Abstract
Patients with interstitial lung disease (ILD), especially those with pulmonary fibrosis, are at increased risk of developing lung cancer. Management of lung cancer in patients with ILD is particularly challenging. Diagnosis can be complicated by difficulty differentiating lung nodules from areas of focal fibrosis, and percutaneous biopsy approaches confer an increased risk of complications in those with pulmonary fibrosis. Lung cancer treatment in these patients pose several specific considerations. The degree of lung function impairment may preclude lobectomy or surgical resection of any type. Surgical resection can trigger an acute exacerbation of the underlying ILD. The presence of ILD confers an increased risk of pneumonitis with radiotherapy, and many of the systemic therapies also carry an increased risk of pneumonitis in this population. The safety of immunotherapy in the setting of ILD remains to be fully elucidated and concerns remain as to triggering pneumonitis. The purpose of this review is to summarize the evidence regarding consideration for tissue diagnosis, chemotherapy and immunotherapy, radiotherapy, and surgery, in this patient population and discuss emerging areas of research. We also propose a multidisciplinary approach and practical considerations for monitoring for ILD progression during lung cancer treatment.
Keywords: Interstitial lung disease, Lung cancer, Pneumonitis
This article summarizes the evidence regarding the risk of developing lung cancer in patients with interstitial lung disease (ILD) and considerations for diagnosis and treatment in this patient population. Practical considerations for monitoring ILD progression during lung cancer treatment are also discussed.
Implications for Practice.
Patients with ILD are at increased risk for developing lung cancer. Treatment of lung cancer in patients with ILD requires specific attention to ILD-related risks of cancer treatment including increased risks of acute exacerbation of the underlying ILD with medical therapies, radiotherapy, and surgery. We recommend that patients with ILD diagnosed with lung cancer undergo multidisciplinary discussion involving pulmonologists to assist in the determination of the risks of treatment and close monitoring for early recognition of treatment-related complications such as pneumonitis or acute exacerbations of the underlying ILD.
Introduction
The term interstitial lung disease (ILD) comprises a diverse group of diffuse parenchymal lung diseases often marked by varying degrees of inflammation and fibrosis. Computed tomography (CT) of the chest is the mainstay of ILD diagnosis. Further phenotyping of the type of ILD hinges on assessment of environmental, occupational, or other exposures (ie, radiation therapy to the thorax, certain medications) and evaluation for systemic diseases that can be associated with ILD (ie, rheumatoid arthritis, systemic sclerosis). In some cases, lung biopsy may be needed to define the underlying histologic abnormalities. Idiopathic pulmonary fibrosis (IPF) is the prototypic form of progressive fibrosing lung disease and is associated with a high morbidity and mortality.1 Patients with ILD, especially fibrotic ILD, are at risk for developing acute exacerbations of their ILD (AE-ILD) which can result in respiratory failure and death.2 Patients with ILD are also at increased risk for comorbidities ranging from pulmonary hypertension to lung cancer.1
Diagnosing and treating lung cancer in patients with ILD can be particularly challenging. Depending on the type of ILD it can be difficult to distinguish a lung nodule from areas of fibrosis. Percutaneous lung needle biopsy carries increased risks of complications in patients with IPF.3 The degree of lung function impairment from ILD may preclude surgical resection for definitive therapy for early-stage lung cancer. For patients who are surgical candidates, surgical resection can trigger an AE-ILD for reasons that are unclear but have been conjectured to include hyperoxia resulting in free radical formation and lung injury induced by mechanical ventilation.4 Patients with ILD are at increased risk for developing pneumonitis from chemotherapy, immunotherapy, and radiotherapy. There is also emerging data that patients with interstitial lung abnormalities (ILA), incidentally detected abnormalities on computed tomography (CT) which may represent ILD in patients for whom an ILD has not been suspected, also have increased risk for developing lung cancer treatment-related complications such as pneumonitis.5 For some of these patients, the findings of ILA on CT of the chest will result in a formal diagnosis of ILD. Whether or not the risk of pneumonitis with lung cancer-directed treatment differs for those with a suspected versus a confirmed diagnosis of ILD or is influenced by the extent and type of ILAs remains to be determined.
Here we summarize the evidence regarding the risk of developing lung cancer in patients with ILD and considerations for diagnosis and treatment in this patient population. We also discuss a multidisciplinary approach and practical considerations aimed to closely monitor for ILD progression during lung cancer treatment.
ILD and Risk of Lung Cancer
The risk of lung cancer is increased in patients with ILD across varying ILD subtypes.6-9 In a meta-analysis of 35 studies, the estimated prevalence of lung cancer in patients with IPF was 13.5%, with higher rates in men and in smokers.9 Squamous cell carcinoma was the most common subtype (37.8%) followed by adenocarcinoma (30.8%). In a retrospective cohort study of 103 patients with IPF, the cumulative incidence rate of lung cancer increased over time from IPF diagnosis; incidence was 3.3%, 15.4% and 54.7% at 1, 5, and 10 years, respectively, and the age at diagnosis of IPF was independently associated with the risk of lung cancer.10 In a larger study of 938 patients with IPF, the strongest predictors for the development of lung cancer were male gender, current smoking, and decline in forced vital capacity (FVC) of ≥10%/year.11 Patients with combined pulmonary fibrosis and emphysema (CPFE) have an estimated odds ratio (OR) of 9.06 for developing squamous cell lung cancer compared with those without underlying lung disease.12
Patients with systemic sclerosis and ILD also have a higher risk of lung cancer.13 In patients under age 60, lung cancer incidence was higher in those with connective tissue disease-associated ILD (CTD-ILD) than in non-CTD-ILD (excluding IPF).14 The risk of lung cancer is increased even the setting of ILA. An analysis of the National Lung Screening Trial showed that 20.2% of the 25 041 participants had ILA and that the presence of ILA were associated with an increased lung cancer incidence (adjusted incidence rate ratio 1.33).15
The outcomes of lung cancer in patients with IPF are worse than in patients with either disease process alone. One study showed that the median survival of patients with lung cancer and IPF was 38 months vs 64 months in patients with IPF without lung cancer.16 Survival is also worse for patients with lung cancer and ILD than in those with lung cancer without ILD even when controlling for cancer stage.17,18 ILD subtype affects survival with survival worse for lung cancer in patients with IPF compared to nonspecific interstitial pneumonia (NSIP) or cryptogenic organizing pneumonia (COP).19 Additionally, the presence of ILA has been associated with greater lung cancer-specific mortality in the National Lung Cancer Trial and shorter overall survival in stage I non–small cell lung cancer (NSCLC).15,20
Diagnosis of Lung Cancer in Patients with ILD
Despite the increased incidence of lung cancer in the setting of ILD, there are no ILD-specific guidelines for lung cancer screening. Lung cancers arising in patients with IPF tend to be peripheral, in the lower lobes, and in areas near fibrosis.21-23 The co-existence of fibrosis can make it difficult to differentiate a potential lung cancer from focal fibrosis. Positron emission tomography-CT (PET/CT) can be helpful in this setting. In a study of 55 participants with IPF, PET/CT had a sensitivity and specificity of 98% and 86%, respectively, for detecting malignant lung nodules.24 PET/CT is also more specific than CT for staging mediastinal lymph nodes in patients with non–small cell lung cancer (NSCLC) and IPF.25 When the decision has been made to proceed with a biopsy, options include CT-guided percutaneous transthoracic needle biopsy (TTNB) or endobronchial ultrasound-guided biopsy. The latter is likely to be helpful if suspicious lymph nodes have been noted on CT or PET/CT.
