Abstract
Despite professional society guidelines recommending that obesity be treated as a chronic disease by emphasizing the use of lifestyle modification in conjunction with pharmacotherapy, antiobesity medications are uncommonly prescribed in most clinical practices. The recent Food and Drug Administration approval of semaglutide 2.4 mg weekly to treat obesity—as well as other forthcoming advancements in diabetes and antiobesity medications—highlights the potential of pharmacotherapy to significantly augment weight loss efforts. In this Expert Endocrine Consult, we review the evolving role of antiobesity pharmacotherapy in clinical practice and suggest a framework for the use of these medications.
Keywords: weight loss, antiobesity pharmacotherapy, obesity
The prevalence of obesity has trended upwards in recent decades in the United States. In 2017-2018, the adult obesity rate passed 40% nationally for the first time, and it is predicted to eclipse 50% of US adults by 2030 [1]. Obesity increases the risk for type 2 diabetes (T2DM), hypertension, cardiovascular disease (CVD), chronic kidney disease, osteoarthritis, nonalcoholic steatohepatitis (NASH), and death from several cancers [2–8].
The rise in obesity and its comorbidities highlights the need for improvements in obesity treatment. Despite the availability of antiobesity medications (AOMs) and data supporting their efficacy and safety, their rate of use is low, with less than 2% of patients who qualify for treatment receiving prescriptions for AOMs [9,10]. Possible explanations include physicians' lack of training in obesity management and use of AOMs, limited time during clinical visits, the high cost of many AOMs with limited insurance coverage, and hesitancy stemming from a history of failure of AOMs after regulatory approval [11].
Exciting advances in weight loss pharmacotherapy are taking place. The significantly greater efficacy of semaglutide and other emerging pharmacologic options compared with previously Food and Drug Administration (FDA)–approved AOMs highlights the need for clear guidelines and clinician and patient education regarding the potential for these therapies to significantly augment weight loss efforts. The purpose of this Expert Endocrine Consult is to introduce an updated approach to the medical treatment of obesity considering recent advances in the field of AOMs. This updated approach is aimed at both the endocrinologist and the primary care physician looking to expand their practice to include pharmacological treatment of obesity.
Clinical Case
A 55-year-old man with a past medical history of T2DM, obesity (body mass index [BMI] 35.5 kg/m2), and depression presents to your primary care clinic. He was diagnosed with T2DM 6 years ago. Current therapy includes semaglutide 1.0 mg weekly and metformin 1000 mg twice daily. He is also on paroxetine 30 mg daily for depression. Before starting semaglutide 1 year ago, his hemoglobin A1c was 8.8%. Since starting semaglutide, his hemoglobin A1C has decreased to 7.3% and he has lost 24 lbs, 10.9 kg. (−4.2% total body weight loss). He is very pleased with these results and is interested in losing more weight. He has worked on lifestyle changes including a calorie-restricted diet but has never enrolled in a structured weight loss program. Although he reports his depressive symptoms are well controlled on paroxetine, he identifies a lack of energy as a barrier to adhering to a daily exercise program. What pharmacologic options might be appropriate as a next therapeutic step?
Lifestyle
Lifestyle modification serves as the foundation for weight loss treatment. Comprehensive lifestyle interventions including a reduced calorie diet, increased physical activity, and behavioral support should be promoted and have been discussed in detail in several guidelines [12–14]. From a dietary perspective, several approaches have been studied, including low-fat, low-carbohydrate, low-glycemic-load, low-fat vegan-style, lacto-ovo-vegetarian, Mediterranean-style, and higher-protein diets, among others, without evidence of the superiority of one approach to another [15, 16]. Dietary interventions show efficacy over the short-term, with studies showing maximal weight loss at 6 months, with small losses maintained up to 2 years. Over the long-term, patients tend to regain most of the weight that was lost.
Intensive lifestyle interventions for patients with T2DM, including both group and individual counseling sessions, a reduced calorie intake with meal replacement products, and at least 175 minutes per week of moderate-intensity physical activity, have been shown to produce modest weight loss over the long-term (6% vs 3% in the placebo group in the 10-year Look AHEAD trial) [17]. However, even these intensive lifestyle interventions failed to maintain goal weight loss (>7%) over the long-term and have not been shown to change cardiovascular outcomes.
The challenges in maintaining long-term weight loss are due to compensatory biological mechanisms that decrease energy expenditure (out of proportion to reduction in body mass) and increase appetite in response to weight loss [18–22]. Because these changes persist after weight loss, lifestyle interventions often need to be augmented by therapies such as AOMs and bariatric surgery that target these compensatory mechanisms [23]. Pharmacotherapy for obesity should be considered for patients who have not met weight loss goals with lifestyle modification alone and in those who have lost weight but struggle to maintain their weight loss [24, 25].
Medications that Cause Weight Gain
In addition to lifestyle counseling, a central point of caring for patients with overweight or obesity involves reviewing their use of medications that promote weight gain and prescribing drugs that are weight neutral or that promote weight loss [24,26]. Common medication classes associated with weight gain include steroids, antipsychotics, antiepileptics, glucocorticoids, and gabapentinoid medications. These medications are often indicated and effective for treating their respective primary disorders. However, if patients experience significant weight gain on these medications, particularly to a degree where the negative effects of weight gain may exceed the positive treatment effects, clinicians should consider switching patients to alternative medications that are weight neutral or that promote weight loss. Adjustment of these medications should be made in a shared decision making process including the patient and prescribing provider (eg, psychiatry, neurology, or other specialists).
Type 2 Diabetes Mellitus
For patients with T2DM and overweight or obesity, clinicians should consider weight-losing medications (ie, metformin, sodium–glucose cotransporter 2 inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists) and weight-neutral medications (ie, dipeptidyl peptidase-4 inhibitors), and, when possible, avoid agents associated with weight gain such as pioglitazone, sulfonylureas, and insulin [14, 24, 27]. For patients requiring insulin therapy, there are strategies to mitigate insulin-associated weight gain. The addition of metformin and GLP-1 agonists has been shown to reduce or, in the case of GLP-1 agonists, even nullify insulin-associated weight gain [28–31]. Clinicians should add 1 of these 2 agents when starting a patient with T2DM on insulin therapy. Among insulin therapies, basal insulin is associated with less weight gain than biphasic or prandial short-acting insulin and should be the first-line option [32, 33].
Psychiatric Disorders
For patients with psychiatric disorders, such as major depression or bipolar disorder, clinicians should choose weight-neutral medications when appropriate. The antidepressant medications paroxetine, amitriptyline, and mirtazapine have been associated with weight gain and should be avoided in favor of bupropion and fluoxetine when possible as they are associated with weight loss [26, 34–36]. Atypical antipsychotics are the drug class associated with the most weight gain. Among this drug class, olanzapine, quetiapine, and risperidone are associated with the largest weight gain and should be avoided in patients with overweight or obesity when possible [26]. While no atypical antipsychotics are associated with weight loss, ziprasidone may be weight neutral and appears to be a better option for patients who are overweight and obese [37].
