Abstract
Background
Mild behavioral impairment (MBI) is a syndrome that uses later‐life emergent and persistent neuropsychiatric symptoms (NPS) to identify a group at high risk for incident dementia. MBI is associated with neurodegenerative disease markers in advance of syndromic dementia. Functional connectivity (FC) correlates of MBI are understudied and could provide further insights into mechanisms early in the disease course. We used resting‐state functional magnetic resonance imaging (rs‐fMRI) to test the hypothesis that FC within the default mode network (DMN) and salience network (SN) of persons with MBI (MBI+) is reduced, relative to those without (MBI–).
Methods
From two harmonized dementia‐free cohort studies, using a score of ≥6 on the MBI Checklist to define MBI status, 32 MBI+ and 63 MBI– individuals were identified (mean age: 71.7 years; 54.7% female). Seed‐based connectivity analysis was implemented in each MBI group using the CONN fMRI toolbox (v20.b), with the posterior cingulate cortex (PCC) as the seed region within the DMN and anterior cingulate cortex (ACC) as the seed within the SN. The average time series from the PCC and ACC were used to determine FC with other regions within the DMN (medial prefrontal cortex, lateral inferior parietal cortex) and SN (anterior insula, supramarginal gyrus, rostral prefrontal cortex), respectively. Age, sex, years of education, and Montreal Cognitive Assessment scores were included as model covariates. The false discovery rate approach was used to correct for multiple comparisons, with a p‐value of .05 considered significant.
Results
For the DMN, MBI+ individuals exhibited reduced FC between the PCC and the medial prefrontal cortex, compared to MBI–. For the SN, MBI+ individuals exhibited reduced FC between the ACC and left anterior insula.
Conclusion
MBI in dementia‐free older adults is associated with reduced FC in networks known to be disrupted in dementia. Our results complement the evidence linking MBI with Alzheimer's disease biomarkers.
Highlights
Resting‐state functional magnetic resonance imaging was completed in 95 dementia‐free persons from FAVR and COMPASS‐ND studies.
Participants were stratified by informant‐rated Mild Behavioral Impairment Checklist (MBI‐C) score, ≥6 for MBI+.
MBI+ participants showed reduced functional connectivity (FC) within the default mode network and salience network.
These FC changes are consistent with those seen in early‐stage Alzheimer's disease.
MBI may help identify persons with early‐stage neurodegenerative disease.
Keywords: Alzheimer's disease, mild behavioral impairment, mild cognitive impairment, neuropsychiatric symptoms, resting‐state functional magnetic resonance imaging
1. INTRODUCTION
Global efforts to prevent or reduce the burden of Alzheimer's disease (AD) dementia have not been successful, partially due to challenges in detecting disease early enough for therapeutic interventions. Mild cognitive impairment (MCI) is an at‐risk state for AD, 1 representing prodromal disease when associated with AD neurobiological changes. 1 , 2 However, many with MCI do not have dementia‐related neurobiological changes and do not progress to dementia. 3 In the absence of overt cognitive symptoms, early detection is even more difficult. Thus, more accurate approaches are required to identify a high‐risk group for dementia.
Increasingly, data support improved sensitivity and specificity of dementia prognostication and early detection by incorporating neuropsychiatric symptoms (NPS) into modeling. Developed and validated explicitly for dementia prognostication, mild behavioral impairment (MBI) selects later‐life emergent and persistent NPS, to identify the high‐risk group. 4 Incorporating MBI assessment (i.e., behavioral risk) into models using cognitive assessments (i.e., cognitive risk) could provide greater specificity in identifying this high‐risk group, improving estimates from those based on cognitive status alone. MCI participants with concurrent MBI have a higher progression rate to dementia and a lower reversion rate to normal cognition (NC) than MCI without MBI. 3 Similarly, in subjective cognitive decline (SCD) and NC, concurrent MBI confers greater risk for incident MCI and dementia. 5 Recent studies have linked MBI with underlying biological changes in early‐stage dementia. MBI in dementia‐free older adults is associated with meaningful changes in amyloid beta, 6 , 7 phosphorylated tau, 8 and neurodegeneration, 9 , 10 consistent with the biological profile of AD. These findings suggest that MBI could serve as a proxy marker for dementia risk, with the MBI group flagged for further clinical and/or biomarker assessment and earlier interventions. However, little research has explored brain functional changes in association with MBI.