TTNB can be an appealing biopsy approach for lung nodules in patients with IPF because these nodules are likely to be peripheral. One study estimated the sensitivity and specificity of CT-guided TTNB for lung nodules in patients with IPF as 90% and 84%, respectively; however, 34% of biopsies returned with nondiagnostic results.4 This study had a high rate of complications at 51%, with a 12% rate of major complications including pneumothorax requiring chest tube placement (8.7%) and AE-IPF within 1 month of the procedure (2%), and the presence of honeycombing along the needle trajectory was associated with an OR of 11.2 for developing a major procedure-related complication. In comparison, a meta-analysis of results of CT-guided TTNB for pulmonary lesions in patients not limited to ILD reported a pooled incidence rate of 3% for pneumothorax requiring chest tube placement.26
Systemic Therapy in Patients with ILD
Systemic therapy has been a component of standard treatment for localized and advanced lung cancer for decades.27 In all-comers with NSCLC, tumor histology (eg, squamous or non-squamous) and molecular characterization are influential factors when deciding among various FDA-approved treatment regimens.28 However, selection of appropriate systemic therapy regimens for patients with NSCLC who have concurrent ILD is a more complex undertaking as chemotherapy, certain targeted therapies, and immunotherapies carry the risk of inducing AE-ILD or pneumonitis. For example, studies suggest that 5%-20% of patients with ILD will experience AE-ILD on chemotherapy and that such flares can prove fatal.29,30 Implementation of standard-of-care systemic therapy for lung cancer treatment in the patient with ILD is not always be feasible due to toxicity considerations. Therefore, optimal systemic therapy strategies for patients with NSCLC with coexisting ILD must balance efficacy and unique safety considerations.
The studies that established the efficacy of existing systemic therapy regimens for treatment of NSCLC uniformly excluded patients with baseline ILD. As a result, much of current understanding of the interplay between chemotherapy and underlying ILD is derived from retrospective reports, meta-analyses, and a handful of single arm phase II and pilot studies which were primarily conducted in Asian populations. The findings from prospective studies are summarized in Table 1. In a meta-analysis of first-line chemotherapy for NSCLC that included 684 patients with pre-existing ILD, the pooled objective response rate was 43%, suggesting that patients with ILD benefit from systemic therapy.37 Additionally, in this meta-analysis the pooled rate of AE-ILD was approximately 8% with chemotherapy based on 644 patients treated in 19 studies. The rate of AE-ILD differed according to chemotherapy regimen, with lower rates (5%) for nab-paclitaxel containing regimens relative to other regimens (12%).
Table 1.
Incidence of interstitial lung disease exacerbation in prospective trials of first-line platinum doublet chemotherapy in non–small cell lung cancer patients with interstitial lung disease.
| Study | Number of patients (Study design) |
Chemotherapy regimen | ILD exacerbation (%) |
|---|---|---|---|
| Kenmotsu et al31 | 94 (phase II) |
Carboplatin + Nab-paclitaxel | 4.3 |
| Asahina et al32 | 36 (phase II) |
Carboplatin + Nab-paclitaxel | 5.6 |
| Minegishi et al33 | 18 (Pilot) |
Carboplatin + Weekly Paclitaxel | 5.6 |
| Fukuizumi et al34 | 35 (phase II) |
Carboplatin + Weekly Paclitaxel | 12.1 |
| Sekine et al35 | 21 (phase II) |
Carboplatin + S-1 | 9.5 |
| Hanibuchi et al36 | 33 (phase II) |
Carboplatin + S-1 | 6.1 |
Abbreviation: ILD, interstitial lung disease.
The combination of carboplatin and nab-paclitaxel has been evaluated as first-line treatment in 2 Japanese single arm phase II studies with NSCLC and ILD.31,32 In the first study which enrolled 94 patients with mild (71%) or moderate (29%) ILD, only 4% patients experienced an AE-ILD within 28 days of completing treatment.31 Notably, one patient suffered a fatal AE-ILD. The rates of ILD were similar in the HOT1302 trial which enrolled 36 patients.32 Specifically, 2 patients (5.6%) experienced pneumonitis, including one patient whose pneumonitis consisted of a grade 5 (fatal) AE-ILD.32 The efficacy and safety of carboplatin in combination with either paclitaxel or the oral fluoropyrimidine derivative S-1 has also been evaluated in treatment-naïve patients with advanced NSCLC and ILD. Patients received carboplatin combined with weekly paclitaxel as part of an initial pilot study (n = 18 patients)33 and a follow-up single arm phase II study (n = 35 patients).34 These 2 Japanese studies reported an AE-ILD rate of 5.6% (pilot study) and 12.1% (phase II study), respectively, with no patients experiencing a fatal AE-ILD.33,34 In 2 Japanese single arm phase II studies where the regimen of S-1 plus carboplatin was assessed, chemotherapy-related AE-ILD occurred in 6.1%-9.5% of patients.35,36 Of note, pemetrexed-based therapy (the most common regimen for all-comers with non-squamous NSCLC) has not been formally assessed in prospective trials in patients with ILD. However, the frequency of AE-ILD in patients treated with pemetrexed alone or as part of combination therapy in small retrospective studies exceeded 10%.38,39
Although with limitations, these studies demonstrate the efficacy of first-line platinum doublet chemotherapy in patients with ILD. While the rates of ILD exacerbation were <10% in most studies, several patients experienced fatal AE-ILD. Retrospective data suggest that chemotherapy paired with antifibrotic agents may protect against the development of AE-ILD. In a small study where patients with IPF received platinum doublet chemotherapy (carboplatin plus either nab-paclitaxel or S-1) with the antifibrotic agent pirfenidone, no cases of ILD exacerbation were observed.40 This hypothesis is being formally tested in the J-SONIC phase III study which is randomizing patients to receive carboplatin and nab-paclitaxel with or without nintedanib.41
As progression on platinum doublet therapy is inevitable, prognosis of lung cancer is not only dependent on durability of response to platinum doublet therapy but also on benefit from other therapies. Rates of AE-ILD have been shown to be considerably high with certain second-line chemotherapies. For example, ILD exacerbation rates for patients with NSCLC and a usual interstitial pneumonia pattern who received docetaxel or gemcitabine were 28% and 43%, respectively.42 In contrast, the rate of ILD exacerbation with vinorelbine was reassuringly low (0%) in a retrospective analysis,42 suggesting that vinorelbine may be a choice therapy in this patient population. However, given the small sample size of patients receiving each regimen across studies, future larger studies are needed for validation.
Finally, although chemotherapy is a longstanding component of treatment for advanced NSCLC, a growing subset of patients whose tumors harbor actionable alterations will preferentially receive oral targeted therapies as initial therapy.27 Targeted therapies, such as EGFR tyrosine kinase inhibitors (TKIs) and anaplastic lymphoma kinase (ALK) inhibitors, are associated with developing pneumonitis,43,44 with some studies suggesting that the risk of developing pulmonary toxicity may be heightened in patients with pre-existing ILD.45,46 In a prospective cohort study that included over 3000 Japanese patients with advanced or recurrent NSCLC, the cumulative incidence of acute ILD was 4% over 12 weeks of gefitinib treatment.47 Additionally, 27% of patients treated with gefitinib had preexisting ILD, and the presence of preexisting ILD was associated with an increased risk of developing acute ILD events. In a meta-analysis that included data from over 2000 patients with advanced NSCLC who received monotherapy with an ALK inhibitor, the overall incidence of pneumonitis was 2.14%.44 There is limited data, however, as to incidence rates of acute exacerbation of ILD for patients with preexisting ILD receiving targeted therapy for NSCLC, and further research is needed to understand the risk for therapy-related pulmonary toxicity in this patient population.