Anticonvulsants and Mood Stabilizers
For patients with seizure disorders, clinicians should engage in shared decision-making with patients to make an informed decision about antiepileptic drug (AED) choice. When appropriate, clinicians should avoid divalproex, carbamazepine, and gabapentin, which are all associated with weight gain. Conversely, zonisamide and topiramate are associated with weight loss and should be considered in patients with recent weight gain or if overweight, when appropriate. Lithium and lamotrigine appear to be weight neutral and should also be favored over AEDs associated with weight gain [26].
Inflammatory Rheumatic Diseases
Obesity is a common comorbidity in patients with inflammatory rheumatic diseases, with a hypothesized causal role due to the proinflammatory nature of adipose tissue [38, 39]. Patients with obesity have higher disease scores and are less likely to respond to treatment with disease-modifying antirheumatic drugs [40]. In patients with inflammatory rheumatic diseases, clinicians should minimize or avoid corticosteroids in favor of nonsteroidal anti-inflammatory drugs and disease-modifying antirheumatic drugs given the tendency of corticosteroids to promote weight gain [41, 42].
Antiobesity Pharmacotherapy
Pharmacotherapy is indicated as an adjunct to caloric restriction and physical activity in adults with a BMI ≥30 kg/m2 or BMI 27 to 29.9 kg/m2 with at least 1 weight-related comorbidity such as diabetes, hypertension, hyperlipidemia, obstructive sleep apnea, nonalcoholic fatty liver disease, or osteoarthritis. Most AOMs target the compensatory increase in appetite that occurs in the setting of weight loss. In counseling patients on AOMs, patients should be educated that the addition of AOMs to a lifestyle program enhances weight loss (as has been shown in many clinical trials) [43–48].
Overview of AOMs
Phentermine
Phentermine was the first FDA-approved AOM and is the most widely prescribed AOM in the United States. Phentermine acts as a sympathomimetic, increasing norepinephrine in the central nervous system, thereby suppressing appetite [49]. It was first approved in 1959 for short-term use for weight loss based on a 36-week trial that showed both continuous and intermittent administration of phentermine led to more weight loss than placebo (−12.2 kg, −13.0 kg, −4.8 kg, respectively) [50]. Two more recent randomized controlled trials from Korea have confirmed the efficacy of phentermine for short-term weight loss, both showing significant weight reduction compared with placebo over the course of 12 weeks [51, 52]. While phentermine is the only AOM that is not FDA-approved for long-term use (approval for ≤3 months), many patients are prescribed the medication long-term [9]. Available phentermine doses are 8 mg 3 times daily, and 15 mg, 30 mg, and 37.5 mg taken once daily.
Statistically significant adverse effects of phentermine in clinical trials include dry mouth (55%) and insomnia (34%), without significant differences in systolic or diastolic blood pressure, insomnia, headache, or palpitations between the treatment and placebo group [51]. Common reasons for discontinuation in the phentermine group included dry mouth and nausea/vomiting, although the phentermine group did not have a higher discontinuation rate than the control group. Although there are no long-term cardiovascular outcome studies assessing the safety of phentermine, a 2019 retrospective analysis of real-world data did not find an increase in cardiovascular risk with long-term use [53]. Phentermine is not recommended for patients with CVD and is contraindicated in patients with hyperthyroidism, glaucoma, or in patients taking monoamine oxidase (MAO) inhibitors. Relative contraindications include patients with poorly controlled hypertension or a history of addiction given its amphetamine-like properties.
Orlistat
Orlistat is an AOM that was FDA-approved for long-term use for weight management in 1999 and approved for over-the-counter use since 2007. Unlike other AOMs, orlistat does not act on appetite, but rather it inhibits triglyceride absorption in the gut. In one of the longest trials on orlistat, the 4-year XENDOS trial randomly assigned 3304 people to placebo or orlistat, with the orlistat-treated patients losing 11% of their bodyweight after 1 year compared with 6% in the placebo-treated group. Both groups experienced weight regain over the following 3 years, with the orlistat group losing 6.9% of their bodyweight at the end of 4 years compared with 4.1% for the placebo-treated group [54]. In addition to effects on weight, the study showed a reduced progression from prediabetes to diabetes in subjects treated with orlistat. Orlistat is available in 60-mg capsules over-the-counter and 120-mg capsules by prescription, both dosed 3 times daily.
Adverse effects of this medication are predictable based on its mechanism of action: gastrointestinal (GI) adverse effects including fatty/oily stool, fecal urgency, and oily spotting are common, with frequency rates ranging from 15% to 30% in most studies [55]. These GI adverse effects are the main reason for discontinuation in studies, with discontinuation rates of 5% in orlistat-treated patients (2% higher than placebo, 95% CI 1-3%). These adverse effects can be reduced by avoiding high-fat diets. It is recommended to supplement multivitamins and beta carotene due to the risk for malabsorption of fat-soluble vitamins.
Phentermine/Topiramate Extended Release
Phentermine can be combined with topiramate for additional weight loss effects. Topiramate is an AED that was FDA approved for seizures in 1996 and migraine prevention in 2004. Although its mechanism of action for weight loss is not entirely known, it is thought to suppress appetite by increasing dopamine release and inhibiting glutamate receptors, as well as modulating neuropeptide-Y, a hormone that increases food consumption [56]. Combined with phentermine, the 2 drugs have additive effects on weight loss. This combination was approved for use in 2012.
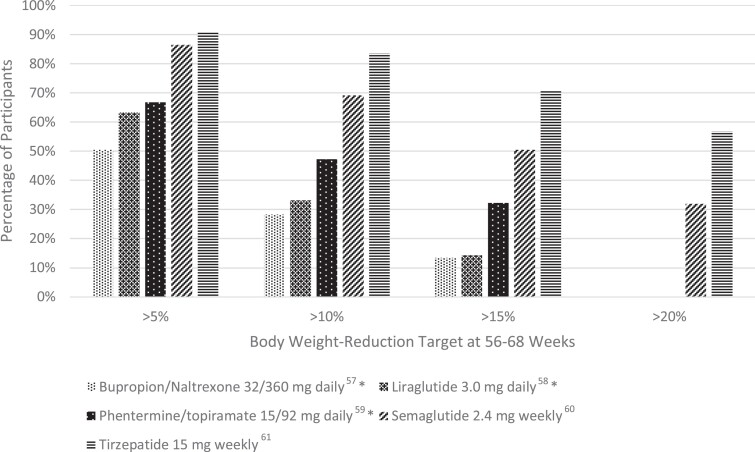
The CONQUER trial was a 56-week, phase 3 trial of 2487 adults who were overweight or obese (age 18-70 years, BMI 27-45 kg/m²) and 2 or more comorbidities (hypertension, dyslipidemia diabetes, or prediabetes, or abdominal obesity) [47]. Patients were randomized to placebo, once daily phentermine 7.5 mg/topiramate ER 46 mg, or once daily phentermine 15 mg/topiramate ER 92 mg in a 2:1:2 ratio. At 56 weeks, body weight change was −1.4 kg, −8.1 kg, and −10.2 kg, respectively. Five percent weight loss was achieved in 21% of patients with placebo, 62% of patients with phentermine 7.5 mg/topiramate 46 mg daily, and 70% of patients with phentermine 15 mg/92 mg daily; the corresponding numbers for 10% weight loss were 7%, 37%, and 48% (see Fig. 1).
Figure 1.