Functional connectivity (FC) is a resting‐state functional magnetic resonance imaging (rs‐fMRI) measure that characterizes spatio‐temporal correlations between distinct brain regions in the absence of an explicit task, together forming resting‐state networks (RSNs). FC disruptions within specific brain RSNs have been implicated in cognitive and neuropsychiatric manifestations of AD dementia and could serve as putative biomarkers for early detection. Specifically, FC disruptions within the default mode network (DMN) and salience network (SN) have been reported in both early‐onset and late‐onset AD. 11 The DMN includes the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), and lateral inferior parietal cortex (LIP). 12 The SN includes the anterior cingulate cortex (ACC), anterior insula (AI), supramarginal gyrus (SMG), and rostral prefrontal cortex (RPFC). 13 FC disruptions in the DMN and SN have been implicated in dementia‐related NPS. Delusions in AD have been associated with DMN hypoconnectivity, 14 and hyperactivity in mild to moderate AD with DMN hypoconnectivity 15 and SN hyperconnectivity. 16 Earlier in the disease course, apathy in amnestic MCI has been associated with DMN disruptions, 17 and affective symptoms in preclinical AD with SN disruptions. 18 Studies on FC disruptions in MBI are limited. Two studies in Parkinson's disease patients, one using task‐based fMRI, found MBI‐associated reduced activation in the prefrontal and posterior parietal cortices, reduced deactivation in the medial temporal regions, 19 and disrupted corticostriatal connectivity. 20 The only rs‐fMRI study of MBI in participants with NC and amnestic MCI reported no MBI‐associated FC changes within the DMN and SN but identified an association between MBI and dysfunction within the fronto‐parietal control network. 21 This study used a self‐report of MBI symptoms with infrequent endorsement of clinically significant MBI. Here, we aimed to investigate FC alterations in the DMN and SN in dementia‐free older adults with MBI compared to those without, using an informant‐rated MBI assessment. We hypothesized that MBI would be associated with reduced connectivity in the DMN and SN. Additionally, to determine whether the association of MBI with FC alterations varied with sex we explored sex‐related differences in FC.
2. METHODS
2.1. Study population
Data were obtained from two referral‐based prospective observational studies: (1) Functional Assessment of Vascular Reactivity (FAVR), 22 and (2) Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS‐ND). 23
FAVR is a study at the University of Calgary consisting of 100 participants with NC, MCI, and AD that investigates the relationship between brain blood flow and memory symptoms. COMPASS‐ND is a Canada‐wide observational study that consists of 1139 participants with NC, SCD, MCI, or dementia; 23 however, only data from 409 participants have been released. Inclusion criteria for both studies were identical, and FAVR was essentially an extension of COMPASS‐ND with more local controls, thus some participants in FAVR were also enrolled in COMPASS‐ND. Both studies have harmonized imaging protocols for rs‐fMRI based on the Canadian Dementia Imaging Protocol (CDIP). 24 The CDIP ensures that all sequence parameters were harmonized across scanners, and imaging parameters were chosen to obtain similar quality in terms of contrast and resolution across sites. The complete list of parameters is available on the CDIP main website (http://www.cdip‐pcid.ca). The core protocol for rs‐fMRI was as follows: a task‐free, eyes open (resting state) fMRI for the assessment of functional networks and pathways using a T2 *‐weighted blood oxygen level‐dependent (BOLD)‐sensitive sequence, with resolution of 3.5 × 3.5 × 3.5 mm3, repetition time (TR) = 2110 msec (gradient echo: 2500 msec), and 300 volumes over time. 24 Institutional ethics boards approved the studies, and informed consent was obtained from all participants at each site affiliated with the study.
2.2. Participant selection and grouping
The inclusion criteria for all participants enrolled in both FAVR and COMPASS‐ND was as follows: (1) a cognitive diagnosis of NC or MCI at baseline (i.e., dementia‐free status); (2) available MBI Checklist (MBI‐C) 25 data; and (3) complete demographic, cognitive, and imaging data. Cognition was measured with the Montreal Cognitive Assessment (MoCA), which provides a global measure of cognitive impairment. 26 Behavior was measured with the MBI‐C, the instrument developed to measure emergent and persistent NPS in dementia‐free older adults in accordance with the MBI criteria. Participants with an MBI‐C score ≥6 were defined as MBI+. 27 Applying the inclusion criteria resulted in a sample of 60 participants from FAVR, with 30 of them cross‐enrolled in COMPASS‐ND, and 35 participants from COMPASS‐ND only. Pooling harmonized data over both cohorts resulted in a final sample of 95 dementia‐free participants (mean age = 71.7; 54.7 females; mean MoCA = 25.0). Of these, 32 were MBI+, and 63 were MBI–.
2.3. MRI acquisition
For participants enrolled in FAVR and the Calgary COMPASS‐ND site, structural and functional imaging data were acquired using a Discovery MR750 3.0 T MRI scanner (GE Healthcare) with a 32‐channel head coil configuration (Nova Medical Inc.). Different scanner types and models were used for the 12 other COMPASS‐ND sites, but scanner type was not controlled for. However, all sites used the harmonized CDIP. 24 Three‐dimensional inversion‐prepared T1‐weighted images were acquired using the following parameters: TR/echo time (TE) = 7.4/3 ms, flip angle = 11°, voxel size = 1 × 1 × 1 mm3, field of view (FOV) = 25.6 cm, slice thickness = 1 mm. The following parameters were used for rs‐fMRI: acquisition time = 500 secs, gradient‐recalled echo planar imaging, interleaved bottom‐up acquisition, TR/TE = 2500/30 ms, voxel size = 3 × 3 × 3 mm3, FOV = 22.4 cm, matrix size = 64 × 64. Participants were told to stay awake, focus on a point, not think of anything, and keep their eyes open.