Immunotherapy in Patients with ILD
Immune checkpoint inhibitors (ICIs) have improved overall survival in patients with lung cancer.48 While immune system activation has critical implications for cancer treatment, concerns around immune-related adverse effects due to non-specific immune activation exist.49 ICI-related pneumonitis is a serious and sometimes fatal adverse side effect.50 However, the risk of ICI-related pneumonitis has not been well studied in patients with ILD, as these patients have been largely excluded from clinical trials with ICIs.51-54 Estimates regarding the incidence of pneumonitis in patients with underlying ILA or ILD have been predominately retrospective in nature and range widely from 7.3% to 42.9% depending on the study population and definition of pneumonitis (Table 2).55-62 Several studies have shown that despite a higher incidence of pneumonitis in patients with pre-existing ILD, most cases of pneumonitis did not result in deaths.57,58,63 Moreover, in a retrospective study of 41 patients with ILD receiving ICIs for different cancers, deaths related to cancer and other non-ILD related causes were far more frequent compared to deaths due to respiratory failure from ILD or ICI-related pneumonitis.62 Some studies have found that ground-glass attenuation and increased fibrosis score are risk factors for the development of ICI-related pneumonitis in patients with pre-existing ILD or ILA, suggesting a need for increased monitoring of these patients.56,59,61,63 Among patients with NSCLC and ILD, response rates, progression-free survival, and overall survival were similar to those without ILD supporting that patients with ILD benefit from ICI treatment.55 Together, these data suggest that despite increased risk of ICI-related pneumonitis in patients with ILD, ICIs should not be uniformly withheld in this population.55,62 Rather, the potential risks and benefits of treatment with immunotherapy should be frankly discussed with NSCLC patients with ILD, allowing for shared decision making. Whether currently available ILD treatments such as nintedanib may reduce the risk of developing pneumonitis remains to be determined.64
Table 2:
Outcomes of patients with interstitial lung disease receiving immune checkpoint inhibitors
| Study | ILD study population | Key findings |
|---|---|---|
| Tasaka et al55 | 416 patients with advanced or recurrent NSCLC, 49 of whom had ILD, treated with nivolumab or pembrolizumab | Increased incidence of pneumonitis in the group with pre-existing ILD compared to the non-ILD group (30.6% compared with 9.5%). Non-inferior progression-free and overall survival in the pre-existing ILD group compared to controls. |
| Nakanishi et al56 | 83 patients with NSCLC, 13 of whom had ILA, treated with nivolumab or pembrolizumab | Incidence of ICI-ILD* was higher in the group of subjects with pre-existing ILA compared with those without ILA (42.9% vs. 10.1%, P = .007). In the ILA group, ground-glass attenuation was associated with increased risk of ICI-ILD. |
| Shimoji et al63 | 199 subjects with non-lung cancers, 37 (18.6%) of whom had ILA, treated with nivolumab or pembrolizumab | Presence of ILA was associated with increased risk of developing ICI-ILD. For those with ILA, the presence of ground-glass attenuation was associated with the development of ICI-ILD. |
| Kanai et al57 | 216 patients with NSCLC, including 26 with ILD, treated with nivolumab. | Higher incidence (31% vs 12%) and severity (19% vs. 5%) of pneumonitis in the ILD group compared with the non-ILD group. No pneumonitis-associated deaths. |
| Fujimoto et al58 | 18 patients with NSCLC and mild IIP defined as vital capacity greater than 80% predicted and a possible UIP or inconsistent with UIP pattern treated with nivolumab. | Two cases of grade 2 pneumonitis. No deaths related to nivolumab treatment. |
| Nishiyama et al59 | 48 patients with advanced NSCLC with ILD treated with nivolumab, pembrolizumab, or atezolizumab | 7 subjects (14.5%) developed AE-ILD. The occurrence of AE-ILD was associated with ground-glass attenuation score prior to ICI administration. |
| Shibaki et al60 | 331 patients with NSCLC, including 17 with ILD, treated with nivolumab or pembrolizumab | Pneumonitis incidence was higher in subjects with underlying ILD compared with those without (29% vs. 10%, P = .027). |
| Yamaguchi et al61 | 123 subjects with NSCLC, including 37 with a fibrosis score ≥ 1, treated with nivolumab or pembrolizumab | 18 patients developed ICI-related pneumonitis (14.6%) with fibrosis score on CT of ≥ 1 being a risk factor for ICI-related pneumonitis |
| Dobre et al62 | 41 patients with ILD and cancer (73.2% with lung cancer) treated with nivolumab or pembrolizumab | 3 subjects (7.3%) developed ICI-related pneumonitis. At 1 year, most deaths were cancer related (69.6%) or due to non-cancer and non-ILD etiologies (17.4%) compared with 13% of deaths related to ILD or ICI pneumonitis. |
Abbreviations: ILD, interstitial lung disease; NSCLC, non–small cell lung cancer; ICI, immune checkpoint inhibitor; ILA, interstitial lung abnormalities; CT, computed tomography; IIP, idiopathic interstitial pneumonia; UIP, usual interstitial pneumonia; AE, acute exacerbation. *Defined as the development of new, bilateral consolidation or ground-glass attenuation.
Radiotherapy in Patients with ILD
Radiotherapy is a critical component of treatment for many patients with lung cancer. While generally well tolerated, side effects from radiotherapy vary significantly based on the cumulative dose, number of treatment fractions, and the size of the treatment field. The risk of pneumonitis is a critical consideration for all patients undergoing thoracic radiotherapy and a particularly significant concern in patients with pre-existing ILD.
Early-stage lung cancer and radiotherapy
A proportion of patients with early-stage NSCLC is considered medically inoperable due to medical comorbidities. For these patients, definitive local therapy with stereotactic body radiotherapy (SBRT) is the standard of care.65 SBRT is defined as high-dose radiotherapy delivered in a highly conformal manner with rapid dose fall-off over up to 5 fractions66 and is preferred over conventionally fractionated radiotherapy for patients with inoperable Stage I-IIA NSCLC. SBRT is typically associated with low rates of pneumonitis67 or decline in pulmonary function, even in patients with impaired baseline pulmonary function.68,69 However, for patients with pre-existing ILD, there is significant hesitation regarding the use of SBRT due to concern for developing of pneumonitis. There are no prospective trials on the use of radiotherapy in patients with ILD. There are also limited retrospective data on the use of radiotherapy in patients undergoing treatment for ILD. Instead, published data largely include patients with subclinical ILD, typically defined as previously diagnosed ILD but not receiving ILD-directed treatment or pre-treatment imaging consistent with ILD without a previous ILD diagnosis.
Even among studies of patients with primarily subclinical ILD, SBRT is associated with significant toxicities (Table 3). In a retrospective study of 504 patients with Stage I lung cancer, of whom 6% had pre-existing ILD, the rate of ≥grade 3 radiation pneumonitis (RP) was significantly higher in patients with ILD (32%) compared to 4% in the overall group.70 Additionally, 21% of patients with ILD experienced grade 5 RP. In a study of 537 patients with Stage I lung cancer treated with SBRT, 39 of whom were identified as having imaging features consistent with ILD, patients with ILD had a significantly higher rate of grade ≥2 RP (20.5% vs. 5.8%) and grade ≥3 RP (10.3% vs. 1.0%).71 Higher rates of extensive RP outside the radiotherapy treatment fields have also been reported in patients with ILD.72
Table 3.
Stereotactic body radiotherapy and rate of radiation pneumonitis in lung cancer patients with interstitial lung disease.
| Study | Number of patients | Number of patients with ILD | Rate of 2+ RP | Rate of Grade 3+ RP | Summary | ||||
|---|---|---|---|---|---|---|---|---|---|
| ILD | Non-ILD | P-value | ILD | Non-ILD | P-value | ||||
| Tsurugai et al73 | 508 | 42 | 19% | 15% | .46 | 12% | 3% | .009 | ILD was associated with increased risk of grade ≥3 RP. |
| Glick et al71 | 537 | 39 | 20.5% | 5.8% | <.01 | 10.3% | 1.0% | <.01 | ILD and mean lung dose were associated with increased risk of RP. |
| Bahig et al70 | 504 | 28 | NA | NA | NA | 32% | 2% | <.001 | ILD was associated with increased risk of grade ≥3 RP with 21% of patients with ILD developing grade 5 RP. |
| Ueki et al74 | 157 | 20 | 55% | 13% | <.0001 | 10% | 1.5% | .02 | ILD was associated with increased risk of RP, including Grade 5 RP. |
| Yoshitake et al75 | 260 | 18* | 50% | 6.7% | NA | 38.9% | 2.2% | NA | Presence of interstitial changes was only factor that associated with risk of grade ≥2 RP. |
| Yamaguchi et al72 | 100 | 16 | 19% | 10% | NA | 19% | 3.5% | NA | Lung dose (V5 – V25 and mean lung dose) but not subclinical ILD were associated with grade ≥2 RP. |
Abbreviations: ILD, interstitial lung disease; RP, radiation pneumonitis; NA, not available.