Percentage of study participants who met the following weight-reduction targets (>5, >10, >15, >20% weight loss) after 56-68 weeks of treatment, sorted by AOM. *Data are not available for >20% weight loss.
The most common adverse effects include dry mouth (13% for the 7.5/46 mg dose, 21% for the 15/92 mg dose), paresthesia (14%, 21% respectively), and constipation (15%, 17% respectively), as well as a small increase in heart rate (0.6-1.6 beats/minute higher than placebo). The CONQUER trial also showed a dose-related increase in anxiety, depression, and cognitive impairment. Similar to phentermine monotherapy, phentermine/topiramate ER is not recommended for patients with CVD and is contraindicated in patients with hyperthyroidism, glaucoma, or in patients taking MAO inhibitors.
Topiramate poses a risk for orofacial clefts in infants exposed in utero. Clinicians should be aware of the risk evaluation mitigation strategy for phentermine/topiramate ER that informs clinicians and patients of the potential for this teratogenic effect, the importance of pregnancy prevention in females of reproductive age receiving this medication, and the need to discontinue the medication immediately if pregnancy occurs.
Naltrexone/Bupropion Sustained Release
The combination tablet of bupropion and naltrexone has been FDA approved for weight loss since 2014. Bupropion is a reuptake inhibitor of dopamine and norepinephrine that is FDA approved for depression and smoking cessation. Among antidepressants, it is least likely to produce weight gain. Naltrexone is an opioid antagonist approved for the treatment of opioid and alcohol dependence. While naltrexone as monotherapy does not appear to cause weight loss, when combined with bupropion it appears to diminish the mu-opioid receptor autoinhibitory feedback loop on anorexigenic hypothalamic neurons activated by bupropion, leading to reduced food intake and weight loss [57, 58]. The initial dose is 1 tablet (8 mg naltrexone and 90 mg of bupropion) daily for 1 week, which over the course of 4 weeks is increased stepwise up to the treatment dose of 2 tablets twice daily (32 mg naltrexone and 360 mg bupropion). Typical weight loss seen with this combination drug is in the 5% to 6% range [59].
The cardiovascular safety of naltrexone/bupropion SR combination was tested in the LIGHT trial, a randomized, placebo-controlled, noninferiority, cardiovascular outcomes trial. The trial was terminated prematurely after the study sponsor publicly released confidential favorable interim results after only 25% of expected vascular events had accrued, making it difficult to interpret the cardiovascular safety of this combination drug [60].
Statistically significant adverse effects of bupropion/naltrexone include nausea (30%), headache (14%), and constipation (15%), without significant differences in depression or suicidality events, insomnia, dizziness, or dry mouth between the treatment and placebo groups [57, 59]. The most common adverse effects leading to discontinuation were nausea (6%) and headache (2.6%) [57]. Contraindications include pregnancy, uncontrolled hypertension, seizure disorder, eating disorder, chronic opioid use, severe hepatic dysfunction, and in patients taking MAO inhibitors.
Liraglutide 3.0 mg Daily
Liraglutide was the first GLP-1 agonist to be approved for weight loss treatment in December 2014. GLP-1 agonists promote weight loss by delaying gastric emptying and suppressing subjective hunger and food intake [61]. The SCALE Obesity and Prediabetes and SCALE Diabetes were both 56-week, randomized, double-blind, placebo-controlled, clinical trials examining the effect of liraglutide 3.0 mg daily on patients with normoglycemia, prediabetes, and diabetes. Both trials demonstrated significantly greater weight loss with liraglutide. In SCALE Obesity and Prediabetes, weight loss was −8.0 ± 6.7% with liraglutide vs −2.6 ± 5.7% with placebo (P < .001), and in SCALE Diabetes weight loss was 6.0% with liraglutide vs 2.0% with placebo (P < .001) [62, 63]. In the former trial, more participants in the liraglutide group achieved a weight reduction of ≥5% (63.2 vs 27.1%), ≥10% (33.1 vs 10.6%), and ≥15% (14.4 vs 3.5%) (see Fig. 1) [62]. Liraglutide is administered subcutaneously once daily, with an initial dose of 0.6 mg daily for 1 week with weekly increases in dose (1.2, 1.8. 2.4, 3 mg) to the recommended 3.0 mg dose.
Similar to other GLP-1 agonists, GI adverse effects are common, including nausea (40%), diarrhea (20%), constipation (20%) and vomiting (16%). GI events were the most common reason for discontinuation in the liraglutide group (6.4% vs 0.7% in placebo group). Serious but less common adverse effects include gallbladder disease (2.5%) and pancreatitis (0.4%). While liraglutide was associated with benign and malignant thyroid C-cell tumors in rodent studies, human trials have not shown evidence of these tumors.
Semaglutide 2.4 mg Weekly
Semaglutide is the newest FDA-approved GLP-1 agonist for chronic weight management. FDA approval was based on results from the STEP trials (see Table 1). In the STEP 1 trial, a total of 1961 adults with obesity or overweight with a weight-related comorbidity (excluding patients with diabetes) were randomized to semaglutide 2.4 mg weekly or placebo as well as lifestyle intervention [64]. The study found the mean change in body weight from baseline to week 68 was −14.9% for the semaglutide group and −2.4% for the placebo group, for an estimated treatment difference of −12.4% (P < .001). When compared with placebo, more participants in the semaglutide group achieved a weight reduction of ≥5% (86.4 vs 31.5%), ≥ 10% (69.1 vs 12.0%), ≥ 15% (50.5 vs 4.9%), and ≥20% (32.0 vs 1.7%) (see Fig. 1). Among participants with prediabetes at baseline, 84% reverted to normoglycemia in the intervention group vs 38% in the control.
Table 1.