RESEARCH IN CONTEXT
Systematic Review: PubMed was searched for preclinical and prodromal dementia studies of functional connectivity (FC) in association with mild behavioral impairment (MBI) or neuropsychiatric symptoms (NPS). The aim was to determine whether MBI or NPS are associated with neurodegenerative disease–related FC changes in advance of syndromic dementia. Very few studies have been published, including one with MBI, which is discussed herein. Little is known about FC associations with behavioral or personality change in advance of dementia.
Interpretation: In a pooled sample of dementia‐free older adults, MBI was associated with reduced FC within the default mode network and salience network, changes consistent with those seen in early‐stage Alzheimer's disease. These findings add to the evidence base that NPS, meeting MBI criteria, may assist in the identification of preclinical or prodromal disease.
Future directions: Longitudinal data with additional imaging and fluid biomarkers are required to further explore the neural correlates of MBI.
2.4. Data pre‐processing
Data processing was performed using the CONN toolbox v20.b (https://www.nitrc.org/projects/conn) running on MATLAB 2020b (MathWorks®). Pre‐processing steps included: (1) realigning, which involved co‐registration of all scans and resampling to the reference image (first scan of the first session) using b‐spline interpolation; (2) centering the functional and matching structural data to zero coordinates (data translation); (3) applying a slice‐timing correction to correct for inter‐slice differences in acquisition times; (4) removal of outlier scan volumes based on a global‐signal z‐value threshold of 5 and a subject‐motion threshold of 0.9 mm using Artifact Detection Tools (ART)‐based outlier detection (www.nitrc.org/projects/artifact_detect/); (5) segmentation, normalization, and registration of functional and structural data to the Montreal Neurological Institute (MNI) space; and (6) applying spatial smoothing with a Gaussian kernel with a full width at half‐maximum (FWHM) of 8 mm. For the ART‐based outlier detection on step 4 of data pre‐processing, an exploratory analysis was performed to test all three thresholds offered: 0.5 mm, 0.9 mm, and 2 mm. A conservative 0.5 mm threshold resulted in dropping 14.89% of our volumes (2829 volumes); the 0.9 mm threshold dropped 4.97% of our volumes (944 volumes), while the 2 mm threshold dropped 0.078% of our volumes (14 volumes). We opted to use the intermediate 0.9 mm threshold to avoid excessive dropping of outlier volumes for a meaningful analysis while excluding those that may introduce unnecessary noise and affect data quality.
Denoising was performed to remove the effect of any residual non‐neural noise, using an anatomical component‐based noise correction (aCompCor) procedure. 28 Potential components that were denoised included signals from (1) white matter and (2) cerebrospinal fluid (CSF); (3) estimated subject‐motion parameters; and (4) outlier data on the signal. Temporal frequency was filtered to be in the range of 0.008 to 0.09 Hz, to capture the typical range of low frequency fluctuations of the rs‐fMRI signal.
2.5. Seed‐based connectivity analysis
Seed‐based connectivity analysis (SCA) is a hypothesis‐driven approach in which the rs‐fMRI time course of an a priori seed region of interest (ROI) is used to obtain brain maps of the estimated FC with the seed ROI. In the present study, the PCC (x,y,z: 1,–61,38) was selected as the seed ROI within the DMN, and the ACC (x,y,z:0,22,35) as the seed within the SN, in accordance with previous studies on the close correspondence of activation patterns and FC maps of the PCC and ACC to those of the DMN and SN, respectively. 12 , 29 , 30 , 31 Other selected ROIs within the DMN were the MPFC (1,55,–3), left LIP (–37,–77,33), and right LIP (47,–67,29). Other ROIs within the SN were the left AI (–44,13,1), right AI (47,14,0), left SMG (–60,–39,31), right SMG (62,–35,32), left RPFC (–32,45,27), and right RPFC (32,46,27). All ROIs within each network were pre‐defined in CONN v20.b with coordinates based on the MNI brain atlas.
