Abstract
The ability to produce words through singing can be preserved in severe aphasia, but the benefits of group-based singing rehabilitation in aphasia are largely unknown. Our aim was to determine the efficacy of a multicomponent singing intervention on communication and speech production, emotional-social functioning and caregiver well-being in aphasia. Fifty-four patients with acquired brain injury and chronic aphasia and their family caregivers (n = 43) were recruited. Using a crossover randomized controlled trial design, participants were randomized to two groups who received a 4-month singing intervention either during the first or second half of the study in addition to standard care. The intervention comprised weekly group-based training (including choir singing and group-level melodic intonation therapy) and tablet-assisted singing training at home. At baseline, 5- and 9-month stages, patients were assessed with tests and questionnaires on communication and speech production, mood, social functioning, and quality of life and family caregivers with questionnaires on caregiver burden. All participants who participated in the baseline measurement (n = 50) were included in linear mixed model analyses. Compared with standard care, the singing intervention improved everyday communication and responsive speech production from baseline to 5-month stage, and these changes were sustained also longitudinally (baseline to 9-month stage). Additionally, the intervention enhanced patients’ social participation and reduced caregiver burden. This study provides novel evidence that group-based multicomponent singing training can enhance communication and spoken language production in chronic aphasia as well as improve psychosocial wellbeing in patients and caregivers.
https://www.clinicaltrials.gov, Unique identifier: NCT03501797.
Keywords: singing, rehabilitation, aphasia, communication, speech production
Siponkoski et al. report that group-based multicomponent singing training can enhance communication and spoken language production in chronic aphasia as well as improve psychosocial wellbeing in patients and caregivers. The findings demonstrate that singing provides a versatile, motivating, scalable, and potentially cost-effective approach to aphasia rehabilitation.
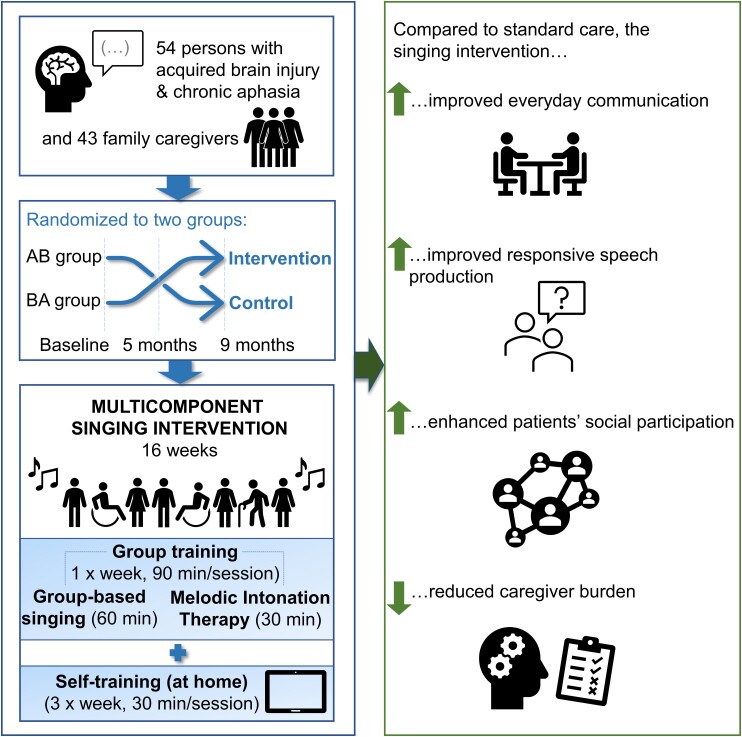
Graphical Abstract
Graphical abstract.
Introduction
Aphasia is a highly debilitating condition that impairs communication abilities, causing social isolation and decreasing emotional wellbeing.1 The leading cause of aphasia is stroke: about 40% of stroke survivors experience aphasia, and in half of them, the communication impairment persists after 1 year post-stroke.2 Aphasia reduces quality of life (QoL) more than other stroke-induced deficits3 or many severe chronic illnesses, including cancer and Alzheimer’s disease.4 Considering the high prevalence of stroke and the sustained burden caused by aphasia on the survivors, their families and the entire society, there is a pressing need for new effective, easily applicable and scalable treatments that target both the communicative and psychosocial needs of persons with aphasia (PWAs). This is particularly true at the chronic stage when PWAs typically no longer receive active treatment, even though it can be effective also at this stage,5,6 and often experience social exclusion7 as well as for their family caregivers (FCs), who face high burden and are at risk of developing depression and anxiety.8
Music is a versatile and effective rehabilitation tool, which can support motor, cognitive and emotional recovery after stroke9–11 but which has thus far not been translated effectively to clinical practice in treating chronic post-stroke aphasia. In aphasia, the ability to vocalize through singing is often preserved,12 and singing can help the motor production of words for example by slowing down the rate of vocal production, entraining it to the musical rhythm and increasing the connectedness between syllables/words.13 Various singing-based aphasia rehabilitation methods have been developed, including the melodic intonation therapy (MIT) where the production of formulaic speech phrases is trained together with a therapist using melodic intoning (singing) and rhythm (hand tapping), following a protocol that progresses from singing to the production of speech with more natural prosody.14,15 MIT has shown promise in enhancing functional communication and expressive language in aphasia,16,17 although larger randomized controlled trials (RCTs) are still needed to provide definite evidence on its clinical efficacy.18 Notably, these methods have mostly been applied in individual-level rehabilitation, which not only requires extensive personnel resources but also overlooks the emotional, communicative and social-interactive power of singing when done in a group.
Group-based singing is a viable and multifaceted approach for aphasia rehabilitation, because it combines verbal production with expressive music making, enjoyable social interaction and peer support. The emotional, social and cognitive benefits of choir singing have been recognized in healthy older adults,19,20 among whom it has become a very popular activity. In PWAs, choir singing has thus far been explored in three small-scale pilot studies. In a within-subject study of nine PWAs, Tamplin et al.21 reported a trend towards reduction of psychological distress after a 20-week community choir intervention (2 h sessions once a week) comprising singing familiar songs, vocal exercises and socialization and led by a music therapist and assisted by volunteers. Qualitatively, positive effects of the choir intervention on confidence, peer support, mood, motivation and communication were observed in interviews of PWAs.21 In a three-arm pilot study of 15 PWAs, Zumbansen et al.22 found no significant improvement in functional communication, expressive language, mood or QoL after a 26-week choir intervention (2 h sessions once a week) compared with a control intervention (drama) or standard care but reported a correlation between attendance to social activities and improvement in functional communication. Recently, Tarrant et al.23 reported a two-arm feasibility study of 36 PWAs in which a 10-week group singing intervention (90 min sessions once a week) led by a community musician and assisted by a PWA volunteer was found to be acceptable and safe for PWAs. These studies provide proof of concept that group singing is a feasible intervention for PWAs and suggest that its clinical efficacy should be explored in a larger clinical trial.24
In summary, previous studies suggest that MIT and choir singing are promising tools for enhancing communicative and psychosocial functioning, respectively, but their synergistic combination within the same group intervention protocol has never been explored. Likewise, previous studies have not considered the role of FC participation and added home training in PWA singing interventions. We developed a new multicomponent singing intervention for PWAs, which (i) combines choir singing and MIT adapted for group-level training to target both communicative and psychosocial outcomes, (ii) is aimed both for PWAs and their FCs to support their interaction and provide enjoyable joint activity and peer support to both, to reduce caregiver burden and to facilitate the translation of practiced functions and skills to the everyday life of the PWAs and (iii) includes tablet-assisted singing training at home to increase the intensity of the intervention and enable the learning of the choir songs. The aims of the multicomponent intervention were to improve communication and spoken language production and emotional, social and functional outcomes in PWAs and psychological well-being in FCs.