*Defined as patients with “interstitial changes” on pretreatment CT.
Locally advanced lung cancer and radiotherapy
Radiotherapy also plays a critical role in the treatment of patients with locally advanced NSCLC. For patients with inoperable stage III NSCLC, standard treatment is chemoradiotherapy followed by adjuvant durvalumab.76 Unlike SBRT, which is typically characterized by small treatment fields, treatment of locally advanced lung cancer requires larger treatment fields due to primary tumor size and lymph node involvement. These larger treatment fields in turn result in increased exposure of lung tissue to radiotherapy. As such, the rates of RP, even in patients without co-existing ILD, are high in patients with locally advanced NSCLC. For example, in the PACIFIC trial, the rate of any grade pneumonitis in the control arm which received chemoradiotherapy alone was 24.8%, and the rate of grade 3 or 4 pneumonitis was 2.6%.77 Given these increased risks, treatment of patients with ILD and locally advanced lung cancer is particularly complicated.
In a retrospective series of 87 patients with subclinical ILD and NSCLC who were treated with ≥50 Gy of conventionally fractionated radiotherapy, the majority of whom had Stage III NSCLC (70.1%) and received sequential, rather than concurrent, chemoradiotherapy (78.2%), the rate of grade ≥2 RP was 51.7%, including a 5.7% rate of grade 5 RP.78 Of the patients with grade 5 RP, 3/5 received concurrent chemoradiotherapy. On multivariate analysis, mean lung dose (MLD) ≥12 Gy was significantly associated with grade ≥2 RP. The rate of grade ≥3 RP was higher in patients with subclinical ILD involving ≥25% of lung volume. Prior receipt of gemcitabine and lung V5 ≥50% was also associated with increased risk of grade ≥3 RP. In an analysis of 84 patients with pre-existing ILD, ILD involvement >10% of lung field predicted the development of acute RP.79 For patients with locally advanced lung cancer, we often favor sequential chemotherapy and radiotherapy rather than concurrent chemoradiotherapy given the increased risk of pneumonitis associated with concurrent chemoradiotherapy.
Proton Beam Therapy
There is emerging data on the use of proton beam therapy (PBT) for the treatment of lung cancer.80 A conceptual advantage of PBT is the steep dose fall-off, which can in turn minimize low-dose bath. With proton therapy, only one to 2 beams are typically required for the treatment of lung tumors. By contrast, with conventional photon radiotherapy, multiple beam angles are used to achieve conformality of the high dose region. Due to the multiple beam angles, there is increased exposure of adjacent tissues to lower doses of radiotherapy (Fig. 1). In patients with ILD, even exposure to lower doses of radiotherapy can have potentially significant risks. In a small, retrospective study of 30 patients with Stage I-II NSCLC and IPF, 8 of whom received PBT and 22 of whom received SBRT using photons with 3-dimensional conformal RT or intensity-modulated RT, the group treated with PBT had a numerically smaller but not statistically significant incidence of treatment-related pulmonary complications compared to the photon therapy group (12.5% vs 40.9%; P = .22).81 However, the role of proton therapy for the treatment of lung cancer is still under investigation. A randomized trial of protons vs intensity-modulated radiotherapy with photons for patients with locally advanced NSCLC did not show a reduction in the incidence of radiation pneumonitis with proton therapy.82 Further research is needed as to the efficacy of PBT for lung cancer and if PBT is associated with a lower risk of RP.
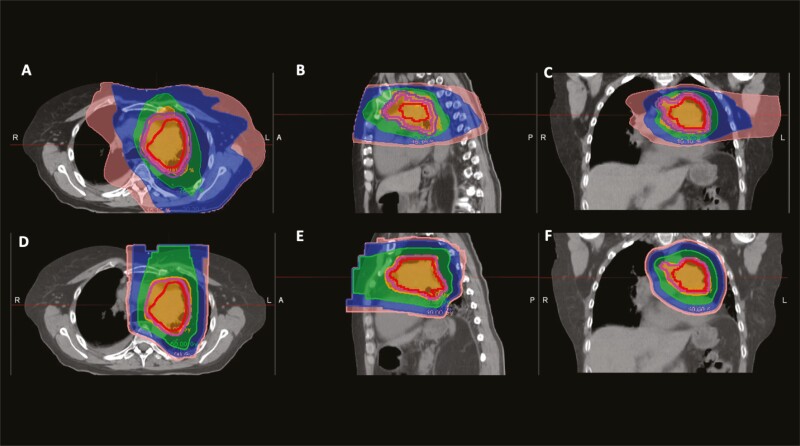
Figure 1.
Photon and proton plans for a patient with a LUL tumor invading the mediastinum. Figure panels A-C are the axial, sagittal, and coronal images from the photon treatment plan using volumetric arc therapy. Figure panels D-F are the axial, sagittal, and coronal images from the proton treatment plan. The internal target volume is in red, clinical target volume is in pink, and planning treatment volume is in purple. The 10 Gy isodose line is pink, the 20 Gy isodose line is blue, the 50 Gy isodose line is green, and the 60 Gy isodose line is in orange. In the photon plan (A-C), the low dose bath is much larger, as can be seen by the larger size of the 10 and 20 Gy isodose line regions compared with the proton plan (D-F).
Abbreviations: R, right; L, left; A, anterior; P, posterior.
Surgical Considerations
While surgical resection may be curative in early-stage lung disease, it is associated with an increased risk of post-operative complications and worse survival in patients with ILD.83 Surgical intervention, both pulmonary and non-pulmonary, in patients with ILD is known to be a risk factor for AE-ILD.84,85 The incidence of post-operative AE-ILD in patients undergoing surgical resection for lung cancer is high, ranging from 9% to 23%.86,87 The implications are significant, as mortality rates of post-operative AE-ILD can exceed 40%.5,87 Patients with ILD are at increased risk of other pulmonary complications, including pneumonia, atelectasis, pneumothorax, and pulmonary embolism, as well as increased post-operative mortality.87-92
Retrospective studies have identified multiple risk factors for post-operative complications in patients with ILD. Both decreased FVC and decreased diffusing capacity of the lung for carbon monoxide (DLCO) are associated with post-operative AE-ILD and increased short-term mortality.87,90,92-98 The pattern of ILD has also been associated with risk of AE-ILD. A UIP pattern on CT is an independent risk factor for post-operative AE-ILD.87 Need for oxygen therapy pre-operatively has also been associated with increased morbidity and mortality in patients with ILD undergoing surgical lung biopsy.99,100 In addition to these ILD-related risk factors, procedural-based factors, need for emergency surgery, longer duration of anesthesia, and extent of surgery (ie, lobectomy vs localized wedge resection), are associated with increased risk of pulmonary complications such as pneumonia.87,91,92,101 While validated ILD-specific models to quantify operative risk are lacking, there are several well-studied general risk-stratification models for post-operative pulmonary complications, including the ARISCAT score.102 The ARISCAT score risk stratifies patients based on a number of factors, including pre-operative oxygen saturations, recent respiratory infection, and expected duration of surgery. An ARISCAT score ≥45 has been shown to predict the development of post-operative AE in patients with ILD.97 There remains an ongoing need for additional models for risk stratification for complications associated with lung cancer treatment in ILD patients extending from post-operative risks to risk of pneumonitis from systemic therapies and radiation therapy.