Summary of STEP trials
| Trial | Population | Intervention | Main outcomes |
|---|---|---|---|
| STEP 1 [64] | 1961 adults with BMI >30 or ≥27 with ≥1 weight-related condition who did not have diabetes | Randomized to semaglutide 2.4 mg weekly vs placebo for 68 weeks. Assigned to intervention or placebo in 2:1 ratio. + counseling on lifestyle for both groups |
Mean weight change at 68 weeks: −14.9% with semaglutide vs −2.4% with placebo. 5% or more weight loss: 86.4% vs 31.5% 10% or more weight loss: 69.1% vs 12.0% 15% or more weight loss: 50.5% vs 4.9% |
| STEP 2 [65] | 1210 adults with type 2 diabetes and obesity | Randomized to semaglutide 2.4 mg vs semaglutide 1.0 mg vs placebo in a 1:1:1 fashion for 68 weeks + counseling on lifestyle for both groups |
Mean weight change at 68 weeks: −9.6% with semaglutide 2.4 mg vs −7.0% with semaglutide 1 mg vs −3.4% with placebo |
| STEP 3 [66] | 611 adults with BMI >30 or ≥27 with ≥1 weight-related condition, without diabetes | Randomized to semaglutide 2.4 mg weekly vs placebo as an adjunct to intensive behavioral therapy (ie, initial low-calorie diet × 8 weeks and 30 counseling sessions) | Mean weight change at 68 weeks: −16.0% semaglutide vs −5.7% placebo 5% or more weight loss: 86.6% vs 47.6% 10% or more weight loss: 75.3% vs 27.0% 15% or more weight loss: 55.8% vs 13.2% |
| STEP 4 [67] | 902 adults with BMI >30 or ≥27 with 1 weight-related co-morbidity, without diabetes | All trial participants were initially started on semaglutide and titrated up to 2.4 mg weekly. At 20 weeks, the 803 participants who reached 2.4 mg weekly were randomized (2:1) to 48 weeks of continued subcutaneous semaglutide (n = 535) or switched to placebo (n = 268), plus lifestyle intervention in both groups. | Mean weight loss after 20-week run-in period was 10.8% Mean weight change from week 20 to week 68: −7.9% with continued semaglutide, +6.9% with switch to placebo (−14.8% difference) |
| STEP 5 [68] | 304 adults with BMI >30 or ≥27 with 1 weight-related comorbidity, without diabetes | Randomized to semaglutide 2.4 mg weekly vs placebo for 104 weeks | Mean weight loss from baseline to week 104 was −15.2% in the semaglutide group vs −2.6% with placebo, an estimated treatment difference of −12.6% |
| STEP 6 [69] | 401 adults from East Asia with BMI >30 or ≥27 with 1 weight-related comorbidity, with or without diabetes | Randomized to 1 of 3 arms: semaglutide 2.4 mg weekly, semaglutide 1.7 mg weekly, or placebo for 68 weeks | Mean weight change at 68 weeks: −13.2% semaglutide 2.4 mg weekly, −9.6% semaglutide 1.7 mg weekly, and −2.1% placebo |
| STEP 7 | Planned enrollment of 375 adults with BMI >30 or ≥27 with 1 weight-related co-morbidity, without diabetes | 44-week trial of semaglutide 2.4 mg weekly vs placebo | Not yet published. Will measure percent change in body weight from baseline |
| STEP 8 [70] | 338 adults with BMI >30 or ≥27 with 1 weight-related comorbidity, without diabetes | Randomized 3:1 and 3:1 for 68 weeks to semaglutide 2.4 mg weekly vs matched placebo, or to liraglutide 3.0 mg weekly vs matched placebo | Mean weight change at 68 weeks: −15.8% semaglutide vs −6.4% liraglutide vs −1.9% pooled placebo |
Abbreviations: BMI, body mass index; STEP, Semaglutide Treatment Effect in People with Obesity.
The STEP 4 trial demonstrated the beneficial effect of long-term treatment with semaglutide for weight loss [67]. The trial included a 20-week lead-in period in which all trial participants received semaglutide 2.4 mg weekly, followed by a 48-week period where the intervention group continued semaglutide 2.4 mg weekly and the control group received a placebo. The semaglutide group continued to lose and then sustained weight loss over the following 48 weeks, whereas the placebo group regained weight and had worsening of cardiovascular risk factors. Semaglutide is administered subcutaneously once weekly, with an initial dose of 0.25 mg daily once weekly for 4 weeks, with the dose increased at 4-week intervals (0.5, 1.0, 1.7, 2.4 mg) to the recommended 2.4 mg dose.
Like other GLP-1 agonists, GI adverse effects are common, occurring more frequently in participants receiving semaglutide than those receiving placebo in the STEP 1 trial (74.2 vs 47.9%). GI events were the most common reason for discontinuation in the semaglutide group (4.5% vs 0.8% in placebo group). Serious but less common adverse effects include gallbladder disease (2.6%) and pancreatitis (0.2%). There was no difference between treatment and placebo groups in the incidence of benign and malignant neoplasms.
Other Medications that Produce Weight Loss
Setmelanotide is a selective agonist of the melanocortin-4-receptor (MC4R) that was FDA approved in November 2020 for the treatment of monogenic obesity due to pro-opiomelanocortin, leptin receptor deficiency, or proprotein convertase subtilisin/kexin type 1 deficiency in individuals ages 6 or older, and was approved in June 2022 for Bardet–Beidel syndrome. The mechanism of action involves the binding of leptin to its receptor, which leads to agonism of the MC4R through the cleavage of peptides. Genetic mutations in this pathway lead to hyperphagia, impaired pubertal development, obesity, and insulin resistance. Setmelanotide acts as an agonist to the MC4R, bypassing the effects of these genetic mutations. A recent trial showed a mean weight loss of 25.6% among individuals with pro-opiomelanocortin deficiency and 12.5% among those with leptin receptor deficiency [71].
Zonisamide is another AED that can produce modest weight loss, although it is not FDA approved for this indication. Studies ranging from 16 weeks to 1 year show 6% to 7% weight loss for zonisamide 400 mg daily [72, 73]. Common reported adverse effects include nausea/vomiting, headaches, anxiety, impaired memory, and language problems.
Recommended Approach to AOMs
Considerations When Selecting an AOM
Unfortunately, medication cost and insurance coverage are the primary drivers in selecting AOMs for an individual patient (see Table 2 for average medication costs). A 2018 review paper examined 136 marketplace health insurance plans, finding only 11% had coverage for AOMs [74]. Medicare excludes drug therapy for obesity, and only 7 state Medicaid programs have AOM coverage. A recent opinion article by Roser et al posits that, with the annual cost of overweight and obesity estimated at $990 billion per annum, it likely would be more cost-effective for insurance companies to cover AOMs than to continue the current limited coverage of obesity therapies [75, 76]. Patient advocacy groups are currently lobbying insurers to pay for AOMs and lobbying Congress to pass a bill (the Treat and Reduce Obesity Act of 2021) that would require Medicare to pay for AOMs [77, 78].
Table 2.
Summary of FDA-approved weight loss medications
| Agent | Mechanism of action | Typical maintenance dose | Average price for 30-day supplya | Weight lossb | Key points |
|---|---|---|---|---|---|
| Phentermine | Norepinephrine release | 8-37.5 mg daily | $11.31 ($37.21) | 3-7% | Approved for short-term use |
| Orlistat | Lipase inhibitor | 60 mg TID (OTC); 120 mg TID (Rx) | OTC (Alli): ∼$45; Rx (Xenical): $808.06 ($688.71) | 3-4% | Available over-the-counter, GI side effects |
| Naltrexone ER/bupropion ER | Opioid antagonist/antidepressant (dopamine) | 16 mg/180 mg BID | $308 ($258.86) | 5-6% | Intermediate in effectiveness and side effects (Nausea/vomiting) |
| Liraglutide 3.0 mg | GLP-1 receptor agonist | 3.0 mg daily | $1064.86 ($1170.80) | 6-7% | Intermediate effectiveness and side effects, possible CVD benefit, very high cost |
| Phentermine/topiramate ER | Sympathomimetic/GABA | 7.5-15 mg/46-92 mg daily | $231.07 ($192.81) | 8-11% | Highly effective, intermediate side effects |
| Semaglutide 2.4 mg | GLP-1 receptor agonist | 2.4 mg weekly | $1576.73 ($1374.27) | 14-15% | Best-in-class effectiveness, CVD benefit, very high cost |
Average retail price (discounted price) as listed on GoodRx the week of 18 July 22.
1-year efficacy, difference vs placebo (P < .05 for all).