In order to determine the clinical efficacy of the intervention, we performed a single-blind crossover RCT in PWAs (N = 54) and their FCs (n = 43) comparing the multicomponent singing intervention to standard care from baseline (T1) to 5-month (T2) and 9-month (T3) follow-up stages. We hypothesized that compared with standard care, the multicomponent singing intervention would enhance communication skills in the PWAs as the primary outcome (from T1 to T2) as well as lead to improvements in spoken language production, verbal memory, mood and QoL in the PWAs and in caregiver burden in the FCs as secondary outcomes.
Materials and methods
Participants and study design
Fifty-four PWAs with a history of cerebrovascular accident (n = 53) or traumatic brain injury (n = 1) leading to aphasia and their FCs (n = 43) were successfully recruited from the Helsinki region during 2017–19 through patient organizations (Helsinki-Uusimaa Stroke Association, Finnish Brain Association) and clinical speech therapists. The FCs were spouses (n = 22), children (n = 8), siblings (n = 2), parents (n = 4) and others (n = 7). The recruiting psychologists interviewed all PWAs interested in the study for evaluating eligibility. The inclusion criteria were (i) age ≥ 18; (ii) Finnish-speaking; (iii) time since stroke/injury > 6 months; (iv) at least mild aphasia [Boston Diagnostic Aphasia Examination (BDAE) Aphasia Severity Rating Scale25 score ≤4 (preliminary assessment based on recruitment interview)]; (v) no subjective hearing deficit; (vi) cognitive ability to give an informed consent; (vii) no neurological/psychiatric co-morbidity or substance abuse and (viii) ability to produce vocal sound through singing/humming. The study was approved by the Ethics committee of the Helsinki-Uusimaa Hospital District, and written informed consents were obtained from all PWAs and FCs.
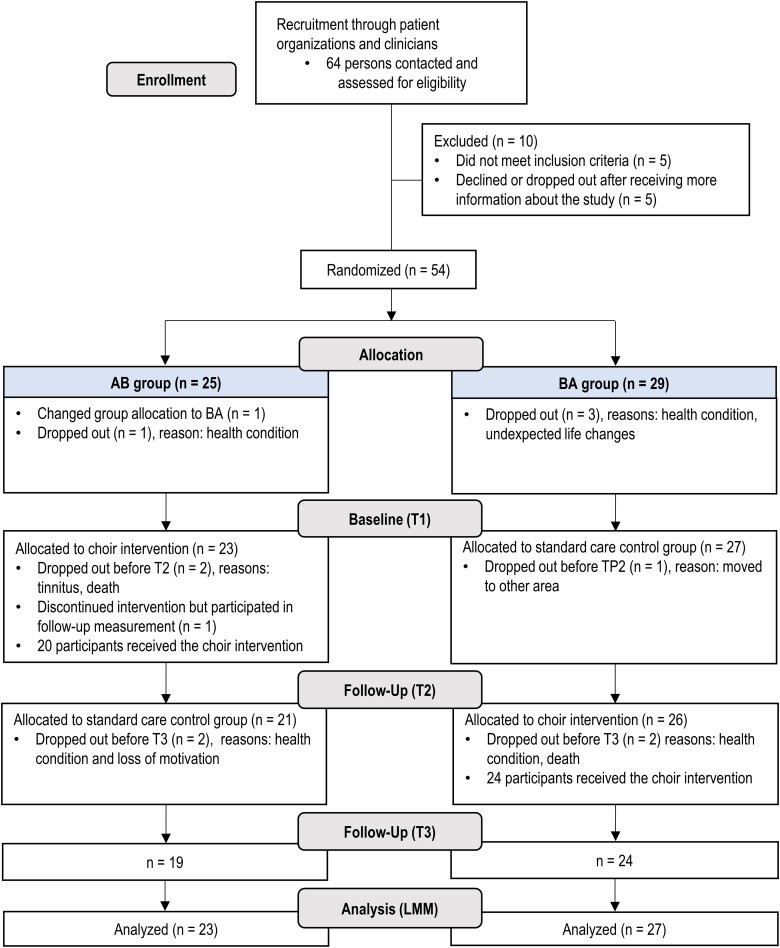
The study was implemented using a crossover RCT in order to enable access to treatment for all participants and maximize the data on intervention experiences. The intervention and the study are reported according to the TIDieR26 and CONSORT27 guidelines. Fig. 1 shows a flowchart of the study design. In two data collection waves (2018: n = 33, 2019: n = 21), the PWA participants were randomly assigned to two groups (AB/BA, A = intervention, B = control) stratified for aphasia severity (preliminary BDAE severity level), FC’s participation in group sessions, sex, age and time since stroke/injury. The randomization was performed for matched pairs using an online random number generator (https://www.random.org) by a researcher not involved in data collection.
Figure 1.
Flowchart outlining the design and progress of the trial. LMM, linear mixed effects model; T1, timepoint 1 (baseline); T2, timepoint 2 (5-month); T3, timepoint 3 (9-month).
The outcome measures, including neuropsychological and language tests and questionnaires, were performed at baseline (T1), 5-month mid-point (T2) and 9-month endpoint (T3). Additionally, MRI and EEG data were gathered from a subgroup of PWAs, in addition to which quantitative and qualitative feedback was collected from PWAs and FCs after the intervention period; these results will be reported separately. AB received the singing intervention during the first 16-week period (T1 and T2) and BA received it during the last 16-week period (T2 and T3). Throughout the trial, both groups received standard care, comprising the standard speech therapy, neuropsychological rehabilitation and physical/occupational therapy provided in public health care.
The drop-out rates and reasons are presented in Fig. 1. Some PWAs discontinued the study due to health problems, and there were two deaths not attributed to the study. One PWA reported that group singing triggered tinnitus, no other adverse effects or harms were reported by the PWAs or FCs.
Additional 23 PWAs were recruited from Southwest Finland for a third study wave and underwent T1 in January–February 2020, but the trial was stopped due to the COVID-19 pandemic in March 2020 and their data had to be excluded from the present study. The sample size was determined based on previous music-intervention studies and practical possibilities in finding participants.
Intervention
The 16-week multicomponent singing intervention was a combination of group training (1 session/week, 1.5 h/session, total 24 h) held at a local aphasia centre (https://www.afasiakeskus.fi/) and home training (target: 3 sessions/week, 30 min/session, total 24 h). Implemented by a two-person team (choir conductor and music therapist, authors E.-R.S. and S.L.), the intervention was held separately for 4 groups of participants (2 AB groups and 2 BA groups; 10–14 PWAs and 6–10 FCs per group). Thirty-two (AB = 14, BA = 18) FCs joined the group sessions; the rest participated only as informants. To enhance treatment conformity, the intervention was administered to all groups by the same two-person team, in accordance with a priori fixed protocol described below.
Group training
Group training sessions comprised 60 min of singing training for PWAs and FCs and 30 min of group-based MIT for PWAs. The singing training was implemented in an encouraging group environment and consisted of breathing and vocal exercises and voice warm-ups (20 min/session), aimed at strengthening voice intensity and syllable-level vocal production, and group singing with choral elements (40 min/session). The group training was implemented in a spacious lobby area, with easy wheel chair access and chairs arranged in a semi-circle around the choir conductor and a screen to which song lyrics were projected during training. The song repertoire (10 songs) mainly consisted of highly familiar Finnish popular and folk songs (for facilitating word retrieval and recall) and a few novel songs (for learning new verbal material), accompanied with piano during the training. The songs were especially arranged for PWAs and FCs, with keys selected for novice singers and tempos slowed down to ease word production, and included also polyphonic arrangements where PWAs sang melody and FCs sang second melody. After each group training session, there was a short voluntary social get together with coffee/tea, which most participants took part in. Each group rehearsed to perform the songs for a small concert held for family and friends in the last session, bringing a goal-oriented element to the training.