The presence of ILD resulting in decreased lung compliance may make patients more vulnerable to ventilator-induced lung injury, particularly with single lung ventilation of the non-operative lung.88,103 To reduce the risk of ventilator-induced lung injury current ventilation methods focus on minimizing hyper-expansion of the non-operative lung and use of low tidal volumes. Higher intra-operative fluid balance also increases the risk of post-operative AE in patients with IPF undergoing lung cancer resection.104 Recent guideline protocols therefore stipulate a goal of euvolemia in the peri- and post-operative period of thoracic surgical intervention.105 Lastly, peri-operative infection and aspiration can both act as a trigger for AE in ILD patients.5
Encouragingly, the implementation of minimally invasive surgical techniques such as video-assisted thoracoscopic surgery (VATS) may decrease surgical morbidity for patients with ILD.106 Similarly, recent work suggests that pursuing less extensive surgical intervention (ie, sublobar as opposed to lobar resection) for patients with ILD and early-stage lung cancers may offer similar survival benefit.107 Unfortunately, regardless of advances that mitigate immediate post-operative risk, patients with ILD still have significantly worse long-term survival after lung resection compared to patients with lung cancer without ILD.83
Percutaneous Ablation of Lung Tumors
Image-guided thermal ablation (IGTA), including radiofrequency ablation (RFA) microwave ablation (MWA), and cryoablation, may be a consideration for patients with ILD and early-stage lung cancer although additional research is needed.108 While data on use of IGTA for management of lung cancer in patients with ILD is limited, efficacy and overall survival of patients treated with IGTA has been shown to be similar to sublobar resection and SBRT in patients with NSCLC.109 In patients age ≥65 years and stage I NSCLC, overall survival was similar between those treated with sublobar resection versus thermal ablation when controlling for demographics and certain clinical variables.109 In a study of 54 patients with inoperable stage IA NSCLC treated with RFA, survival rates at 2 years were in line with those historically reported for SBRT.110
The development of AE-ILD is also concern for patients with ILD who undergo IGTA. In retrospective analysis of 420 patients including patients with ILD who underwent RFA for primary lung cancer or pulmonary metastases, 3 of 4 deaths were due to AE-ILD.111 A systematic review of 3 retrospective studies and a total of 46 inoperable patients treated with RFA for early-stage NSCLC or oligo metastases reported an ILD-specific toxicity of 25% and 9% mortality.112 Data on cryoablation is limited. A conference abstract reported AE-ILD and subsequent death in 2 of 11 patients with IPF (18%) after percutaneous cryoablation of T1N0M0 NSCLC.113 In the absence of a detailed report, the potential role of cryoablation in this population remains to be determined.
Practical Considerations
For some patients, the initial diagnosis of ILD or ILA may occur at the time of lung cancer diagnosis. Given that ILD and ILA increase the risks associated with lung cancer treatment, providers should pay particular attention to CT findings that may suggest an ILD (Figs. 2 and 3). These CT findings include the presence of ground-glass opacities, reticular markings, traction bronchiectasis, or honeycombing. For patients with a suspected diagnosis of ILD, we recommend pulmonary evaluation as knowledge of the type of ILD may help with balancing risks and benefits of lung cancer treatments with prognosis of the underlying lung disease.
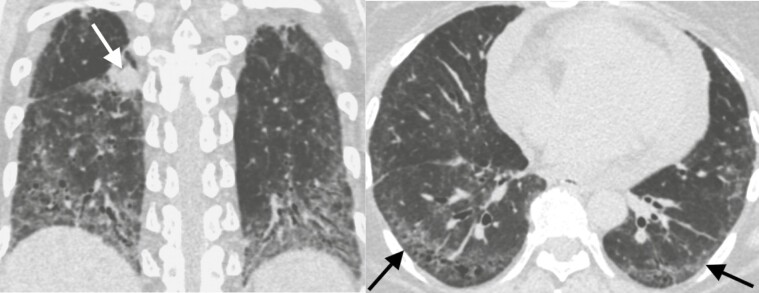
Figure 2.
Forty-four-year-old woman with systemic sclerosis-associated interstitial lung disease and an enlarging right lower lobe solid nodule corresponding to a biopsy proven non-small cell lung cancer. Left. Coronal CT image demonstrates the lung cancer (arrow) and lower lung predominant fibrosis marked by volume loss, ground-glass opacities, and traction bronchiectasis. Right. Axial CT image of the lung bases demonstrates peripheral predominant ground glass, reticular markings, and traction bronchiectasis (arrows) most consistent with a non-specific interstitial pneumonitis pattern.
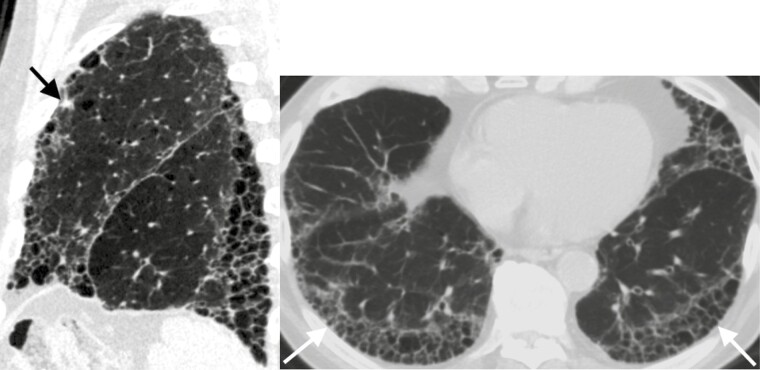
Figure 3.
Eighty-two-year-old man with idiopathic pulmonary fibrosis and an indeterminate subpleural solid left upper lobe nodule. Left: Sagittal CT image demonstrates the nodule (arrow) and honeycombing with an apical basilar gradient consistent with a usual interstitial pneumonia pattern. Right: Axial CT image of the lung bases demonstrates stacked cystic structures (arrows) consistent with honeycombing.
For a patient with ILD we recommend that multi-disciplinary discussions include a pulmonologist prior to starting lung cancer treatment and throughout therapy. This multidisciplinary approach encourages frequent discussion between providers which makes adjusting and implementing changes to patient treatment plans more efficient especially if pneumonitis develops. Where possible we recommend that patients with ILD undergo pulmonary function tests (PFTs) prior to treatment initiation, including spirometry, lung volumes, and DLCO. This allows for the grading of the severity of lung function impairment which may inform what treatment options may be available (for example, surgical candidacy). PFTs should be measured periodically throughout treatment to assess for interval changes to signify ILD progression. We also recommend interval monitoring of oxygen levels at rest and with exertion to assess the need for supplemental oxygen or adjustment to existing supplemental oxygen flow rates, especially in the setting of worsening dyspnea. Lastly, as lung cancer treatment may exacerbate pre-existing respiratory symptoms early referral to palliative care may be beneficial.
Conclusions and Future Directions
Patients with ILD carry unique considerations for diagnosis and treatment of lung cancer and are at increased risk for adverse effects from all modalities of lung cancer treatment. Despite this, treating lung cancer can prolong survival in this population. Central to the care of patients with ILD and lung cancer is close multidisciplinary collaboration. Opportunities exist to further understand the risk factors leading to pneumonitis in patients with ILD and ILA and if ILD subtype or radiologic pattern may assist with refining the risks of treatment. Whether the use of antifibrotic therapies mitigates the risk of developing pneumonitis or AE-ILD in the setting of lung cancer treatment remains to be determined.40,114
Contributor Information
Angela J Frank, Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Boston, MA, USA.
Ibiayi Dagogo-Jack, Massachusetts General Hospital Cancer Center, Massachusetts General Hospital, Boston, MA, USA.