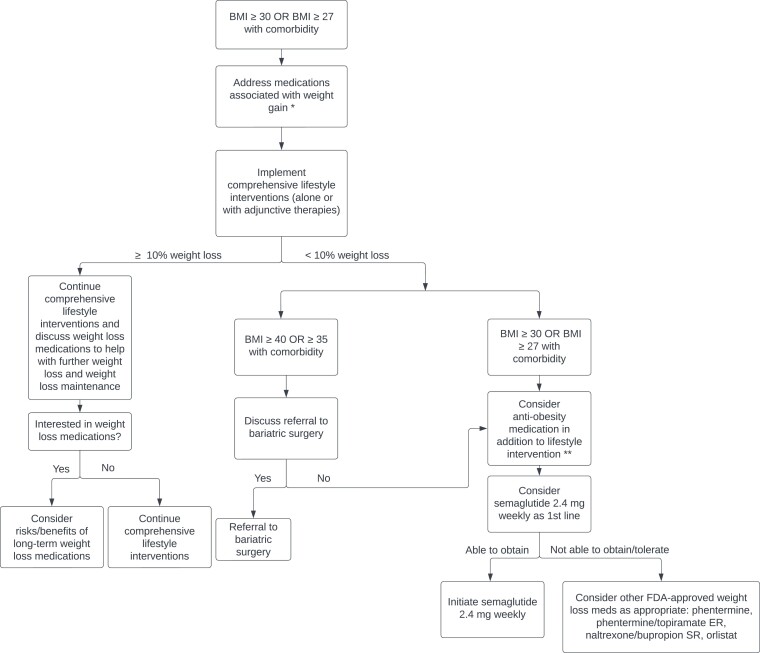
Ideally, cost would not play a significant role in AOM selection, and clinicians would primarily consider the degree of weight loss desired and the effect on comorbid conditions when selecting an AOM. Guidelines produced by the American Heart Association, American College of Cardiology and The Obesity Society (AHA/ACC/TOS), and the Endocrine Society both recommend a goal of ≥5% weight loss [12, 24]. However, data show that higher magnitudes of weight loss (≥10%) lead to greater and more clinically meaningful improvements in weight-related comorbidities, including greater relative risk reduction for cardiovascular events, improvements in NASH histology, decreased disease activity in patients with inflammatory rheumatic disease, and improvements in osteoarthritis, obstructive sleep apnea, and cancer risk [79–83]. With recent data showing a significantly greater degree of weight loss with semaglutide (∼15%% vs 5-10% for the other FDA-approved AOMs, with 69% of participants achieving ≥10% weight loss and 50% of participants achieving >15% weight loss), we recommend semaglutide 2.4 mg weekly as the first line AOM for obesity management (see Fig. 2). In addition, we propose that weight loss goals for most individuals with obesity should be ≥10%, as this is now achievable with current AOMs.
Figure 2.
Recommended approach to initiating AOM therapy in patients with BMI ≥30 kg/m2 or ≥27 kg/m2 with a weight-related comorbidity. *For treatment strategies specific to patients with T2DM, please see “Medications that Cause Weight Gain” “Type 2 Diabetes Mellitus”. **Engage in shared decision-making, considering patient-specific factors such as preferences on modes of delivery.
Practical Tips for Success With GLP-1 Agonists
When starting GLP-1 agonists, there are several strategies that promote success and decrease risk of discontinuation. Strategies to minimize adverse effects include slow dose escalation, counseling on expected adverse effects and their duration, and utilizing a multidisciplinary team approach (primary care provider, pharmacists, nurses, and medical assistants) to provide regular follow-up and guidance as patients initiate the medication. It is particularly important to discuss GI adverse effects, as patients who are not expecting these adverse effects may prematurely discontinue the medication.
Routine follow-up can come in many forms, including virtual visits, phone calls, pharmacist check-ins, or even portal messages at routine intervals. This type of follow-up can increase communication with the patient, normalizing adverse effects and allowing tighter dose titration, while also reducing the number of clinical visits a patient has to make, thereby reducing primary care provider burden and overall health care costs. Other strategies include a dose escalation period, with 1-week dose pauses when adverse effects are encountered, which may minimize nausea/vomiting. GI adverse effects may also be reduced by avoiding high fat foods and focusing on small meals.
Future Directions in Antiobesity Pharmacotherapy
Tirzepatide
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide and GLP-1 receptor agonist that was FDA approved for T2DM in May 2022. Two sets of trials have examined the efficacy of tirzepatide in treating T2DM and reducing body weight. The SURPASS trials examined the effect of tirzepatide in patients with T2DM. SURPASS-1 compared 3 different doses of tirzepatide (5 mg, 10 mg, 15 mg) to placebo for 40 weeks, finding significant mean hemoglobin A1C (−1.87%, −1.89%, −2.07%) and body weight reductions (−7.9%, −9.3%, −11.0%) for all tirzepatide doses compared with the placebo group [84]. SURPASS-2 compared the same doses of tirzepatide (5 mg, 10 mg, 15 mg) with semaglutide 1.0 mg weekly, finding more effective and dose-dependent reductions in body weight, blood pressure, and hemoglobin A1C [85].
The recently published SURMOUNT-1 trial examined the efficacy of tirzepatide in producing weight loss in patients with obesity who do not have diabetes [86]. The phase 3, double-blind, randomized controlled trial assigned 2539 patients in a 1:1:1:1 ratio to receive weekly tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. Average weight loss at week 72 was of an unprecedented magnitude: −15.0% for the 5-mg weekly dose, −19.5% for the 10-mg dose, −20.9% for the 15-mg dose, and −3.1% for the placebo (P < .001 for all comparisons with placebo). Notably, 50% and 57% of participants in the 10- and 15-mg groups had a reduction in body weight of 20% or more, compared with 3% in the placebo group (P < .001 for all comparisons with placebo) (see Fig. 1).
Together, these trials show promise for tirzepatide as an effective and safe medication for both weight reduction and glycemic control in patients with obesity and/or T2DM. Typical adverse effects are similar to GLP-1 agonists and include nausea, vomiting, and diarrhea. No clinically significant hypoglycemia was reported in any of the trials.
Cagrilintide
Cagrilintide, a long-acting amylin analogue, is another emerging AOM. The medication mimics natural amylin, a pancreatic hormone that induces satiety. When used alone at higher doses of 2.4 and 4.5 mg weekly, resulting weight loss was 9.7% and 10.8% at 26 weeks, respectively, compared with 3.0% with placebo and 9.0% with liraglutide 3.0 mg/day [87]. Common adverse effects included nausea, diarrhea, and injection site reactions. Cagrilintide also shows promise as an adjunct to current AOMs; recent phase 1b trials show promising results for cagrilintide (0.16-4.5 mg weekly) combined with semaglutide 2.4 mg weekly when compared with placebo combined with semaglutide 2.4 mg weekly [88]. Larger trials of this combination are planned.