Adapted from the original MIT,14,15 the group-based MIT (30 min/session) comprised singing-based training of formulaic speech phrases, incorporating the key elements of MIT [simple melodic structure, simultaneous tapping with the non-paretic (left) hand, stepwise progression from modelling and unison production to repetition]. In our adapted MIT protocol, the training followed a simple five-step cycle for each phrase: (i) thinking (mental preparation), (ii) sung production with melodic intonation, (iii) sung production with melodic intonation and rhythmic pacing (hand tapping for stressed syllables), (iv) spoken production with rhythmic pacing and (v) natural spoken production (without pacing). In this cycle, the therapist first provides a model for each step, which the PWA then performs. Augmentative and Alternative Communication pictures depicting the phrases were also used as visual aids during the MIT training.
The group-based MIT was used in 14 sessions (excluding first and last session). In the first 7 sessions, the PWAs rehearsed a set of 10 formulaic phrases with the music therapist using the MIT protocol, while the FCs trained the second melody of polyphonic songs with the choir conductor in another room. In the latter seven sessions, once the PWAs had internalized the MIT protocol, the FCs joined the PWAs and they trained together using MIT in reciprocal dialogue situations themed around everyday life (e.g. having dinner with guests, cleaning the house). For this, the participants were split to two groups with lead singers. In the first group, the lead singer produced a melodically intoned phrase (e.g. ‘Welcome!’) which the first group then repeated. After this, the lead singer of the second group produced a dialogic response to the first phrase (e.g. ‘Thank you!’), correspondingly repeated by the second group. Using this cycle, the groups had short conversations, aimed at translating the MIT protocol to daily life.
Home training
Singing in a choir usually entails self-training of the song material at home. To facilitate the learning of the song material and to increase the intensity of the training, a tablet-based training application called Singalonger was developed together with a Finnish company (Outloud). Singalonger was used on a tablet computer (Samsung Galaxy Tab 4) and a headset microphone (Logitech H151), provided to each participant. The application included all the songs that were in the choir repertoire and had three options for training aids that the participants could select when singing along to each song: (i) an instrumental auditory model or a sung (female/male) auditory model, (ii) karaoke-type printed lyrics running on the screen (in time with melody) and (iii) a video showing the mouth movements of the model female/male singer (helping to imitate the movements). Singalonger automatically recorded the singing of the participant and analysed the pitch and length of each sung note, which enabled providing online feedback (star-rating) to encourage and motivate training. The patients were trained in using the tablet and application in the first group session and then and instructed to train the song material by themselves using Singalonger three times a week (30 min/session) for the following 16 weeks. The participants also received easy-to-follow pictorial instructions and had technical support available throughout the intervention on how to use the tablet and application.
Outcome measures
The neuropsychological and language assessments were conducted by trained psychologists (authors S.-T.S., A.P. and E.P.) at the Cognitive Brain Research Unit. All spoken language production tasks were recorded. The questionnaires were sent prior to the testing session to PWAs and FCs and were returned to the psychologist. FCs were instructed to help the PWA only in reading the questions without giving guidance in answering. The researchers conducting the assessments and analysing the data were blinded to the group allocation of the participants until the final statistical analysis when the AB/BA groups were compared with each other.
Our primary outcome was change in communication ability from T1 to T2. Secondary outcomes were change in communication ability from T1 to T3 and changes in spoken language production and verbal skills and emotional, social and functional outcome from T1 to T2 and T1 to T3.
Communication ability
Communication ability was measured with the Communicative Activity Log (CAL)28 and the Communication subscale of the Stroke Impact Scale 3.0 (SIS),29 which were both administered to PWAs (self-report) and FCs (informant-report). These measures were chosen to capture changes in daily communication induced by the intervention in an ecologically valid way. In order to gain a comprehensive picture of communication skills in the sample, independent of aphasia severity level, and to pool measures to reduce the amount of analysis and the risk of type I errors,30 we calculated a common communication index by averaging the percentage scores (score/total × 100) of the PWAs and FCs in the CAL communication total score and SIS communication subscale score (reversed to match the CAL).
Spoken language production and verbal skills
Spontaneous speech was assessed with the Spontaneous speech index of the Western Aphasia Battery (WAB).31 The Repetition and Naming indices of WAB were used to assess more automatic and stimulus-dependent spoken language production. They were averaged together to form a Responsive speech index, similar to previous studies.32 At baseline, we also calculated the WAB Aphasia Quotient (AQ),31 indicating the overall severity level of the aphasia, from the Spontaneous speech, Repetition, Naming and Comprehension (estimated based on the Sequential commands subtest) indices. Additionally, we evaluated motor speech production (apraxia of speech) using the articulatory agility subtest of BDAE25 and a verbal memory index from the average of the percentage scores (score/total × 100) of the Logical Memory and Word Lists subtests of Wechsler Memory Scale III,33 and the Finnish KAT verbal working memory task.34 Parallel versions of the memory tasks were used for the T1-T2-T3 measurements, and their orders were randomized and balanced between AB/BA groups.
Emotional, social and functional outcomes
Functional impairment of PWAs was assessed with four SIS subscales: physical functioning (average of ADL/IADL, strength, hand function, and mobility), emotion, memory and thinking, and participation and role function. The percentage scores of the PWAs and FCs were averaged together. PWAs’ self-evaluated mood (depression) and social support were measured using the percentage scores of the Center for Epidemiological Studies-Depression (CES-D)35 and Social Provision Scale (SPS),36 respectively. The General Health Questionnaire (GHQ-12)37 and Zarit Burden Interview (ZBI-22)38 were administered to the FCs and their average percentage score was used as an index of caregiver burden.
Statistical analysis
The statistical analyses were conducted using IBM SPSS Statistics 26.
Intention-to-treat analysis
Main analyses were conducted using linear mixed effects model (LMM) on the whole sample of participants who participated in the first measurement (n = 50). This approach utilizes all available data according to the intention-to-treat (ITT) principle.39 Time × Group interactions were analysed between T1 and T2 using repeated measures analysis (restricted maximum likelihood method), in which Group and Time were included in the model as fixed effects and within-subject variation as a random effect. Compound symmetry was selected as the covariance structure based on Schwarz’s Bayesian Criterion. For measures yielding significant effects in LMM, the long-term effects were further investigated within both AB and BA groups over T1–T3. Direct comparisons between groups were not conducted between T2 and T3 due to the possible carry-over effect in the AB group. Effect sizes were approximated using the repeated measures ANOVA, because the LMM procedure does not produce effect sizes in SPSS, and the chosen LMM model and covariance structure were very similar with traditional ANOVA.
Per-protocol analysis
To evaluate the sensitivity of our results in a dataset of subjects who adhered to study protocol and participated in all measurement points (between T1 and T2: AB = 20, BA = 26), we performed a per-protocol (PP) analysis for the significant measures from the LMM analysis using repeated measures ANOVA. One PWA who participated in T1 and T2 measurements, but dropped out of the intervention, was excluded from the analysis due to protocol violation. Additionally, pre- and post-intervention (AB: T1 and T2, BA: T2 and T3) scores were compared for the significant measures using paired t-tests.