Ioana A Dobre, Queen’s University School of Medicine, Kingston, ON, Canada.
Sarah Tait, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Lana Schumacher, Department of Surgery, Massachusetts General Hospital, Boston, MA, USA.
Florian J Fintelmann, Department of Radiology, Division of Thoracic Imaging and Intervention, Massachusetts General Hospital, Boston, MA, USA.
Leah M Fingerman, Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Boston, MA, USA.
Florence K Keane, Department of Radiation Oncology, Massachusetts General Hospital, Boston, MA, USA.
Sydney B Montesi, Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Boston, MA, USA.
Conflict of Interest
Ibiayi Dagogo-Jack: Pfizer, AstraZeneca, Xcovery, Bristol Myers Squibb, Genentech, Bayer, Syros, Novocyre, BostonGene, Sanifi-Genzyme, Janssen, Boehringer Ingelheim, Bayer, Catalyst, (C/A), Array, Genentech, Novartis, Pfizer, Guardant Health (RF), Foundation Medicine, Creative Education Concepts, OncLive, ASCO Post, DAVA Oncology, Medscape, Total Health Conferencing, American Lung Association (H), Array, Pfizer (travel expenses); Florian J. Fintelmann: William M. Wood Foundation, Pfizer (RF); Florence K. Keane: Siemens (C/A, H); Sydney B. Montesi: DevPro Biopharma, Gilead Sciences, Roche (C/A), NIH/National Heart, Lung, and Blood Institute [K23HL15033] to SBM, Pliant Therapeutics, Merck, United Therapeutics (RF), APIE Therapeutics (SAB), Wolters Kluwer (royalties). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: A.J.F., I.D.J., L.M.F., S.B.M. Manuscript writing: All authors. Final approval of manuscript: All authors.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Lederer DJ, Martinez FJ.. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811-1823. 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 2. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. an international working group report. Am J Resp Crit Care 2016;194(3):265-275. [DOI] [PubMed] [Google Scholar]
- 3. Shin YJ, Yun G, Yoon SH, et al. Accuracy and complications of percutaneous transthoracic needle lung biopsy for the diagnosis of malignancy in patients with idiopathic pulmonary fibrosis. Eur Radiol. 2021;31(12):9000-9011. 10.1007/s00330-021-08038-x. [DOI] [PubMed] [Google Scholar]
- 4. Amundson WH, Racila E, Allen T, et al. Acute exacerbation of interstitial lung disease after procedures. Resp Med 2019;150:30-37. [DOI] [PubMed] [Google Scholar]
- 5. Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Medicine 2020;8(7):726-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi W-I, Park SH, Park BJ, Lee CW.. Interstitial lung disease and lung cancer development: a 5-year nationwide population-based study. Cancer Res Treat 2018;50(2):374-381. 10.4143/crt.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. Am J Resp Crit Care 2000;161(1):5-8. [DOI] [PubMed] [Google Scholar]
- 8. Matsushita H, Tanaka S, Saiki Y, et al. Lung cancer associated with usual interstitial pneumonia. Pathol Int. 1995;45(12):925-932. [DOI] [PubMed] [Google Scholar]
- 9. Jafarinezhad A, Yektakooshali MH.. Lung cancer in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. PLoS One. 2018;13(8):e0202360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009;14(5):723-728. [DOI] [PubMed] [Google Scholar]
- 11. Yoo H, Jeong B-H, Chung MJ, et al. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med. 2019;19(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koo HJ, Do K-H, Lee JB, et al. Lung cancer in combined pulmonary fibrosis and emphysema: a systematic review and meta-analysis. PLoS One. 2016;11(9):e0161437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morrisroe K, Hansen D, Huq M, et al. Incidence, risk factors, and outcomes of cancer in systemic sclerosis. Arthrit Care Res 2020;72(11):1625-1635. [DOI] [PubMed] [Google Scholar]
- 14. Choi W-I, Lee DY, Choi H-G, et al. Lung Cancer development and mortality in interstitial lung disease with and without connective tissue diseases: a five-year Nationwide population-based study. Respir Res. 2019;20(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown S-AW, Padilla M, Mhango G, et al. Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial. Chest 2019;156(6):1195-1203. [DOI] [PubMed] [Google Scholar]
- 16. Tomassetti S, Gurioli C, Ryu JH, et al. The Impact of Lung Cancer on Survival of Idiopathic Pulmonary Fibrosis. Chest 2015;147(1):157-164. [DOI] [PubMed] [Google Scholar]
- 17. Alomaish H, Ung Y, Wang S, et al. Survival analysis in lung cancer patients with interstitial lung disease. PLoS One. 2021;16(9):e0255375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee T, Park JY, Lee HY, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: Clinical characteristics and impact on survival. Resp Med 2014;108(10):1549-1555. [DOI] [PubMed] [Google Scholar]
- 19. Kreuter M, Ehlers-Tenenbaum S, Schaaf M, et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis Official J Wasog World Assoc Sarcoidosis Other Granulomatous Disord 2014;31(4):266-274. [PubMed] [Google Scholar]
- 20. Hida T, Hata A, Lu J, et al. Interstitial lung abnormalities in patients with stage I non-small cell lung cancer are associated with shorter overall survival: the Boston lung cancer study. Cancer Imaging 2021;21(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato E, Takayanagi N, Takaku Y, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. Erj Open Res 2018;4(1):00111-02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Zhu M, Geng J, et al. Incidence and radiologic-pathological features of lung cancer in idiopathic pulmonary fibrosis. Clin Respir J 2018;12(4):1700-1705. [DOI] [PubMed] [Google Scholar]
- 23. Lee KJ, Chung MP, Kim YW, et al. Prevalence, risk factors and survival of lung cancer in the idiopathic pulmonary fibrosis. Thorac Cancer 2012;3(2):150-155. [DOI] [PubMed] [Google Scholar]
- 24. Lee SH, Sung C, Lee HS, et al. Is 18F-FDG PET/CT useful for the differential diagnosis of solitary pulmonary nodules in patients with idiopathic pulmonary fibrosis? Ann Nucl Med. 2018;32(7):492-498. [DOI] [PubMed] [Google Scholar]
- 25. Jeon TY, Lee KS, Yi CA, et al. Incremental value of PET/CT over CT for mediastinal nodal staging of non–small cell lung cancer: comparison between patients with and without idiopathic pulmonary fibrosis. Am J Roentgenol 2010;195(2):370-376. [DOI] [PubMed] [Google Scholar]
- 26. Han Y, Kim HJ, Kong KA, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0191590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398(10299):535-554. [DOI] [PubMed] [Google Scholar]
- 28. Reck M, Rabe KF.. Precision diagnosis and treatment for advanced non-small-cell lung cancer. New Engl J Medicine 2017;37(9)7:849-861. [DOI] [PubMed] [Google Scholar]
- 29. Ikeda S, Kato T, Kenmotsu H, et al. Current treatment strategies for non-small-cell lung cancer with comorbid interstitial pneumonia. Cancers 2021;13(16):3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Minegishi Y, Takenaka K, Mizutani H, et al. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Internal Med 2009;48(9):665-672. [DOI] [PubMed] [Google Scholar]
- 31. Kenmotsu H, Yoh K, Mori K, et al. Phase II study of nab-paclitaxel + carboplatin for patients with non-small-cell lung cancer and interstitial lung disease. Cancer Sci. 2019;110(12):3738-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Asahina H, Oizumi S, Takamura K, et al. A prospective phase II study of carboplatin and nab-paclitaxel in patients with advanced non-small cell lung cancer and concomitant interstitial lung disease (HOT1302). Lung Cancer 2019;138:65-71. [DOI] [PubMed] [Google Scholar]
- 33. Minegishi Y, Sudoh J, Kuribayasi H, et al. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer 2011;71(1):70-74. [DOI] [PubMed] [Google Scholar]