Emerging Therapies
Several medications with multi-receptor action are under development and in early trials, including oxyntomodulin, cotadutide, and retatrutide. Oxyntomodulin is a peptide hormone that activates both the glucagon-like peptide-1 receptor and the glucagon receptor and, when administered exogenously, can improve glucose tolerance and result in weight loss [89]. Phase 1b trials have shown favorable safety profiles and body weight and glucose-lowering effects using synthetic peptide analogues of oxyntomodulin [90]. Cotadutide is another GLP-1 and glucagon receptor agonist with a similar safety and efficacy profile currently undergoing phase 2 trials [91]. Retatrutide is a “triple-G agonist” (GLP-1, glucagon receptor, and glucose-dependent insulinotropic polypeptide receptor agonist) that is currently undergoing 2 phase 2 trials, 1 for adults with overweight or obesity and 1 for adults with T2DM. Phase 1 trials showed the drug was well tolerated with promising weight loss after 12 weeks of treatment in patients with T2DM [92].
Weight Loss Maintenance
The AHA/ACC/TOS guidelines outline 4 pillars of weight maintenance therapy:
monthly or more frequent face-to-face or telephone contact [12, 93];
200 to 300 minutes of physical activity per week;
weekly or more frequent monitoring of body weight;
continued consumption of a reduced-calorie diet.
Frequent Face-to-Face or Telephone Contact
Forms of frequent face-to-face or telephone contact with medical professionals, such as monthly multidisciplinary programs focusing on lifestyle change, can prevent weight regain [93]. Evidence supports the aphorism coined by Hall and Kahan [94]: “long term benefits require long term attention.” Continuing a comprehensive weight loss program (on site or by telephone) for periods of up to 2.5 years after initial weight loss reduction reduces weight regain, as compared with the provision of minimal intervention (ie, usual care) [94]. Example programs include community weight management groups like the Diabetes Prevention Program and commercial programs like Weight Watchers or Jenny Craig.
Physical Activity for Weight Maintenance
Physical activity plays an important role in mitigating weight regain after weight loss, more so than for initial weight loss [20, 95]. However, a significant quantity is necessary—evidence shows that high-volume exercise (>1800-2500 kcal per week) is associated with sustained weight loss [96–98]. The type of exercise (aerobic vs resistance training) appears to matter less than the quality, although aerobic exercise may have a small, if clinically insignificant, advantage over resistance training [99].
Frequent Monitoring of Body Weight
Self-weighing is associated with weight loss maintenance, with consistent self-weighing (≥6 or all 7 days/week) associated with successful weight loss maintenance [100]. Successful weight loss maintainers use self-weighing as a method to set goals and acceptable boundaries for weight variation and to prompt small corrective actions for slight weight regain before significant weight gain has occurred [101]. Other themes from qualitative studies highlight self-weighing as a tool that fosters a sense of remaining on track, reinforces healthy habits, and serves as a prompt for clear relapse protocols [102].
Continuing Dietary Changes and AOMs
If weight loss is successful with pharmacotherapy, it is recommended to continue AOMs long-term to ameliorate comorbidities and augment adherence to behavioral change [24]. Crossover trials of AOMs have shown that the weight loss effects of these medications attenuate if discontinued, like medications for other chronic disease like hypertension and T2DM [103, 104].
Patients should be encouraged to sustain the dietary changes that led to initial weight loss. Other strategies that have been associated with weight maintenance include higher dietary protein intake—which may increase satiety and possibly increase overall energy expenditure—and adhering to a diet that aligns with a patient's food preferences [105–108].
Bariatric Surgery
For a subset of bariatric surgery patients, lifestyle change may not be sufficient to prevent weight regain and worsening of obesity-related comorbidities like T2DM. For this subset of patients, pharmacotherapy may attenuate weight regain and manage hyperglycemia [109, 110]. Several AOMs, including phentermine (alone or with topiramate), sodium–glucose cotransporter 2 inhibitors, and GLP-1 agonists have been shown to promote weight loss and reduction in hemoglobin A1C in patients with T2DM after bariatric surgery [111–116]. These therapies should be considered in patients who have not achieved goal weight reduction or are experiencing weight regain after bariatric surgery.
Weight Loss Maintenance—Final Thoughts
For clinicians, it is important to acknowledge that weight management is a lifelong challenge and to be prepared to assist patients with addressing small weight gains before they become larger ones, emphasizing the above strategies.
Clinical Case Resolution
This patient with T2DM and obesity has several possible options for AOM therapy. His current GLP-1 agonist, semaglutide 1.0 mg weekly, provides some weight loss benefit (−6.0 kg in SUSTAIN FORTE) [117] but there are AOMs that could be added to his regimen for greater weight loss potential. For instance, recently available semaglutide 2.0 mg weekly for T2DM has slightly greater weight loss than 1.0 mg weekly (−6.9 kg vs −6.0 kg, respectively) [117]. This dose increase could be considered to augment his weight loss, although it is not clear from available trial data how likely it is that he might reach his ≥10% weight loss goal. You check with your pharmacist, who finds the patient's insurance will not cover semaglutide 2.4 mg or a switch to tirzepatide.
In this scenario, a good option would be phentermine/topiramate, which has been shown to result in 7% to 11% weight loss [47]. This patient does not have a history of CVD or hypertension. His depression is well controlled and he denies suicidal thoughts. You decide to start him on phentermine/topiramate ER 7.5/46 mg daily. You discuss with the patient that AOM therapy should be done in conjunction with a comprehensive lifestyle intervention, focusing on sustainable physical activity with a goal of at least 150 minutes per week, as well as dietary changes that increase protein and fiber intake and decrease processed food. After discussions with the patient, he decides to try Weight Watchers.
You also re-evaluate his antidepressant paroxetine, which is associated with weight gain and anticholinergic effects that may contribute to his lower energy levels. More activating antidepressants associated with weight loss include bupropion and fluoxetine. While bupropion has more data regarding weight loss, it has sympathomimetic properties similar to phentermine that may increase the risk of hypertension, palpitations, and tachyarrhythmias when taken together. You decide to switch the patient from paroxetine to fluoxetine.
At 16-week follow-up he has achieved an additional 4% body weight loss on phentermine/topiramate ER 7.5/46 mg daily (−8.2% overall since starting semaglutide for glycemic control), so you continue at this dose. At 6-month follow-up, he reports he has lost 7 kg (−9.3%) body weight. You congratulate him on his success and encourage continued sustainable lifestyle changes, emphasizing the importance of finding an exercise program that he enjoys and can continue long-term. You schedule follow-up in another 6 months.
Conclusion
Exciting recent advancements in the field of AOMs brings hope for a new paradigm in medical obesity management. With the emergence of more effective medications, we have proposed an updated goal of ≥10% weight loss as the threshold to gauge success with AOM treatment. Further work is needed to make these drugs more affordable and easily implementable, and to study their use over longer time periods. With further research, clinician education, and advocacy regarding AOM accessibility, the above paradigm can become widely implemented.
Abbreviations
- AED
antiepileptic drug
- AOM
antiobesity medication
- BMI
body mass index
- CVD
cardiovascular disease
- FDA
Food and Drug Administration
- GI
gastrointestinal
- MAO
monoamine oxidase
- MC4R
melanocortin-4-receptor
- NASH
nonalcoholic steatohepatitis
- T2DM
type 2 diabetes
Contributor Information
Connor Enright, Department of Medicine, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Elizabeth Thomas, Division of Endocrinology, Metabolism and Diabetes, University of Colorado School of Medicine, Aurora, CO 80045, USA; Endocrinology Section, Rocky Mountain Veterans Affairs Medical Center, Aurora, CO 80045, USA.