Results
Clinical and demographic characteristics of the PWAs are presented in Table 1, and their adherence to the intervention and amount of other rehabilitation (standard care) received during the study period in Table 2. The AB and BA groups did not differ significantly in any of these variables. Also, the demographic characteristics of the FCs (n = 43, 30 females, mean age 61.4 years) did not differ between AB/BA.
Table 1.
Baseline clinical and demographic background information of the PWAs
| All (n = 50) | AB (n = 23) | BA (n = 27) | Difference between groups (P value) | |
|---|---|---|---|---|
| Demographic information | ||||
| Age | 64.0 (12.3) | 63.5 (10.3) | 64.5 (14.0) | 0.787 (t) |
| Sex (female/male) | 28/22 | 11/12 | 17/10 | 0.283 (χ2) |
| Handedness (right/left) | 42/8 | 21/2 | 21/6 | 0.261 (F) |
| Education levela | 2.9 (1.4) | 3.0 (1.4) | 2.9 (1.4) | 0.965 (t) |
| Clinical information | ||||
| Aetiology of injury (ischaemic/haemorrhagic/both/TBI) | 28/16/3/1 | 14/6/2/0 | 14/10/1/1 | 0.641 (F) |
| Time since injury (months) | 73.3 (68.4) | 76.0 (69.5) | 71.0 (68.7) | 0.789 (t) |
| Aphasia severity (mild/moderate or severe)b | 34/16 | 14/9 | 20/7 | 0.318 (χ2) |
| Musical background | ||||
| Choir singing years | 3.1 (9.2) | 4.0 (11.4) | 2.0 (5.2) | 1.000 (U) |
| Singing lessons years | 0.5 (2.8) | 0.1 (0.5) | 0.8 (3.7) | 0.671 (U) |
| Instrument lessons years | 1.4 (3.2) | 0.6 (1.4) | 2.3 (4.2) | 0.201 (U) |
Data are mean (SD) unless otherwise stated.
t, independent-samples t-test; F, Fisher’s exact test; U, Mann–Whitney U-test; χ2, Chi-squared test.
Education level according to the UNESCO International Standard Classification of Education: range 1 (primary education) to 6 (doctoral or equivalent level).
Aphasia severity based on the WAB AQ rate, Score 0–50 = severe, Score 51–100 = mild/moderate.
Table 2.
Amount of standard rehabilitation and group and home training
| All (n = 50) | AB (n = 23) | BA (n = 27) | Difference between groups (P value) | |
|---|---|---|---|---|
| T1–T3 | ||||
| Home traininga | 11.9 (9.8) | 13.7 (11.0) | 10.4 (8.6) | 0.321 (U) |
| Group training (attendance rate) | 90.1% (14.0) | 89.0% (18.0) | 92.4% (9.4) | 0.426 (t) |
| Speech therapy | 9.75 (12.4) | 7.8 (12.4) | 11.3 (12.5) | 0.232 (U) |
| Physical therapy | 8.9 (15.0) | 9.2 (16.2) | 8.8 (14.4) | 0.848 (U) |
| Occupational therapy | 1.8 (4.3) | 1.8 (5.2) | 1.7 (3.6) | 0.740 (U) |
| Neuropsychological rehabilitation | 0.2 (0.8) | 0.1 (0.4) | 0.3 (1.0) | 0.663 (U) |
| T1–T2 | ||||
| Speech therapy | 6.5 (8.6) | 4.2 (2.6) | 7.3 (7.5) | 0.332 (U) |
| Physical therapy | 6.2 (9.0) | 3.7 (5.4) | 5.1 (7.3) | 0.778 (U) |
| Occupational therapy | 1.4 (3.7) | 0.1 (4.1) | 0.9 (1.9) | 0.751 (U) |
| Neuropsychological rehabilitation | 0.4 (1.6) | 0.0 (0.0) | 0.3 (1.0) | 0.724 (U) |
| T2–T3 | ||||
| Speech therapy | 4.1 (5.8) | 4.4 (6.5) | 5.1 (5.8) | 0.288 (U) |
| Physical therapy | 4.1 (7.4) | 3.8 (6.1) | 4.2 (8.4) | 0.566 (U) |
| Occupational therapy | 0.5 (2.0) | 0.1 (0.2) | 1.1 (2.8) | 0.386 (U) |
| Neuropsychological rehabilitation | 0.1 (0.2) | 0.1 (0.4) | 1.0 (0.0) | 0.258 (U) |
Data are mean (SD) in hours unless otherwise stated.
Based on Singalonger log files. Abbreviations: U = Mann–Whitney U-test; t = independent-samples t-test; T1, timepoint 1 (baseline); T2, timepoint 2 (5-month); T3, timepoint 3 (9-month).
Communication and spoken language production outcome
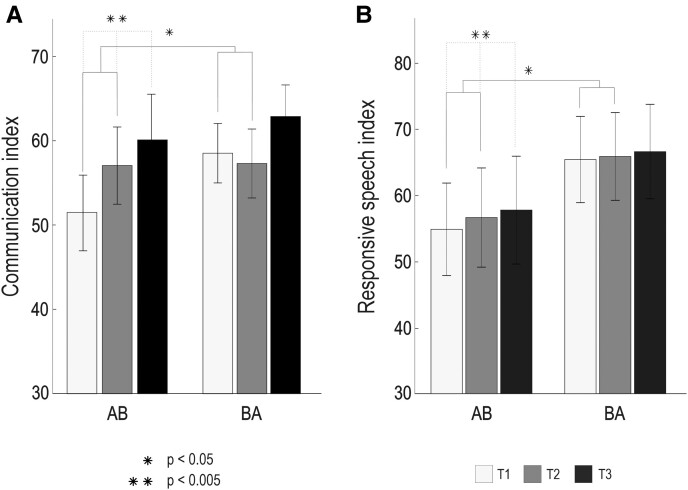
The ITT results from communication and spoken language production Time × Group LMM analysis (T1 and T2) are presented in Table 3 and Fig. 2. There were significant improvements in the AB group compared with the BA group between T1 and T2 in the Communication index [F(1,45) = 7.08, P = 0.011, ηp2 = 0.140] and in the Responsive speech index [F(1,45) = 4.10, P = 0.049, ηp2 = 0.084]. No significant effects were observed in the other measures.
Table 3.
Communication and spoken language outcome results from LMM analysis
| Measure | Group | T1 mean (SD) | T2 mean (SD) | T3 mean (SD) | Observations T1/T2/T3 | Baseline diff. | Δ T1–T2 (F value) | Δ T1–T2 (P value) |
|---|---|---|---|---|---|---|---|---|
| Communication | ||||||||
| Communication index (percentage)a | AB | 51.6 (21.6) | 57.1 (21.0) | 60.2 (22.4) | 50/47/40 | 0.219 | 7.082 | 0.011 |
| BA | 58.6 (18.4) | 57.4 (20.9) | 63.0 (18.0) | |||||
| Spoken language production | ||||||||
| Spontaneous speech index (percentage)a | AB | 60.7 (32.8) | 62.6 (32.7) | 63.4 (32.4) | 50/47/42 | 0.223 | 0.264 | 0.610 |
| BA | 70.2 (30.0) | 71.5 (30.3) | 72.4 (29.8) | |||||
| Responsive speech index (percentage)a | AB | 55.0 (33.5) | 56.7 (34.3) | 57.9 (34.5) | 50/47/40 | 0.276 | 4.100 | 0.049 |
| BA | 65.5 (33.8) | 65.9 (33.7) | 66.7 (33.4) | |||||
| Articulatory agility (percentage)a | AB | 49.0 (29.5) | 49.7 (31.5) | 47.4 (31.2) | 49/47/42 | 0.442 | 0.308 | 0.582 |
| BA | 55.6 (33.8) | 54.1 (33.5) | 58.7 (34.5) | |||||
| Verbal memory | ||||||||
| Verbal memory index (percentage)a | AB | 27.1 (15.6) | 26.6 (16.6) | 29.4 (16.5) | 50/47/42 | 0.370 | 2.022 | 0.191 |
| BA | 30.1 (15.5) | 31.9 (16.0) | 33.2 (18.7) | |||||
Higher score indicates better outcome.
