614G spike.3,4 Despite the widespread administration of booster vaccines, the effect of a booster injection with new bivalent vaccines on the neutralizing-antibody response in humans remains unknown.
Therefore, we collected serum samples from participants who had received three doses of either of the original monovalent mRNA vaccines followed by one dose of a bivalent vaccine targeting BA.4–BA.5 (bivalent-booster group), with each booster produced by the two original manufacturers. (The BA.4–BA.5 subvariants are often grouped together because they have the same spike protein.) Details regarding the methods that were used in this study and the recruitment and follow-up of the participants are provided in the Supplementary Appendix (available with the full text of this letter at NEJM.org).
We compared neutralizing-antibody levels in these samples with levels in samples obtained from three other groups of participants: those who had received either three or four doses of monovalent mRNA vaccines (three-dose and four-dose monovalent groups) and those who had a history of BA.4–BA.5 breakthrough infection after three or four doses of monovalent mRNA vaccine (convalescent group). We used pseudovirus neutralization assays to test all serum samples against the D614G strain and against omicron sublineages BA.1, BA.2, BA.4–BA.5, BA.4.6, BA.2.75, and BA.2.75.2. To further assess the extent of antibody response, we also measured neutralizing-antibody levels against several related sarbecoviruses, including SARS-CoV, GD-pangolin, GX-pangolin, and WIV1.
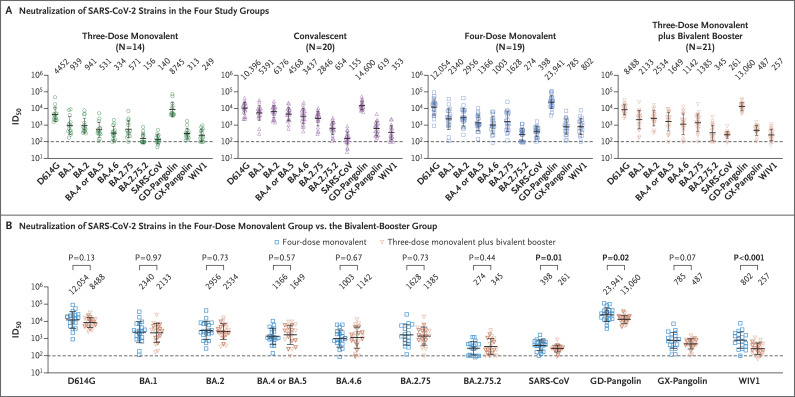
Clinical details are summarized for all groups in Table S1 in the Supplementary Appendix and are listed for each participant in Table S2. The participants in the four-dose group were older than those in the bivalent-booster group (mean age, 55.3 years vs. 36.4 years). Serum was collected from the four-dose and bivalent-booster groups at a similar interval after the last dose of vaccine (mean, 24.0 days in the four-dose group and 26.4 days in the bivalent-booster group); the interval was longer after vaccination in the three-dose group (39.2 days) and after infection in the convalescent group (31.8 days). All four groups had the highest neutralizing-antibody titers (measured as the 50% inhibitory dilution [ID50]) against the D614G strain (Figure 1A). Geometric mean ID50 titers against each of the tested SARS-CoV-2 variants were lowest in the three-dose monovalent group and highest in the convalescent group. The between-group difference in neutralization of any SARS-CoV-2 variant tested was not significant between the four-dose monovalent group and the bivalent-booster group (Figure 1B). ID50 titers against three related sarbecoviruses (SARS-CoV, GD-pangolin, and WIV1) were slightly but significantly higher in the four-dose monovalent group than in the bivalent-booster group.
Figure 1. Neutralization Profiles of Serum Samples against SARS-CoV-2 Variants and Other Sarbecoviruses.
Panel A shows the 50% inhibitory dilution (ID50) titers of serum samples obtained from participants who had received either three or four doses of a monovalent messenger RNA (mRNA) vaccine (three-dose and four-dose monovalent groups), those who had BA.4–BA.5 breakthrough infection after three or four doses of monovalent mRNA vaccine (convalescent group), and those who had received three doses of a monovalent mRNA vaccine followed by one dose of a bivalent vaccine targeting BA.4–BA.5 variants (bivalent-booster group). Panel B shows the antibody response induced by a fourth dose of the original monovalent mRNA vaccine as compared with a fourth dose of a BA.4–BA.5 bivalent booster. Fourth doses of the mRNA-1273 vaccines are indicated by bold symbols; nonbold symbols indicate fourth doses of the BNT162b2 vaccines. Between-group comparisons were performed with the use of Mann–Whitney tests. In Panels A and B, values above the data points indicate the geometric mean ID50 titers, the dotted horizontal lines indicate the lower limit of detection of the assay, and 𝙸 bars indicate the standard deviation.
Boosting with new bivalent mRNA vaccines targeting both the BA.4–BA.5 variant and the D614G strain did not elicit a discernibly superior virus-neutralizing peak antibody response as compared with boosting with the original monovalent vaccines. Limitations of our study include the small sample size and follow-up period of our groups. We also note that the between-group comparisons were not controlled for factors such as age, vaccine type, and health status, which may have had an effect on antibody responses. These findings may be indicative of immunologic imprinting,5 although follow-up studies are needed to determine whether antibody responses will deviate over time, including after the administration of a second bivalent booster.
Supplementary Appendix
Disclosure Forms
This letter was published on January 11, 2023, at NEJM.org.
Footnotes
Supported by the SARS-CoV-2 Assessment of Viral Evolution (SAVE) Program of the National Institutes of Health and by a contract (75N93019C00051, to Dr. Gordon) from the National Institute of Allergy and Infectious Diseases.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022;608:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. August 31, 2022. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use).
- 3.Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med 2022;387:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheaffer SM, Lee D, Whitener B, et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 omicron variant in mice. Nat Med 2022. October 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheatley AK, Fox A, Tan H-X, et al. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol 2021;42:956-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.