In first‐level analyses, for each participant, a seed‐based FC map was obtained by computing pairwise Fisher‐transformed correlation coefficients between the seed ROI and the other pre‐defined DMN and SN ROIs in CONN. Subject‐level FC was then contrasted between the MBI+ and MBI– groups using a general linear model, adjusted for age, sex, education, and MoCA score. MoCA was included as a continuous variable to control for neurodegenerative disease stage and place each participant on the pre‐dementia cognitive spectrum. To identify potential sex differences, groups were stratified into males and females, and an MBI‐by‐sex interaction term was included in the general linear model. The a priori statistical plan was to fit the sex‐stratified regression model if the interaction term was statistically significant, otherwise the analysis would be repeated without the interaction term. Maps were corrected for multiple comparisons using a false discovery rate (FDR) of 0.05. The final FC group difference maps were converted to T‐statistics and overlaid onto the standard MNI template for visualization purposes. Analyses for the DMN and SN seeds were performed separately.
3. RESULTS
Table 1 shows participant demographics. MBI groups did not differ by sex or years of education. However, they did differ by age (p=.039), MoCA (p<.001), and cognitive status (p<.001), with a higher mean age, lower mean MoCA score, and higher percentage of MCI in the MBI+ group. For the DMN analysis, the MBI‐by‐sex interaction term was not significant (β = –0.09, p = .25). A significant cluster exhibiting reduced FC in the MBI+ group relative to the MBI– group was observed in the MPFC (β = –0.15, p‐FDR = .04; Figure 1). No other ROI exhibited a significant group difference (Table 2 presents p‐FDR statistics for all DMN ROIs).
TABLE 1.
Participant demographics
| All (n = 95) | MBI+ (n = 32) | MBI– (n = 63) | Statistic | ||
|---|---|---|---|---|---|
| Cognitive status (%) | NC | 60 (63.2) | 9 (28.1) | 51 (81) | χ 2 = 25.45 (p < .001)* |
| MCI | 35 (36.8) | 23 (71.9) | 12 (19) | ||
| Mean age (SD) | 71.7 (7.4) | 73.9 (8.1) | 70.6 (6.7) | t = 2.09 (p = .039) * | |
| Sex (%) | Male | 43 (45.3) | 18 (56.3) | 25 (39.7) | χ 2 = 2.35 (p = .13) |
| Female | 52 (54.7) | 14 (43.7) | 38 (60.3) | ||
| Mean years of education (SD) | 15.6 (3.8) | 14.9 (4.4) | 15.9 (3.4) | t = –1.25 (p = .21) | |
| Mean MoCA (SD) | 25.0 (3.2) | 22.8 (3.3) | 26.3 (2.6) | t = –5.69 (p < .001)* |
*Statistically significant at p < .05.
Abbreviations: MBI, mild behavioral impairment; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; NC, normal cognition; SD, standard deviation.
FIGURE 1.
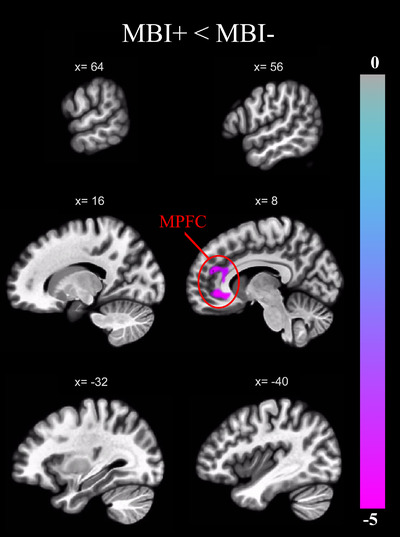
Brain regions exhibiting a significant group difference (p‐FDR = 0.05) in FC with the PCC of the DMN, expressed as a t‐statistic and corrected for age, sex, years of education, and MoCA score. FC with the MPFC was reduced in the MBI+ group compared to MBI–. DMN, default mode network; FC, functional connectivity; FDR, false discovery rate; MBI, mild behavioral impairment; MoCA, Montreal Cognitive Assessment; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex
For the SN, the MBI‐by‐sex interaction term was also not significant (β = –0.06, p = .17). A significant cluster exhibiting reduced FC in the MBI+ group relative to the MBI– group was observed in the left AI (β = –0.12, p‐FDR = .03; Figure 2). No other ROI exhibited a significant group difference (Table 2 presents p‐FDR statistics for all SN ROIs).
FIGURE 2.
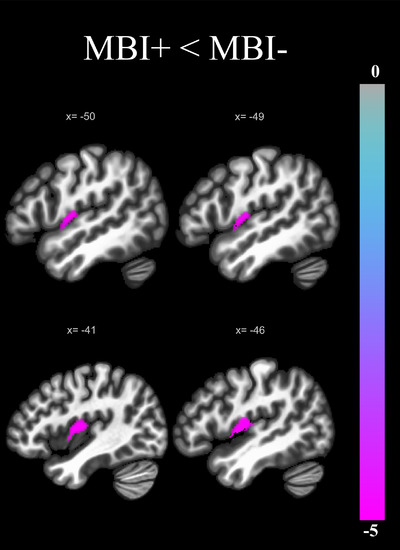
Brain regions exhibiting a significant group difference (p‐FDR = 0.05) in FC with the ACC of the SN, expressed as a t‐ statistic and corrected for age, sex, years of education, and MoCA score. FC with the left anterior insula was reduced in the MBI+ group compared to MBI–. ACC, anterior cingulate cortex; FC, functional connectivity; FDR, false discovery rate; MBI, mild behavioral impairment; MoCA, Montreal Cognitive Assessment; SN, salience network
TABLE 2.
