Abstract
The monarchE Cohort 1 patient population was enrolled based on high-risk clinicopathological features that can easily be identified as part of routine clinical breast cancer evaluation. Efficacy data from Cohort 1 demonstrate substantial evidence of benefit for adjuvant abemaciclib+ET in patients with HR+, HER2− early breast cancer at high risk of recurrence (ClinicalTrials.gov: NCT03155997 [monarchE]).
Keywords: abemaciclib, adjuvant, CDK4/6, early breast cancer, Ki-67
This article presents efficacy data in Cohort 1 of the monarchE study, a patient population that was enrolled based on high-risk clinicopathological features that can easily be identified during routine breast cancer evaluation, and highlights the evidence for the benefit of adding adjuvant abemaciclib to endocrine therapy for patients with node positive HR+, HER2- early breast cancer at a high risk of recurrence.
Introduction
The monarchE study achieved statistical significance for the primary endpoint of invasive disease-free survival (IDFS) at the second interim analysis (HR = 0.747, 95% CI 0.598, 0.932; P = .0096).1 With longer follow-up time, abemaciclib continued to demonstrate robust and clinically meaningful treatment benefit in IDFS and distant relapse-free survival (DRFS).2 Abemaciclib is the first and only CDK4 & 6 inhibitor approved for the adjuvant treatment of patients with HR+, HER2– node-positive, high-risk early breast cancer (EBC), with the specific indicated population varying by geography. Specifically, global regulators have granted approvals ranging from the intent to treat population to various subpopulations (Cohort 1, or Cohort 1 with Ki-67 index of ≥20%).1,2 Here we present efficacy data in Cohort 1, a monarchE subpopulation accounting for 91% of all enrolled patients and defined by high-risk clinicopathological features (Supplementary Fig. S1) that has been recently approved for the adjuvant treatment of patients with high risk EBC in Japan and Europe.3,4
Methods
Patients (n = 5637) in the monarchE (NCT03155997) phase III, global, clinical trial were randomly assigned (1:1) to receive ET ± abemaciclib for 2 years, with ET prescribed for at least 5 years. The detailed study design and statistical analyses were previously disclosed.1,2 The study included 2 cohorts. Patients in cohort 1 had high-risk features defined as: either ≥4 positive axillary lymph nodes (pALN), or 1-3 pALN and grade 3 disease and/or tumor ≥5 cm. Cohort 2, including patients with 1-3 pALN, grade <3, tumor <5 cm, and Ki-67 ≥20%, started enrollment later, and thus the data remain immature. Efficacy data in Cohort 1 presented here were evaluated at the additional follow-up 1 analysis (AFU1; data cutoff: 1 April 2021), at the request of global health authorities.
Results
Cohort 1 included 5120 patients; 2555 assigned to receive abemaciclib + ET and 2565 to ET alone. Baseline demographics and disease characteristics reflected the high risk clinicopathologic features defined for Cohort 1 (Table 1). Choice of initial ET was well balanced between treatment arms. The median follow-up time for Cohort 1 was 28 months, at which point over 90% of patients had completed the 2-year study treatment period (abemaciclib + ET: N = 1872; ET alone: N = 1883) or discontinued (abemaciclib + ET: N = 455; ET alone: N = 435).
Table 1.
Demographics and baseline characteristics in the monarchE Cohort 1 and intent-to-treat (ITT) populations.