David R Saxon, Email: david.saxon@cuanschutz.edu, Division of Endocrinology, Metabolism and Diabetes, University of Colorado School of Medicine, Aurora, CO 80045, USA; Endocrinology Section, Rocky Mountain Veterans Affairs Medical Center, Aurora, CO 80045, USA.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Funding
None
Disclosure Statement
The authors have nothing to disclose.
References
- 1. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–2450. [DOI] [PubMed] [Google Scholar]
- 2. Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8(7):e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang L, Rong J, Wang Y, et al. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78(2):150–155. [DOI] [PubMed] [Google Scholar]
- 6. Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes. 2018;11:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med. 2017;15(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saxon DR, Iwamoto SJ, Mettenbrink CJ, et al. Antiobesity medication use in 2.2 million adults across eight large health care organizations: 2009-2015. Obesity. 2019;27(12):1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Claridy MD, Czepiel KS, Bajaj SS, Stanford FC. Treatment of obesity: pharmacotherapy trends of office-based visits in the United States from 2011 to 2016. Mayo Clin Proc. 2021;96(12):2991–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. [DOI] [PubMed] [Google Scholar]
- 12. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joseph JJ, Deedwania P, Acharya T, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation. 2022;145(9):e722–e759. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Introduction: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S1–S2. [DOI] [PubMed] [Google Scholar]
- 15. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. [DOI] [PubMed] [Google Scholar]
- 17. Hill JO, Horton ES, Hubbard VS, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldsmith R, Joanisse DR, Gallagher D, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R79–R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–912. [DOI] [PubMed] [Google Scholar]
- 20. Pasman WJ, Saris WH, Muls E, Vansant G, Westerterp-Plantenga MS. Effect of exercise training on long-term weight maintenance in weight-reduced men. Metabolism. 1999;48(1):15–21. [DOI] [PubMed] [Google Scholar]
- 21. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. [DOI] [PubMed] [Google Scholar]
- 22. Leibel RL, Seeley RJ, Darsow T, Berg EG, Smith SR, Ratner R. Biologic responses to weight loss and weight regain: report from an American Diabetes Association research symposium. Diabetes. 2015;64(7):2299–2309. [DOI] [PubMed] [Google Scholar]
- 23. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. [DOI] [PubMed] [Google Scholar]
- 24. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. [DOI] [PubMed] [Google Scholar]
- 25. Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14(1):12–24. [DOI] [PubMed] [Google Scholar]
- 26. Domecq JP, Prutsky G, Leppin A, et al. Drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(2):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goudswaard AN, Furlong NJ, Rutten GE, Stolk RP, Valk GD. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2004;2004(1):CD003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia. 1999;42(4):406–412. [DOI] [PubMed] [Google Scholar]
- 30. Chow CC, Tsang LW, Sorensen JP, Cockram CS. Comparison of insulin with or without continuation of oral hypoglycemic agents in the treatment of secondary failure in NIDDM patients. Diabetes Care. 1995;18(3):307–314. [DOI] [PubMed] [Google Scholar]
- 31. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103. [DOI] [PubMed] [Google Scholar]
- 32. Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–1730. [DOI] [PubMed] [Google Scholar]
- 33. Raslová K, Tamer SC, Clauson P, Karl D. Insulin detemir results in less weight gain than NPH insulin when used in basal-bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index. Clin Drug Investig. 2007;27(4):279–285. [DOI] [PubMed] [Google Scholar]
- 34. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–1272. [DOI] [PubMed] [Google Scholar]
- 35. Rosenzweig-Lipson S, Beyer CE, Hughes ZA, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113(1):134–153. [DOI] [PubMed] [Google Scholar]
- 36. Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17(S1):S37–S41. [DOI] [PubMed] [Google Scholar]
- 37. Komossa K, Rummel-Kluge C, Hunger H, et al. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009; (4):CD006569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu B, Hiraki LT, Sparks JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheumat Dis. 2014;73(11):1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Hair MJ, Landewé RB, van de Sande MG, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis. 2013;72(10):1654–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lupoli R, Pizzicato P, Scalera A, et al. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta-analysis. Arthritis Res Ther. 2016;18(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Da Silva JA, Jacobs JW, Bijlsma JW. Revisiting the toxicity of low-dose glucocorticoids: risks and fears. Ann N Y Acad Sci. 2006;1069(1):275–288. [DOI] [PubMed] [Google Scholar]
- 42. Prummel MF, Mourits MP, Blank L, Berghout A, Koornneef L, Wiersinga WM. Randomized double-blind trial of prednisone versus radiotherapy in graves’ ophthalmopathy. Lancet. 1993;342(8877):949–954. [DOI] [PubMed] [Google Scholar]
- 43. Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutr Diet. 2004;17(4):293–316. [DOI] [PubMed] [Google Scholar]
- 44. Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–2120. [DOI] [PubMed] [Google Scholar]
- 45. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067–3077. [DOI] [PubMed] [Google Scholar]
- 47. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. [DOI] [PubMed] [Google Scholar]
- 48. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–256. [DOI] [PubMed] [Google Scholar]
- 49. Rothman RB, Baumann MH, Dersch CM, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse (New York, NY). 2001;39(1):32–41. [DOI] [PubMed] [Google Scholar]
- 50. Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968;1(5588):352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim KK, Cho HJ, Kang HC, Youn BB, Lee KR. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei Med J. 2006;47(5):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010;12(10):876–882. [DOI] [PubMed] [Google Scholar]
- 53. Lewis KH, Fischer H, Ard J, et al. Safety and effectiveness of Longer-term phentermine use: clinical outcomes from an electronic health record cohort. Obesity (Silver Spring, Md). 2019;27(4):591–602. [DOI] [PubMed] [Google Scholar]
- 54. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. [DOI] [PubMed] [Google Scholar]
- 55. Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2004;2003(3):CD004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cosentino G, Conrad AO, Uwaifo GI. Phentermine and topiramate for the management of obesity: a review. Drug Des Devel Ther. 2011;7:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring, Md). 2013;21(5):935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res. 2014;84:1–11. [DOI] [PubMed] [Google Scholar]
- 59. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. [DOI] [PubMed] [Google Scholar]
- 60. Nissen SE, Wolski KE, Prcela L, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990. [DOI] [PubMed] [Google Scholar]
- 61. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(6):784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. [DOI] [PubMed] [Google Scholar]
- 63. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687. [DOI] [PubMed] [Google Scholar]
- 64. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. [DOI] [PubMed] [Google Scholar]
- 65. Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. [DOI] [PubMed] [Google Scholar]
- 66. Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kadowaki T, Isendahl J, Khalid U, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206. [DOI] [PubMed] [Google Scholar]
- 70. Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clément K, van den Akker E, Argente J, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960–970. [DOI] [PubMed] [Google Scholar]