Group difference effects (MBI+ < MBI–) for FC within the DMN and salience network SN
| Seed region | ROI (x, y, z) | Cluster p ‐value (FDR) |
|---|---|---|
| PCC (DMN) | MPFC (1, 55, –3) | .0037* |
| LP (L) (–37, –77, 33) | .54 | |
| LP (R) (47, –67, 29) | .54 | |
| ACC (SN) | AI (L) (–44, 13, 1) | .028* |
| SMG (L) (–60, –39, 31) | .075 | |
| SMG (R) ( 62, –35, 32) | .11 | |
| AI (R) (47, 14, 0) | .11 | |
| RPFC (L) (–32, 45, 27) | .90 | |
| RPFC (R) (32, 46, 27) | .93 |
*Cluster p‐FDR <.05.
Abbreviations: ACC, anterior cingulate cortex; AI, anterior insula; DMN, default mode network; FC, functional connectivity; FDR, false discovery rate; L, left; LP, lateral parietal; MBI, mild behavioral impairment; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; R, right; ROI, region of interest; RPFC, rostral prefrontal cortex; SMG, supramarginal gyrus; SN, salience network.
4. DISCUSSION
In this cross‐sectional analysis of 95 dementia‐free participants comparing MBI+ and MBI– groups, MBI was associated with reduced PCC–MPFC FC within the DMN and reduced ACC–AI (L) FC within the SN. No sex differences were observed.
The DMN is a distributed network, active during task‐negative/idle states but reduces activity during task‐positive states. From a cognitive standpoint the DMN, particularly the PCC, has been implicated in memory, for example, successfully recollecting previously studied items. 12 The ventral MPFC within the DMN receives sensory information from the external world and the body, and relays it to other subcortical structures such as the hypothalamus and amygdala. 12 The DMN, particularly its MPFC node, plays a key role in social and affective behaviors. 32 As such, dysfunction of the DMN may be associated with affective symptoms, captured by MBI in our study. Furthermore, the PCC and MPFC are anatomically connected via the cingulum tract. 33 Reduced white matter (WM) integrity along the cingulum has been associated with reduced FC in the DMN and impaired cognition. 34 A diffusion tensor imaging (DTI) study has demonstrated associations between microstructural changes in the cingulum and agitation and irritability in MCI/AD. 35 WM alterations in the cingulum have also been identified in early‐stage AD 36 and considering that MBI is an early‐stage construct, these early structural changes in the cingulum could be associated with MBI. The only MBI DTI study, in 165 dementia‐free and 38 AD dementia participants, demonstrated an association between reduced WM integrity in the cingulum and the MBI impulse dyscontrol domain. 10 Based on these findings, MBI‐associated changes in the cingulum tract may explain the reduced FC between the PCC and MPFC in MBI in our sample.
The DMN is known to be affected in AD and has been the focus of interest in numerous studies in MCI and AD. In the mild phase of AD dementia, the DMN has been implicated with reports of reduced FC between the hippocampus and MPFC, ventral ACC, right cuneus/precuneus, and PCC. 37 In MCI a meta‐analysis of seven studies involving the DMN demonstrated reduced PCC FC to be the most consistent group difference relative to healthy controls. 38 However, studies of DMN FC in preclinical AD have received less attention. One study in a cognitively healthy older adult sample reported greater PCC amyloid burden associated with lower DMN FC. 39 These findings provide support for DMN disruptions as a marker early in the disease course.
In AD, few studies have explored associations between NPS and DMN FC. One study found a significant correlation between lower FC of the DMN, specifically the MPFC, and hyperactivity syndrome. 15 Another study involving AD patients with and without delusions identified that patients with delusions showed significantly reduced FC within the DMN compared to those without. 14 These findings provide evidence for a potential association of NPS with poorer DMN FC in AD. One study has reported on NPS and DMN FC in advance of dementia. In 50 MCI participants a correlation between increasing Apathy Inventory score and lower DMN FC in the PCC was found. 40 Thus, the field remains relatively uninformed on associations between NPS and DMN FC in dementia‐free older adults, with our study being one of few.