| Category | Cohort 1 | ITT populationd | ||
|---|---|---|---|---|
| Abemaciclib + ET | ET alone | Abemaciclib + ET | ET alone | |
| N = 2555, n (%) a | N = 2565, n (%) a | N = 2808, n (%) a | N = 2829, n (%) a | |
| Age, years, median (range) | 51 (23-89) | 51 (22-86) | 51 (23-89) | 51 (22-86) |
| <65 | 2150 (84.1) | 2190 (85.4) | 2371 (84.4) | 2416 (85.4) |
| ≥65 | 405 (15.9) | 375 (14.6) | 437 (15.6) | 413 (14.6) |
| Female | 2535 (99.2) | 2553 (99.5) | 2787 (99.3) | 2814 (99.5) |
| Male | 20 (0.8) | 12 (0.5) | 21 (0.7) | 15 (0.5) |
| Hormone receptor status | ||||
| Estrogen receptor positive | 2537 (99.3) | 2548 (99.3) | 2786 (99.2) | 2810 (99.3) |
| Estrogen receptor negative | 13 (0.5) | 16 (0.6) | 16 (0.6) | 17 (0.6) |
| Progesterone receptor positive | 2208 (86.4) | 2226 (86.8) | 2426 (86.4) | 2456 (86.8) |
| Progesterone receptor negative | 268 (10.5) | 269 (10.5) | 298 (10.6) | 295 (10.4) |
| Menopausal statusb,c | ||||
| Premenopausal | 1108 (43.4) | 1112 (43.4) | 1221 (43.5) | 1232 (43.5) |
| Postmenopausal | 1447 (56.6) | 1453 (56.6) | 1587 (56.5) | 1597 (56.5) |
| Prior chemotherapyb | ||||
| Neoadjuvant chemotherapy | 932 (36.5) | 930 (36.3) | 1039 (37.0) | 1048 (37.0) |
| Adjuvant chemotherapy | 1500 (58.7) | 1504 (58.6) | 1642 (58.5) | 1647 (58.2) |
| No chemotherapy | 123 (4.8) | 131 (5.1) | 127 (4.5) | 134 (4.7) |
| Region b | ||||
| North American/Europe | 1323 (51.8) | 1330 (51.9) | 1470 (52.4) | 1479 (52.3) |
| Asia | 522 (20.4) | 523 (20.4) | 574 (20.4) | 582 (20.6) |
| Other | 710 (27.8) | 711 (27.7) | 764 (27.2) | 768 (27.1) |
| Positive axillary lymph nodes | ||||
| 0 | 6 (0.2) | 6 (0.2) | 7 (0.2) | 7 (0.2) |
| 1-3 | 873 (34.2) | 888 (34.6) | 1118 (39.8) | 1142 (40.4) |
| ≥4 | 1675 (65.6) | 1671 (65.1) | 1682 (59.9) | 1680 (59.4) |
| Histopathological grade at diagnosis | ||||
| Grade 1 | 186 (7.3) | 190 (7.4) | 209 (7.4) | 216 (7.6) |
| Grade 2 | 1181 (46.2) | 1193 (46.5) | 1377 (49.0) | 1395 (49.3) |
| Grade 3 | 1063 (41.6) | 1050 (40.9) | 1086 (38.7) | 1064 (37.6) |
| Grade cannot be assessed | 117 (4.6) | 122 (4.8) | 126 (4.5) | 141 (5.0) |
| Pathologic tumor size | ||||
| <2 cm | 676 (26.5) | 656 (25.6) | 781 (27.8) | 767 (27.1) |
| 2-5 cm | 1233 (48.3) | 1278 (49.8) | 1372 (48.9) | 1419 (50.2) |
| ≥5 cm | 600 (23.5) | 606 (23.6) | 607 (21.6) | 610 (21.6) |
| Ki-67 index | ||||
| <20% | 946 (37.0) | 968 (37.7) | 953 (33.9) | 974 (34.4) |
| ≥20% | 1017 (39.8) | 986 (38.4) | 1262 (44.9) | 1233 (43.6) |
| TNM stage (derivede) | ||||
| IA | 1 (0.0) | 0 (0.0) | 2 (0.1) | 1 (0.0) |
| IIA | 229 (9.0) | 248 (9.7) | 324 (11.5) | 353 (12.5) |
| IIB | 281 (11.0) | 286 (11.2) | 392 (14.0) | 387 (13.7) |
| IIIA | 1023 (40.0) | 1018 (39.7) | 1029 (36.6) | 1026 (36.3) |
| IIIB | 96 (3.8) | 84 (3.3) | 99 (3.5) | 88 (3.1) |
| IIIC | 915 (35.8) | 925 (36.1) | 950 (33.8) | 963 (34.0) |
Where values do not add up to 100%, remaining data are missing, unavailable, or could not be assessed.
Per interactive web response system.
Menopausal status is at the time of diagnosis and all males are considered postmenopausal.
Thirty-eight patients were found not to meet the high risk criteria and hence were considered ineligible but were included in the ITT population.
Derived TNM stage based on the pathological tumor size and number of positive lymph nodes following primary surgery.
Abbreviations: ET, endocrine therapy; n, number of patients; N, number of patients in population; TNM, tumor, node, metastasis.