- 34. Fukuizumi A, Minegishi Y, Omori M, et al. Weekly paclitaxel in combination with carboplatin for advanced non-small-cell lung cancer complicated by idiopathic interstitial pneumonias: a single-arm phase II study. Int J Clin Oncol. 2019;24(12):1543-1548. [DOI] [PubMed] [Google Scholar]
- 35. Sekine A, Satoh H, Baba T, et al. Safety and efficacy of S-1 in combination with carboplatin in non-small cell lung cancer patients with interstitial lung disease: a pilot study. Cancer Chemoth Pharm 2016;77(6):1245-1252. [DOI] [PubMed] [Google Scholar]
- 36. Hanibuchi M, Kakiuchi S, Atagi S, et al. A multicenter, open-label, phase II trial of S-1 plus carboplatin in advanced non-small cell lung cancer patients with interstitial lung disease. Lung Cancer 2018;125:93-99. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Miao L, Hu Y, et al. The efficacy and safety of first-line chemotherapy in patients with non-small cell lung cancer and interstitial lung disease: a systematic review and meta-analysis. Frontiers Oncol 2020;10:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kato M, Shukuya T, Takahashi F, et al. Pemetrexed for advanced non-small cell lung cancer patients with interstitial lung disease. Bmc Cancer 2014;14(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamada S, Ichiyasu H, Ikeda T, et al. Protective effect of bevacizumab on chemotherapy-related acute exacerbation of interstitial lung disease in patients with advanced non-squamous non-small cell lung cancer. BMC Pulm Med. 2019;19(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamamoto Y, Yano Y, Kuge Tet al. Safety and effectiveness of pirfenidone combined with carboplatin-based chemotherapy in patients with idiopathic pulmonary fibrosis and non-small cell lung cancer: a retrospective cohort study. Thorac Cancer 2020;11(11):3317-3–325.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Otsubo K, Kishimoto J, Kenmotsu H, et al. Treatment rationale and design for J-SONIC: a randomized study of carboplatin plus Nab-paclitaxel with or without nintedanib for advanced non-small-cell lung cancer with idiopathic pulmonary fibrosis. Clin Lung Cancer. 2017;19(1):e5-e9. [DOI] [PubMed] [Google Scholar]
- 42. Kenmotsu H, Naito T, Kimura M, et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 2011;6(7):1242-1246. [DOI] [PubMed] [Google Scholar]
- 43. Ding PN, Lord SJ, Gebski V, et al. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: a meta-analysis of clinical trials of gefitinib, erlotinib and afatinib in advanced EGFR-mutated non-small-cell lung cancer. J Thorac Oncol Official Publ Int Assoc Study Lung Cancer 2016;12(4):633-643. [DOI] [PubMed] [Google Scholar]
- 44. Suh CH, Kim KW, Pyo J, et al. The incidence of ALK inhibitor-related pneumonitis in advanced non-small-cell lung cancer patients: a systematic review and meta-analysis. Lung Cancer 2019;132:79-86. [DOI] [PubMed] [Google Scholar]
- 45. Hotta K, Kiura K, Takigawa N, et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer: The Okayama Lung Cancer Study Group Experience. J Thorac Oncol 2010;5(2):179-184. [DOI] [PubMed] [Google Scholar]
- 46. Johkoh T, Sakai F, Kusumoto M, et al. Association between baseline pulmonary status and interstitial lung disease in patients with non-small-cell lung cancer treated with erlotinib--a cohort study. Clin Lung Cancer. 2014;15(6):448-454. [DOI] [PubMed] [Google Scholar]
- 47. Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in japanese patients with lung cancer. Am J Resp Crit Care 2008;177(12):1348-1357. [DOI] [PubMed] [Google Scholar]
- 48. Shen X, Zhao B.. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. Bmj 2018;362:k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sears CR, Peikert T, Possick JD, et al. Knowledge gaps and research priorities in immune checkpoint inhibitor–related pneumonitis. an Official American Thoracic Society Research Statement. Am J Resp Crit Care 2019;200(6):e31-e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. New Engl J Medicine 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. New Engl J Medicine 2015;373(2):123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horn L, Spigel DR, Vokes EEet al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35(35):3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reck M, Rodríguez-Abreu D, Robinson AGet al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. New Engl J Medicine 2016;375(19):1823-1–833.. [DOI] [PubMed] [Google Scholar]
- 55. Tasaka Y, Honda T, Nishiyama N, et al. Non-inferior clinical outcomes of immune checkpoint inhibitors in non-small cell lung cancer patients with interstitial lung disease. Lung Cancer 2021;155:120-126. [DOI] [PubMed] [Google Scholar]
- 56. Nakanishi Y, Masuda T, Yamaguchi K, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Investigation 2019;57(5):451-459. [DOI] [PubMed] [Google Scholar]
- 57. Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018;9(7):847-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fujimoto D, Yomota M, Sekine A, et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: a multicenter, open-label single-arm phase II trial. Lung Cancer 2019;134:274-278. [DOI] [PubMed] [Google Scholar]
- 59. Nishiyama N, Honda T, Sema M, et al. The utility of ground-glass attenuation score for anticancer treatment-related acute exacerbation of interstitial lung disease among lung cancer patients with interstitial lung disease. Int J Clin Oncol. 2020;25(2):282-291. [DOI] [PubMed] [Google Scholar]
- 60. Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother. 2020;69(1):15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer 2018;125:212-217. [DOI] [PubMed] [Google Scholar]
- 62. Dobre IA, Frank AJ, D’silva KM, et al. Outcomes of patients with interstitial lung disease receiving programmed cell death 1 inhibitors: a retrospective case series. Clin Lung Cancer. 2021;22(5):e738-e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shimoji K, Masuda T, Yamaguchi K, et al. Association of preexisting interstitial lung abnormalities with immune checkpoint inhibitor–induced interstitial lung disease among patients with nonlung cancers. JAMA Netw Open 2020;3(11):e2022906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamakawa H, Oba T, Ohta H, et al. Nintedanib allows retreatment with atezolizumab of combined non-small cell lung cancer/idiopathic pulmonary fibrosis after atezolizumab-induced pneumonitis: a case report. BMC Pulm Med. 2019;19(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non-small cell lung cancer, Version 2.2021. J National Compr Cancer Netw Jnccn 2021;19(3):254-266. [DOI] [PubMed] [Google Scholar]
- 66. Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7(1):44-54. [DOI] [PubMed] [Google Scholar]
- 67. Verma V, Simone CB, Allen PK, et al. Multi-institutional experience of stereotactic ablative radiation therapy for stage i small cell lung cancer. Int J Radiat Oncol Biology Phys 2017;97(2):362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biology Phys 2014;88(5):1092-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol Official Publ Int Assoc Study Lung Cancer 2009;4(7):838-844. [DOI] [PubMed] [Google Scholar]
- 70. Bahig H, Filion E, Vu T, et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol 2016;6(5):367-374. [DOI] [PubMed] [Google Scholar]
- 71. Glick D, Lyen S, Kandel S, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival in patients treated with lung stereotactic body radiation therapy (SBRT). Clin Lung Cancer. 2018;19(2):e219-e226. [DOI] [PubMed] [Google Scholar]
- 72. Yamaguchi S, Ohguri T, Ide S, et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis. Lung Cancer 2013;82(2):260-265. [DOI] [PubMed] [Google Scholar]
- 73. Tsurugai Y, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for lung cancer patients with idiopathic interstitial pneumonias. Radiother Oncol. 2017;125(2):310-316. [DOI] [PubMed] [Google Scholar]
- 74. Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol 2015;10(1):116-125. [DOI] [PubMed] [Google Scholar]