The SN allows for the integration of salient signals to guide behavior and shows consistent activation in response to emotionally significant internal and external stimuli. 13 Studies have described SN changes across the cognitive spectrum, although fewer in pre‐dementia samples. In AD dementia, one study identified regions of enhanced connectivity to be a part of the SN. 41 In MCI, the SN was reported to play a modulating role between the DMN and central executive network. 42 This modulating role of the SN was reported to be impaired with worse SN disruption, especially the dorsal salience subsystem, which correlated with poorer cognitive performance on the MoCA. The authors suggested this disruption may be a neural basis for cognitive decline. 43 In healthy older controls, one study reported significant reductions in gray matter volume and intra‐network FC in SN ROIs compared to healthy younger controls, suggesting that SN FC and volume differences can begin early in the course of neurodegenerative disease, when cognition remains normal. 44
Very few studies have described SN changes in association with NPS. In a small study of AD dementia (n = 20), NPS were associated with increased FC between SN regions, notably the ACC and insula. 16 Increased SN activation has also been proposed as a neurobiological mechanism for anxiety in AD, due to the release of (and thus greater activity in) emotion‐generating structures along the AI–ACC network. 45 In a dementia‐free population, one study reported that depressed elderly participants with high apathy had decreased FC between the rostral AI and dorsal ACC and subcortical components of the SN, compared to non‐apathetic–non‐depressed participants and healthy controls. 46 Our study builds upon this scant prior literature by incorporating the neurobehavioral syndrome MBI.
Our study of dementia‐free older adults demonstrated MBI‐associated FC reductions in SN components, specifically the ACC and AI (L). Strong bilateral connections exist between the ACC and AI, and these regions have been implicated in neuropsychiatric and behavioral features such as agitation, anxiety, apathy, empathy, hallucinations, irritability, and social behavior. 47 , 48 , 49 The AD literature suggests that connectivity changes with disease progression. Longitudinal data in prodromal AD demonstrate global cortical hyperconnectivity, which then progressively disappears through the onset of dementia and progression. Specific to the SN, over the course of preclinical AD, a hyperconnectivity phase is described, with a subsequent transition to hypoconnectivity as a function of increasing tau. 50 This early‐stage hyperconnectivity may reflect compensatory mechanisms of the brain in response to pathology‐induced neuronal injury, with a surge of neurotransmitters in areas affected by the pathological process. With disease progression and extended neuronal death, which is closely associated with tau pathology, these compensatory mechanisms are overwhelmed, leading to subsequent hypoconnectivity. 50 Thus, SN hypoconnectivity reflects more advanced disease, with greater neuropathological burden. In our study, the association between MBI+ status and SN hypoconnectivity relative to MBI– status is consistent with the literature, which has determined MBI+ to be a marker of greater neuropathological burden, relative to MBI–. 51 Our dementia‐free sample included both NC and MCI, the latter of which likely reflected more advanced disease stage, for which we adjusted by including MoCA score as a covariate. Our insular signal was unilateral, with ACC–AI (L) hypoconnectivity associating with MBI. The left anterior insula is involved in the maintenance of social and emotional working memory, 52 cognitive domains shown to be affected in a cognitively normal sample with MBI. 53 However, additional studies are required to explore laterality further, given trend‐level hypoconnectivity for the right AI. Overall, our findings support the use of MBI status as a simple clinical approach to identify a group at higher risk of having dementia‐related brain changes. MBI and FC, separately and collectively, could be leveraged as monitoring biomarkers for AD or as surrogate markers for prevention trials. However, further exploration of the utility of this biomarker combination in longitudinal studies with larger sample size is required.
5. LIMITATIONS
Despite the novelty of the study and the use of the informant‐rated MBI‐C, there are some limitations to consider. A cross‐sectional approach was used for group comparisons, and causality cannot be determined. Future studies may resolve this limitation with longitudinal data to assess changes in FC, cognition, and behavior over time. MBI domain analyses were precluded by the size of the sample, providing information on global MBI status but not domain‐level analyses. However, notwithstanding the five different domains with varying frequencies and severities, the observed FC disruptions in the DMN and SN in our study may indicate a certain degree of convergence of FC mechanisms across domains. With larger samples, MBI domain‐specific analyses should be included to inform domain‐level FC mechanisms. The inability to perform two‐by‐two analyses of MBI status and cognitive group (NC/MCI) due to insufficient group sample sizes might be considered a limitation. However, rather than dichotomizing NC and MCI (and losing statistical power), we pooled NC and MCI groups and adjusted for global cognitive status with MoCA scores. This approach precisely accounts for the gradual progression of cognitive impairment and underlying disease, from ostensibly NC through to MCI, an approach consistent with previous studies. 2 , 16 With future COMPASS‐ND data releases and continued recruitment into FAVR, this analysis may become feasible. Variation in scanner types and models across COMPASS‐ND sites may be another limitation of the present study. However, imaging protocols were harmonized to ensure data consistency across COMPASS‐ND participant sites. The identified FC disruptions within the DMN and SN have not only been implicated in AD but also in other dementia subtypes such as frontotemporal dementia. 54 Specific disease cannot be determined in our sample, but future data releases including fluid biomarker results may assist in this determination. Last, the use of psychotropic medications may have differed across MBI groups in our study, and it could have an impact on the MBI‐associated FC changes. However, concurrent medication use was not reliably reported in our datasets, precluding the use of psychotropic drug use as a covariate.