Values in bold indicate statistically significant results. T1, timepoint 1 (baseline); T2, timepoint 2 (5-month); T3, timepoint 3 (9-month).
Figure 2.
Communication and speech production results from the LMM analysis (N = 50). (A) Communication index (F = 7.082, P = 0.011). (B) Responsive speech index (F = 4.100, P = 0.049). AB received the intervention from T1 to T2, and BA received the intervention from T2 to T3. The statistical method used in this study was the LMM analysis. The bar plots (mean—SEM) show changes in test scores over the three time-points (T1–T3) presented group-wise (AB/BA). Significant Time × Group interactions are shown with solid lines and significant within-group Time main effects are shown with dashed lines. LMM, linear mixed effects model; SEM, standard error of the mean; T1, timepoint 1 (baseline); T2 timepoint 2 (5-month); T3 timepoint 3 (9-month).
Within-group longitudinal analyses showed that the Time main effect (T1–T3) in the AB group was significant in the Communication index [F(2,37) = 6.44, P = 0.004, ηp2 = 0.308] and in the Responsive speech index [F(2,37) = 6.87, P = 0.003, ηp2 = 0.222]. Post hoc pairwise comparisons indicated that in both the Communication and the Responsive speech index, the outcome improved between T1 and T2 (P = 0.013 and P = 0.001) and between T1 and T3 (P = 0.002 and P = 0.009) but did not change between T2 and T3 (P = 0.337 and P = 0.608) in the AB group, suggesting that the gains from the intervention were maintained at the longitudinal follow-up. These comparisons survived after false discovery rate (FDR)-correction. Within the BA group, there were no significant Time (T1–T3) main effects in either index [Communication: F(2,47) = 2.02, P = 0.144, ηp2 = 0.079; Responsive speech: F(2,46) = 1.567, P = 0.220, ηp2 = 0.068], but the changes were in a positive direction.
Functional, emotional and social outcome
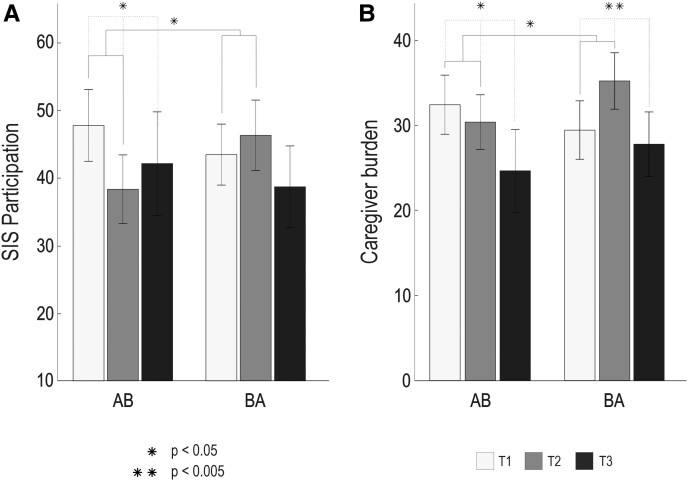
The ITT results from functional, emotional and social outcome measures are shown in Table 4 and Fig. 3. In the Time × Group LMM analysis, a significant improvement in the AB versus BA group between T1 and T2 was found in the SIS participation and role function subscale [F(1,43) = 6.44, P = 0.015, ηp2 = 0.139] and in the Caregiver burden index [F(1,40) = 6.77, P = 0.014, ηp2 = 0.177]. No significant effects were found in the other measures.
Table 4.
Functional, social, mood and cognitive outcomes analysed using LMM
| Measure | Group | T1 mean (SD) | T2 mean (SD) | T3 mean (SD) | Observations T1/T2/T3 | Baseline diff. | Δ T1–T2 (F value) | Δ T1–T2 (P value) |
|---|---|---|---|---|---|---|---|---|
| Functional impairment | ||||||||
| SIS physical functioning (percentage) | AB | 37.7 (23.7) | 37.2 (25.0) | 35.5 (26.1) | 48/47/40 | 0.613 | 0.223 | 0.639 |
| BA | 34.1 (23.7) | 34.9 (25.2) | 32.6 (22.6) | |||||
| SIS emotion (percentage) | AB | 29.3 (17.2) | 27.1 (14.2) | 23.9 (19.3) | 50/47/40 | 0.703 | 0.799 | 0.376 |
| BA | 27.6 (10.3) | 27.3 (10.4) | 23.5 (12.0) | |||||
| SIS memory and thinking (percentage) | AB | 30.1 (19.8) | 27.6 (20.4) | 25.7 (20.9) | 50/47/40 | 0.386 | 1.312 | 0.258 |
| BA | 25.5 (17.5) | 25.8 (19.9) | 23.2 (17.5) | |||||
| SIS participation and role function (percentage) | AB | 47.7 (24.9) | 38.3 (23.3) | 42.1 (31.5) | 47/47/40 | 0.537 | 6.440 | 0.015 |
| BA | 43.4 (22.5) | 46.3 (26.5) | 38.7 (29.0) | |||||
| Mood (depression) | ||||||||
| CES-D total score (percentage) | AB | 27.8 (13.1) | 25.2 (14.7) | 22.14 (12.6) | 50/47/40 | 0.613 | 0.136 | 0.715 |
| BA | 29.5 (11.2) | 28.0 (10.6) | 29.9 (12.4) | |||||
| Social support | ||||||||
| SPS total score (percentage)a | AB | 82.21(12.6) | 80.1 (12.1) | 79.2 (11.8) | 50/46/40 | 0.325 | 0.136 | 0.677 |
| BA | 79.0 (12.0) | 78.1 (10.6) | 76.1 (9.5) | |||||
| Caregiver burden | ||||||||
| Caregiver burden index (percentage) | AB | 32.4 (14.3) | 30.4 (12.1) | 24.7 (16.19) | 37/36/28 | 0.548 | 6.765 | 0.014 |
| BA | 29.5 (15.4) | 35.2 (15.6) | 27.8 (15.6) | |||||
Higher score indicates better outcome.
Values in bold indicate statistically significant results. T1, timepoint 1 (baseline); T2, timepoint 2 (5-month); T3, timepoint 3 (9-month).
Figure 3.
Functional outcome and caregiver burden results from the LMM analysis (N = 50). (A) SIS participation and role function (F = 6.440, P = 0.015). (B) Caregiver burden as measured by GHQ-12 and ZBI-22 (F = 6.765, P = 0.014). AB received the intervention from T1 to T2, and BA received the intervention from T2 to T3. The statistical method used in this study was the LMM analysis. The bar plots (mean—SEM) show changes in test scores over the three time-points (T1–T3) presented group-wise (AB/BA). Significant Time × Group interactions are shown with solid lines. Time main effects are shown with dashed lines. GHQ-12, General Health Questionnaire 12; LMM, linear mixed effects model; SEM, standard error of the mean; SIS, Stroke Impact Scale; T1, timepoint 1 (baseline); T2, timepoint 2 (5-month); T3, timepoint 3 (9-month); ZBI-22, Zarit Burden Interview 22.
