To the Editor: Waning immunity after messenger RNA (mRNA) vaccination and the emergence of variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have led to reduced mRNA vaccine efficacy against symptomatic infection and severe disease.1,2 Bivalent mRNA boosters (manufactured by Pfizer–BioNTech and Moderna) expressing the spike protein of the B.1.1.529 (omicron) BA.5 sublineage and the ancestral WA1/2020 strain have been developed because BA.5 substantially evades neutralizing antibodies.3 However, the immunogenicity of the BA.5-containing bivalent mRNA boosters remains unknown.
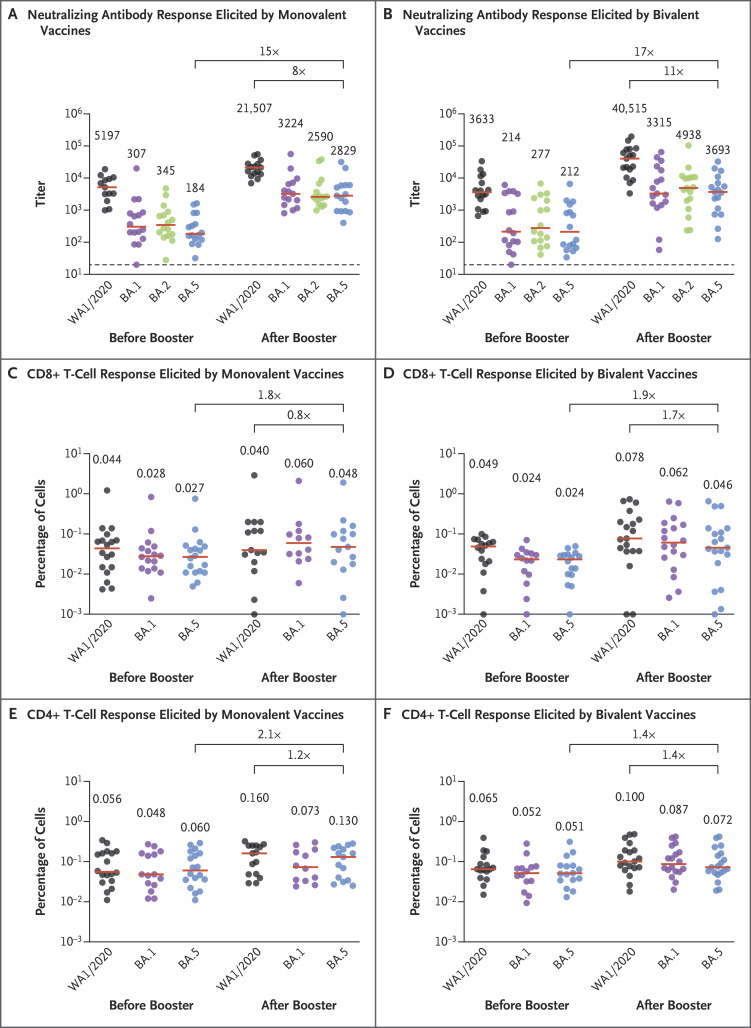
We evaluated immune responses in 15 participants who had received the original monovalent mRNA boosters and in 18 participants who had received the bivalent mRNA boosters of the two vaccines (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). The participants received a median of three doses of vaccine against SARS-CoV-2, and 33% had documentation of SARS-CoV-2 infection during the omicron surge, although it is likely that the majority of the participants had hybrid immunity before boosting, given the high incidence of BA.5 infection during the summer and fall of 2022. Both the monovalent and bivalent mRNA boosters led to preferential expansion of WA1/2020 neutralizing antibody titers and lower BA.5 neutralizing antibody titers (Figure 1A and 1B and Fig. S1). The median BA.5 neutralizing antibody titer increased from 184 to 2829 after monovalent mRNA boosting and from 211 to 3693 after bivalent mRNA boosting. Binding antibody responses were similar after monovalent and bivalent mRNA boosting by enzyme-linked immunosorbent and electrochemiluminescence assays (Fig. S2 and S3).
Figure 1. Neutralizing Antibody and T-Cell Responses after Monovalent or Bivalent mRNA Boosting.
Shown are the results of luciferase-based pseudovirus neutralization assays among participants who received booster doses of either monovalent (Panel A) or bivalent (Panel B) mRNA vaccines. Also shown are spike-specific CD8+ T-cell responses on intracellular cytokine staining assays among participants who received booster doses of either monovalent (Panel C) or bivalent (Panel D) mRNA vaccines, along with corresponding CD4+ T-cell responses with the two types of vaccines (Panels E and F). Responses were measured against the ancestral strain of SARS-CoV-2 (WA1/2020) and against omicron sublineages BA.1, BA.2, and BA.5. Red horizontal bars indicate median values, as shown above the data points. The values above the brackets are the numerical factor differences in the response against the BA.5 variant after boosting as compared with the response before boosting (upper brackets) and the factor differences after boosting for the response against the ancestral strain as compared with the response against the BA.5 sublineage (lower brackets).
Spike-specific CD8+ and CD4+ T-cell responses increased modestly after monovalent and bivalent mRNA boosting. The median BA.5 CD8+ T-cell response increased from 0.027% to 0.048% after monovalent mRNA boosting and from 0.024% to 0.046% after bivalent mRNA boosting (Figure 1C and 1D). The median BA.5 CD4+ T-cell response increased from 0.060% to 0.130% after monovalent mRNA boosting and from 0.051% to 0.072% after bivalent mRNA boosting (Figure 1E and 1F). The median BA.5 memory B-cell response was 0.079% after monovalent mRNA boosting and 0.091% after bivalent mRNA boosting (Fig. S4).
Our data indicate that both monovalent and bivalent mRNA boosters markedly increased antibody responses but did not substantially augment T-cell responses. Neutralizing antibody titers against the ancestral strain of SARS-CoV-2 were higher than titers against BA.5 after both monovalent and bivalent boosting. The median BA.5 neutralizing antibody titer was similar after monovalent and bivalent mRNA boosting, with a modest trend favoring the bivalent booster by a factor of 1.3. It is possible that larger studies may show a greater between-group difference, but any such comparative studies between monovalent and bivalent mRNA boosters would need to enroll the two cohorts within the same time frame and after the BA.5 surge, because negative results on nucleocapsid serologic analysis would not exclude all infected participants. These data are consistent with the modest benefits observed with a BA.1-containing bivalent mRNA booster.4 Our findings suggest that immune imprinting by previous antigenic exposure5 may pose a greater challenge than is currently appreciated for inducing robust immunity against SARS-CoV-2 variants.
Supplementary Appendix
Disclosure Forms
This letter was published on January 11, 2023, at NEJM.org.
Footnotes
Supported by a grant (CA260476) from the National Institutes of Health (NIH) and by grants from the Massachusetts Consortium for Pathogen Readiness and the Ragon Institute (all to Dr. Barouch), as well as a grant (AI69309, to Dr. Collier) from the NIH.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Lin D-Y, Gu Y, Xu Y, et al. Association of primary and booster vaccination and prior infection with SARS-CoV-2 infection and severe COVID-19 outcomes. JAMA 2022;328:1415-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collie S, Nayager J, Bamford L, Bekker LG, Zylstra M, Gray G. Effectiveness and durability of the BNT162b2 vaccine against omicron sublineages in South Africa. N Engl J Med 2022;387:1332-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med 2022;387:86-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med 2022;387:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds CJ, Pade C, Gibbons JM, et al. Immune boosting by B.1.1.529 (omicron) depends on previous SARS-CoV-2 exposure. Science 2022;377(6603):eabq1841-eabq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.