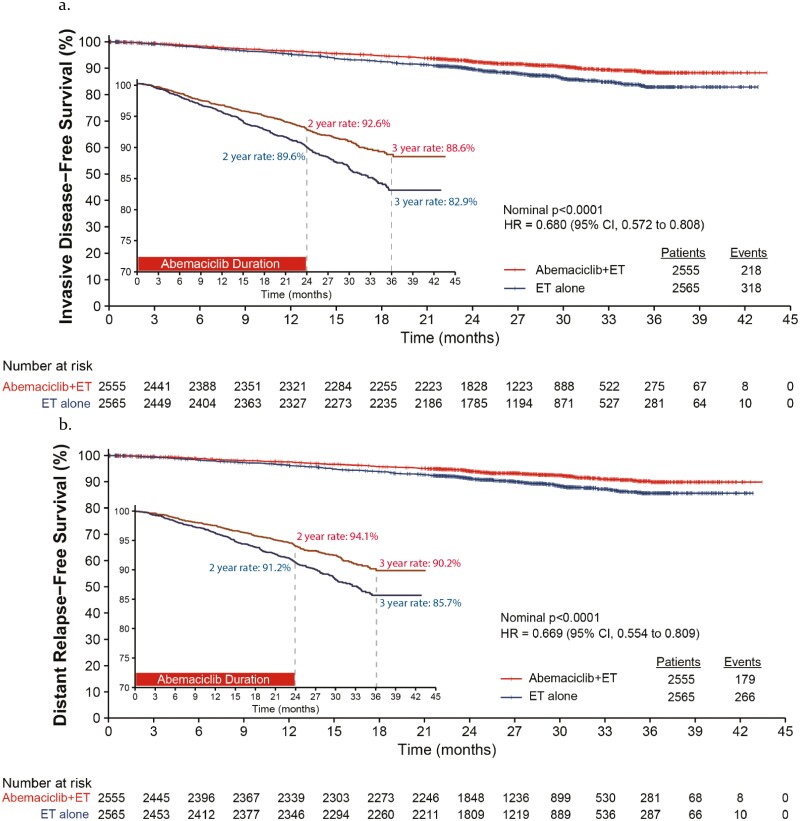
Abemaciclib + ET reduced the risk of developing an IDFS event by 32% (Fig. 1; HR = 0.680, 95% CI 0.572-0.808; nominal P-value < .0001) with an absolute improvement of 5.7% at 3 years. Additionally, there was a 33% reduction in the risk of developing a DRFS event (Fig. 1; HR = 0.669, 95% CI 0.554-0.809; nominal P-value < .0001) and a 4.5% absolute improvement at 3 years. Consistent treatment benefit in IDFS and DRFS was seen across subgroups of patient and disease characteristics (Supplementary Figs. S2, S3). Efficacy by initial ET is presented in Supplementary Table S1. The majority of the first recurrence events were distant recurrences and common sites included bone, liver, and lung, with fewer recurrences in the abemaciclib arm (Supplementary Table S2). An infographic summary of efficacy data for Cohort 1 is presented in Supplementary Fig. S1. Safety data, conducted on the safety population for the entire study were previously disclosed2,5 and consistent with the safety profile of abemaciclib.
Figure 1.
Invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS) in the Cohort 1 subpopulation at additional follow-up 1 (AFU1). (a) Kaplan-Meier curve of IDFS in Cohort 1. (b) Kaplan-Meier curve of DRFS in Cohort 1. A zoomed in version is provided within each Kaplan-Meier curve to allow better visualization of curve separation.
Discussion
The monarchE trial was designed to identify patients at a higher risk of developing recurrences during the first few years of adjuvant ET,6 using common pathologic features,7 and has proven that addition of abemaciclib to endocrine therapy (ET) can be effective in reducing the risk of recurrences, especially in bone and liver metastases. Consistent with this intent, the 17% risk of recurrence observed at 3 years in the control arm confirms the high-risk nature of this patient population, as supported by real-world data8 and highlights the need to improve curative intent treatment options for these patients. These data demonstrate clinically meaningful benefit in reducing the risk of developing invasive disease, and in particular, incurable and life-threatening metastatic disease. The robust treatment benefit in IDFS and DRFS observed within this subpopulation was consistent across subgroups including the initial ET patients received. Since the vast majority of the patients and the majority of the recurrence events come from Cohort 1, results in this subpopulation drove the robust efficacy results in the intent-to-treat population.
The benefit of abemaciclib when combined with standard-of-care ET in patients with HR+, HER2– EBC at a high risk of recurrence represents the first major advance in the treatment of these patients in almost 2 decades. Recent regulatory approvals based on the Cohort 1 population3 highlight the robust and clinically meaningful results in this subpopulation, allowing oncologists to easily identify patients with HR+, HER2− EBC at high risk of recurrence based on clinicopathological features who can benefit from the addition of abemaciclib.
Conclusion
In conclusion, adjuvant abemaciclib combined with ET demonstrated substantial benefit for patients with HR+, HER2− EBC at high risk of recurrence who were identified easily based on clinicopathological features that are commonly assessed as part of routine clinical care.