In the SIS participation and role function, there was a significant Time main effect over T1–T3 in the AB group [F(2,37) = 3.34, P = 0.047, ηp2 = 0.129]. Post hoc testing showed that outcome improved between T1 and T2 (P = 0.016) but not between T1 and T3 (P = 0.095) or T2 and T3 (P = 0.539), suggesting that the positive effect of the intervention on the social participation of the PWAs was short-term. These comparisons survived FDR-correction. Within the BA group, there was no significant Time main effect [F(2,45) = 0.66, P = 0.520, ηp2 = 0.019], but the changes were in a positive direction.
In the Caregiver burden index, there were significant within-group T1–T3 changes in both AB [F(2,24) = 3.49, P = 0.047, ηp2 = 0.226] and BA [F(2,34) = 7.07, P = 0.003, ηp2 = 0.277] groups. Pairwise comparisons showed that the burden score decreased significantly in the AB group between T1 and T3 (P = 0.015), whereas in the BA group it increased between T1 and T2 (P = 0.012) and decreased between T2 and T3 (P = 0.001), indicating positive effects of the intervention in both groups. These comparisons survived FDR-correction.
PP analysis
A PP analysis using repeated measures ANOVA was conducted for all the outcome measures that showed significant effects in the ITT analyses (T1 and T2 Time × Group; see above). The PP results were in line with the LMM approach, yielding a Time (T1 and T2)×Group (AB versus BA) interaction that was significant for the Communication index [F(1,41) = 5.75, P = 0.021, ηp2 = 0.123] and marginally significant for the Responsive speech index [F(1,44) = 3.76, P = 0.059, ηp2 = 0.079]. Additionally, paired t-tests comparing pre- and post-intervention scores (AB: T1 and T2, BA: T2 and T3) across both groups revealed a significant improvement in both the Communication index (t42 = −3.80, P < 0.001) and the Responsive speech index (t41 = −2.85, P = 0.007). In contrast, changes over the control period (AB: T2 and T3, BA: T1 and T2) were not significant (t42 = −0.16, P = 0.874; t43 = −0.73, P = 0.469). Together, these findings support the ITT results on the efficacy of the intervention on communication and spoken language production.
Discussion
This crossover RCT explored the clinical efficacy of a novel multicomponent singing intervention in chronic aphasia. Our main results between T1 and T2 showed that compared with standard care, the singing intervention (i) enhanced PWAs’ everyday communication ability and spoken language production in tasks involving responsive speech (repetition, naming), (ii) improved PWAs’ social participation and (iii) reduced caregiver burden in FCs. These findings indicate that singing-based rehabilitation, which includes both group- and self-training elements and in which also the FCs can actively participate, can have positive effects on both language functions and psychosocial wellbeing, providing social and emotional support for PWAs and their family members. These findings are clinically important because they provide novel evidence that singing-based interventions coupled with standard care, independent of health care resources, may support recovery of chronic aphasia compared with standard care only.
Most previous studies on group-based singing interventions in aphasia21–24 have utilized non-randomized designs or have been feasibility studies and limited by small sample sizes and they have not included an extensive assessment of communication or spoken language production outcomes. The results of the present trial show for the first time that singing-based rehabilitation that includes both group- and self-training elements can improve everyday communication ability (CAL, SIS Communication) and responsive speech (WAB Repetition and Naming) in chronic aphasia. Previously, similar benefits have been observed after intensive MIT or other individual-level singing-based interventions in aphasia.14–18,40,41 Our evidence for improvement in communication comes from self- and FC-reports, which are naturally not blinded. However, in aphasia care and research, it is important to include the patient’s own view as an indicator of subjectively experienced outcome,42 and FCs can provide valuable complementary information on the everyday functioning and communication of the PWAs, especially in more severe aphasia.43 Together with the improved responsive speech observed in standardized tests performed by a blinded investigator, our results capture the broad effects of singing on language function in aphasia. Importantly, the positive effects on both communication ability and responsive speech were maintained 5 months after the cessation of the intervention, which indicates that the verbal benefits induced by the intervention were robust and durable. No significant changes were found in other tasks measuring spontaneous speech, articulatory agility or verbal memory. One potential explanation for the lack of findings in these tasks could be that singing strengthens more automatic phonological language skills, which are linked to left temporoparietal regions in aphasia, while more motor and cognitive elements of connected speech are linked to left frontal regions in aphasia.44
Regarding social functioning, the singing intervention showed a positive effect in the SIS participation and role function subscale, in line with previous studies reporting psychosocial benefits of choir singing in healthy seniors19,20 and in PWAs.21 This effect was short-term and could reflect the increased activity level and the opportunities for engagement, social interaction, and peer support experienced by the PWAs in an enriched communicative environment.45 No effects were observed on the PWAs’ self-reported mood (CES-D) or social support (SPS) or in more generic functional outcome (other SIS scales), which is somewhat surprising, because music-based interventions have previously been linked to mood and QoL benefits in healthy19,20 and neurological9–11 populations. This may reflect the difficulty of questionnaire-based measurement of subjective emotional wellbeing in aphasic patients and the need for a larger sample size to detect effects.24
Finally, we observed a long-term reduction in caregiver burden following the singing intervention, in line with similar findings of FCs in dementia.46 This may be related to the positive self-experienced emotional impact of choir singing,19,20 the increased interaction with the PWA and other FCs (including peer support) during the intervention, or to the intervention-induced communicative and psychosocial improvements of the PWA. This finding is important given the high prevalence of mood disorders in the FCs of PWAs.8
Regarding the commitment and adherence of the patients to the intervention protocol, the attendance rates for group training were high (around 90%), whereas the amount of home training was more variable. Originally, the patients were instructed to have three 30 min home training sessions each week (total 24 h over 16 weeks), but the realized total amount of home training was markedly lower (on average 11.9 h of using the Singalonger; however, there was a lot of individual variability, with some patients training almost 40 h with the app). There are likely a number of factors contributing to this variability (e.g. motivation, cognitive problems, attitudes, technical issues), which will be separately analysed and reported along with other intervention and usability feedback as well as dosage issues, important for the applicability of the intervention model.
The present study has following potential limitations. First, while being the largest study to date on group-based singing in aphasia, our sample is moderate in size and comprises PWAs of varying severity; in future, large-scale studies are warranted to determine the efficacy of singing across different aphasia types and severity levels. Second, while there was a statistically significant improvement in everyday communication abilities with a large effect size, the direct clinical relevance of this change is not known as there are no standardized estimates for minimal clinically important difference for CAL. Third, although the multicomponent nature of the intervention likely contributes to its broad efficacy in both communicative and psychosocial domains, it also precludes making inferences about the contribution of each component (group-based singing, group-MIT, tablet-based home training) on the outcomes. Fourth, the crossover design bears some methodological considerations: due to the possible carry-over effect in the AB group between T2 and T3, intervention effects in the BA group could not be reliably estimated (compared with standard care). To gain a more comprehensive understanding of the changes over the entire follow-up period (T1–T3), we performed within-group analysis in both intervention groups. Whereas the AB group showed significant findings consistent with our hypothesis between T1 and T2 (with treatment-induced gains in communication, responsive speech and caregiver burden maintained also longitudinally up to T3), the changes in the BA group between T2 and T3 were in a positive direction, but did not reach statistical significance, with the exception of caregiver burden. However, a pooled analysis of the AB and BA groups showed significant improvement in communication and responsive speech over the intervention period whereas there were no significant changes during the control period. It is possible that motivational factors play some role, as the BA group had to wait 5 months (and undergo two assessment points) before receiving the intervention. Furthermore, while the groups did not show significant differences in clinical or biographical background information associated with therapy response in chronic aphasia,47 there might be specific biographical, neuropsychological, or neurobiological factors influencing treatment response to singing-based interventions in chronic aphasia that have remained yet uncharted. Future multimodal studies exploring the predictors of therapy response to singing interventions are needed and would help clinicians to individualize treatment strategies to optimize recovery.