6. CONCLUSION
We identified the FC correlates of MBI in a dementia‐free sample of older adults. These data suggest that MBI is associated with early‐stage changes in functional networks, seen in the progression to AD. These findings complement research linking MBI with AD proteinopathies in advance of dementia and may provide further biological support for the use of MBI as a quick, inexpensive, and simple‐to‐implement clinical construct to help identify the AD process earlier. Incorporation of the MBI‐C into longitudinal imaging studies will further inform the sensitivity and specificity of FC changes in MBI and the associated risk of dementia to ultimately improve detection and test early interventions.
CONFLICT OF INTEREST
All authors report no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
The Canadian Consortium on Neurodegeneration in Aging is supported by a grant from the Canadian Institutes of Health Research with funding from several partners.
Ghahremani M, Nathan S, Smith EE, McGirr A, Goodyear B, Ismail Z. Functional connectivity and mild behavioral impairment in dementia‐free elderly. Alzheimer's Dement. 2023;9:e12371. 10.1002/trc2.12371
REFERENCES
- 1. DeCarli C, Frisoni GB, Clark CM, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108‐115. [DOI] [PubMed] [Google Scholar]
- 2. Munro CE, Donovan NJ, Guercio BJ, et al. Neuropsychiatric symptoms and functional connectivity in mild cognitive impairment. J Alzheimers Dis. 2015;46:727‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGirr A, Nathan S, Ghahremani M, Gill S, Smith EE, Ismail Z. Progression to dementia or reversion to normal cognition in mild cognitive impairment as a function of late‐onset neuropsychiatric symptoms. Neurology. 2022;98:e2132‐e2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ismail Z, McGirr A, Gill S, Hu S, Forkert ND, Smith EE. Mild behavioral impairment and subjective cognitive decline predict cognitive and functional decline. J Alzheimers Dis. 2021;80:459‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lussier FZ, Pascoal TA, Chamoun M, et al. Mild behavioral impairment is associated with beta‐amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement. 2020;16:192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miao R, Chen HY, Gill S, et al. Plasma beta‐amyloid in mild behavioural impairment ‐ neuropsychiatric symptoms on the Alzheimer's continuum. J Geriatr Psychiatry Neurol. 2022;35:434‐441.doi: 10.1177/08919887211016068 [DOI] [PubMed] [Google Scholar]
- 8. Johansson M, Stomrud E, Insel PS, et al. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer's disease. Transl Psychiatry. 2021;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matuskova V, Ismail Z, Nikolai T, et al. Mild behavioral impairment is associated with atrophy of entorhinal cortex and hippocampus in a memory clinic cohort. Front Aging Neurosci. 2021;13:643271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gill S, Wang M, Mouches P, et al. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int J Geriatr Psychiatry. 2021;36:1398‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Badhwar A, Tam A, Dansereau C, Orban P, Hoffstaedter F, Bellec P. Resting‐state network dysfunction in Alzheimer's disease: a systematic review and meta‐analysis. Alzheimers Dement (Amst). 2017;8:73‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015;38:433‐447. [DOI] [PubMed] [Google Scholar]
- 13. Uddin LQ. Salience network of the human brain. 1st ed Academic Press;2016. [Google Scholar]
- 14. Qian W, Fischer CE, Churchill NW, Kumar S, Rajji T, Schweizer TA. Delusions in Alzheimer disease are associated with decreased default mode network functional connectivity. Am J Geriatr Psychiatry. 2019;27:1060‐1068. [DOI] [PubMed] [Google Scholar]
- 15. Lee JS, Kim JH, Lee S‐K. The relationship between neuropsychiatric symptoms and default‐mode network connectivity in alzheimer's disease. Psychiatry Investig. 2020;17:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balthazar ML, Pereira FR, Lopes TM, et al. Neuropsychiatric symptoms in Alzheimer's disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp. 2014;35:1237‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joo SH, Lee CU, Lim HK. Apathy and intrinsic functional connectivity networks in amnestic mild cognitive impairment. Neuropsychiatr Dis Treat. 2017;13:61‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fredericks CA, Sturm VE, Brown JA, et al. Early affective changes and increased connectivity in preclinical Alzheimer's disease. Alzheimers Dement (Amst). 2018;10:471‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin Yoon E, Ismail Z, Kathol I, et al. Patterns of brain activity during a set‐shifting task linked to mild behavioral impairment in Parkinson's disease. Neuroimage Clin. 2021;30:102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang S, Yoon EJ, Kibreab M, et al. Mild behavioral impairment in Parkinson's disease is associated with altered corticostriatal connectivity. Neuroimage Clin. 2020;26:102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuoka T, Ueno D, Ismail Z, et al. Neural correlates of mild behavioral impairment: a functional brain connectivity study using resting‐state functional magnetic resonance imaging. J Alzheimers Dis. 2021;83:1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Subotic A, McCreary CR, Saad F, et al. Cortical thickness and its association with clinical cognitive and neuroimaging markers in cerebral amyloid angiopathy. J Alzheimers Dis. 2021;81:1663‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chertkow H, Borrie M, Whitehead V, et al. The comprehensive assessment of neurodegeneration and dementia: Canadian cohort study. Can J Neurol Sci. 2019;46:499‐511. [DOI] [PubMed] [Google Scholar]