Supplementary Material
Acknowledgments
The authors sincerely thank the patients who participated in the trial, as well as their families, and all those who provided support to the participants. The authors thank the investigators and their support staff who participated in this work. The authors are very grateful for the time and efforts of the monarchE Executive and Steering Committees. The authors would like to thank Eglantine Julle-Daniere for her writing and editorial assistance.
Contributor Information
Masakazu Toi, Department of Breast Surgery, Kyoto University Hospital, Kyoto, Japan.
Frances Boyle, Patricia Ritchie Centre for Cancer Care and Research, Mater Hospital, Sydney, Australia; University of Sydney, Sydney, Australia.
Young-Hyuck Im, Division of Hematology/Medical Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Mattea Reinisch, Breast Unit, Kliniken Essen-Mitte, Essen, Germany.
David Molthrop, Hematology and Oncology, Florida Cancer Specialists & Research Institute, Orlando, FL, USA.
Zefei Jiang, Department of Breast Oncology, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China.
Ran Wei, Lilly Corporate Center, Eli Lilly and Company, Indianapolis, IN, USA.
Francisco Sapunar, Lilly Corporate Center, Eli Lilly and Company, Indianapolis, IN, USA.
Brenda R Grimes, Lilly Corporate Center, Eli Lilly and Company, Indianapolis, IN, USA.
Sarah Cassidy Nabinger, Lilly Corporate Center, Eli Lilly and Company, Indianapolis, IN, USA.
Stephen R D Johnston, Medical Oncology, Breast Unit, Royal Marsden NHS Foundation Trust, London, UK.
Funding
This study was sponsored by Eli Lilly and Company.
Conflict of Interest
Masakazu Toi reports grants and personal fees for research and honoraria from Chugai, Takeda, Pfizer, Kyowa-Kirin, Taiho, Eisai, Daiichi-Sankyo, AstraZeneca, Shimadzu, Yakult, and Nippon Kyaku; other fees for advisory role for drug development from Kyowa-Kirin and Daiichi-Sankyo; research grants from JBCRG association, Astellas, AFI Technologies, Shionogi, and GL Science; personal and other fees from Eli Lilly and Company for advisory role; personal fees for honoraria from MSD, Exact Science, and Novartis; personal fees and other for honoraria and advisory role from Konica Minolta, Bristol Myers Squibb; other fees for advisory role from Athenex Oncology, Bertis Terumo, and Kansai Medical Net; and serves as a board of director for JBCRG Association, Organisation for Oncology and Translational Research, and Kyoto Breast Cancer Research Network, outside the submitted work. Frances Boyle reports personal fees for honoraria and advisory role for Roche, Pfizer, Eli Lilly and Company, and Novartis outside the submitted work. Mattea Reinisch reports personal fees from AstraZeneca, MSD, Eli Lilly and Company, Roche, Daichi Sankyo, Seagen, and Somatex, as well as personal fees and non-financial support from Novartis and Pfizer, outside the submitted work. Ran Wei, Francisco Sapunar, Brenda R. Grimes, and Sarah Cassidy Nabinger are full-time employees of Eli Lilly and Company and/or Eli Lilly and company shareholders. Stephen R.D. Johnston reports personal fees and other from AstraZeneca, Eli Lilly and Company, Novartis, Pfizer, Eisai, and Roche/Genentech outside the submitted work. Young-Hyuck Im, David Molthrop, and Zefei Jiang indicated no financial relationships.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the online instructions.
References
- 1. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987-3998. 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571-1581. [DOI] [PubMed] [Google Scholar]
- 3. VERZENIO™ (abemaciclib) Pharmaceuticals and Medical Devices Agency FY (April 2021-March 2022). 2021. Japan: Pharmaceuticals and Medical Devices Agency (PMDA). [Google Scholar]
- 4. VERZENIOS™ (abemaciclib) European Medicines Agency. 2022. Amsterdam, Netherlands: European Medicines Agency. [Google Scholar]
- 5. Rugo HS, O’Shaughnessy J, Boyle F, et al. Adjuvant abemaciclib combined with endocrine therapy for high risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann Oncol. 2022;33(6):616-627. [DOI] [PubMed] [Google Scholar]
- 6. Forbes JF, Cuzick J, Buzdar A, et al. ; Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists' Group. Arimidex, Tamoxifen Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45-53. 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 7. Group EBCTC. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341-1352. [DOI] [PubMed] [Google Scholar]
- 8. Sheffield KM, Peachey JR, Method M, et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol. 2022;18(21):2667-2682. 10.2217/fon-2022-0310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the online instructions.