Despite these limitations, the observed effects are encouraging in suggesting that singing may be a potential tool to promote communicative and psychosocial outcome even in chronic post-stroke aphasia as well as provide a meaningful joint activity for PWAs and FCs that can also alleviate the burden experienced by the caregivers. Importantly, these positive findings (i) provide further support for recent evidence that rehabilitation interventions can achieve significant improvements in core outcomes, such as motor, cognitive or verbal abilities, still in the chronic stage, years after stroke5,6,48 and that (ii) singing-based interventions can be a powerful tool to unlock communicative skills in chronic aphasia, possibly mediated by the largely bilateral engagement of vocal-motor and auditory brain regions associated with singing.13,49–51 Notably, the multicomponent singing intervention used in the present study included elements of choir singing, singing-based speech training, tablet-assisted home training, and PWA-FC interaction and was implemented by two professionals with expertise on music therapy in neurological patients and on choir conduction and singing instruction. Our results demonstrate this type of novel intervention model provides a versatile, motivating, scalable and potentially cost-effective approach to aphasia rehabilitation.
Acknowledgements
We thank Andrea Norton and Jeanette Tamplin for their valuable help in designing the intervention, and Olli Ström, Mikko Koivisto and Ulla Sergejeff from Outloud for the development of the training software (Singalonger). First and foremost, we warmly thank all PWAs and FCs for their participation and invaluable input in this project.
Abbreviations
- ADL =
activities of daily living
- BDAE =
Boston Diagnostic Aphasia Examination
- CAL =
Communicative Activity Log
- CES-D =
Center for Epidemiological Studies—Depression
- CONSORT =
Consolidated Standards of Reporting Trials
- FC =
family caregiver
- FDR =
false discovery rate
- GHQ-12 =
General Health Questionnaire
- IADL =
instrumental activities of daily life
- ITT =
intention-to-treat
- LMM =
linear mixed effects model
- MIT =
melodic intonation therapy
- PP =
per-protocol
- PWA =
person with aphasia
- QoL =
quality of life
- RCT =
randomized controlled trial
- SIS =
Stroke Impact Scale
- SPS =
Social Provision Scale
- TIDieR =
Template for Intervention Description and Replication
- T1 =
timepoint 1 (baseline)
- T2 =
timepoint 2 (5-month)
- T3 =
timepoint 3 (9-month)
- WAB =
Western Aphasia Battery
- ZBI-22 =
Zarit Burden Interview
Contributor Information
Sini-Tuuli Siponkoski, Cognitive Brain Research Unit, Department of Psychology and Logopedics, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland; Centre of Excellence in Music, Mind, Body and Brain, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland.
Anni Pitkäniemi, Cognitive Brain Research Unit, Department of Psychology and Logopedics, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland; Centre of Excellence in Music, Mind, Body and Brain, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland.
Sari Laitinen, Centre of Excellence in Music, Mind, Body and Brain, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland; Espoo Hospital, 00029 HUS, Espoo, Finland.
Essi-Reetta Särkämö, Private choir conductor, 01300 Vantaa, Finland.
Emmi Pentikäinen, Cognitive Brain Research Unit, Department of Psychology and Logopedics, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland; Centre of Excellence in Music, Mind, Body and Brain, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland.
Heidi Eloranta, Helsinki-Uusimaa Stroke Association, 00610 Helsinki, Finland.
Leena Tuomiranta, Department of Psychology and Logopedics, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland.
Susanna Melkas, Department of Neurology, University of Helsinki and Helsinki University Central Hospital, 00029 HUS, Helsinki, Finland.
Gottfried Schlaug, Department of Neurology, UMass Medical School, Springfield & Department of Biomedical Engineering and Institute of Applied Life Sciences, UMass Amherst, Amherst, MA 01003, USA.
Aleksi J Sihvonen, Cognitive Brain Research Unit, Department of Psychology and Logopedics, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland; Centre of Excellence in Music, Mind, Body and Brain, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland; Department of Neurology, University of Helsinki and Helsinki University Central Hospital, 00029 HUS, Helsinki, Finland; School of Health and Rehabilitation Sciences, Queensland Aphasia Research Centre and UQ Centre for Clinical Research, The University of Queensland, QLD 4029, Brisbane, Australia.
Teppo Särkämö, Cognitive Brain Research Unit, Department of Psychology and Logopedics, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland; Centre of Excellence in Music, Mind, Body and Brain, University of Helsinki, 00014 Helsingin yliopisto, Helsinki, Finland.
Funding
Financial support for the work was provided by the Academy of Finland (grants 299044, 306625 and 327996), Fundació La Marató de TV3 (grant 201729.32), and European Research Council (grant 803466).
Competing interests
The authors have no conflicts of interest to disclose.
Data availability
Anonymized data reported in this manuscript are available from the corresponding author upon reasonable request and subject to approval by the appropriate regulatory committees and officials.