- 24. Duchesne S, Chouinard I, Potvin O, et al. The Canadian dementia imaging protocol: harmonizing national cohorts. J Magn Reson Imaging. 2019;49:456‐465. [DOI] [PubMed] [Google Scholar]
- 25. Ismail Z, Agüera‐Ortiz L, Brodaty H, et al. The Mild Behavioral Impairment Checklist (MBI‐C): a rating scale for neuropsychiatric symptoms in pre‐dementia populations. J Alzheimers Dis. 2017;56:929‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. [DOI] [PubMed] [Google Scholar]
- 27. Kassam F, Chen H, Nosheny R, et al. Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int Psychogeriatr. 2022:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579‐588. [DOI] [PubMed] [Google Scholar]
- 31. Craig AD, How do you feel–now?The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59‐70. [DOI] [PubMed] [Google Scholar]
- 32. Li W, Mai X, Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci. 2014;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greicius MD, Supekar K, Menon V, Dougherty RF. Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andrews‐Hanna JR, Snyder AZ, Vincent JL, et al. Disruption of large‐scale brain systems in advanced aging. Neuron. 2007;56:924‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tighe SK, Oishi K, Mori S, et al. Diffusion tensor imaging of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer's dementia. J Neuropsychiatry Clin Neurosci. 2012;24:484‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wen Q, Mustafi SM, Li J, Risacher SL, et al. White matter alterations in early‐stage Alzheimer's disease: a tract‐specific study. Alzheimers Dement (Amst). 2019;11:576‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang L, Zang Y, He Y, et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496‐504. [DOI] [PubMed] [Google Scholar]
- 38. Eyler LT, Elman JA, Hatton SN, et al. Resting state abnormalities of the default mode network in mild cognitive impairment: a systematic review and meta‐analysis. J Alzheimers Dis. 2019;70:107‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ingala S, Tomassen J, Collij LE, et al. Amyloid‐driven disruption of default mode network connectivity in cognitively healthy individuals. Brain Commun. 2021;3:fcab201. doi: 10.1093/braincomms/fcab201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joo SH, Lee CU, Lim HK. Apathy and intrinsic functional connectivity networks in amnestic mild cognitive impairment. Neuropsychiatr Dis Treat. 2017;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menon V. Large‐scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483‐506. [DOI] [PubMed] [Google Scholar]
- 43. Chand GB, Wu J, Hajjar I, Qiu D. Interactions of the salience network and its subsystems with the default‐mode and the central‐executive networks in normal aging and mild cognitive impairment. Brain Connect. 2017;7:401‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He X, Qin W, Liu Y, et al. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2014;35:3446‐3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mendez MF. The relationship between anxiety and Alzheimer's disease. J Alzheimers Dis Rep. 2021:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yuen GS, Gunning‐Dixon FM, Hoptman MJ, et al. The salience network in the apathy of late‐life depression. Int J Geriatr Psychiatry. 2014;29:1116‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer's disease: what might be associated brain circuits? Mol Aspects Med. 2015;43:25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215‐222. [DOI] [PubMed] [Google Scholar]
- 49. Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer's disease: a systematic review of symptom‐general and–specific lesion patterns. Mol Neurodegener. 2021;16:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schultz AP, Chhatwal JP, Hedden T, et al. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and tau in clinically normal individuals. J Neurosci. 2017;37:4323‐4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ruthirakuhan M, Ismail Z, Herrmann N, Gallagher D, Lanctot KL. Mild behavioral impairment is associated with progression to Alzheimer's disease: a clinicopathological study. Alzheimers Dement. 2022;18:2199‐2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith R, Lane RD, Alkozei A, et al. Maintaining the feelings of others in working memory is associated with activation of the left anterior insula and left frontal‐parietal control network. Soc Cogn Affect Neurosci. 2017;12:848‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Creese B, Brooker H, Ismail Z, et al. Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019;27:823‐834. [DOI] [PubMed] [Google Scholar]
- 54. Kamalian A, Khodadadifar T, Saberi A, et al. Convergent regional brain abnormalities in behavioral variant frontotemporal dementia: A neuroimaging meta‐analysis of 73 studies. Alzheimers Dement (Amst). 2022;14:e12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