- 75. Yoshitake T, Shioyama Y, Asai K, et al. Impact of interstitial changes on radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Anticancer Res. 2015;35(9):4909-4913. [PubMed] [Google Scholar]
- 76. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. New Engl J Med 2018;379(24):2342-2350. [DOI] [PubMed] [Google Scholar]
- 77. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. New Engl J Medicine 2017;377(20):1919-1929. [DOI] [PubMed] [Google Scholar]
- 78. Li F, Liu H, Wu H, et al. Risk factors for radiation pneumonitis in lung cancer patients with subclinical interstitial lung disease after thoracic radiation therapy. Radiat Oncol Lond Engl 2021;16(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ozawa Y, Abe T, Omae M, et al. Impact of preexisting interstitial lung disease on acute, extensive radiation pneumonitis: retrospective analysis of patients with lung cancer. PLoS One. 2015;10(10):e0140437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vyfhuis MAL, Onyeuku N, Diwanji T, et al. Advances in proton therapy in lung cancer. Ther Adv Respir Dis 2018;12:1753466618783878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim H, Pyo H, Noh JM, et al. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: comparison between X-ray and proton therapy. Radiat Oncol Lond Engl 2019;14(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liao Z, Lee JJ, Komaki Ret al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non–small-cell lung cancer. J Clin Oncol 2018; 36(18):1813-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sekihara K, Aokage K, Oki T, et al. Long-term survival after complete resection of non-small-cell lung cancer in patients with interstitial lung disease. Interact Cardiov Th 2017;26(4):638-643. [DOI] [PubMed] [Google Scholar]
- 84. Watanabe A, Kawaharada N, Higami T.. Postoperative acute exacerbation of IPF after lung resection for primary lung cancer. Pulm Medicine 2011;2011:960316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ghatol A, Ruhl AP, Danoff SK.. Exacerbations in idiopathic pulmonary fibrosis triggered by pulmonary and nonpulmonary surgery: a case series and comprehensive review of the literature. Lung 2012;190(4):373-380. [DOI] [PubMed] [Google Scholar]
- 86. Sato S, Shimizu Y, Goto T, et al. Survival after repeated surgery for lung cancer with idiopathic pulmonary fibrosis: a retrospective study. BMC Pulm Med. 2018;18(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2013;147(5):1604-1611.e3. [DOI] [PubMed] [Google Scholar]
- 88. Patel NM, Kulkarni T, Dilling D, et al. Preoperative evaluation of patients with interstitial lung disease. Chest 2019;156(5):826-833. [DOI] [PubMed] [Google Scholar]
- 89. Hutchinson JP, Fogarty AW, Mckeever TM, et al. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Resp Crit Care 2016;193(10):1161-1167. [DOI] [PubMed] [Google Scholar]
- 90. Park J, Kim H, Kim K, et al. Prediction of acute pulmonary complications after resection of lung cancer in patients with preexisting interstitial lung disease. Thorac Cardiovasc Surg. 2011;59(3):148-152. [DOI] [PubMed] [Google Scholar]
- 91. Choi SM, Lee J, Park YS, et al. Postoperative pulmonary complications after surgery in patients with interstitial lung disease. Respir Int Rev Thorac Dis 2014;87(4):287-293. [DOI] [PubMed] [Google Scholar]
- 92. Kumar P, Goldstraw P, Yamada K, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg. 2003;125(6):1321-1327. [DOI] [PubMed] [Google Scholar]
- 93. Shintani Y, Ohta M, Iwasaki T, et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg. 2010;58(4):182-185. [DOI] [PubMed] [Google Scholar]
- 94. Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J. 2001;17(2):175-179. [DOI] [PubMed] [Google Scholar]
- 95. Park JH, Kim DK, Kim DS, et al. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardio-thorac 2007;31(6):1115-1119. [DOI] [PubMed] [Google Scholar]
- 96. Kushibe K, Kawaguchi T, Takahama M, et al. Operative indications for lung cancer with idiopathic pulmonary fibrosis. Thorac Cardiovasc Surg. 2007;55(8):505-508. [DOI] [PubMed] [Google Scholar]
- 97. Hosoki K, Mikami Y, Urushiyama H, et al. Predictors of postoperative acute exacerbation of interstitial lung disease: a case-control study. Bmj Open Respir Res 2020;7(1):e000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Durheim MT, Kim S, Gulack BC, et al. Mortality and respiratory failure after thoracoscopic lung biopsy for interstitial lung disease. Ann Thorac Surg. 2017;104(2):465-470. [DOI] [PubMed] [Google Scholar]
- 99. Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83(3):1140-1144. [DOI] [PubMed] [Google Scholar]
- 100. Rotolo N, Imperatori A, Dominioni L, et al. Efficacy and safety of surgical lung biopsy for interstitial disease. Experience of 161 consecutive patients from a single institution in Italy. Sarcoidosis Vasc Diffuse Lung Dis Official J Wasog World Assoc Sarcoidosis Other Granulomatous Disord 2015;32(3):251-258. [PubMed] [Google Scholar]
- 101. Furuya K, Sakamoto S, Takai Y, et al. Acute exacerbation of idiopathic interstitial pneumonia after nonpulmonary surgery under general anesthesia: a retrospective study. Sarcoidosis Vasc Diffuse Lung Dis Official J Wasog 2017;34(2):156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014;121(2):219-231. [DOI] [PubMed] [Google Scholar]
- 103. Kondoh Y, Taniguchi H, Kitaichi M, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Resp Med 2006;100(10):1753-1759. [DOI] [PubMed] [Google Scholar]
- 104. Mizuno Y, Iwata H, Shirahashi K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardio-thorac 2012;41(6):e161-e165. [DOI] [PubMed] [Google Scholar]
- 105. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). European J Cardio-thoracic Surg Official J European Assoc Cardio-thoracic Surg 2019;55(1):91-115. [DOI] [PubMed] [Google Scholar]
- 106. Pastre J, Khandhar S, Barnett S, et al. Surgical lung biopsy for interstitial lung disease. Safety and Feasibility at a Tertiary Referral Center. Ann Am Thorac Soc 2021;18(3):460-467. [DOI] [PubMed] [Google Scholar]
- 107. Tsutani Y, Mimura T, Kai Y, et al. Outcomes after lobar versus sublobar resection for clinical stage I non−small cell lung cancer in patients with interstitial lung disease. J Thorac Cardiovasc Surg. 2017;154(3):1089-1096.e1. [DOI] [PubMed] [Google Scholar]
- 108. Murphy MC, Wrobel MM, Fisher DA, et al. Update on image-guided thermal lung ablation: society guidelines, therapeutic alternatives, and postablation imaging findings. Am J Roentgenol 2022;219(3):471-485. [DOI] [PubMed] [Google Scholar]
- 109. Kwan SW, Mortell KE, Talenfeld AD, et al. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non–small cell lung cancer. J Vasc Interv Radiol. 2014;25(1):1-9.e1. [DOI] [PubMed] [Google Scholar]
- 110. Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial: Radiofrequency Ablation of Stage IA NSCLC. Cancer 2015;121(19):3491-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center’s experiences. Am J Roentgenol 2011;197(4):W576-W580. [DOI] [PubMed] [Google Scholar]
- 112. Chen H, Senan S, Nossent EJ, et al. Treatment-related toxicity in patients with early-stage non-small cell lung cancer and coexisting interstitial lung disease: a systematic review. Int J Radiat Oncol Biology Phys 2017;98(3):622-631. [DOI] [PubMed] [Google Scholar]
- 113. Ohtsuka T, Asakura K, Masai K, et al. OA12.03 percutaneous cryoablation for lung cancer patients for whom surgery or radiotherapy is contraindicated due to idiopathic pulmonary fibrosis. J Thorac Oncol 2017;12(1):S290. [Google Scholar]
- 114. Dy GK, Prasad D, Kumar P, et al. A phase 2 randomized, double-blind, placebo-controlled study evaluating nintedanib versus placebo as prophylaxis against radiation pneumonitis in patients with unresectable NSCLC undergoing chemoradiation therapy. J Thorac Oncol 2021;16(3):e19-e20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.