References
- 1. Doogan C, Dignam J, Copland D, Leff A. Aphasia recovery: When, how and who to treat? Curr Neurol Neurosci Rep. 2018;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedersen PM, Stig Jørgensen H, Nakayama H, et al. Aphasia in acute stroke: Incidence, determinants, and recovery. Ann Neurol. 1995;38:659–666. [DOI] [PubMed] [Google Scholar]
- 3. Hilari K. The impact of stroke: Are people with aphasia different to those without? Disabil Rehabil. 2011;33:211–218. [DOI] [PubMed] [Google Scholar]
- 4. Lam JMC, Wodchis WP. The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in Ontario hospital-based long-term care residents. Med Care. 2010;48:380–387. [DOI] [PubMed] [Google Scholar]
- 5. Johnson L, Basilakos A, Yourganov G, et al. Progression of aphasia severity in the chronic stages of stroke. Am J Speech Lang Pathol. 2019;28:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleming V, Brownsett S, Krason A, et al. Efficacy of spoken word comprehension therapy in patients with chronic aphasia: A cross-over randomised controlled trial with structural imaging. J Neurol Neurosurg Psychiatry. 2021;92:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parr S. Living with severe aphasia: Tracking social exclusion. Aphasiology. 2007;21:98–123. [Google Scholar]
- 8. Hu P, Yang Q, Kong L, et al. Relationship between the anxiety/depression and care burden of the major caregiver of stroke patients. Medicine (Baltimore). 2018;97:e12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grau-Sánchez J, Münte TF, Altenmüller E, et al. Potential benefits of music playing in stroke upper limb motor rehabilitation. Neurosci Biobehav Rev. 2020;112:585–599. [DOI] [PubMed] [Google Scholar]
- 10. Särkämö T, Tervaniemi M, Laitinen S, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain. 2008;131:866–876. [DOI] [PubMed] [Google Scholar]
- 11. Sihvonen AJ, Särkämö T, Leo V, et al. Music-based interventions in neurological rehabilitation. Lancet Neurol. 2017;16:648–660. [DOI] [PubMed] [Google Scholar]
- 12. Yamadori A, Osumi Y, Masuhara S, Okubo M. Preservation of singing in Broca’s aphasia. J Neurol Neurosurg Psychiatry. 1977;40:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan CY, Rüber T, Hohmann A, Schlaug G. The therapeutic effects of singing in neurological disorders. Music Percept. 2010;27:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch Neurol. 1973;29:130–131. [DOI] [PubMed] [Google Scholar]
- 15. Norton A, Zipse L, Marchina S, Schlaug G. Melodic intonation therapy: Shared insights on how it is done and why it might help. Ann N Y Acad Sci. 2009;1169:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Meulen I, van de Sandt-Koenderman WME, Heijenbrok-Kal MH, et al. The efficacy and timing of melodic intonation therapy in subacute aphasia. Neurorehabil Neural Repair. 2014;28:536–544. [DOI] [PubMed] [Google Scholar]
- 17. Haro-Martínez AM, Lubrini G, Madero-Jarabo R, et al. Melodic intonation therapy in post-stroke nonfluent aphasia: A randomized pilot trial. Clin Rehabil. 2019;33:44–53. [DOI] [PubMed] [Google Scholar]
- 18. Haro-Martínez A, Pérez-Araujo CM, Sanchez-Caro JM, et al. Melodic intonation therapy for post-stroke non-fluent aphasia: Systematic review and meta-analysis. Front Neurol. 2021;12:700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coulton S, Clift S, Skingley A, Rodriguez J. Effectiveness and cost-effectiveness of community singing on mental health-related quality of life of older people: Randomised controlled trial. Br J Psychiatry. 2015;207:250–255. [DOI] [PubMed] [Google Scholar]
- 20. Pentikäinen E, Pitkäniemi A, Siponkoski ST, et al. Beneficial effects of choir singing on cognition and well-being of older adults: Evidence from a cross-sectional study. PLoS ONE. 2021;16:e0245666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamplin J, Baker FA, Jones B, et al. “Stroke a chord”: The effect of singing in a community choir on mood and social engagement for people living with aphasia following a stroke. NeuroRehabilitation. 2013;32:929–941. [DOI] [PubMed] [Google Scholar]
- 22. Zumbansen A, Peretz I, Anglade C, et al. Effect of choir activity in the rehabilitation of aphasia: A blind, randomised, controlled pilot study. Aphasiology. 2017;31:879–900. [Google Scholar]
- 23. Tarrant M, Carter M, Dean SG, et al. Singing for people with aphasia (SPA): Results of a pilot feasibility randomised controlled trial of a group singing intervention investigating acceptability and feasibility. BMJ Open. 2021;11:e040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monroe P, Halaki M, Kumfor F, Ballard KJ. The effects of choral singing on communication impairments in acquired brain injury: A systematic review. Int J Lang Commun Disord. 2020;55:303–319. [DOI] [PubMed] [Google Scholar]
- 25. Goodglass H, Kaplan E. The assessment of aphasia and related disorders, 2nd edn.Lea & Febiger; 1983. [Google Scholar]
- 26. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 27. Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 Statement: Extension to randomised crossover trials. BMJ. 2019;366:l4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pulvermüller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–1626. [DOI] [PubMed] [Google Scholar]
- 29. Duncan PW, Bode RK, Lai SM, Perera S. Rasch analysis of a new stroke-specific outcome scale: The stroke impact scale. Arch Phys Med Rehabil. 2003;84:950–963. [DOI] [PubMed] [Google Scholar]
- 30. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kertesz A. Western aphasia battery. Psychological Corporation; 1982. [Google Scholar]
- 32. Stockert A, Wawrzyniak M, Klingbeil J, et al. Dynamics of language reorganization after left temporo-parietal and frontal stroke. Brain. 2020;143:844–861. [DOI] [PubMed] [Google Scholar]
- 33. Wechsler D. WMS-III—Wechsler Memory Scale. 3rd edn.Psychological Corporation; 1997. [Google Scholar]
- 34. Manninen R-L, Pietilä M-L, Setälä P, Laitinen V. KAT-testi: Kielelliset arviointitehtävät lievien häiriöiden määrittämiseksi aikuisilla. Puheterapeuttien Kustannus; 2015. [Google Scholar]
- 35. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. [DOI] [PubMed] [Google Scholar]
- 36. Cutrona CE, Russell DW. The provisions of social relationships and adaptation to stress. In: Jones WH, Perlman D, eds. Advances in personal relationships. JAI Press; 1987:37-67. [Google Scholar]
- 37. Cuéllar-Flores I, Sánchez-López MP, Limiñana-Gras RM, Colodro-Conde L. The GHQ-12 for the assessment of psychological distress of family caregivers. Behav Med. 2014;40:65–70. [DOI] [PubMed] [Google Scholar]
- 38. Siegert RJ, Jackson DM, Tennant A, Turner-Stokes L. Factor analysis and Rasch analysis of the Zarit Burden Interview for acquired brain injury carer research. J Rehabil Med. 2010;42:302–309. [DOI] [PubMed] [Google Scholar]
- 39. Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magee WL, Clark I, Tamplin J, Bradt J. Music interventions for acquired brain injury. Cochrane Database Syst Rev. 2017;1:CD006787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Q, Li W, Yin Y, et al. The effect of music therapy on language recovery in patients with aphasia after stroke: A systematic review and meta-analysis. Neurol Sci. 2022;43:863–872. [DOI] [PubMed] [Google Scholar]
- 42. Oczkowski C, O’Donnell M. Reliability of proxy respondents for patients with stroke: A systematic review. J Stroke Cerebrovasc Dis. 2010;19:410–416. [DOI] [PubMed] [Google Scholar]
- 43. de Jong-Hagelstein M, Kros L, Lingsma HF, et al. Expert versus proxy rating of verbal communicative ability of people with aphasia after stroke. J Int Neuropsychol Soc. 2012;18:1064–1070. [DOI] [PubMed] [Google Scholar]
- 44. Alyahya RSW, Halai AD, Conroy P, Lambon MA. A unified model of post-stroke language deficits including discourse production and their neural correlates. Brain. 2020;143:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hengst JA, Duff MC, Jones TA. Enriching communicative environments: Leveraging advances in neuroplasticity for improving outcomes in neurogenic communication disorders. Am J Speech Lang Pathol. 2019;28:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Särkämö T, Tervaniemi M, Laitinen S, et al. Cognitive, emotional, and social benefits of regular musical activities in early dementia: Randomized controlled study. Gerontologist. 2014;54:634–650. [DOI] [PubMed] [Google Scholar]
- 47. Kristinsson S, den Ouden DB, Rorden C, et al. Predictors of therapy response in chronic aphasia: Building a foundation for personalized aphasia therapy. J Stroke. 2022;24(2):189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teasell R, Mehta S, Pereira S, et al. Time to rethink long-term rehabilitation management of stroke patients. Top Stroke Rehabil. 2012;19:457–462. [DOI] [PubMed] [Google Scholar]
- 49. Kleber B, Veit R, Birbaumer N, et al. The brain of opera singers: Experience-dependent changes in functional activation. Cereb Cortex. 2010;20:1144–1152. [DOI] [PubMed] [Google Scholar]
- 50. Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33:628–635. [DOI] [PubMed] [Google Scholar]
- 51. Whitehead JC, Armony JL. Singing in the brain: Neural representation of music and voice as revealed by fMRI. Hum Brain Mapp. 2018;39:4913–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data reported in this manuscript are available from the corresponding author upon reasonable request and subject to approval by the appropriate regulatory committees and officials.