- 72. Gadde KM, Franciscy DM, Wagner HR, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289(14):1820. [DOI] [PubMed] [Google Scholar]
- 73. Gadde KM, Kopping MF, Wagner HR, Yonish GM, Allison DB, Bray GA. Zonisamide for weight reduction in obese adults: a 1-year randomized controlled trial. Arch Intern Med. 2012;172(20):1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gomez G, Stanford FC. US Health policy and prescription drug coverage of FDA-approved medications for the treatment of obesity. Int J Obes (Lond). 2018;42(3):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roser P, Bajaj SS, Stanford FC. International lack of equity in modern obesity therapy: the critical need for change in health policy. Int J Obes (Lond). 2022;46(9):1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. World Obesity Day: ‘All countries significantly off track to meet 2025 WHO targets on Obesity’. World Obesity Federation. https://www.worldobesity.org/news/world-obesity-day-all-countries-significantly-off-track-to-meet-2025-who-targets-on-obesity
- 77. Kolata G. The doctor prescribed an obesity drug. Her insurer called it ‘Vanity.’ The New York Times. May 31 2022. https://www.nytimes.com/2022/05/31/health/obesity-drugs-insurance.html
- 78. H.R.1577—117th Congress (2021-2022): Treat and reduce obesity act of 2021. March 4 2021. https://www.congress.gov/bill/117th-congress/house-bill/1577
- 79. Gregg EW, Jakicic JM, Blackburn G, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Do A, Kuszewski EJ, Langberg KA, Mehal WZ. Incorporating weight loss medications into hepatology practice for nonalcoholic steatohepatitis. Hepatology. 2019;70(4):1443–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Weijers JM, Müskens WD, van Riel PLCM. Effect of significant weight loss on disease activity: reason to implement this non-pharmaceutical intervention in daily clinical practice. RMD Open. 2021;7(1):e001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tahrani AA, Morton J. Benefits of weight loss of 10% or more in patients with overweight or obesity: a review. Obesity (Silver Spring, Md). 2022;30(4):802–840. [DOI] [PubMed] [Google Scholar]
- 83. Carneiro-Barrera A, Amaro-Gahete FJ, Guillén-Riquelme A, et al. Effect of an interdisciplinary weight loss and lifestyle intervention on obstructive sleep apnea severity: the INTERAPNEA randomized clinical trial. JAMA Netw Open. 2022; 5(4):e228212.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155. [DOI] [PubMed] [Google Scholar]
- 85. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515. [DOI] [PubMed] [Google Scholar]
- 86. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. [DOI] [PubMed] [Google Scholar]
- 87. Lau DCW, Erichsen L, Francisco AM, et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398(10317):2160–2172. [DOI] [PubMed] [Google Scholar]
- 88. Enebo LB, Berthelsen KK, Kankam M, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397(10286):1736–1748. [DOI] [PubMed] [Google Scholar]
- 89. Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3(3):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ji L, Jiang H, An P, Deng H, Liu M. IBI362 (LY3305677), a weekly-dose GLP-1 and glucagon receptor dual agonist, in Chinese adults with overweight or obesity: a randomised, placebo-controlled, multiple ascending dose phase 1b study. eClinicalMedicine. 2021;39(101088):101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ali MM, Hafez A, Abdelgalil MS, et al. Impact of cotadutide drug on patients with type 2 diabetes mellitus: a systematic review and meta-analysis. BMC Endocr Disord. 2022;22(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. ClinicalTrials.gov. (2021). A study of ly3437943 in participants with type 2 diabetes mellitus (T2DM). January 8 2023. https://clinicaltrials.gov/ct2/show/NCT04143802
- 93. Tsai AG, Felton S, Wadden TA, Hosokawa PW, Hill JO. A randomized clinical trial of a weight loss maintenance intervention in a primary care population. Obesity (Silver Spring). 2015;23(10):2015–2021. [DOI] [PubMed] [Google Scholar]
- 94. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang X, Lyles MF, You T, Berry MJ, Rejeski WJ, Nicklas BJ. Weight regain is related to decreases in physical activity during weight loss. Med Sci Sports Exerc. 2008;40(10):1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168(14):1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85(4):954–959. [DOI] [PubMed] [Google Scholar]
- 98. Ostendorf DM, Caldwell AE, Creasy SA, et al. Physical activity energy expenditure and total daily energy expenditure in successful weight loss maintainers. Obesity (Silver Spring). 2019;27(3):496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis. 2018;61(2):206–213. [DOI] [PubMed] [Google Scholar]
- 100. Brockmann AN, Eastman A, Ross KM. Frequency and consistency of self-weighing to promote weight-loss maintenance. Obesity (Silver Spring). 2020;28(7):1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Greaves C, Poltawski L, Garside R, Briscoe S. Understanding the challenge of weight loss maintenance: a systematic review and synthesis of qualitative research on weight loss maintenance. Health Psychol Rev. 2017;11(2):145–163. [DOI] [PubMed] [Google Scholar]
- 102. Carrard I, Kruseman M. Qualitative analysis of the role of self-weighing as a strategy of weight control for weight-loss maintainers in comparison with a normal, stable weight group. Appetite. 2016;105:604–610. [DOI] [PubMed] [Google Scholar]
- 103. Fanghänel G, Cortinas L, Sánchez-Reyes L, Berber A. Second phase of a double-blind study clinical trial on sibutramine for the treatment of patients suffering essential obesity: 6 months after treatment cross-over. Int J Obes Relat Metab Disord. 2001;25(5):741–747. [DOI] [PubMed] [Google Scholar]
- 104. Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167–172. [DOI] [PubMed] [Google Scholar]
- 105. Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28(1):57–64. [DOI] [PubMed] [Google Scholar]
- 106. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101(6):1320S–1329S. [DOI] [PubMed] [Google Scholar]
- 107. Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307(24):2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gibson AA, Sainsbury A. Strategies to improve adherence to dietary weight loss interventions in research and real-world settings. Behav Sci (Basel). 2017;7(3):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kheniser KG, Kashyap SR. Diabetes management before, during, and after bariatric and metabolic surgery. J Diabetes Complicat. 2018;32(9):870–875. [DOI] [PubMed] [Google Scholar]
- 110. Rye P, Modi R, Cawsey S, Sharma AM. Efficacy of high-dose liraglutide as an adjunct for weight loss in patients with prior bariatric surgery. Obes Surg. 2018;28(11):3553–3558. [DOI] [PubMed] [Google Scholar]
- 111. Istfan NW, Anderson WA, Hess DT, Yu L, Carmine B, Apovian CM. The mitigating effect of phentermine and topiramate on weight regain after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring). 2020;28(6):1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schwartz J, Chaudhry UI, Suzo A, et al. Pharmacotherapy in conjunction with a diet and exercise program for the treatment of weight recidivism or weight loss plateau post-bariatric surgery: a retrospective review. Obes Surg. 2016;26(2):452–458. [DOI] [PubMed] [Google Scholar]
- 113. Kashyap SR, Kheniser K, Aminian A, Schauer P, Le Roux C, Burguera B. Double-blinded, randomized, and controlled study on the effects of canagliflozin after bariatric surgery: a pilot study. Obes Sci Pract. 2020;6(3):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gorgojo-Martínez JJ, Feo-Ortega G, Serrano-Moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis. 2016;12(10):1856–1863. [DOI] [PubMed] [Google Scholar]
- 115. Suliman M, Buckley A, Al Tikriti A, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab. 2019;21(6):1498–1501. [DOI] [PubMed] [Google Scholar]
- 116. Miras AD, Pérez-Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–559. [DOI] [PubMed] [Google Scholar]
- 117. Frías JP, Auerbach P, Bajaj HS, et al. Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9(9):563–574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.